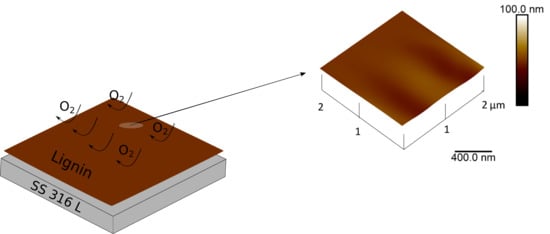
From Waste to Valuable Resource: Lignin as a Sustainable Anti-Corrosion Coating
Abstract
1. Introduction
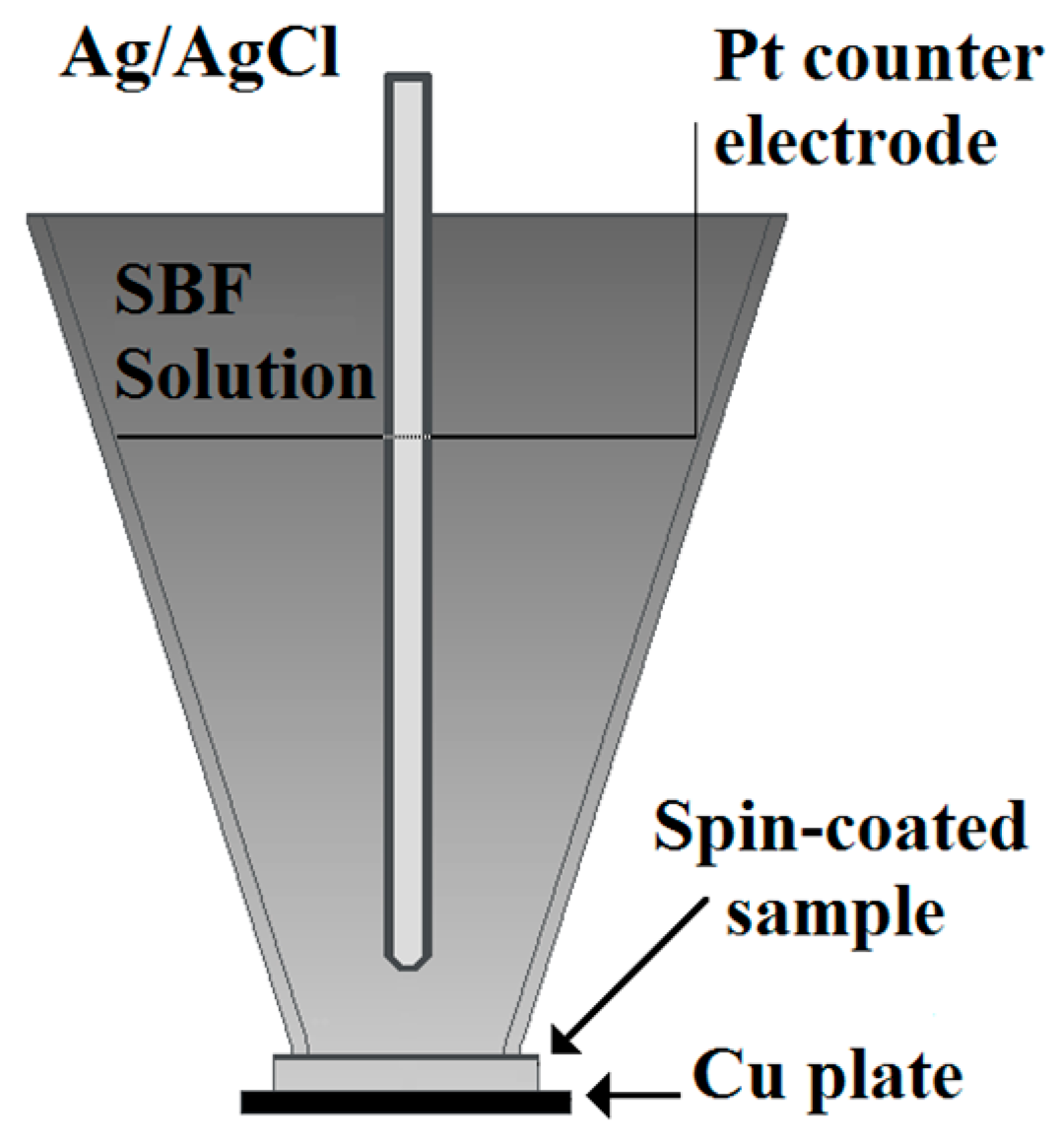
2. Materials and Methods
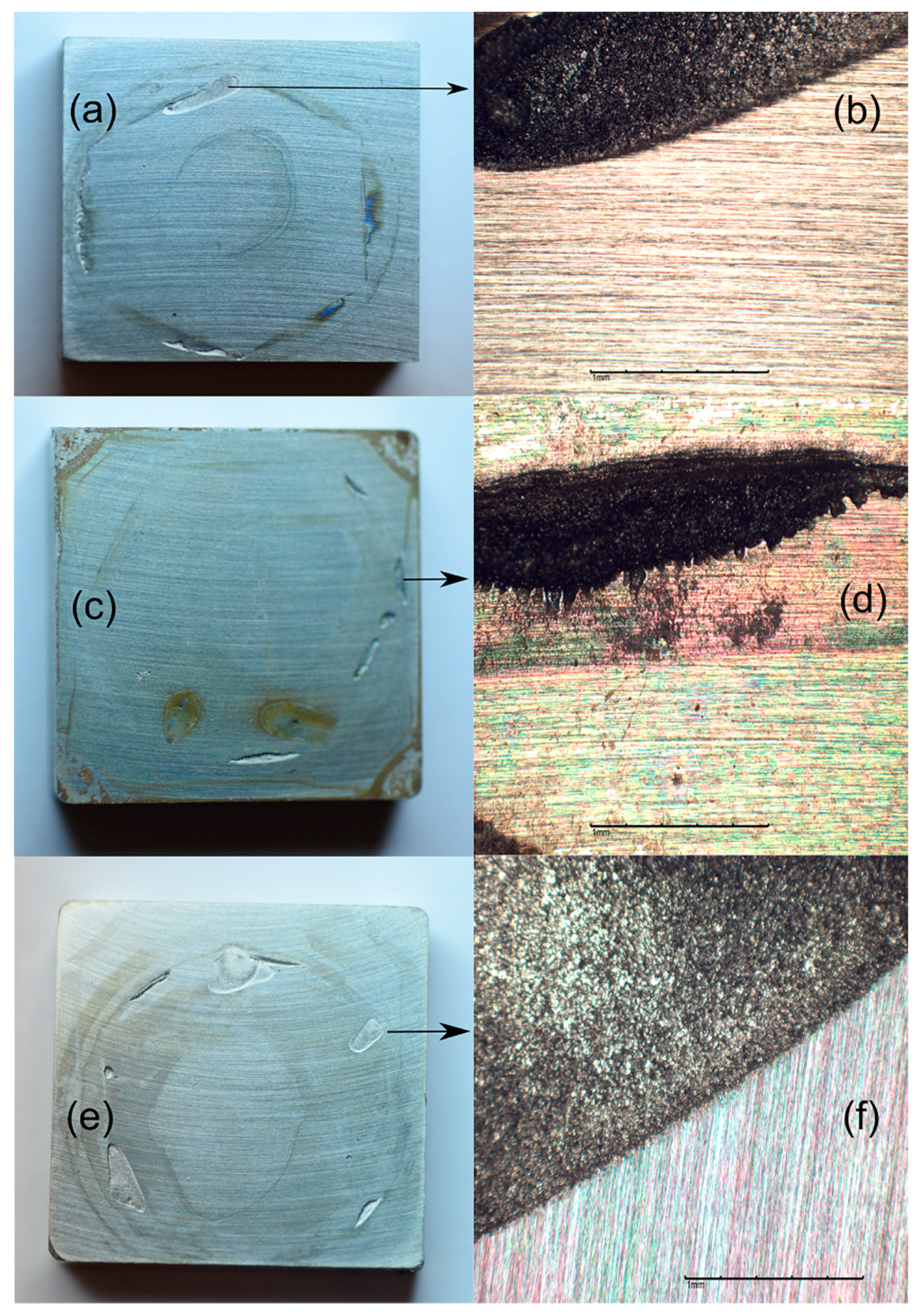
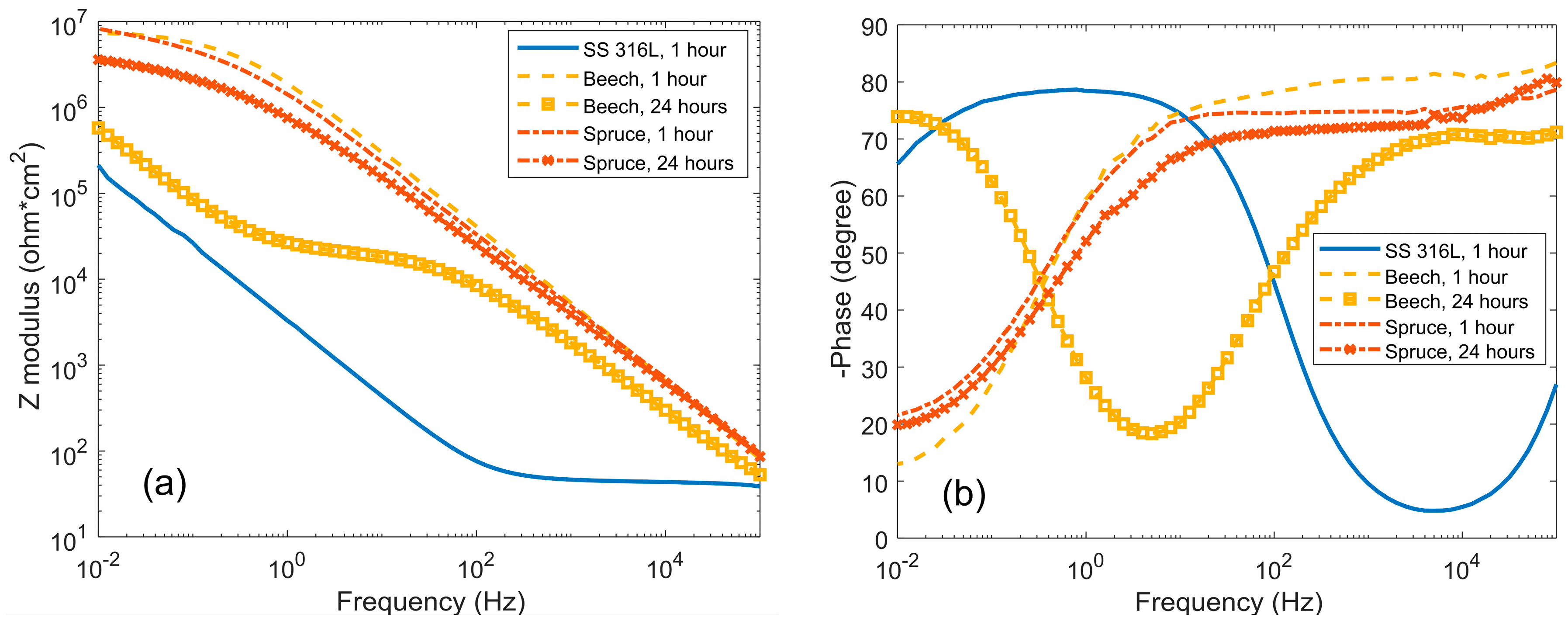
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Element | wt % |
|---|---|
| C | <0.03 |
| Si | <1 |
| Mn | <2 |
| Ni | 10 |
| Cr | 18 |
| Mo | 3 |
| S | <0.03 |
| P | <0.045 |
| Fe | Balance |
| Ion | Solution Concentrations (mM) | |
|---|---|---|
| SBF | PBS | |
| Na+ | 142 | 137 |
| K+ | 5 | 4.2 |
| Mg2+ | 1.5 | – |
| Ca2+ | 2.5 | – |
| Cl− | 147.8 | 139.6 |
| HCO3− | 4.2 | – |
| H2PO43− | – | 1.5 |
| HPO42− | 1 | 8.1 |
| SO42− | 0.5 | – |
| pH (25 °C) | 7.4 | 7.4 |
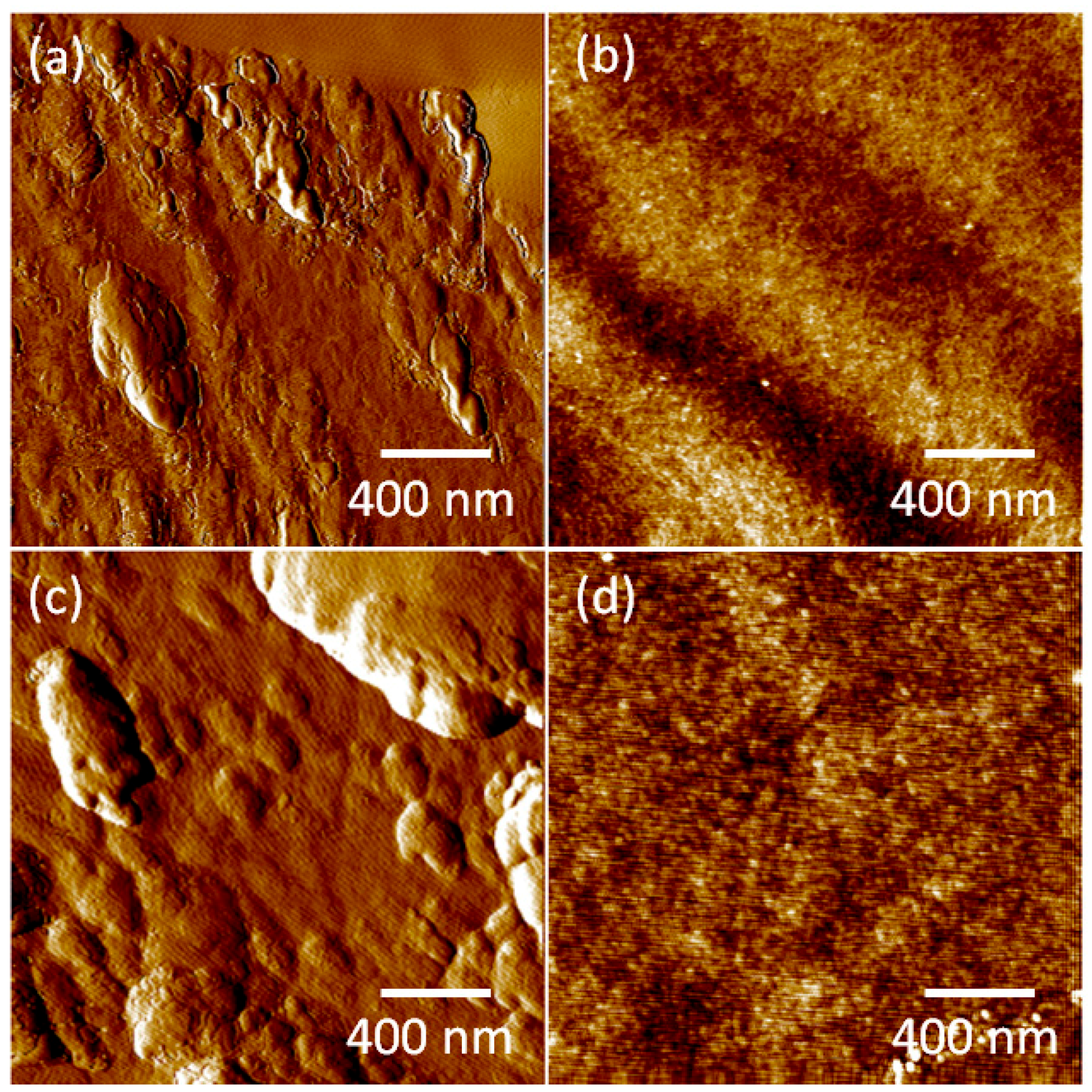
| No. | Sample | RQ (nm) | RA (nm) | RMax (nm) | |
|---|---|---|---|---|---|
| 1 | SS 316L | 2.99 | 2.28 | 22.46 | |
| 2 | Beech | Non-Annealed | 2.54 | 1.79 | 21.8 |
| 3 | Spruce | Non-Annealed | 6.62 | 4.97 | 46.1 |
| 4 | Beech | Annealed | 0.349 | 0.273 | 4.8 |
| 5 | Spruce | Annealed | 0.5 | 0.397 | 7.05 |

References
- Gargulak, J.D.; Lebo, S.E.; McNall, T.J. Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons Inc.: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Harb, S.V.; Cerrutti, B.M.; Pulcinelli, S.H.; Santilli, C.V.; Hammer, P. Siloxane–PMMA hybrid anti-corrosion coatings reinforced by lignin. Surf. Coat. Technol. 2015, 275, 9–16. [Google Scholar] [CrossRef]
