Effect of Graphene Oxide/ZSM-5 Hybrid on Corrosion Resistance of Waterborne Epoxy Coating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
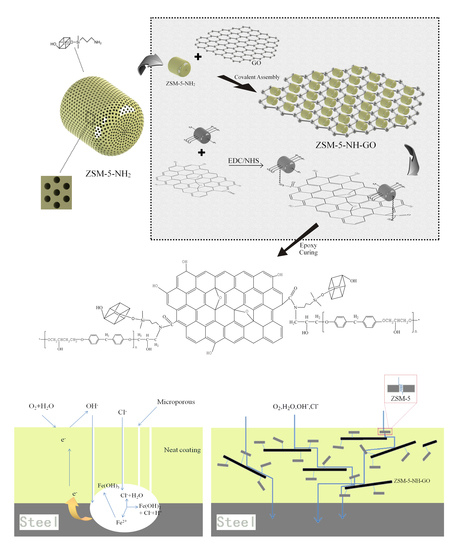
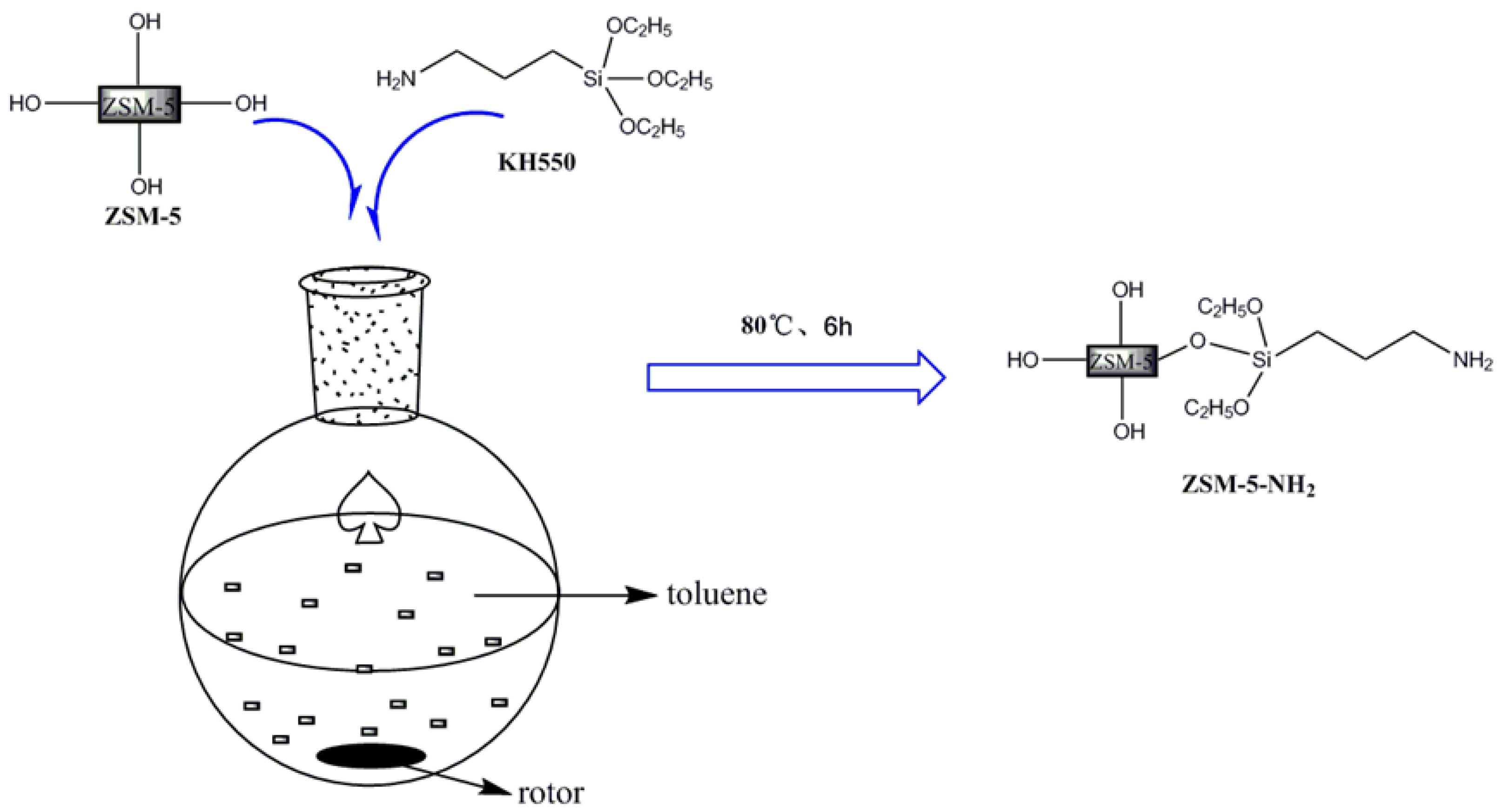
2.2. Preparation of the Amino-Functionalized Zeolite Molecular Sieves (ZSM-5-NH2)
2.3. Integration of the Zeolite Molecular Sieves with Graphene Oxide (ZSM-5-NH-GO)
2.4. Preparation of the ZSM-5-NH-GO Composite Coating
2.5. Characterization
2.5.1. Characterization of ZSM-5-NH-GO
2.5.2. Corrosion Performance Tests of the Anti-Corrosion Coatings
3. Results and Discussions
3.1. Characterization of GO, ZSM-5, ZSM-5-NH2 and ZSM-5-NH-GO
3.1.1. FT-IR Spectroscopy
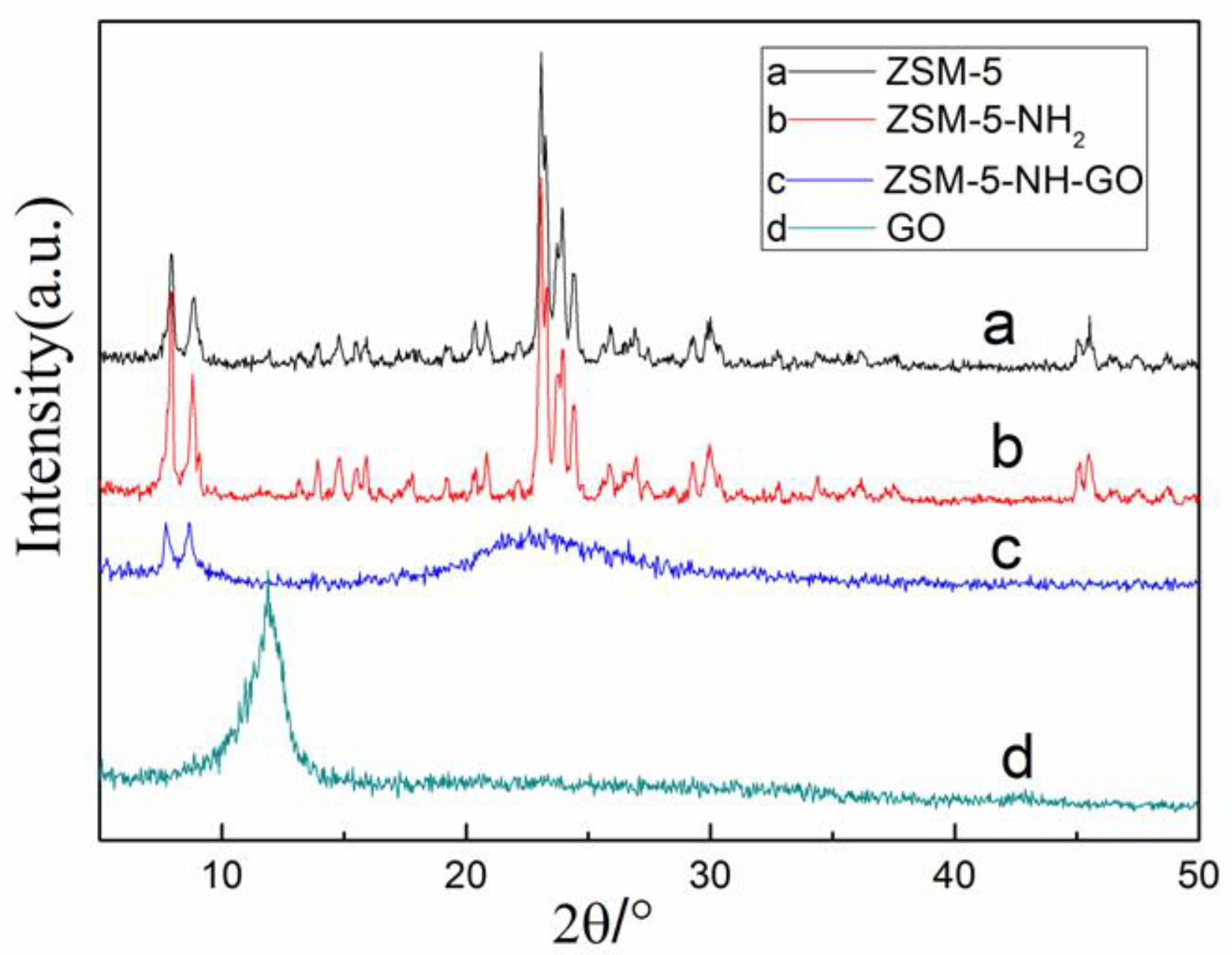
3.1.2. X-ray Diffraction
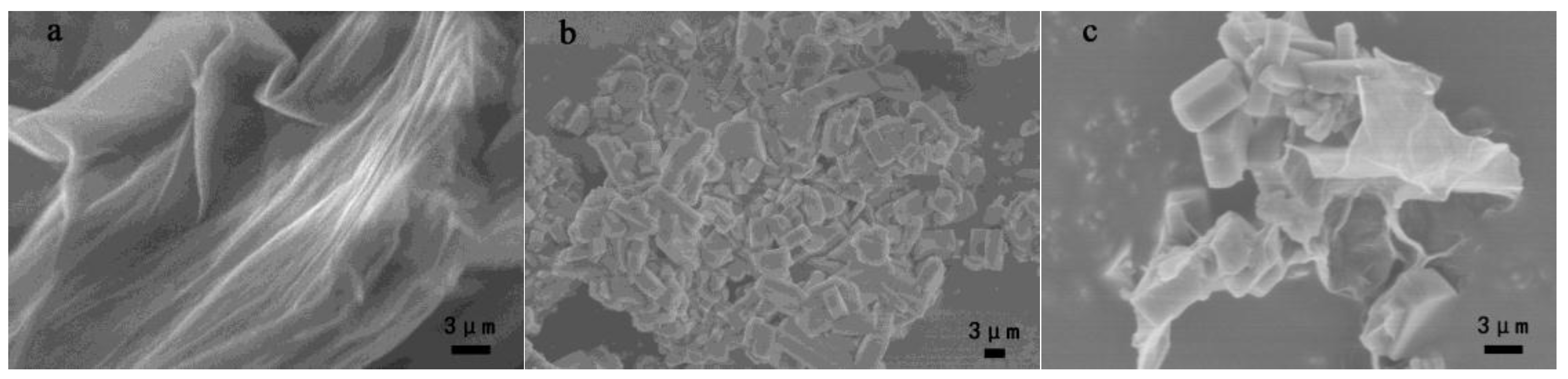
3.1.3. SEM Observations
3.2. Characterization of the Composite Coatings
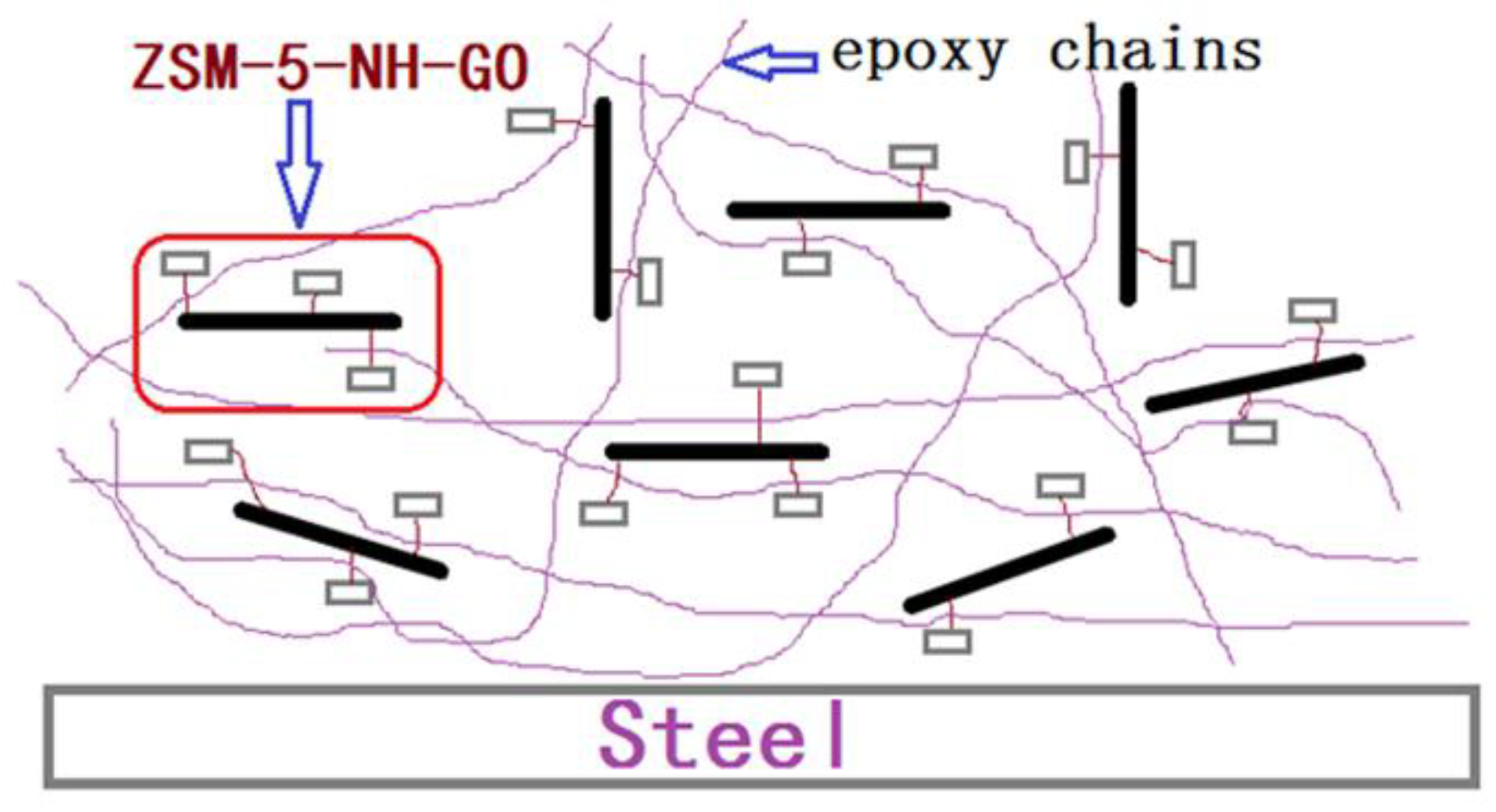
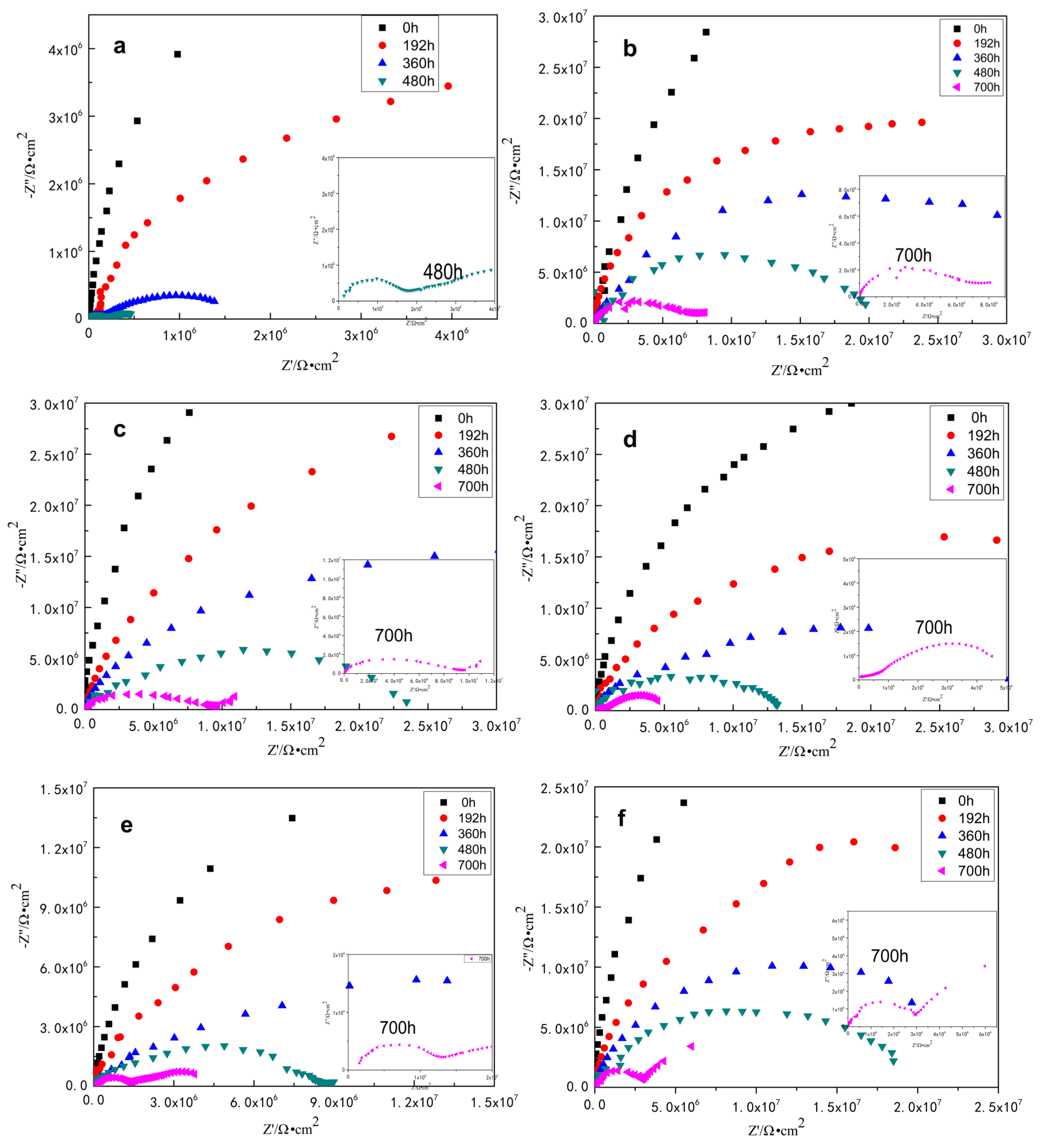
