Superhydrophobic Bilayer Coating Based on Annealed Electrospun Ultrathin Poly(ε-caprolactone) Fibers and Electrosprayed Nanostructured Silica Microparticles for Easy Emptying Packaging Applications
Abstract
:1. Introduction
2. Experimental
2.1. Materials
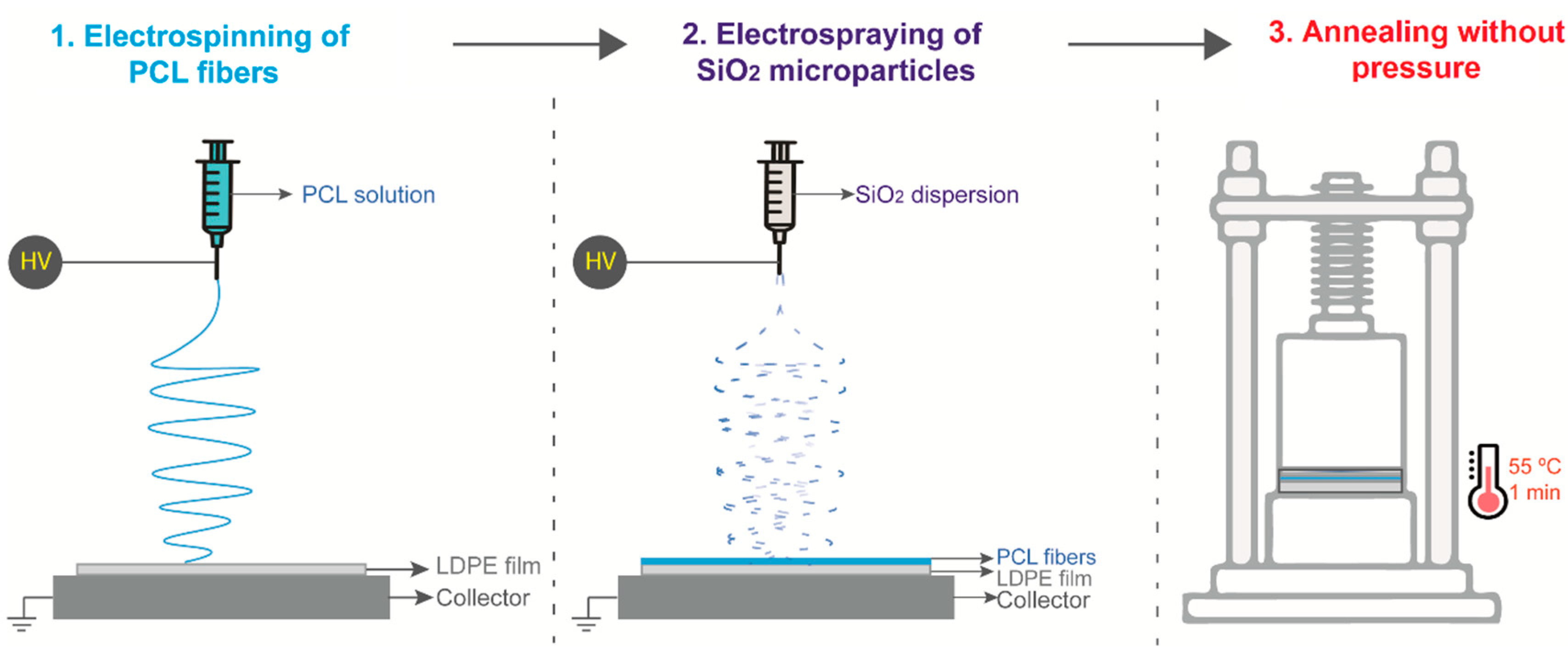
2.2. Fabrication of the Nanostructured Coating
2.2.1. Electrospinning of PCL Fibers onto LDPE Films
2.2.2. Electrospraying of SiO2 Particles onto LDPE/PCL Films
2.2.3. Thermal Post-Treatment
2.3. Film Characterization
2.3.1. Optical and Scanning Electron Microscopy
2.3.2. Water Contact Angle Measurements
2.3.3. Permeability Test
2.3.4. Thermogravimetric Analysis
2.3.5. Differential Scanning Calorimetry
3. Results and Discussion
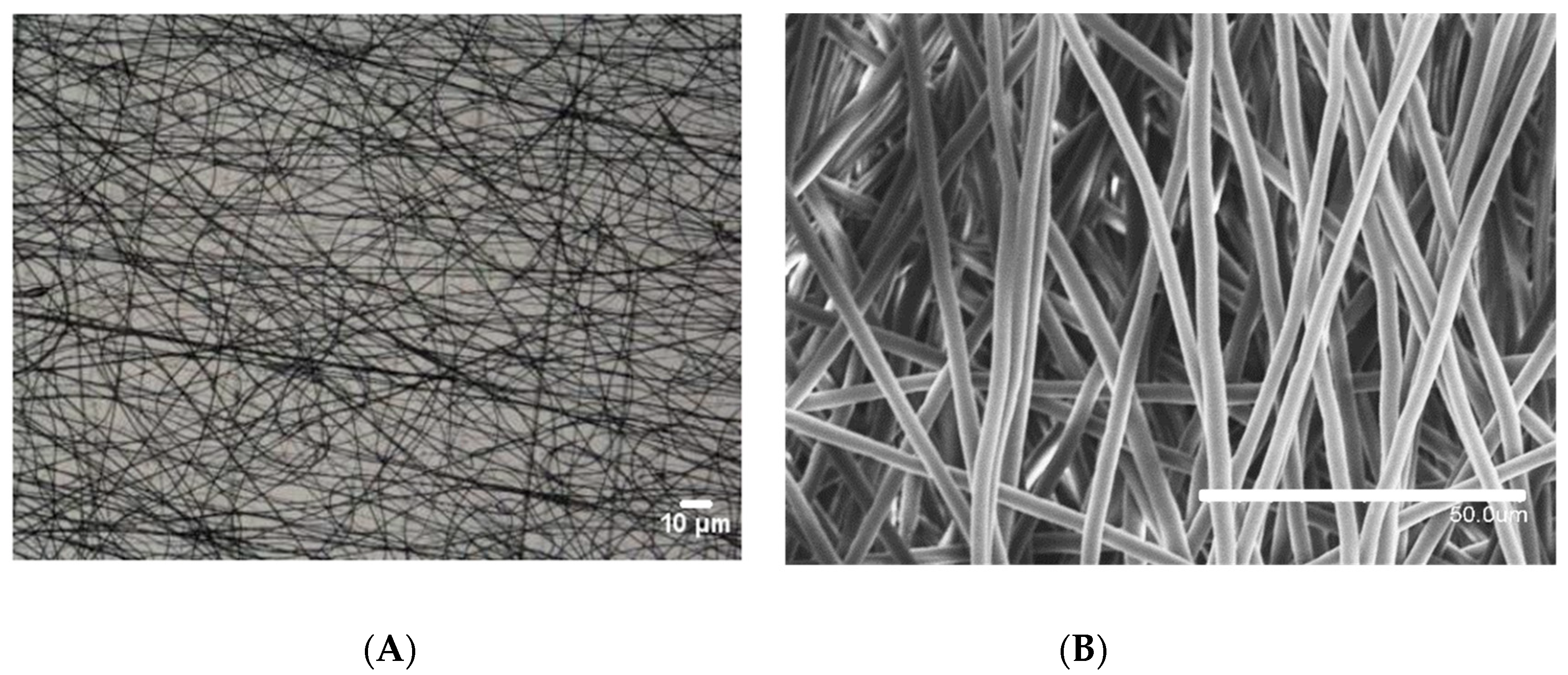
3.1. Optimization of the Thermal Post-Treatment on the PCL Coating
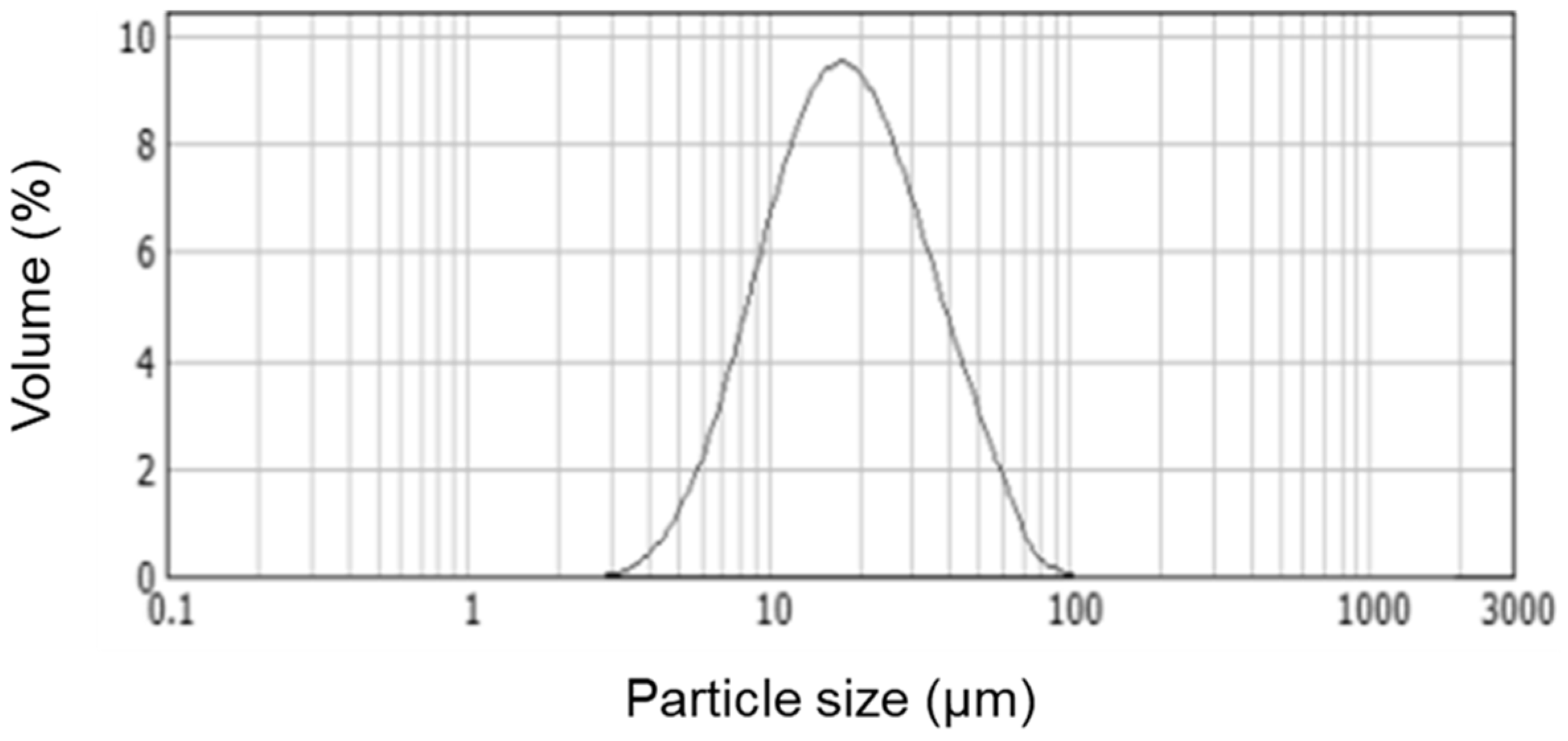
3.2. Deposition of Electrosprayed SiO2
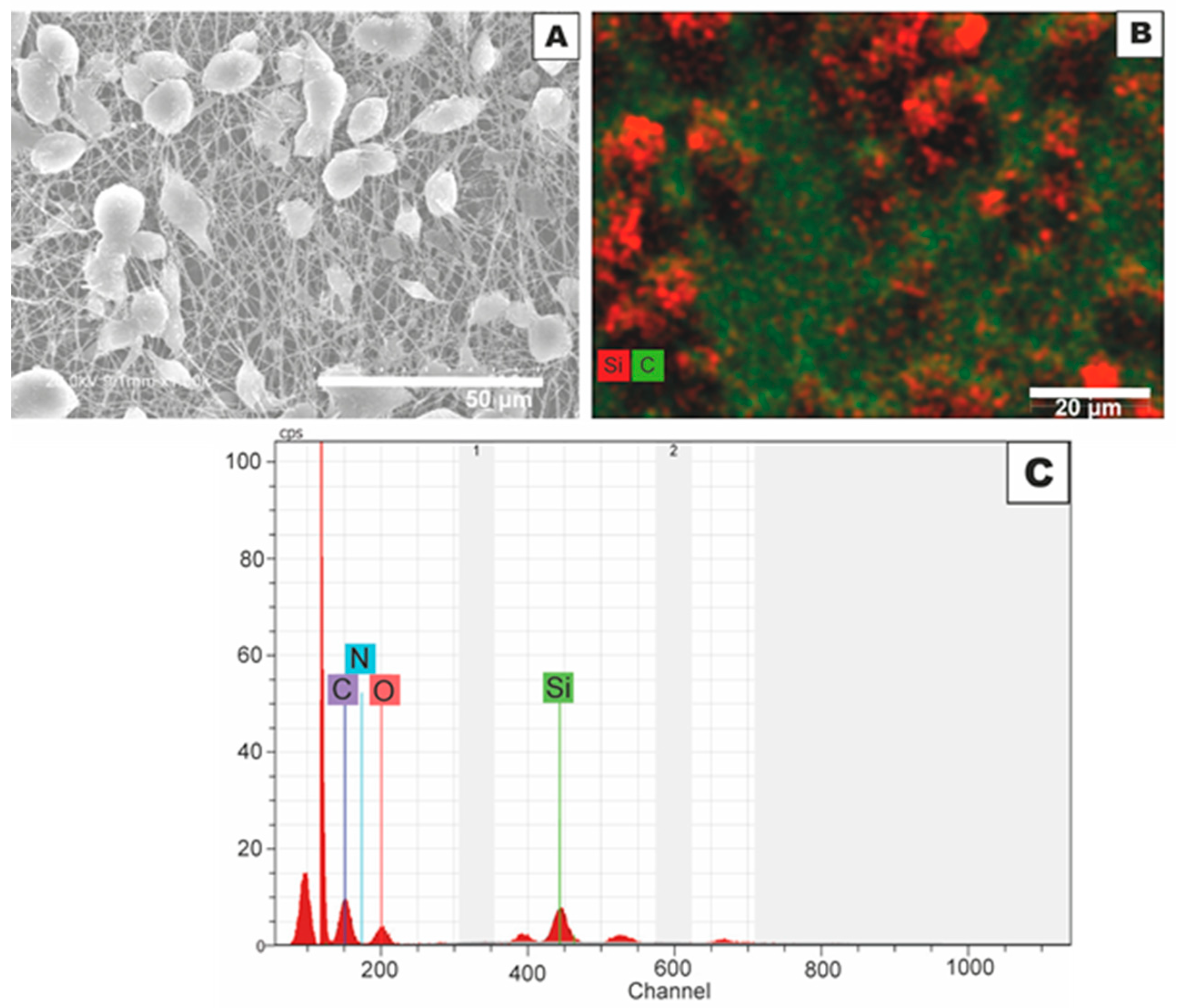
3.3. Morphology and Effect of SiO2 Electrospray Time onto LDPE/PCL Films
3.4. Surface Wettability of Increasing SiO2 Electrospraying Time over LDPE/PCL
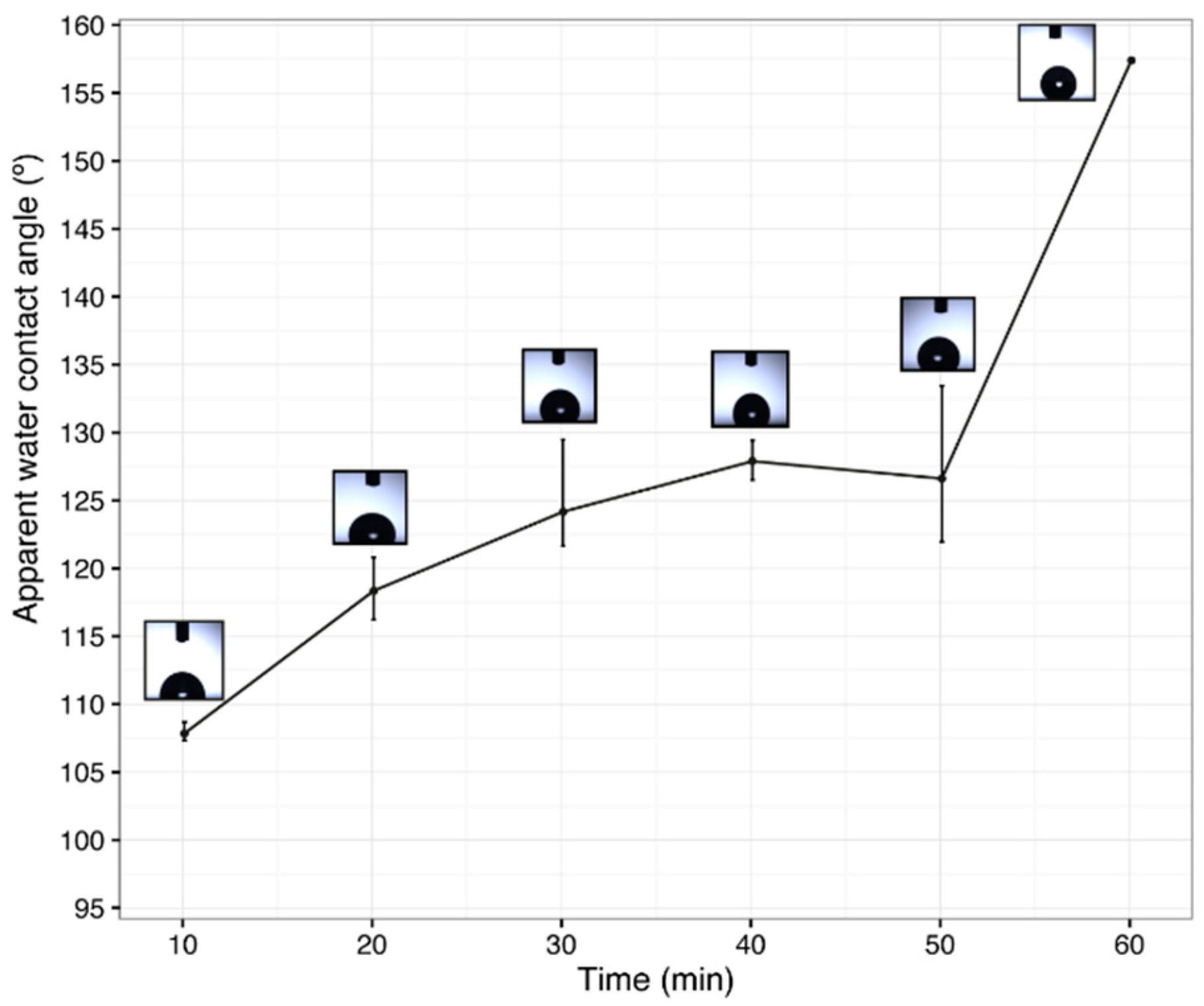
3.5. Effect of the Electrospun PCL Deposition Time on Surface Wettability
3.6. Barrier Properties
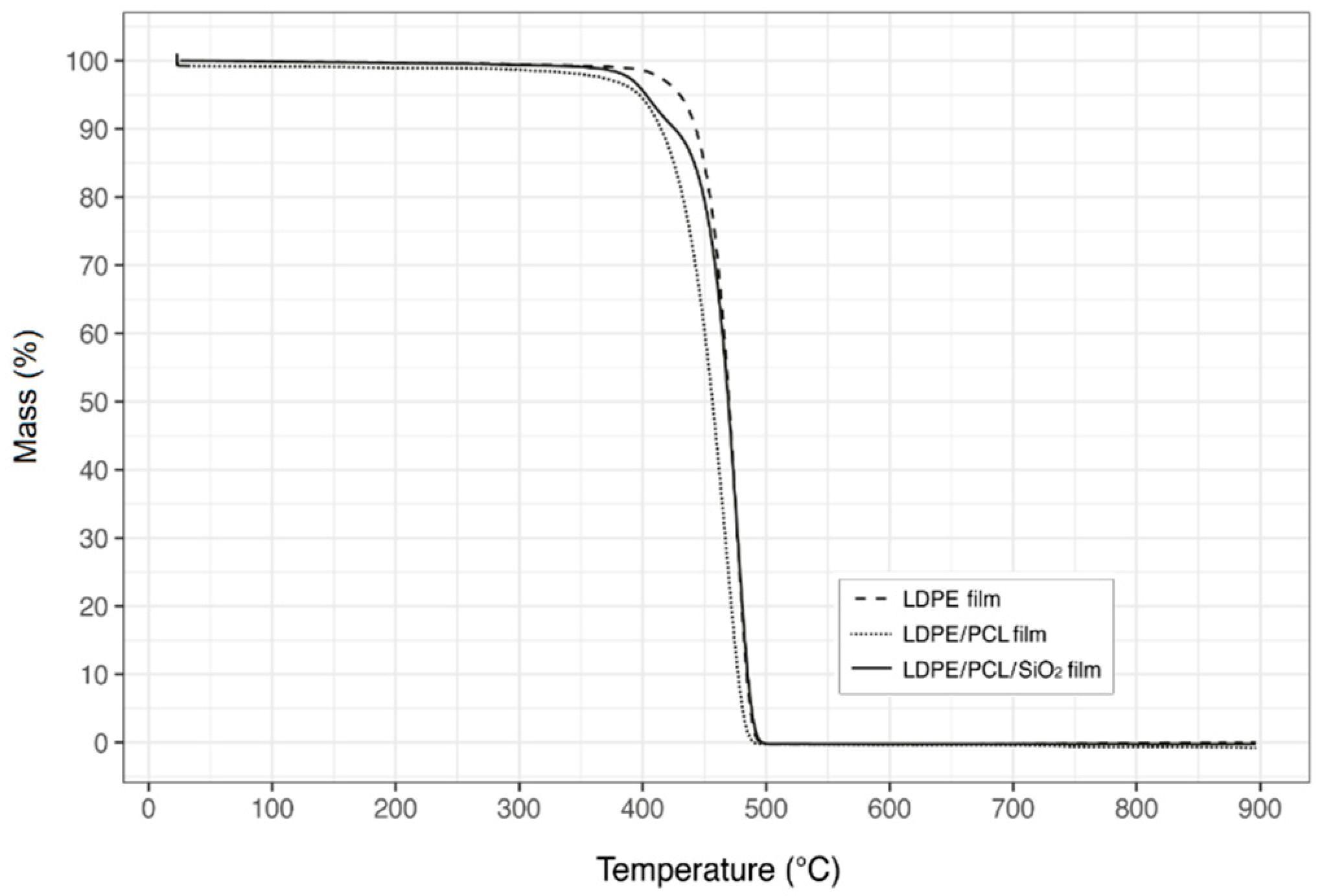