- Global Lignin Products Market—Segmented by Product Type, Source, Application, and Geography—Trends and Forcasts (2015–2020)—Reportlinker Review. Cision PR Newswire Website. Available online: https://www.prnewswire.com/news-releases/global-lignin-products-market---segmented-by-product-type-source-application-and-geography---trends-and-forecasts-2015-2020---reportlinker-review-300145371.html (accessed on 18 October 2018).
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Hult, E.; Ropponen, J.; Poppius-Levlin, K.; Ohra-Aho, T.; Tamminen, T. Enhancing the barrier properties of paper board by a novel lignin coating. Ind. Crops Prod. 2013, 50, 694–700. [Google Scholar] [CrossRef]
- Nair, S.S.; Zhu, J.Y.; Deng, Y.; Ragauskas, A.J. High performance green barriers based on nanocellulose. Sustain. Chem. Process. 2014, 2, 23. [Google Scholar] [CrossRef]
- Bajpai, P. Carbon Fiber from Lignin; Springer: Singapore, 2017; p. 11. [Google Scholar]
- Hakala, T.; Kruus, K. The Making of Bioeconomy and Transformation; VTT: Helsinki, Finland, 2017. [Google Scholar]
- Eraković, S.; Janković, A.; Veljović, D.; Palcevskis, E.; Mitrić, M.; Stevanović, T.; Janaćković, D.; Mišković-Stanković, V. Corrosion stability and bioactivity in simulated body fluid of silver/hydroxyapatite and silver/hydroxyapatite/lignin coatings on titanium obtained by electrophoretic deposition. J. Phys. Chem. B 2013, 117, 1633–1643. [Google Scholar] [CrossRef]
- Akbarzadeh, E.; Ibrahim, M.N.M.; Rahim, A.A. Corrosion inhibition of mild steel in near neutral solution by kraft and soda lignins extracted from oil palm empty fruit bunch. Int. J. Electrochem. Sci. 2011, 6, 5396–5416. [Google Scholar]
- Hussin, M.H.; Rahim, A.A.; Mohamad Ibrahim, M.N.; Brosse, N. Improved corrosion inhibition of mild steel by chemically modified lignin polymers from Elaeis guineensis agricultural waste. Mater. Chem. Phys. 2015, 163, 201–212. [Google Scholar] [CrossRef]