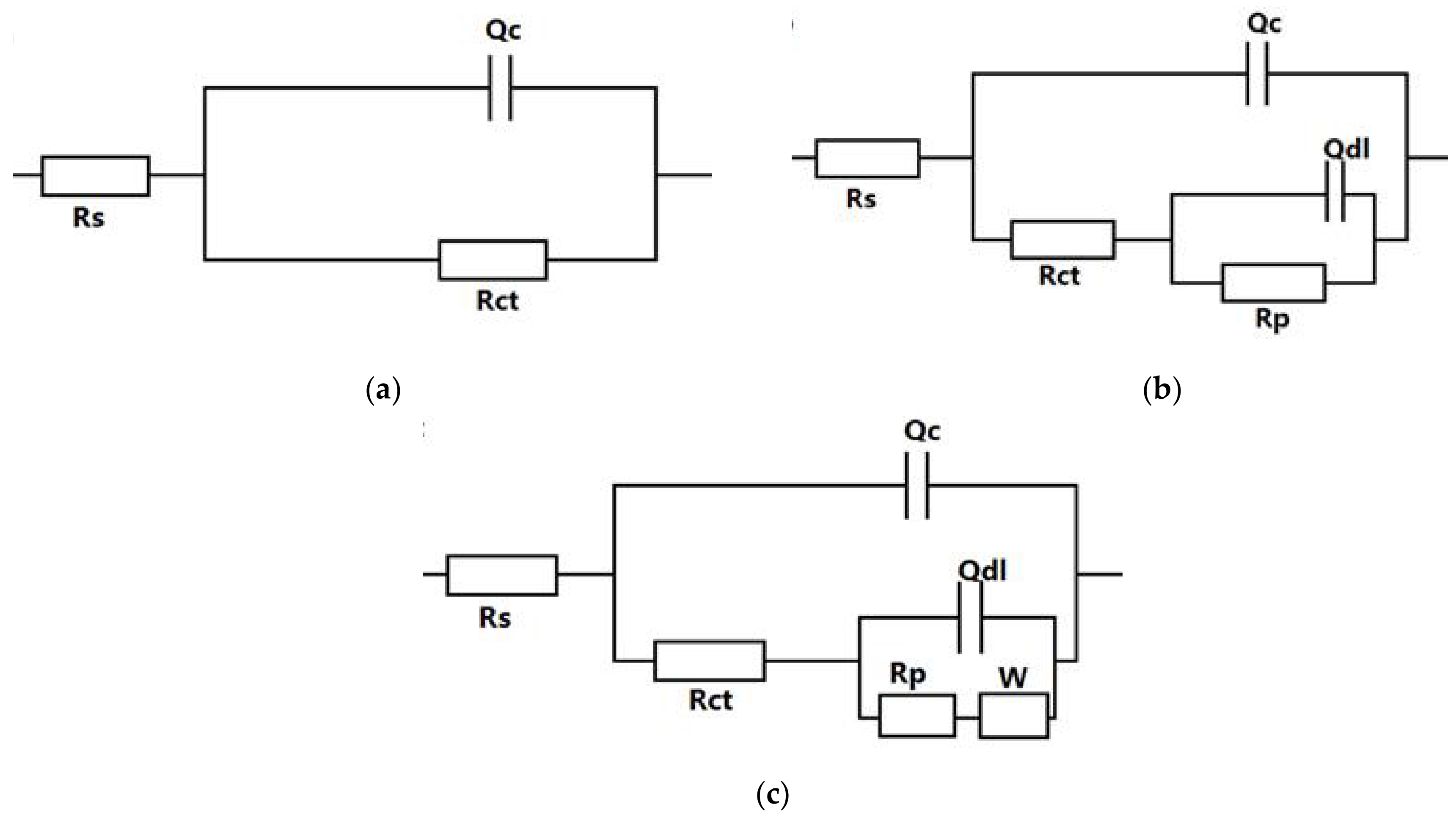
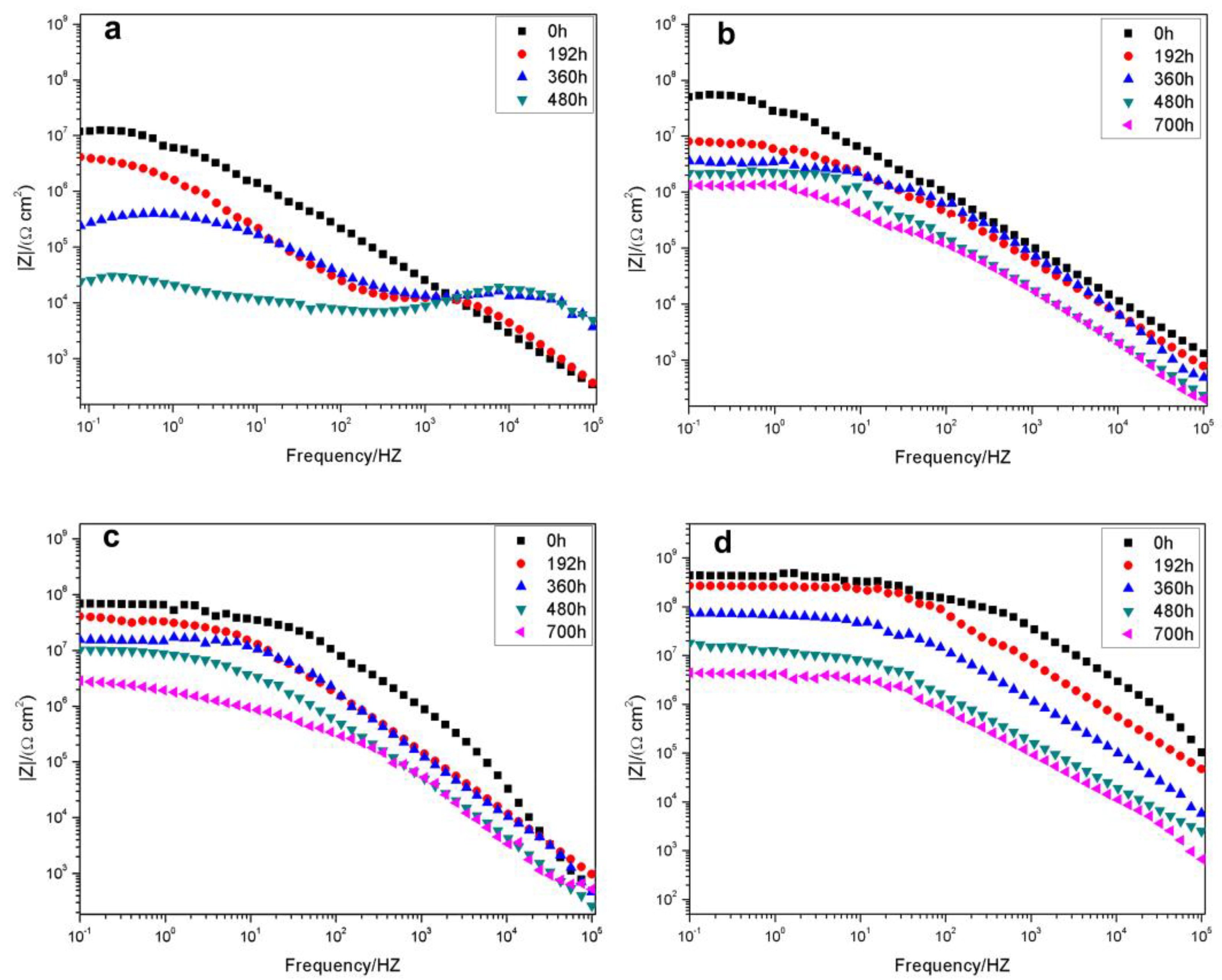
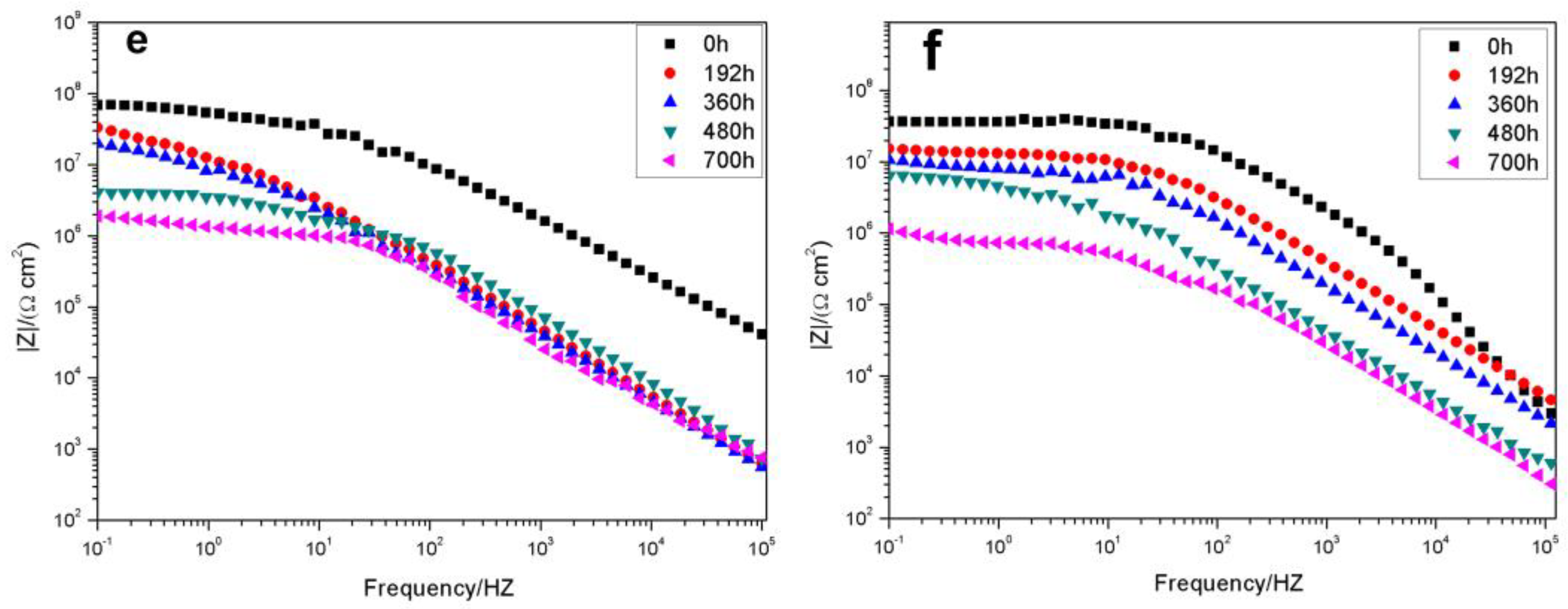
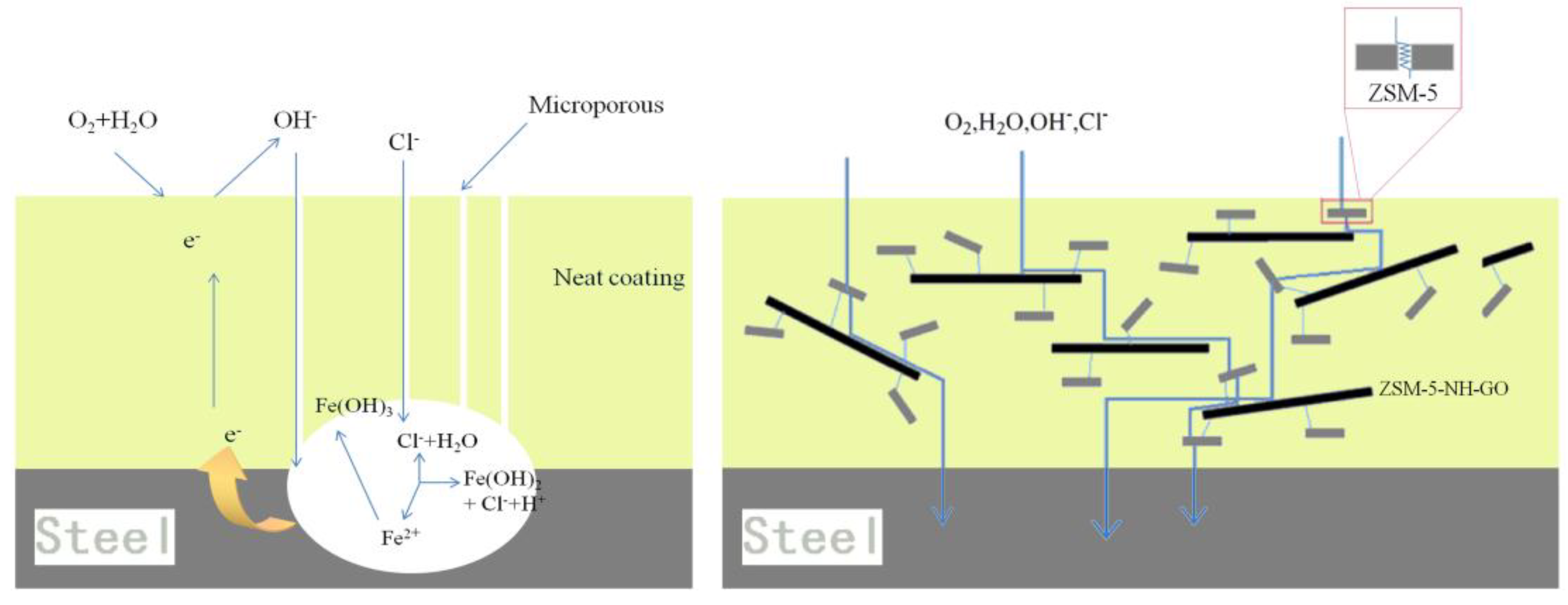
3.2.1. Electrochemical Impedance Spectroscopy of the Coatings
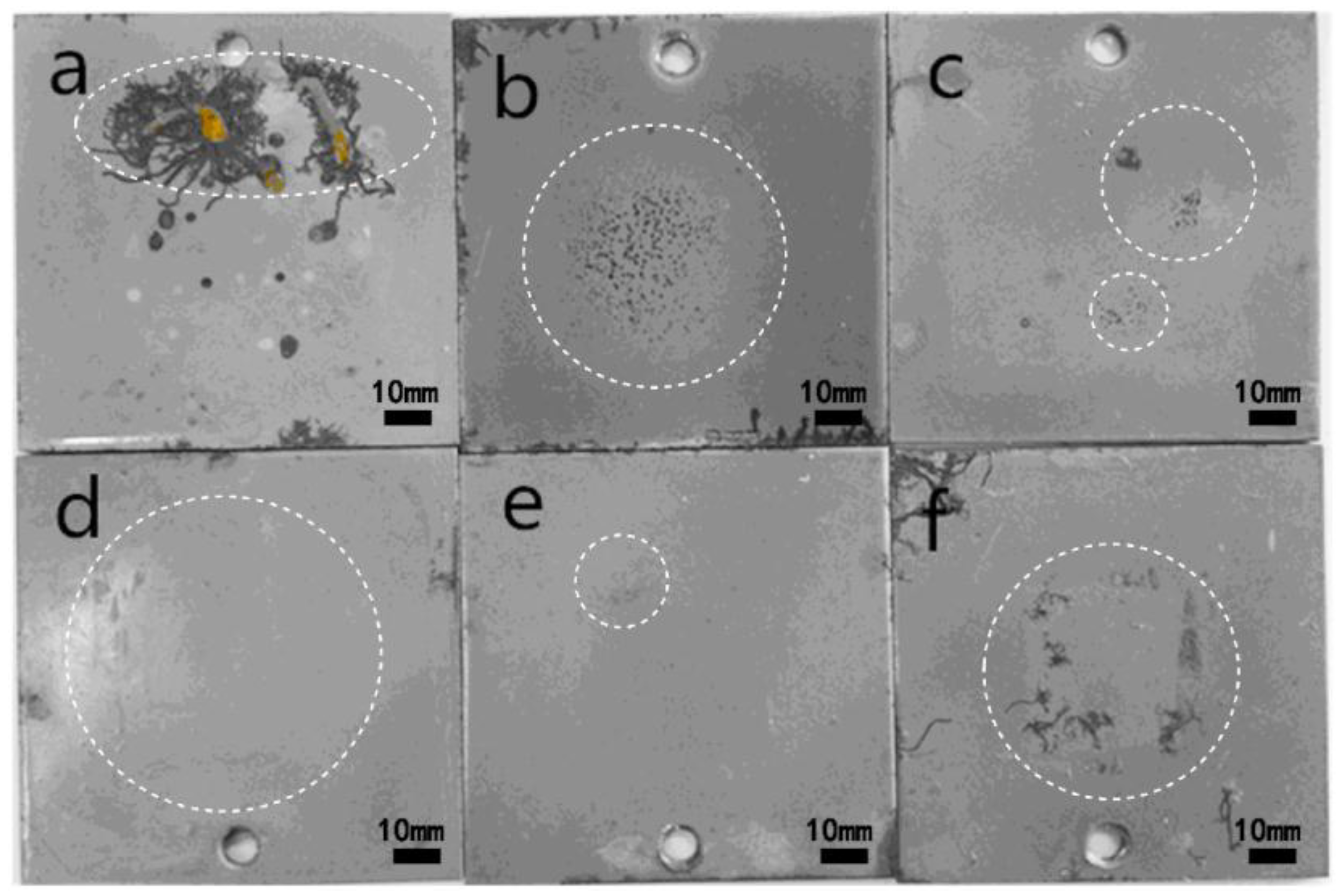
3.2.2. Salt Spray Test
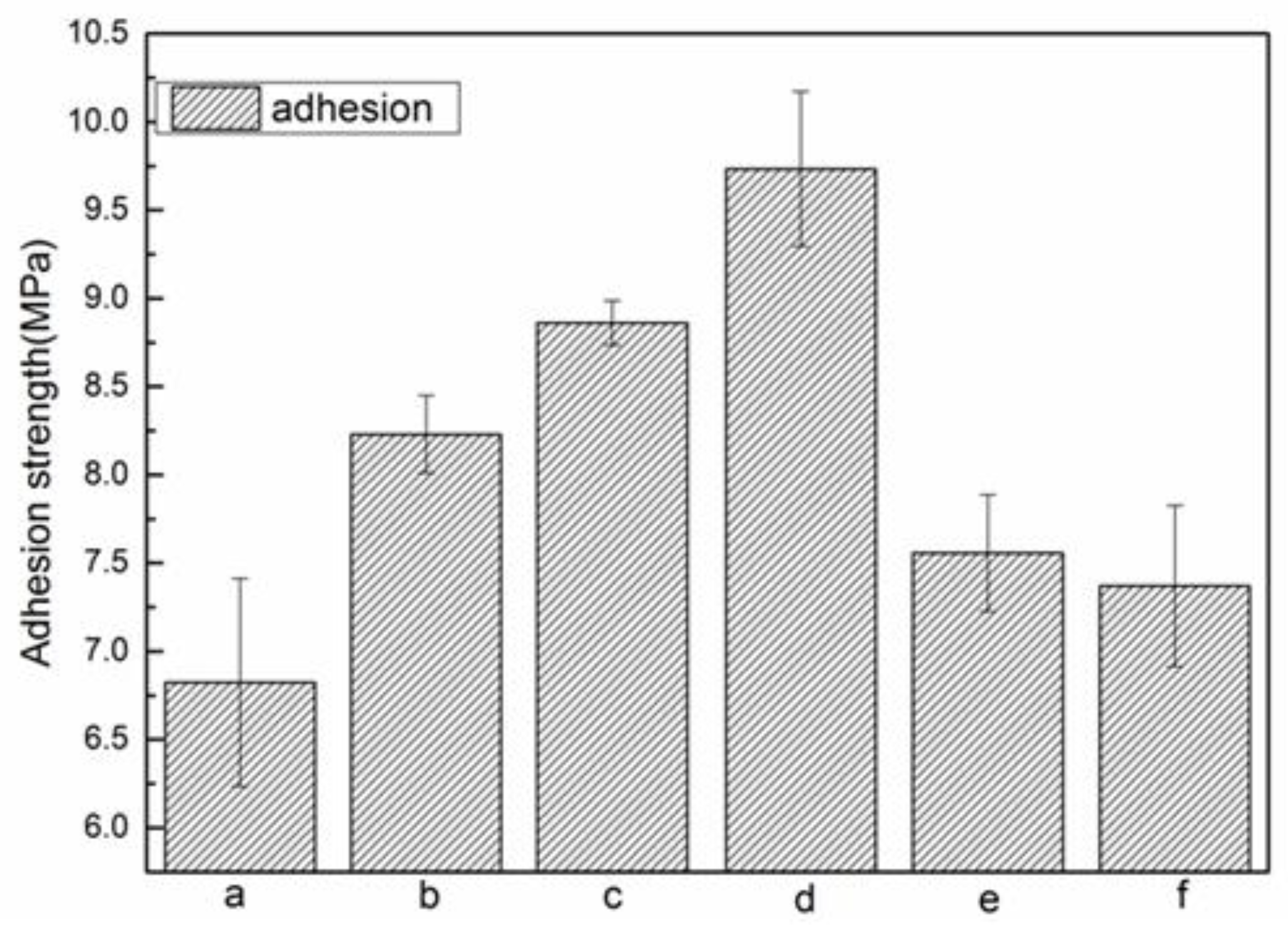
3.2.3. Adhesion Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ambrosi, A.; Pumera, M. The structural stability of graphene anticorrosion coating materials is compromised at low potentials. Chem. Eur. J. 2015, 21, 7896–7901. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Zhang, S.; Dong, J.; Ke, W. Atmospheric corrosion resistance of MnCuP weathering steel in simulated environments. Corros. Sci. 2011, 53, 4187–4192. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, H.; Ma, Y.; Yi, T.; Mao, X.; Lin, A.; Gan, F. Corrosion protection of stainless steel by separate polypyrrole electrode in acid solutions. Mater. Corros. 2011, 62, 68–73. [Google Scholar] [CrossRef]
