Microstructure Evolution of a Magnesium Phosphate Protective Layer on Concrete Structures in a Sulfate Environment
Abstract
:1. Introduction
2. Experimental Program
2.1. Materials
2.1.1. Concrete Materials
2.1.2. MPC Protection Layer Materials
2.2. Concrete Mixture
2.3. MPC Protection Layer Mixture
2.4. Concrete Specimens
2.5. Testing and Characterizations
2.5.1. Compressive Strength
2.5.2. Morphology Observation of Protection Layers
2.5.3. Microstructure Test
3. Results and Discussion
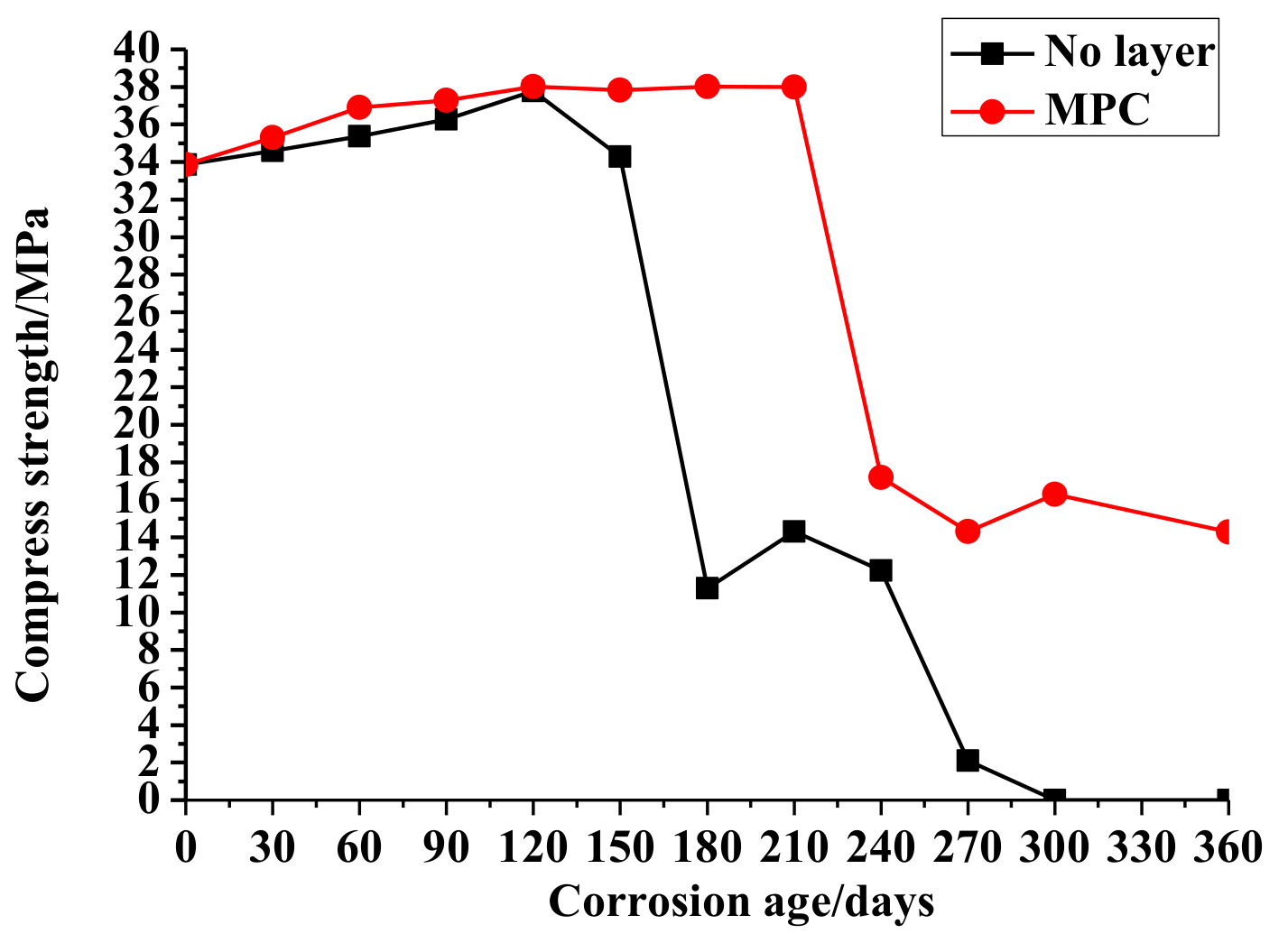
3.1. Compressive Strength
3.1.1. No Layer Concrete
3.1.2. MPC Layer Concrete
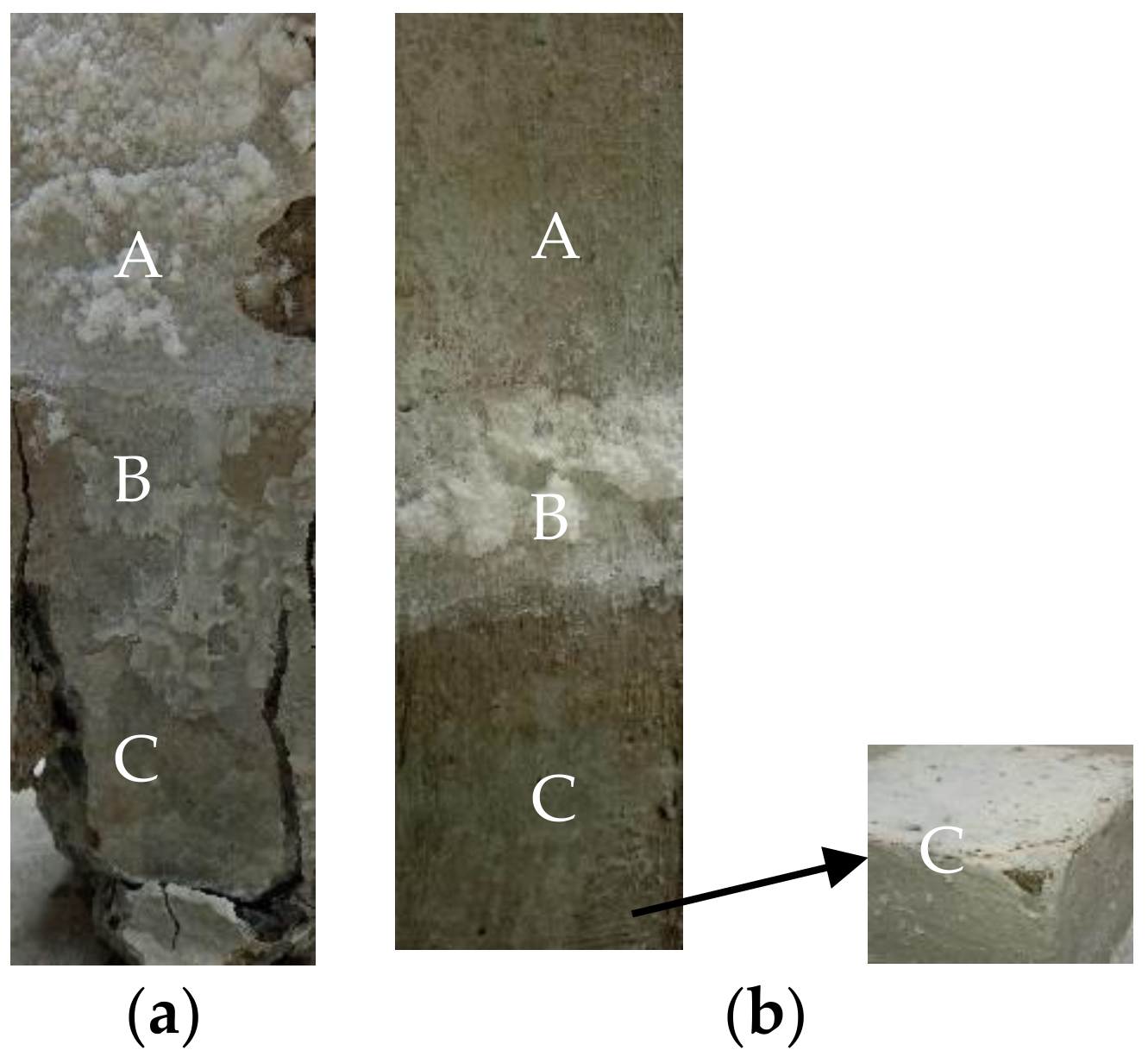
3.2. Appearances and Morphologies of Coated Concretes
3.2.1. 90 Days
3.2.2. 210 Days
3.2.3. 360 Days
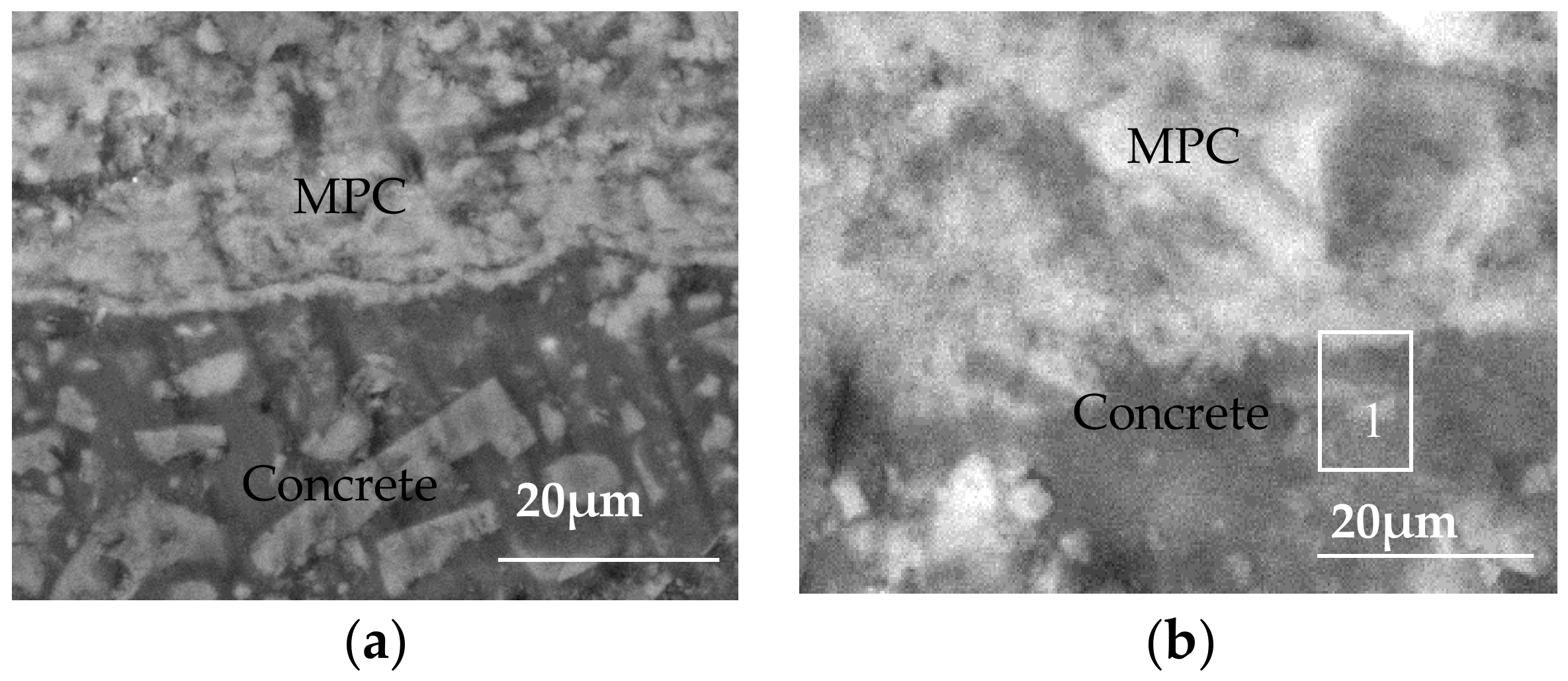
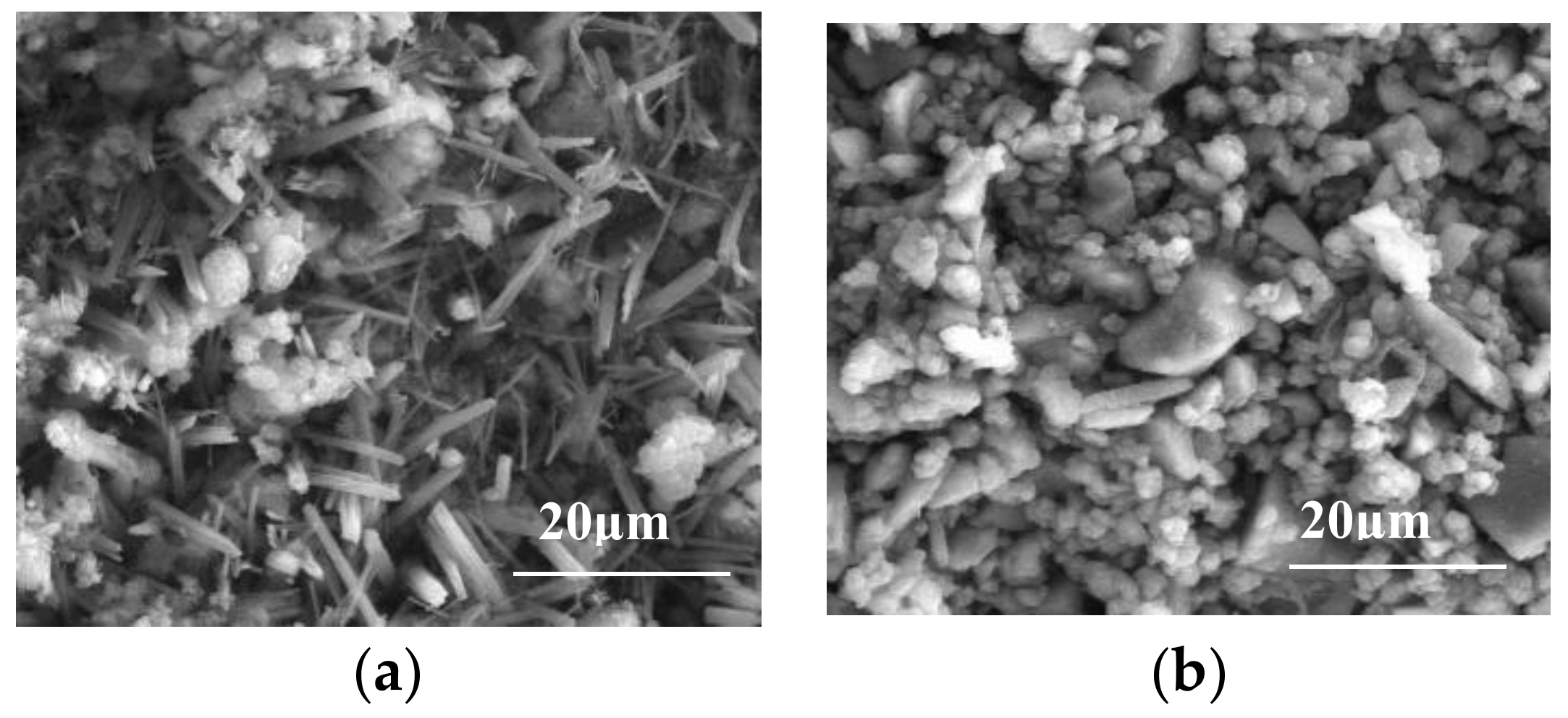
3.3. Evolution of Microstructure of MPC Protective Layer
3.3.1. Changes in MPC Protective Layer
3.3.2. Structure of MPC Protective Layer
4. Conclusions
- (1)
- The surface of the concrete structure was divided into three parts after being exposed to the sulfate erosion environment, namely the atmosphere, adsorption, and soaking regions. Corrosion was mainly concentrated in the adsorption and soaking regions, but the corrosion features in them were quite different. That is, the damage caused by sulfate to the concrete in the soaking region was more intense than that in the adsorption region.
- (2)
- The comparison of concrete compressive strength changes demonstrates the ability of the MPC layer to improve the compressive strength and external appearance of the specimens in the sulfate environment.