- Ren, Y.; Luo, Y.; Zhang, K.; Zhu, G.; Tan, X. Lignin terpolymer for corrosion inhibition of mild steel in 10% hydrochloric acid medium. Corros. Sci. 2008, 50, 3147–3153. [Google Scholar] [CrossRef]
- Shivakumar, M.; Dharmaprakash, M.S.; Manjappa, S.; Nagashree, K.L. Corrosion inhibition performance of lignin extracted from black liquor on mild steel in 0.5 M H2SO4 acidic media. Port. Electrochim. Acta 2017, 35, 351–359. [Google Scholar] [CrossRef]
- Hussin, M.H.; Mohd Shah, A.; Rahim, A.; Ibrahim, M.; Perrin, D.; Brosse, N. Antioxidant and anti corrosive properties of oil palm frond lignins extracted with different techniques. Ann. For. Sci. 2014, 72, 17–26. [Google Scholar] [CrossRef]
- Raschip, I.E.; Vasile, C.; Ciolacu, D.; Cazacu, G. Semi-interpenetrating polymer networks containing polysaccharides. I xanthan/lignin networks. High Perform. Polym. 2007, 19, 603–620. [Google Scholar] [CrossRef]
- Derkacheva, O.; Sukhov, D. Investigation of lignins by FTIR spectroscopy. Macromol. Symp. 2008, 265, 61–68. [Google Scholar] [CrossRef]
- Revie, R.W.; Uhlig, H.H. Corrosion and Corrosion Control, 3rd ed.; John Wiley & Sons Inc.: New York, NY, USA, 1985. [Google Scholar]
- Lo, K.H.; Shek, C.H.; Lai, J.K.L. Recent developments in stainless steels. Mater. Sci. Eng. 2009, 65, 39–104. [Google Scholar] [CrossRef]
- Houska, C. Stainless Steel in Architecture, Building and Construction; Reference Book Series No. 11024; Nickel Institute: Toronto, ON, Canada, 2014. [Google Scholar]
- Guidelines for Nickel Stainless Steels for Marine Environments Natural Waters and Brines; Reference Book Series No. 11003; Nickel Institute: Toronto, ON, Canada, 1987.