- Zhao, Y.; Ren, H.; Dai, H.; Jin, W. Composition and expansion coefficient of rust based on X-ray diffraction and thermal analysis. Corros. Sci. 2011, 53, 1646–1658. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Cao, Y.; Yadama, V.; Xian, M.; Zhang, J. Study of dextrin-derived curing agent for waterborne epoxy adhesive. Carbohydr. Polym. 2011, 83, 1180–1184. [Google Scholar] [CrossRef]
- Erdmenger, T.; Guerrero-Sanchez, C.; Vitz, J.; Hoogenboom, R.; Schubert, U.S. Recent developments in the utilization of green solvents in polymer chemistry. Chem. Soc. Rev. 2010, 39, 3317–3333. [Google Scholar] [CrossRef] [PubMed]
- Atta, A.M.; El-Saeed, A.M.; El-Mahdy, G.M.; Al-Lohedan, H.A. Application of magnetite nano-hybrid epoxy as protective marine coatings for steel. RSC Adv. 2015, 5, 101923–101931. [Google Scholar] [CrossRef]
- Prasai, D.; Tuberquia, J.C.; Harl, R.R.; Jennings, G.K.; Bolotin, K.I. Graphene: Corrosion-inhibiting coating. ACS Nano. 2012, 6, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Okafor, P.A.; Singh-Beemat, J.; Iroh, J.O. Thermomechanical and corrosion inhibition properties of graphene/epoxy ester–siloxane–urea hybrid polymer nanocomposites. Prog. Org. Coat. 2015, 88, 237–244. [Google Scholar] [CrossRef]
- Sahu, S.C.; Samantara, A.K.; Seth, M.; Parwaiz, S.; Singh, B.P.; Rath, P.C.; Jena, B.K. A facile electrochemical approach for development of highly corrosion protective coatings using graphene nanosheets. Electrochem. Commun. 2013, 32, 22–26. [Google Scholar] [CrossRef]
- Zong, P.; Fu, J.; Chen, L.; Yin, J.; Dong, X.; Yuan, S.; Deng, W. Effect of aminopropylisobutyl polyhedral oligomeric silsesquioxane functionalized graphene on the thermal conductivity and electrical insulation properties of epoxy composites. RSC Adv. 2016, 6, 10498–10506. [Google Scholar] [CrossRef]