3.7. Thermal Stability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, J.; Li, X.; Wu, Y.; Liao, G.; Johnston, P.; Topham, P.D.; Wang, L. Rinse-resistant superhydrophobic block copolymer fabrics by electrospinning, electrospraying and thermally-induced self-assembly. Appl. Surf. Sci. 2017, 422, 769–777. [Google Scholar] [CrossRef]
- Darband, G.B.; Aliofkhazraei, M.; Khorsand, S.; Sokhanvar, S.; Kaboli, A. Science and engineering of superhydrophobic surfaces: Review of corrosion resistance, chemical and mechanical stability. Arab. J. Chem. 2018. [Google Scholar] [CrossRef]
- Martin-Polo, M.; Voilley, A.; Blond, G.; Colas, B.; Mesnier, M.; Floquet, N. Hydrophobic films and their efficiency against moisture transfer. 2. Influence of the physical state. J. Agric. Food Chem. 1992, 40, 413–418. [Google Scholar] [CrossRef]
- Gao, J.; Huang, X.; Xue, H.; Tang, L.; Li, R.K.Y. Facile preparation of hybrid microspheres for super-hydrophobic coating and oil-water separation. Chem. Eng. J. 2017, 326, 443–453. [Google Scholar] [CrossRef]
- Han, W.; Wu, D.; Ming, W.; Niemantsverdriet, J.W.; Thüne, P.C. Direct catalytic route to superhydrophobic polyethylene films. Langmuir 2006, 22, 7956–7959. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhao, Y.; Zhai, J. A lotus-leaf-like superhydrophobic surface: A porous microsphere/nanofiber composite film prepared by electrohydrodynamics. Angew. Chem. 2004, 116, 4438–4441. [Google Scholar] [CrossRef]