- Shih, C.C.; Shih, C.M.; Chen, Y.; Su, Y.Y.; Shih, J.S.; Kwok, C.F.; Lin, S.J. Growth inhibition of cultured smooth muscle cells by corrosion products of 316 L stainless steel wire. J. Biomed. Mater. Res. 2001, 57, 200–207. [Google Scholar] [CrossRef]
- Hansen, D.C. Metal corrosion in the human body: The ultimate bio-corrosion scenario. Electrochem. Soc. Interface 2008, 17, 31–34. [Google Scholar]
- Chew, K.; Zein, S.; Ahmad, A. The corrosion scenario in human body: Stainless steel 316L orthopaedic implants. Nat. Sci. 2012, 4, 144–148. [Google Scholar] [CrossRef]
- Malik, A.U.; Mayan Kutty, P.C.; Siddiqi, N.A.; Andijani, I.N.; Ahmed, S. The influence of pH and chloride concentration on the corrosion behaviour of AISI 316L steel in aqueous solutions. Corros. Sci. 1992, 33, 1809–1827. [Google Scholar] [CrossRef]
- Heise, S.; Wirth, T.; Höhlinger, M.; Hernández, Y.T.; Ortiz, J.A.R.; Wagener, V.; Virtanen, S.; Boccaccini, A.R. Electrophoretic deposition of chitosan/bioactive glass/silica coatings on stainless steel and WE43 mg alloy substrates. Surf. Coat. Technol. 2018, 344, 553–563. [Google Scholar] [CrossRef]
- Zhitomirsky, I.; Pang, X. Fabrication of chitosan-hydroxyapatite coatings for biomedical applications. ECS Trans. 2007, 3, 15–22. [Google Scholar] [CrossRef]
- Huang, J.; Lyu, S.; Fu, F.; Wu, Y.; Wang, S. Green preparation of a cellulose nanocrystals/polyvinyl alcohol composite superhydrophobic coating. RSC Adv. 2017, 7, 20152–20159. [Google Scholar] [CrossRef]
- Rahman, O.U.; Shi, S.; Ding, J.; Wang, D.; Ahmad, S.; Yu, H. Lignin nanoparticles: Synthesis, characterization and corrosion protection performance. New J. Chem. 2018, 42, 3415–3425. [Google Scholar] [CrossRef]
- Naseem, A.; Tabasum, S.; Zia, K.M.; Zuber, M.; Ali, M.; Noreen, A. Lignin-derivatives based polymers, blends and composites: A review. Int. J. Biol. Macromol. 2016, 93, 296–313. [Google Scholar] [CrossRef]
- Seyed Shahabadi, S.I.; Kong, J.; Lu, X. Aqueous-only, green route to self-healable, UV-resistant, and electrically conductive polyurethane/graphene/lignin nanocomposite coatings. ACS Sustain. Chem. Eng. 2017, 5, 3148–3157. [Google Scholar] [CrossRef]
- Erakovic, S.; Jankovic, A.; Tsui, C.G.; Tang, C.; Miskovic-Stankovic, V.; Stevanovic, T. Novel bioactive antimicrobial lignin containing coatings on titanium obtained by electrophoretic deposition. Int. J. Mol. Sci. 2014, 15, 12294–12322. [Google Scholar] [CrossRef]
- Vagin, M.Y.; Trashin, S.A.; Karyakin, A.A. Corrosion protection of steel by electropolymerized lignins. Electrochem. Commun. 2006, 8, 60–64. [Google Scholar] [CrossRef]
- Chen, Q.; de Larraya, U.P.; Garmendia, N.; Lasheras-Zubiate, M.; Cordero-Arias, L.; Virtanen, S.; Boccaccini, A.R. Electrophoretic deposition of cellulose nanocrystals (CNs) and CNs/alginate nanocomposite coatings and free standing membranes. Colloids Surf. B 2014, 118, 41–48. [Google Scholar] [CrossRef]
- 316/316L Stainless Steel; Product Data Bulletin; AK Steel Corporation: West Chester, OH, USA, 2016.
- Alonso Frank, M.; Meltzer, C.; Braunschweig, B.; Peukert, W.; Boccaccini, A.R.; Virtanen, S. Functionalization of steel surfaces with organic acids: Influence on wetting and corrosion behavior. Appl. Surf. Sci. 2017, 404, 326–333. [Google Scholar] [CrossRef]
- Rossberg, C.; Janzon, R.; Saake, B.; Leschinsky, M. Effect of process parameters in pilot scale operation on properties of organosolv lignin. BioResources 2018. submitted. [Google Scholar]
- Ragauskas, A. Typical H:G:S Ratio for Lignin from Biomass. Available online: http://biorefinery.utk.edu/technical_reviews/lignin%20HGS.pdf (accessed on 18 October 2018).
- Lê, H.Q.; Zaitseva, A.; Pokki, J.; Ståhl, M.; Alopaeus, V.; Sixta, H. Solubility of organosolv lignin in γ-valerolactone/water binary mixtures. ChemSusChem 2016, 9, 2939–2947. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Annual Book of ASTM Standards, Wear and Erosion, Metal Corrosion (03.02); ASTM International: West Conshohocken, PA, USA, 1993.
- EN ISO 2409:2013 Paints and Varnishes—Cross-cut Test; ISO: Geneva, Switzerland, 2013.