- Cai, K.; Zuo, S.; Luo, S.; Yao, C.; Liu, W.; Ma, J.; Li, Z. Preparation of polyaniline/graphene composites with excellent anti-corrosion properties and their application in waterborne polyurethane anticorrosive coatings. RSC Adv. 2016, 6, 95965–95972. [Google Scholar] [CrossRef]
- Aneja, K.S.; Bohm, S.; Khanna, A.S.; Bohm, H.M. Graphene based anticorrosive coatings for Cr (VI) replacement. Nanoscale 2015, 7, 17879–17888. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cui, J.; Yang, J.; Ma, Y.; Qiu, H.; Yang, J. Silanized graphene oxide reinforced organofunctional silane composite coatings for corrosion protection. Prog. Org. Coat. 2016, 99, 443–451. [Google Scholar] [CrossRef]
- Wang, N.; Cheng, K.; Wu, H.; Wang, C.; Wang, Q.; Wang, F. Effect of nano-sized mesoporous silica MCM-41 and MMT on corrosion properties of epoxy coating. Prog. Org. Coat. 2012, 75, 386–391. [Google Scholar] [CrossRef]
- Balaskas, A.C.; Kartsonakis, I.A.; Tziveleka, L.A.; Kordas, G.C. Improvement of anti-corrosive properties of epoxy-coated AA 2024-T3 with TiO2 nanocontainers loaded with 8-hydroxyquinoline. Prog. Org. Coat. 2012, 74, 418–426. [Google Scholar] [CrossRef]
- Ruhi, G.; Bhandari, H.; Dhawan, S.K. Designing of corrosion resistant epoxy coatings embedded with polypyrrole/SiO2 composite. Prog. Org. Coat. 2014, 77, 1484–1498. [Google Scholar] [CrossRef]
- Rashvand, M.; Ranjbar, Z. Effect of nano-ZnO particles on the corrosion resistance of polyurethane-based waterborne coatings immersed in sodium chloride solution via EIS technique. Prog. Org. Coat. 2013, 76, 1413–1417. [Google Scholar] [CrossRef]
- Wang, N.; Fu, W.; Zhang, J.; Li, X.; Fang, Q. Corrosion performance of waterborne epoxy coatings containing polyethylenimine treated mesoporous-TiO2 nanoparticles on mild steel. Prog. Org. Coat. 2015, 89, 114–122. [Google Scholar] [CrossRef]
- Wang, N.; Wu, Y.H.; Cheng, K.Q.; Zhang, J. Investigation on anticorrosion performance of polyaniline-mesoporous MCM-41 composites in new water-based epoxy coating. Mater. Corros. 2014, 65, 968–976. [Google Scholar] [CrossRef]
- Megalai, S.M.; Manjula, Y.P.; Manonmani, K.N.; Kavitha, N.; Baby, N. Metronidazole: A corrosion Inhibitor for mild dteel in squeous rnvironment. Port. Electrochim. Acta 2012, 30, 395–403. [Google Scholar] [CrossRef]
- ASTM D4541-02 Standard Test Method for Pull-Off Strength of Coatings Using Portable Adhesion Testers; ASTM International: West Conshohocken, PA, USA, 2002.
- Sadeghimeresht, E.; Markocsan, N.; Nylen, P. A comparative study of corrosion resistance for HVAF-sprayed Fe- and Co-based coatings. Coatings 2016, 6, 16. [Google Scholar] [CrossRef]
- ASTM B117 Standard Practice for Operating Salt Spray (Fog) Apparatus; ASTM International: West Conshohocken, PA, USA, 2003.
