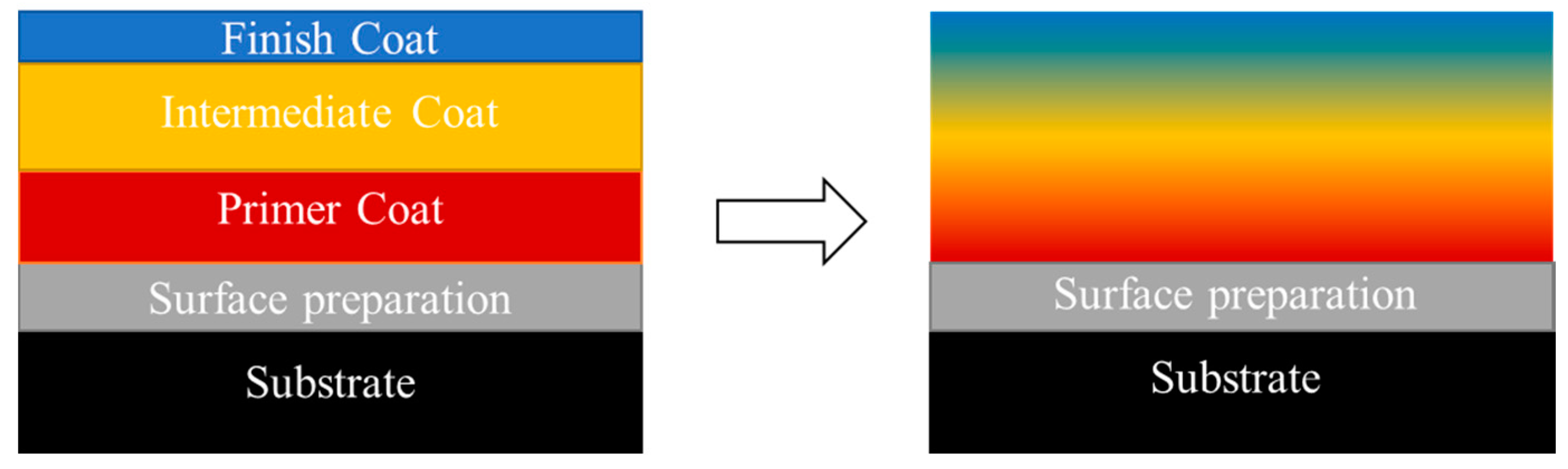
3.1. Stratification Study
After mixing and before application as a film, the four-component system (epoxy and silicone resins, curing agent and phosphate additive) produced homophase solutions whatever the phosphate concentration. After drying and curing, the formation of separated in-layers coating structure through the film thickness was checked and adhesion properties were evaluated (
Table 1).
The reference system without any phosphate additive shows perfect stratification (type I pattern) and the best adhesion rating (5B) on polycarbonate: the hydrophobic layer (the silicone medium) migrated to the upper part of the coating whereas the epoxy system was found at the interface with the substrate.
In presence of liquid FR fillers, nice coatings free of bubbles or defaults were obtained in all cases. They even show slight improvement in terms of surface aspect (less rough and glossier) than the reference coating. The 5B adhesion rating is maintained after the incorporation of RDP in the system, for both amounts tested, whereas the addition of BDP slightly impacts the adhesion of the coating (4B rating is obtained).