- Hansen, A.T.M.; Hidayat, J.B.; Mogensen, K.K.; Jeppesen, D.M.; Jorgensen., B.; Johansen, S.K.; Thygesen, G.L. Enzyme affinity to cell types in wheat straw (Triticum aestivum L.) before and after hydrothermal pretreatment. Biotechnol. Biofuels 2013, 6, 54. [Google Scholar] [CrossRef]
- Brodin, I. Chemical Properties and Thermal Behaviour of Kraft Lignins. Licentiate Thesis, KTH, Stockholm, Sweden, 2009. [Google Scholar]
- Pandey, K.K. A study of chemical structure of soft and hardwood and wood polymers by FTIR spectroscopy. J. Appl. Polym. Sci. 1999, 71, 1969–1975. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood: Chemistry, Ultrastructure, Reactions; Walter de Gruyter & Co.: Berlin, Germany, 1989. [Google Scholar]
- Kline, L.M.; Hayes, D.G.; Womac, A.R.; Labbe, N. Simplified determination of lignin content in hard and soft woods via uv-spectrophotometric analysis of biomass dissolved in ionic liquids. BioResources 2010, 5, 1366–1383. [Google Scholar]
- Malherbe, F.E.; Bernstein, H.J. The infrared and Raman spectra of p-dioxane. J. Am. Chem. Soc. 1952, 74, 4408–4410. [Google Scholar] [CrossRef]
- Zhang, Z. Lignin Modification and Degradation for Advanced Composites and Chemicals. Ph.D. Thesis, Georgia Institute of Technology, Atlanta, GA, USA, 2017. [Google Scholar]
- Notley, M.S.; Norgen, M. Surface energy and wettability of spin-coated thin films of lignin isolated from wood. Langmuir 2010, 26, 5484–5490. [Google Scholar] [CrossRef]
- Liukkonen, A. Contact angle of water on paper components: Sessile drops versus environmental scanning electron microscope measurements. Scanning 1997, 19, 411–415. [Google Scholar] [CrossRef]
- Young, R.A. Wettability of wood pulp fibers applicability of methodology. Wood Fibers Sci. 1976, 8, 120–128. [Google Scholar]