- (3)
- An MPC layer can greatly enhance the resistance of concrete to sulfate attacks in sulfate environments, and is achieved by forming new complexes through reaction with sulfate ions. In this context, the layer structure is more compact than before while the bond strength between the layer and the concrete is strengthened accordingly. The resistance of the MPC layer concrete to sulfate attacks is thus enhanced significantly.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wu, C.; Chen, W.; Zhang, H.; Yu, H.; Zhang, W.; Jiang, N.; Liu, L. The hydration mechanism and performance of Modified magnesium oxy sulfate cement by tartaric acid. Constr. Build. Mater. 2017, 144, 516–524. [Google Scholar] [CrossRef]
- Li, Y.; Bai, W.; Shi, T. A study of the bonding performance of magnesium phosphate cement on mortar and concrete. Constr. Build. Mater. 2017, 142, 459–468. [Google Scholar] [CrossRef]
- Ramey, G.E.; Moore, R.K.; Parker, F., Jr.; Strickland, A.M. Laboratory evaluation of four rapid-setting concrete patching materials. Transp. Res. Rec. 1985, 1041, 47–52. [Google Scholar]
- Michałowski, T.; Pietrzyk, A. A thermodynamic study of struvite + water system. Talanta 2006, 68, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.Y.; Chen, C.M. Influence of admixtures on the dissolution rate of chloride ion in magnesia cement. J. Wuhan Univ. Technol. 2010, 32, 37–40. [Google Scholar]
- Li, Y.; Li, Y.; Shi, T.; Li, J. Experimental study on mechanical properties and fracture toughness of magnesium phosphatecement. Constr. Build. Mater. 2015, 96, 345–352. [Google Scholar] [CrossRef]
- Li, Y.; Chen, B. Factors that affect the properties of magnesium phosphate cement. Constr. Build. Mater. 2013, 47, 977–983. [Google Scholar] [CrossRef]
- Soudee, E.; Pera, J. Influence of magnesia surface on the setting time of magnesia-phosphate cement. Cem. Concr. Res. 2002, 32, 153–157. [Google Scholar] [CrossRef]
- Tan, Y.; Yu, H.; Li, Y.; Bi, W.; Yao, X. The effect of slag on the properties of magnesium potassium phosphate cement. Constr. Build. Mater. 2016, 126, 313–320. [Google Scholar] [CrossRef]
- Ding, Z. Research of Magnesium Phosphosilicate Cement. Ph.D. Thesis, Hong Kong University of Science and Technology, Hong Kong, China, January 2005; pp. 253–286. [Google Scholar]
- Yang, Q.; Zhu, B.; Wu, X. Characteristics and durability test of magnesium phosphate cement-based material for rapid of concrete. Mater. Struct. 2000, 33, 229–234. [Google Scholar] [CrossRef]
- Abutaha, F.; Abdul Razak, H.; Ibrahim, H.A. Effect of coating palm oil clinker aggregate on the engineering properties of normal grade concrete. Coatings 2017, 7, 175. [Google Scholar] [CrossRef]
- Carmona-Quiroga, P.M.; Jacobs, R.M.; Viles, H.A. Weathering of two anti-graffiti protective coatings on concrete paving slabs. Coatings 2017, 7, 1. [Google Scholar] [CrossRef]
- Climent, M.Á.; Carmona, J.; Garcés, P. Graphite-cement paste: A new coating of reinforced concrete structural elements for the application of electrochemical anti-corrosion treatments. Coatings 2016, 6, 32. [Google Scholar] [CrossRef]
- Mors, R.; Jonkers, H. Effect on concrete surface water absorption upon addition of lactate derived agent. Coatings 2016, 7, 51. [Google Scholar] [CrossRef]
- Safiuddin, M. Concrete damage in field conditions and protective sealer and coating systems. Coatings 2016, 7, 99. [Google Scholar] [CrossRef]
- Nikoo, M.; Sadowski, Ł.; Nikoo, M. Prediction of the corrosion current density in reinforced concrete using a self-organizing feature map. Coatings 2017, 7, 160. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, S.; Wu, X. Deicer-scaling resistance of phosphate cement-based binder for rapid of concrete. Cem. Concr. Res. 2002, 32, 165–168. [Google Scholar] [CrossRef]