- Tao, Y.; Kanoh, H.; Kaneko, K. ZSM-5 monolith of uniform mesoporous channels. J. Am. Chem. Soc. 2003, 125, 6044–6045. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; González, A.J.; Heeralal, V.B.; Wang, D.Y. Covalent assembly of MCM-41 nanospheres on graphene oxide for improving fire retardancy and mechanical property of epoxy resin. Compos. Part B Eng. 2017, 138, 101–112. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Attar, M.M.; Farzam, M. Corrosion performance of a hot-dip galvanized steel treated by different kinds of conversion coatings. Surf. Coat. Technol. 2010, 205, 874–884. [Google Scholar] [CrossRef]
- Mekeridis, E.D.; Kartsonakis, I.A.; Kordas, G.C. Multilayer organic-inorganic coating incorporating TiO2 nanocontainers loaded with inhibitors for corrosion protection of AA2024-T3. Prog. Org. Coat. 2012, 73, 142–148. [Google Scholar] [CrossRef]
- Khalajabadi, S.Z.; Abu, A.H.; Ahmad, N.; Kadir, M.R.A.; Ismail, A.F.; Nasiri, R.; Haider, W.; Redzuan, N.B.H. Biodegradable Mg/HA/TiO2 nanocomposites coated with MgO and Si/MgO for orthopedic applications: A study on the corrosion, surface characterization, and biocompatability. Coatings 2017, 7, 154. [Google Scholar] [CrossRef]
- Szociński, M.; Darowicki, K.; Schaefer, K. Identification and localization of organic coating degradation onset by impedance imaging. Polym. Degrad. Stab. 2010, 95, 960–964. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Ghasemi, E.; Mahdavian, M.; Changizi, E.; Moghadam, M.M. Covalently-grafted graphene oxide nanosheets to improve barrier and corrosion protection properties of polyurethane coatings. Carbon 2015, 93, 555–573. [Google Scholar] [CrossRef]
- Suleiman, R.; Dafalla, H.; El Ali, B. Novel hybrid epoxy silicone materials as efficient anticorrosive coatings for mild steel. RSC Adv. 2015, 5, 39155–39167. [Google Scholar] [CrossRef]



| Sample | Waterborne Expoxy/g | Pigment/g | Curing Agent/g | Water/g |
|---|---|---|---|---|
| Neat epoxy | 12 | – | 6 | 6 |
| 0.2% ZSM-5-NH-GO | 12 | 0.024 | 6 | 6 |
| 0.5% ZSM-5-NH-GO | 12 | 0.06 | 6 | 6 |
| 0.7% ZSM-5-NH-GO | 12 | 0.084 | 6 | 6 |
| 1% ZSM-5-NH-GO | 12 | 0.12 | 6 | 6 |
| 2% ZSM-5-NH-GO | 12 | 0.24 | 6 | 6 |
| Sample | Rs (Ω·cm2) | Rct (Ω·cm2) | Qc (F·cm−2) | Rp (Ω·cm2) | W (Ω·cm2) | |
|---|---|---|---|---|---|---|
| EP | 0 h | 1.582 × 104 | 4.284 × 109 | 7.414 × 10-12 | ||
| 192 h | 5.868 × 103 | 6.157 × 107 | 4.781 × 10-9 | |||
| 360 h | 2.655 × 103 | 8.251 × 106 | 9.202 × 10-11 | 1.756 × 107 | ||
| 480 h | 1.531 × 102 | 1.416 × 105 | 9.525 × 10-10 | 8.766 × 104 | 4.244 × 10-6 | |
| 0.2% ZSM-5-NH-GO/EP | 0 h | 2.184 × 103 | 4.541 × 1010 | 1.994 × 10-11 | ||
| 192 h | 1.373 × 103 | 1.511 × 109 | 1.248 × 10-10 | |||
| 360 h | 1.000 × 102 | 6.749 × 108 | 1.984 × 10-11 | 1.218 × 108 | ||
| 480 h | 1.000 × 102 | 2.266 × 108 | 1.074 × 10-10 | 8.207 × 107 | ||
| 700 h | 6.916 × 103 | 5.937 × 107 | 2.202 × 10-12 | 2.885 × 107 | 6.354 × 10-5 | |
| 0.5% ZSM-5-NH-GO/EP | 0 h | 1.713 × 104 | 5.861 × 1010 | 1.483 × 10-12 | ||
| 192 h | 2.602 × 103 | 2.871 × 109 | 1.382 × 10-12 | |||
| 360 h | 6.157 × 102 | 3.391 × 108 | 6.338 × 10-12 | |||
| 480 h | 1.000 × 102 | 1.527 × 108 | 3.722 × 10-10 | 1.070 × 108 | ||
| 700 h | 1.000 × 102 | 5.019 × 107 | 6.835 × 10-11 | 3.035 × 107 | 3.191 × 10-8 | |
| 0.7% ZSM-5-NH-GO/EP | 0 h | 6.875 × 103 | 3.266 × 1012 | 1.283 × 10-10 | ||
| 192 h | 1.000 × 103 | 1.000 × 1010 | 1.769 × 10-12 | |||
| 360 h | 5.303 × 102 | 1.274 × 109 | 7.835 × 10-12 | |||
| 480 h | 8.001 × 102 | 1.105 × 108 | 1.646 × 10-9 | 1.286 × 106 | ||
| 700 h | 1.000 × 102 | 2.990 × 107 | 8.474 × 10-11 | 3.394 × 107 | ||
| 1.0% ZSM-5-NH-GO/EP | 0 h | 1.113 × 104 | 9.525 × 109 | 1.553 × 10-11 | ||
| 192 h | 3.436 × 103 | 8.728 × 108 | 7.573 × 10-11 | |||
| 360 h | 1.561 × 103 | 3.565 × 108 | 1.759 × 10-12 | |||
| 480 h | 3.181 × 102 | 4.686 × 106 | 3.393 × 10-11 | 3.476 × 107 | ||
| 700 h | 1.471 × 102 | 9.593 × 106 | 1.468 × 10-12 | 7.453 × 106 | 9.902 × 10-3 | |
| 2.0% ZSM-5-NH-GO/EP | 0 h | 1.093 × 104 | 5.094 × 108 | 9.782 × 10-12 | ||
| 192 h | 1.061 × 104 | 4.221 × 108 | 1.352 × 10-11 | 3.047 × 109 | ||
| 360 h | 1.996 × 103 | 3.534 × 107 | 1.217 × 10-11 | 2.718 × 108 | ||
| 480 h | 2.099 × 103 | 1.420 × 107 | 1.541 × 10-10 | 2.915 × 107 | ||
| 700 h | 1.985 × 102 | 6.204 × 106 | 1.493 × 10-10 | 2.498 × 107 | 7.811 × 10-8 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Gao, H.; Zhang, J.; Kang, P. Effect of Graphene Oxide/ZSM-5 Hybrid on Corrosion Resistance of Waterborne Epoxy Coating. Coatings 2018, 8, 179. https://doi.org/10.3390/coatings8050179
Wang N, Gao H, Zhang J, Kang P. Effect of Graphene Oxide/ZSM-5 Hybrid on Corrosion Resistance of Waterborne Epoxy Coating. Coatings. 2018; 8(5):179. https://doi.org/10.3390/coatings8050179
Chicago/Turabian StyleWang, Na, Huiying Gao, Jing Zhang, and Ping Kang. 2018. "Effect of Graphene Oxide/ZSM-5 Hybrid on Corrosion Resistance of Waterborne Epoxy Coating" Coatings 8, no. 5: 179. https://doi.org/10.3390/coatings8050179
APA StyleWang, N., Gao, H., Zhang, J., & Kang, P. (2018). Effect of Graphene Oxide/ZSM-5 Hybrid on Corrosion Resistance of Waterborne Epoxy Coating. Coatings, 8(5), 179. https://doi.org/10.3390/coatings8050179