- Yang, Q.; Pan, X. Correlation between lignin physicochemical properties and inhibition to enzymatic hydrolysis of cellulose. Biotechnol. Bioeng. 2016, 113, 1213–1224. [Google Scholar] [CrossRef]
- Deflorian, F.; Fedrizzi, L. Adhesion characterization of protective organic coatings by electrochemical impedance spectroscopy. J. Adhes. Sci. Technol. 1999, 13, 629–645. [Google Scholar] [CrossRef]
- Metallic Materials. Handbook of Materials for Medical Devices; Davis, J.R., Ed.; ASM International: Materials Park, OH, USA, 2003; p. 21. [Google Scholar]
- Deng, Y.; Bai, W.; Chen, J.; Zhang, X.; Wang, S.; Lin, J.; Xu, Y. Bio-inspired electrochemical corrosion coatings derived from graphene/natural lacquer composites. RSC Adv. 2017, 7, 45034–45044. [Google Scholar] [CrossRef]
- Kim, J.Y.; Hwang, H.; Oh, S.; Kim, Y.S.; Kim, U.J.; Choi, J.W. Investigation of structural modification and thermal characteristics of lignin after heat treatment. Int. J. Biol. Macromol. 2014, 66, 57–65. [Google Scholar] [CrossRef]
- Boeriu, C.G.; Bravo, D.; Gosselink, R.J.A.; Van Dam, J.E.G. Characterization of structure-dependent functional properties of lignin with infrared spectroscopy. Ind. Crops Prod. 2004, 20, 205–218. [Google Scholar] [CrossRef]
- Anaee, R. Corrosion Behavior of Some Implant Alloys in Simulated Human Body Environment. Master’s Thesis, Iraq University of Technology, Baghdad, Iraq, 2010. [Google Scholar]
- Dariva, C.G.; Galio, A.F. Corrosion inhibitors-principles, mechanisms and applications. In Developments in Corrosion Protection; Aliofkhazraei, M., Ed.; InTech: Vienna, Austria, 2014; p. 365. [Google Scholar]
- Altweiq, A.M.; Khouri, S.J.; Al-luaibi, S.; Lehmann, R.; DrǕcker, H.; Vogt, C. The role of extracted alkali lignin as corrosion inhibitor. J. Mater. Environ. Sci. 2011, 2, 259–270. [Google Scholar]