- Ma, M.; Gupta, M.; Li, Z.; Zhai, L.; Gleason, K.K.; Cohen, R.E.; Rubner, M.F.; Rutledge, G.C. Decorated electrospun fibers exhibiting superhydrophobicity. Adv. Mater. 2007, 19, 255–259. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Pérez-Masiá, R.; Lagaron, J.M. A review on electrospun polymer nanostructures as advanced bioactive platforms. Polym. Eng. Sci. 2016, 56, 500–527. [Google Scholar] [CrossRef]
- Torres-Giner, S. 5—Electrospun nanofibers for food packaging applications. In Multifunctional and Nanoreinforced Polymers for Food Packaging; Lagarón, J.-M., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 108–125. [Google Scholar]
- Agarwal, S.; Horst, S.; Bognitzki, M. Electrospinning of fluorinated polymers: Formation of superhydrophobic surfaces. Macromol. Mater. Eng. 2006, 291, 592–601. [Google Scholar] [CrossRef]
- Kang, M.; Jung, R.; Kim, H.-S.; Jin, H.-J. Preparation of superhydrophobic polystyrene membranes by electrospinning. Colloids Surf. Physicochem. Eng. Asp. 2008, 313, 411–414. [Google Scholar] [CrossRef]
- Lasprilla-Botero, J.; Álvarez-Láinez, M.; Lagaron, J.M. The influence of electrospinning parameters and solvent selection on the morphology and diameter of polyimide nanofibers. Mater. Today Commun. 2018, 14, 1–9. [Google Scholar] [CrossRef]
- Khan, M.K.I.; Schutyser, M.A.I.; Schroën, K.; Boom, R. The potential of electrospraying for hydrophobic film coating on foods. J. Food Eng. 2012, 108, 410–416. [Google Scholar] [CrossRef]
- Zhou, Z.; Wu, X.-F. Electrospinning superhydrophobic–superoleophilic fibrous PVDF membranes for high-efficiency water-oil separation. Mater. Lett. 2015, 160, 423–427. [Google Scholar] [CrossRef]