- Yang, Q.; Zhu, B.; Zhang, S.; Wu, X. Properties and applications of magnesia-phosphate cement mortar for rapid of concrete. Cem. Concr. Res. 2000, 30, 1807–1813. [Google Scholar] [CrossRef]
- Zhu, D.; Zongjin, L. High early strength magnesium phosphosilicate cement. Chin. J. Mater. Res. 2006, 20, 141–147. (In Chinese) [Google Scholar]
- Yu, B.; Chen, Z.; Yu, L. Water-resisting ability of cemented broken rocks. Int. J. Min. Sci. Technol. 2016, 26, 449–454. [Google Scholar] [CrossRef]
- Yang, J. Construction Method of Repairing Protective Layer of Reinforced Concrete Structure. China Invention Patent ZL 2015104528590, 11 November 2015. [Google Scholar]
- Yang, J. Preparation and Method of Magnesium Potassium Phosphate Cement Fireproof Protection Layer for Steel Structure. China Invention Patent ZL 2016109076541, 15 March 2017. [Google Scholar]
- Zhu, C.; Chang, X.; Men, Y.; Luo, X. Modeling of grout crack of rockbolt grouted system. Int. J. Min. Sci. Technol. 2015, 25, 73–77. [Google Scholar]
- Jun, L.; Yong-sheng, J.; Guodong, H.; Cheng, J. Retardation and reaction mechanisms of magnesium phosphate cement mixed with glacial acetic acid. RSC Adv. 2017, 7, 46852–46857. [Google Scholar] [CrossRef]
- JGJ55-2011 Specification for Mix Proportion Design of Ordinary Concrete; Ministry of Housing and Urban-Rural Construction of the People’s Republic of China: Beijing, China, 2011. (In Chinese)
- GB 50107-2010 Standard for Test and Evaluation of Concrete Compression Strength; Ministry of Housing and Urban-Rural Construction of the People’s Republic of China: Beijing, China, 2010. (In Chinese)
- Liu, Z.; Xiao, J.; Huang, H.; Yuan, Q.; Deng, D. Physicochemical study on the interface zone of concrete exposed to different slfate attack. J. Wuhan Univ. Technol. Mater. Sci. 2006, 1, 167–174. [Google Scholar]



| Raw Materials | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | SO3 | Other Ingredients |
|---|---|---|---|---|---|---|---|---|---|
| Cement | 24.55 | 7.77 | 3.62 | 54.59 | 2.68 | 0.31 | 1.50 | 2.24 | 1.2 |
| Raw Materials | MgO | SiO2 | CaO | Fe2O3 |
|---|---|---|---|---|
| Cement | 92.85 | 2.68 | 3.14 | 1.2 |
| Cement | Fine Aggregate | Coarse Aggregate | Water-to-Cement Ratio | Compressive Strength (MPa) | Equivalent Flexural Strength (MPa) |
|---|---|---|---|---|---|
| 1 | 1.24 | 2.52 | 0.4 | 48.1 | 8.3 |
| mMgO | mMgO/mP | mB/mMgO | Water-to-MPC Ratio |
|---|---|---|---|
| 1 | 4 | 0.05 | 0.12 |
| Specimens (mm3) | Protection Layer Category | Brushing Times | Quantity of Specimens |
|---|---|---|---|
| 100 × 100 × 400 | MPC | 2 | 6 |
| No layer | 2 | 6 | |
| 100 × 100 × 100 | MPC | 2 | 36 |
| No layer | 2 | 36 |
| Ca | P | Mg | N | O | Na | Si |
|---|---|---|---|---|---|---|
| 20.6 | 8.2 | 14.4 | 6.4 | 33.4 | 4.83 | 7.35 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Ji, Y.; Huang, G.; Zhang, L. Microstructure Evolution of a Magnesium Phosphate Protective Layer on Concrete Structures in a Sulfate Environment. Coatings 2018, 8, 140. https://doi.org/10.3390/coatings8040140
Li J, Ji Y, Huang G, Zhang L. Microstructure Evolution of a Magnesium Phosphate Protective Layer on Concrete Structures in a Sulfate Environment. Coatings. 2018; 8(4):140. https://doi.org/10.3390/coatings8040140
Chicago/Turabian StyleLi, Jun, Yongsheng Ji, Guodong Huang, and Linglei Zhang. 2018. "Microstructure Evolution of a Magnesium Phosphate Protective Layer on Concrete Structures in a Sulfate Environment" Coatings 8, no. 4: 140. https://doi.org/10.3390/coatings8040140
APA StyleLi, J., Ji, Y., Huang, G., & Zhang, L. (2018). Microstructure Evolution of a Magnesium Phosphate Protective Layer on Concrete Structures in a Sulfate Environment. Coatings, 8(4), 140. https://doi.org/10.3390/coatings8040140