- Yahya, S.; Othman, N.K.; Daud, A.R.; Jalar, A. Surface morphology studies of low carbon steel treated in aqueous lignin. Sains Malays. 2013, 42, 1793–1798. [Google Scholar]
- Bryant, M.; Hu, X.; Farrar, R.; Brummitt, K.; Freeman, R.; Neville, A. Crevice corrosion of biomedical alloys: A novel method of assessing the effects of bone cement and its chemistry. J. Biomed. Mater. Res. 2013, 101, 792–803. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Kim, J.; Kim, W. Effect of the crevice former on the corrosion behavior of 316L stainless steel in chloride-containing synthetic tap water. Met. Mater. Int. 2018, 24, 516–524. [Google Scholar] [CrossRef]
- Gonzalez-Garcia, Y.; Gonzalez, S.; Souto, R.M. Electrochemical and structural properties of polyurethane coating on steel substrates for corrosion protection. Corros. Sci. 2007, 49, 3514–3526. [Google Scholar] [CrossRef]
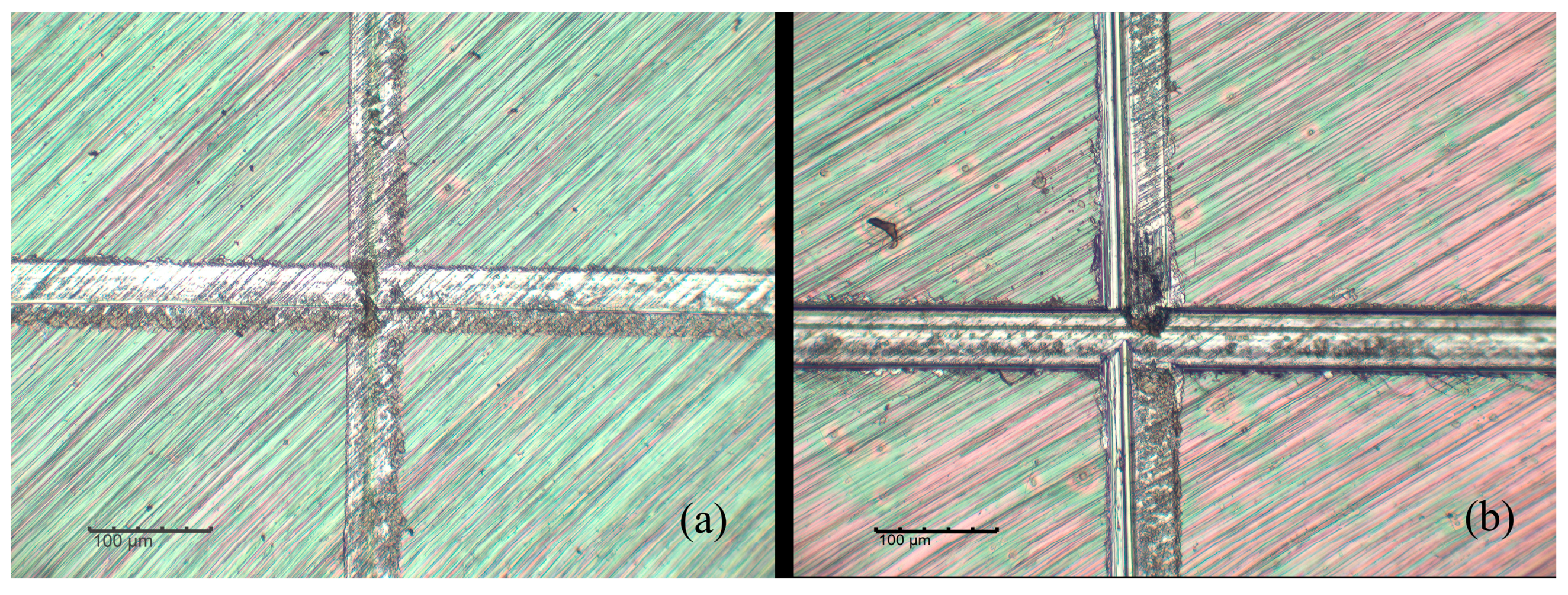

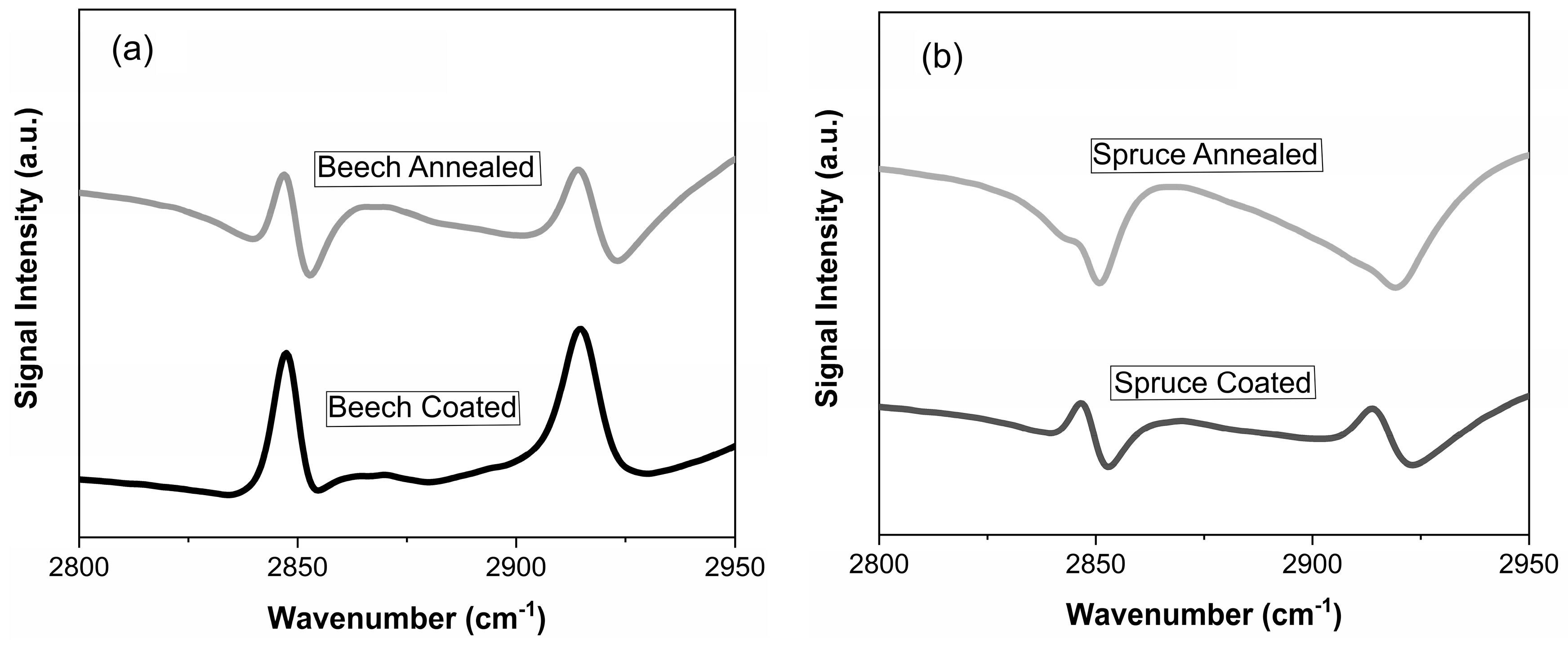
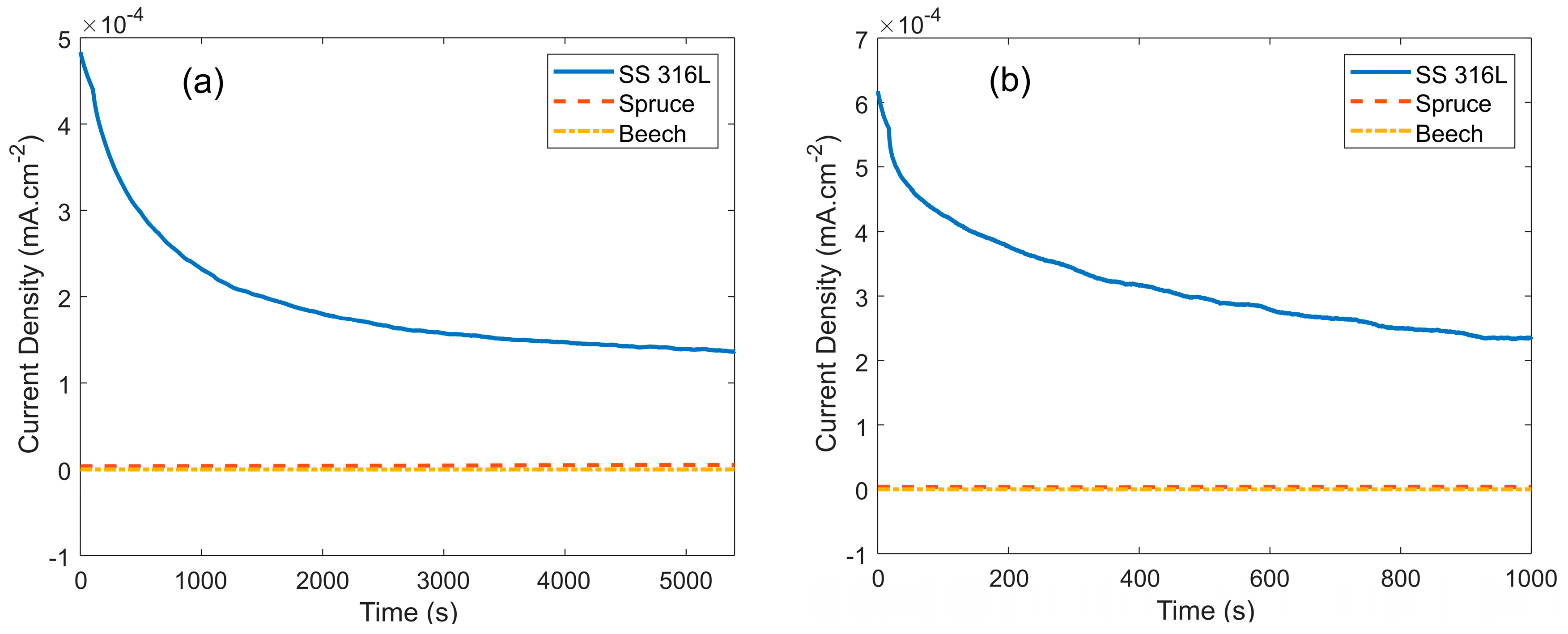
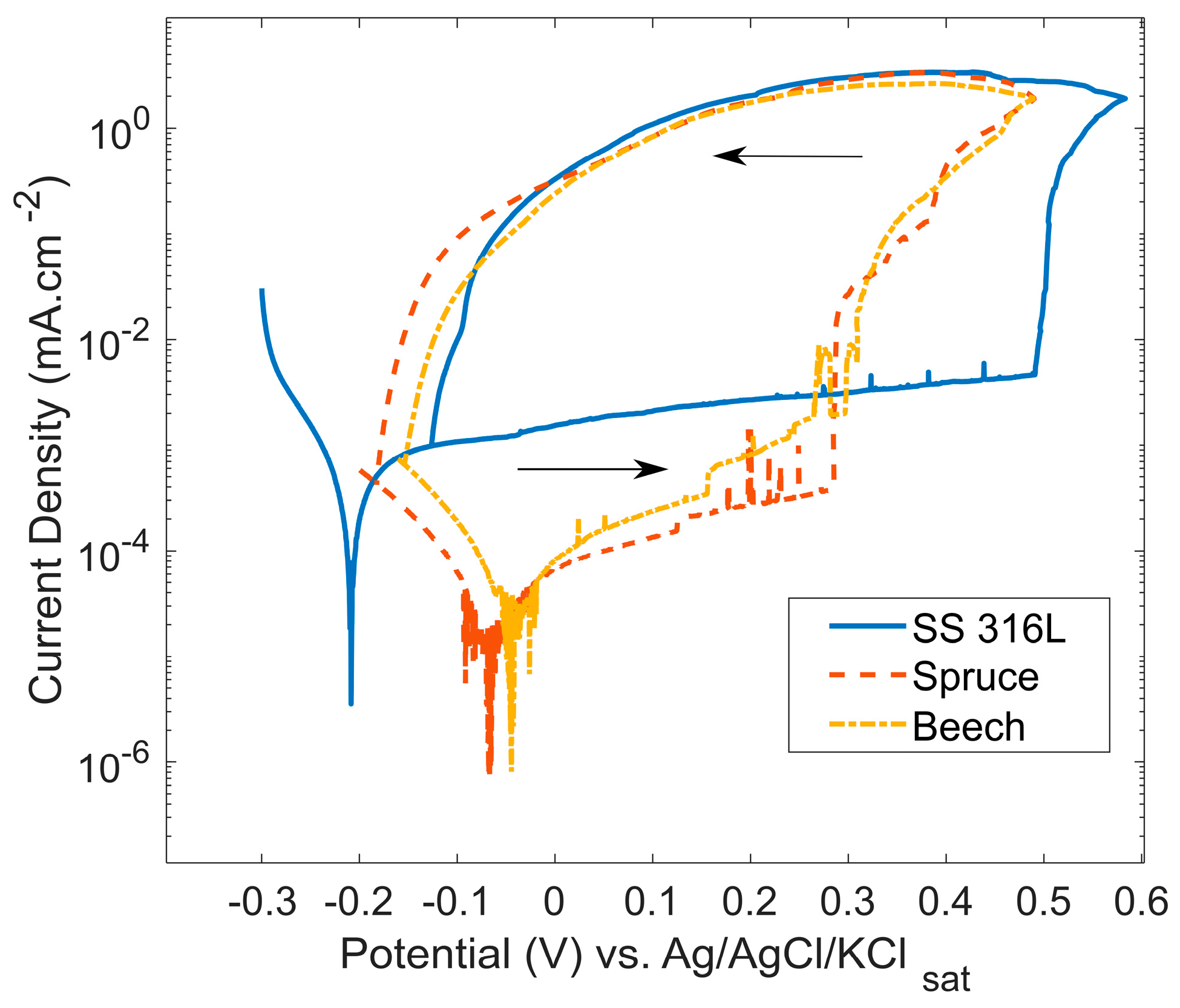
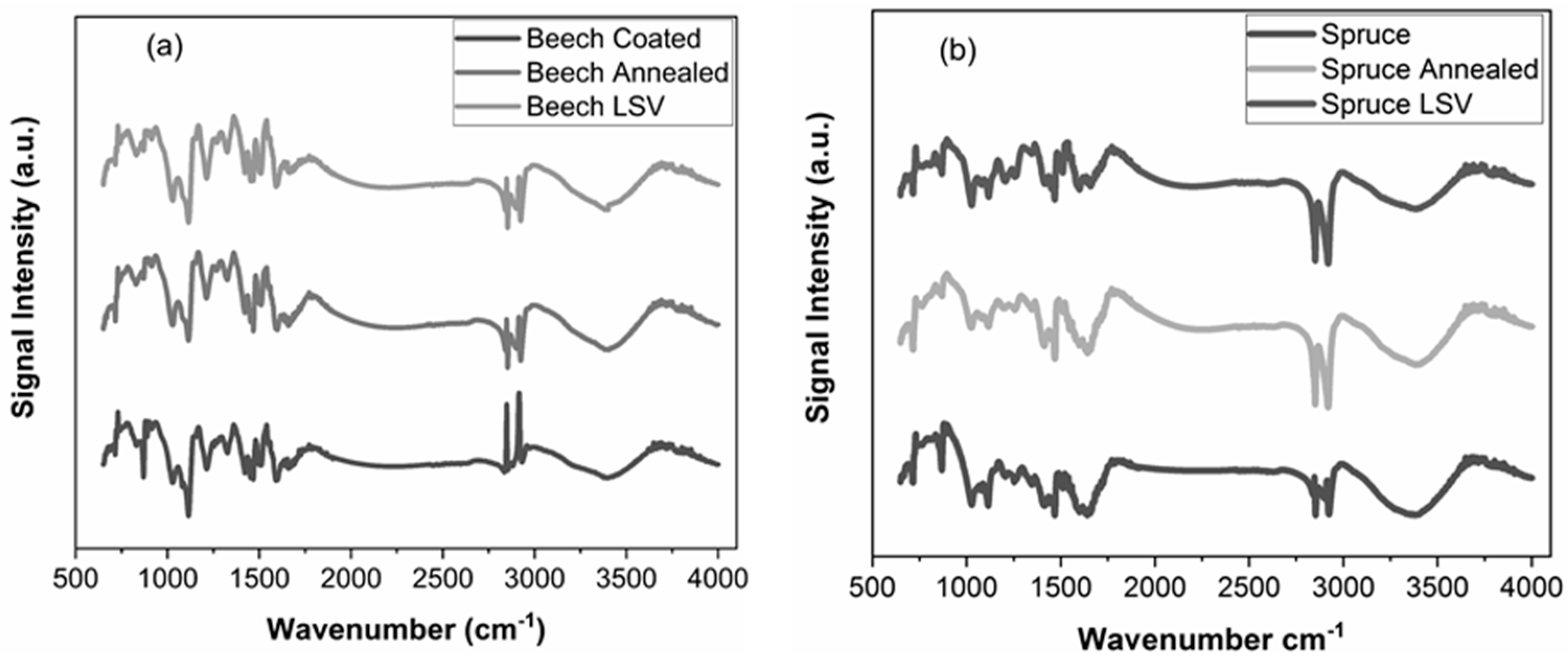
| Plant Source | G | S | H |
|---|---|---|---|
| spruce (softwood) | 94 | 1 | 5 |
| beech (hardwood) | 43.2 | 55.4 | 1.4 |
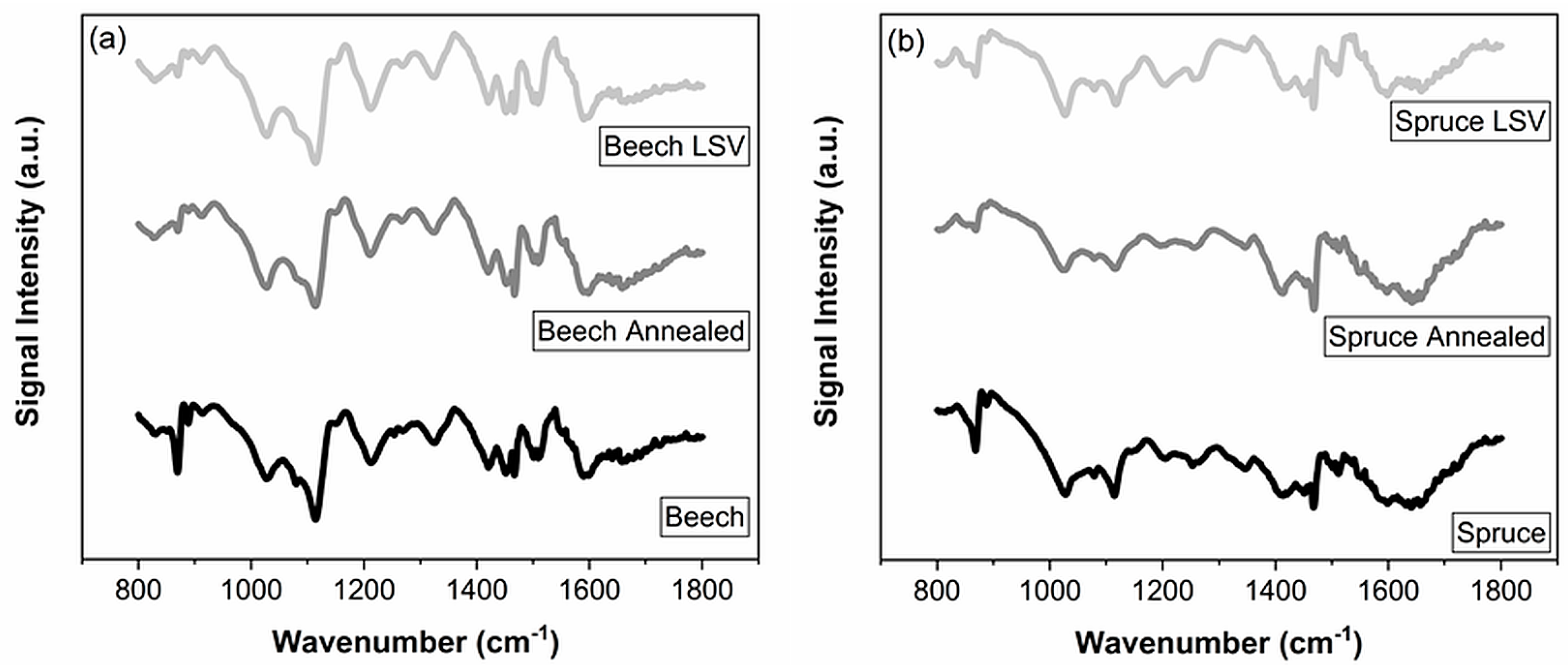
| No. | Spruce | Beech | Assignment |
|---|---|---|---|
| Band Position (cm−1) | Band Position (cm−1) | ||
| 1 | 1599 | 1590 | Aromatic ring vibration [44,45] |
| C=C stretch of aromatic ring [46] | |||
| 2 | 1501–1512 | 1500–1510 | Aromatic ring vibration [44,45] |
| 3 | 1467 | 1467 | C–OH deformation [44] |
| C–H asymmetric deformation [45] | |||
| C–H bend methyl/methylene [46] | |||
| 4 | 1420 | 1420 | C–OH in-plane deformation (aromatic stretch) [44] |