- Lavielle, N.; Hébraud, A.; Schlatter, G.; Thöny-Meyer, L.; Rossi, R.M.; Popa, A.-M. Simultaneous electrospinning and electrospraying: A straightforward approach for fabricating hierarchically structured composite membranes. ACS Appl. Mater. Interfaces 2013, 5, 10090–10097. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Li, Y.; Dai, Y.; Gao, F.; Tang, K.; Cao, H. Fabrication of three-dimensional superhydrophobic membranes with high porosity via simultaneous electrospraying and electrospinning. Mater. Lett. 2016, 170, 67–71. [Google Scholar] [CrossRef]
- Lim, J.-M.; Moon, J.H.; Yi, G.-R.; Heo, C.-J.; Yang, S.-M. Fabrication of one-dimensional colloidal assemblies from electrospun nanofibers. Langmuir 2006, 22, 3445–3449. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Zhang, X. Ultrafine polyacrylonitrile/silica composite fibers via electrospinning. Mater. Lett. 2008, 62, 2161–2164. [Google Scholar] [CrossRef]
- Siddiqa, A.J.; Chaudhury, K.; Adhikari, B. Hydrophilic low density polyethylene (LDPE) films for cell adhesion and proliferation. Res. Rev. J. Med. Chem. 2015, 1, 43–54. [Google Scholar]
- Fresnais, J.; Benyahia, L.; Chapel, J.P.; Poncin-Epaillard, F. Polyethylene ultrahydrophobic surface: Synthesis and original properties. Eur. Phys. J. Appl. Phys. 2004, 26, 209–214. [Google Scholar] [CrossRef]