| C–H deformation [46] | |||
| 5 | 1322 | 1324 | C=O stretch syringyl units [44,45,46] |
| 6 | 1263 | – | C–O stretch of guaiacyl units [44,45,46] |
| 7 | 1205 | 1211 | C–C/C–O/C=O stretch guaiacyl units [46] |
| 8 | 1114 | – | Aromatic C–H or guaiacyl unit [46] |
| 9 | – | 1115 | C–H deformation syringyl units [46] |
| 10 | 1079 | 1079 | C–O stretch of secondary alcohol [46] |
| 11 | 1027 | 1027 | C–O stretch of primary alcohol [46] |
| 12 | – | 913 | C–H bending syringyl units, aromatic ring [46] |
| 13 | 869 | 869 | C–H out of plane [44] |
| 14 | – | 825 | C–H bending of syringyl units [46] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dastpak, A.; Yliniemi, K.; De Oliveira Monteiro, M.C.; Höhn, S.; Virtanen, S.; Lundström, M.; Wilson, B.P. From Waste to Valuable Resource: Lignin as a Sustainable Anti-Corrosion Coating. Coatings 2018, 8, 454. https://doi.org/10.3390/coatings8120454
Dastpak A, Yliniemi K, De Oliveira Monteiro MC, Höhn S, Virtanen S, Lundström M, Wilson BP. From Waste to Valuable Resource: Lignin as a Sustainable Anti-Corrosion Coating. Coatings. 2018; 8(12):454. https://doi.org/10.3390/coatings8120454
Chicago/Turabian StyleDastpak, Arman, Kirsi Yliniemi, Mariana Cecilio De Oliveira Monteiro, Sarah Höhn, Sannakaisa Virtanen, Mari Lundström, and Benjamin P. Wilson. 2018. "From Waste to Valuable Resource: Lignin as a Sustainable Anti-Corrosion Coating" Coatings 8, no. 12: 454. https://doi.org/10.3390/coatings8120454
APA StyleDastpak, A., Yliniemi, K., De Oliveira Monteiro, M. C., Höhn, S., Virtanen, S., Lundström, M., & Wilson, B. P. (2018). From Waste to Valuable Resource: Lignin as a Sustainable Anti-Corrosion Coating. Coatings, 8(12), 454. https://doi.org/10.3390/coatings8120454