- Echegoyen, Y.; Fabra, M.J.; Castro-Mayorga, J.L.; Cherpinski, A.; Lagaron, J.M. High throughput electro-hydrodynamic processing in food encapsulation and food packaging applications: Viewpoint. Trends Food Sci. Technol. 2017, 60, 71–79. [Google Scholar] [CrossRef]
- ASTM E96–95 Standard Test Methods for Water Vapor Transmission of Materials; ASTM International: West Conshohocken, PA, USA, 2011.
- Sanchez-Garcia, M.D.; Gimenez, E.; Lagaron, J.M. Morphology and barrier properties of solvent cast composites of thermoplastic biopolymers and purified cellulose fibers. Carbohydr. Polym. 2008, 71, 235–244. [Google Scholar] [CrossRef]
- Zaccaria, M.; Fabiani, D.; Cannucciari, G.; Gualandi, C.; Focarete, M.L.; Arbizzani, C.; De Giorgio, F.; Mastragostino, M. Effect of silica and tin oxide nanoparticles on properties of nanofibrous electrospun separators. J. Electrochem. Soc. 2015, 162, A915–A920. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Bormashenko, E. Progress in understanding wetting transitions on rough surfaces. Adv. Colloid Interface Sci. 2015, 222, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Friberg, S.E. Wetting of real surfaces, by Edward Yu. Bormashenko. J. Dispers. Sci. Technol. 2015, 36, 160. [Google Scholar] [CrossRef]
- Mittal, K. A guide to the equilibrium contact angles maze. In Contact Angle, Wettability and Adhesion; Mittal, K.L., Ed.; CRC Press: London, UK, 2009; Volume 6. [Google Scholar]
- Li, Y.; Quéré, D.; Lv, C.; Zheng, Q. Monostable superrepellent materials. Proc. Natl. Acad. Sci. USA 2017, 114, 3387–3392. [Google Scholar] [CrossRef] [PubMed]
- De Gennes, P.-G.; Brochard-Wyart, F.; Quere, D. Capillarity and Wetting Phenomena: Drops, Bubbles, Pearls, Waves; Springer: New York, NY, USA, 2004. [Google Scholar]
- Zhu, Z.; Liu, Y.; Hou, H.; Shi, W.; Qu, F.; Cui, F.; Wang, W. Dual-bioinspired design for constructing membranes with superhydrophobicity for direct contact membrane distillation. Environ. Sci. Technol. 2018, 52, 3027–3036. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Xu, Y.; Zhou, Y.; Gao, W.; Zhao, H.; Wang, W. Preparation of superhydrophobic nanocomposite fiber membranes by electrospinning poly(vinylidene fluoride)/silane coupling agent modified SiO2 nanoparticles. J. Appl. Polym. Sci. 2016, 134. [Google Scholar] [CrossRef]
- Číhal, P.; Vopicka, O.; Pilnacek, K.; Poustka, J.; Friess, K.; Hajslova, J.; Dobias, J.; Dole, P. Aroma scalping characteristics of polybutylene succinate based films. Polym. Test. 2015, 46, 108–115. [Google Scholar] [CrossRef]
- Hadj-Hamou, A.S.; Metref, F.; Yahiaoui, F. Thermal stability and decomposition kinetic studies of antimicrobial PCL/nanoclay packaging films. Polym. Bull. 2017, 74, 3833–3853. [Google Scholar] [CrossRef]
- Bolbukh, Y.; Kuzema, P.; Tertykh, V.; Laguta, I. Thermal degradation of polyethylene containing antioxidant and hydrophilic/hydrophobic silica. J. Therm. Anal. Calorim. 2008, 94, 727–736. [Google Scholar] [CrossRef]

| Sample | Thermal Treatment | Apparent Water Contact Angle (°) |
|---|---|---|
| LDPE | – | 67.3 ± 1.3 |
| LDPE/PCL | – | 128.5 ± 0.5 |
| LDPE/PCL | 55° for 1 min | 118.5 ± 0.1 |
| LDPE/PCL | 55° for 5 min | 88.3 ± 0.7 |
| LDPE/PCL | 65° for 5 min | 74.2 ± 1.2 |
| LDPE/PCL | 70° for 5 min | 73.1 ± 2.3 |
| LDPE/PCL | 80° for 5 min | 66.6 ± 1.5 |
| LDPE/PCL | 90° for 5 min | 67.1 ± 0.7 |
| Coating Time | SiO2 Layer | |
|---|---|---|
| Cross-section View | Top View | |
| 10 min |  |  |
| 20 min |  |  |
| 30 min |  |  |
| 40 min |  |  |
| 50 min |  |  |
| 60 min |  |  |
| Coating Time | PCL Layer | |
|---|---|---|
| Cross-section View | Top view | |
| 10 min |  | 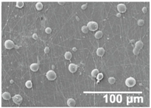 |
| 30 min |  |  |
| 60 min |  | 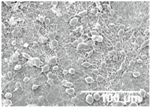 |
| Sample * | Deposition Time of PCL (min) | Thermal Treatment ** | Apparent Water Contact Angle (°) |
|---|---|---|---|
| LDPE/PCL10/SiO2 | 10 | Yes | 98.8 ± 0.9 |
| LDPE/PCL30/SiO2 | 30 | Yes | 131.6 ± 0.6 |
| LDPE/PCL60/SiO2 | 60 | Yes | 133.2 ± 0.6 |
| LDPE/PCL120/SiO2 | 120 | Yes | 157.4 ± 0.1 |
| Film Sample | Thickness (µm) | d-Limonene Permeance × 109 (kg·Pa−1·s−1·m−2) |
|---|---|---|
| LDPE | 150 ± 1 | 1.44 ± 0.08 |
| LDPE/PCL | 183 ± 1 | 1.35 ± 0.19 |
| LDPE/PCL/SiO2 | 189 ± 2 | 1.48 ± 0.13 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lasprilla-Botero, J.; Torres-Giner, S.; Pardo-Figuerez, M.; Álvarez-Láinez, M.; M. Lagaron, J. Superhydrophobic Bilayer Coating Based on Annealed Electrospun Ultrathin Poly(ε-caprolactone) Fibers and Electrosprayed Nanostructured Silica Microparticles for Easy Emptying Packaging Applications. Coatings 2018, 8, 173. https://doi.org/10.3390/coatings8050173
Lasprilla-Botero J, Torres-Giner S, Pardo-Figuerez M, Álvarez-Láinez M, M. Lagaron J. Superhydrophobic Bilayer Coating Based on Annealed Electrospun Ultrathin Poly(ε-caprolactone) Fibers and Electrosprayed Nanostructured Silica Microparticles for Easy Emptying Packaging Applications. Coatings. 2018; 8(5):173. https://doi.org/10.3390/coatings8050173
Chicago/Turabian StyleLasprilla-Botero, Juliana, Sergio Torres-Giner, Maria Pardo-Figuerez, Mónica Álvarez-Láinez, and Jose M. Lagaron. 2018. "Superhydrophobic Bilayer Coating Based on Annealed Electrospun Ultrathin Poly(ε-caprolactone) Fibers and Electrosprayed Nanostructured Silica Microparticles for Easy Emptying Packaging Applications" Coatings 8, no. 5: 173. https://doi.org/10.3390/coatings8050173
APA StyleLasprilla-Botero, J., Torres-Giner, S., Pardo-Figuerez, M., Álvarez-Láinez, M., & M. Lagaron, J. (2018). Superhydrophobic Bilayer Coating Based on Annealed Electrospun Ultrathin Poly(ε-caprolactone) Fibers and Electrosprayed Nanostructured Silica Microparticles for Easy Emptying Packaging Applications. Coatings, 8(5), 173. https://doi.org/10.3390/coatings8050173