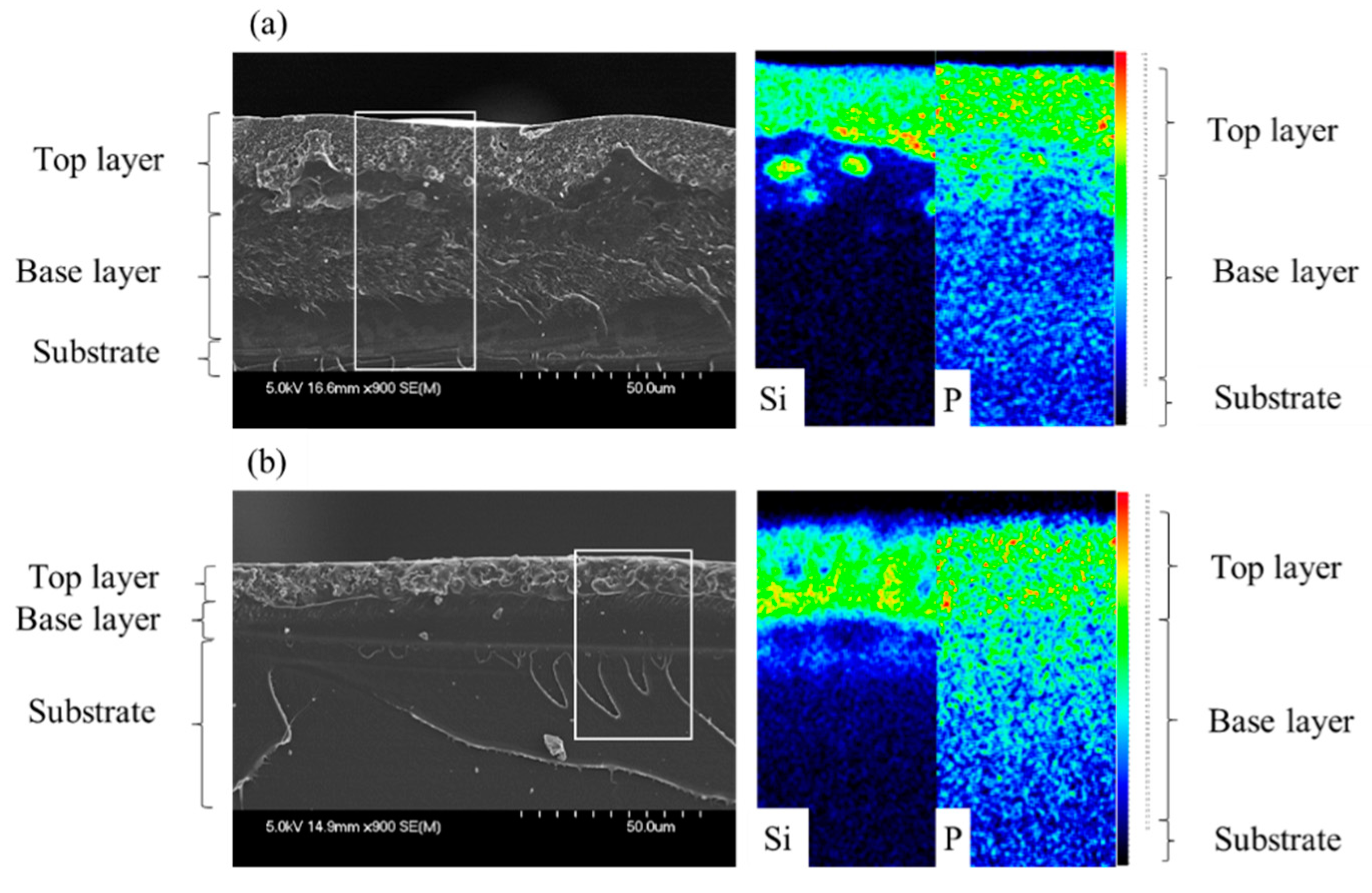
Unlike the results mentioned in the literature, it appears that neither RDP nor BDP affect the layering process when incorporated at 5 wt.% in the formulation: a type I pattern is obtained with fully separated layers, as observed with the unfilled system (
Figure 2).
Both additives migrate to the upper layer of the film, in the silicone medium, meaning that RDP and BDP have a higher affinity with silicone than with epoxy. This migration is rather a positive phenomenon in this case as it allows concentrating the additives’ properties (i.e., flame retardant properties) in the upper part of the coating, where it is the most needed in case of fire. With RDP, some inhomogeneities are noticeable along the film near the two resins interface region: some island shaped regions rich in silicone have not totally migrated to the upper layer of the film. A concentration gradient of phosphorus is also detectable through the film thickness, although the major part of RDP has migrated to the upper layer. With BDP, the same behavior can be observed: the silicone resin and the filler are mainly concentrated in the upper part of the coating. However, the concentration gradient of phosphorus is more pronounced through the thickness than with RDP. The difference could be explained by a higher affinity of RDP with the silicone resin, promoting the migration of the additive to the air interface. However, the concentration gradient is much more noticeable with phosphorus than with silicium: this means that the affinity between the silicone resin and the filler is not strong enough to allow its complete migration toward the top layer. A difference in viscosity could also explain the difference in concentration gradient between BDP and RDP containing formulations. Indeed, BDP is much more viscous than RDP (17,000 CPS [
22] compared to 12,450 CPS for RDP [
23]. As its viscosity is higher, its migration to the upper layer may be slowed down. It is noteworthy to notice that even if the additive has not enough time to completely migrate to the upper layer of the film, it does not impair the stratification process: the layering of the silicone to the top of the coating remains perfect.
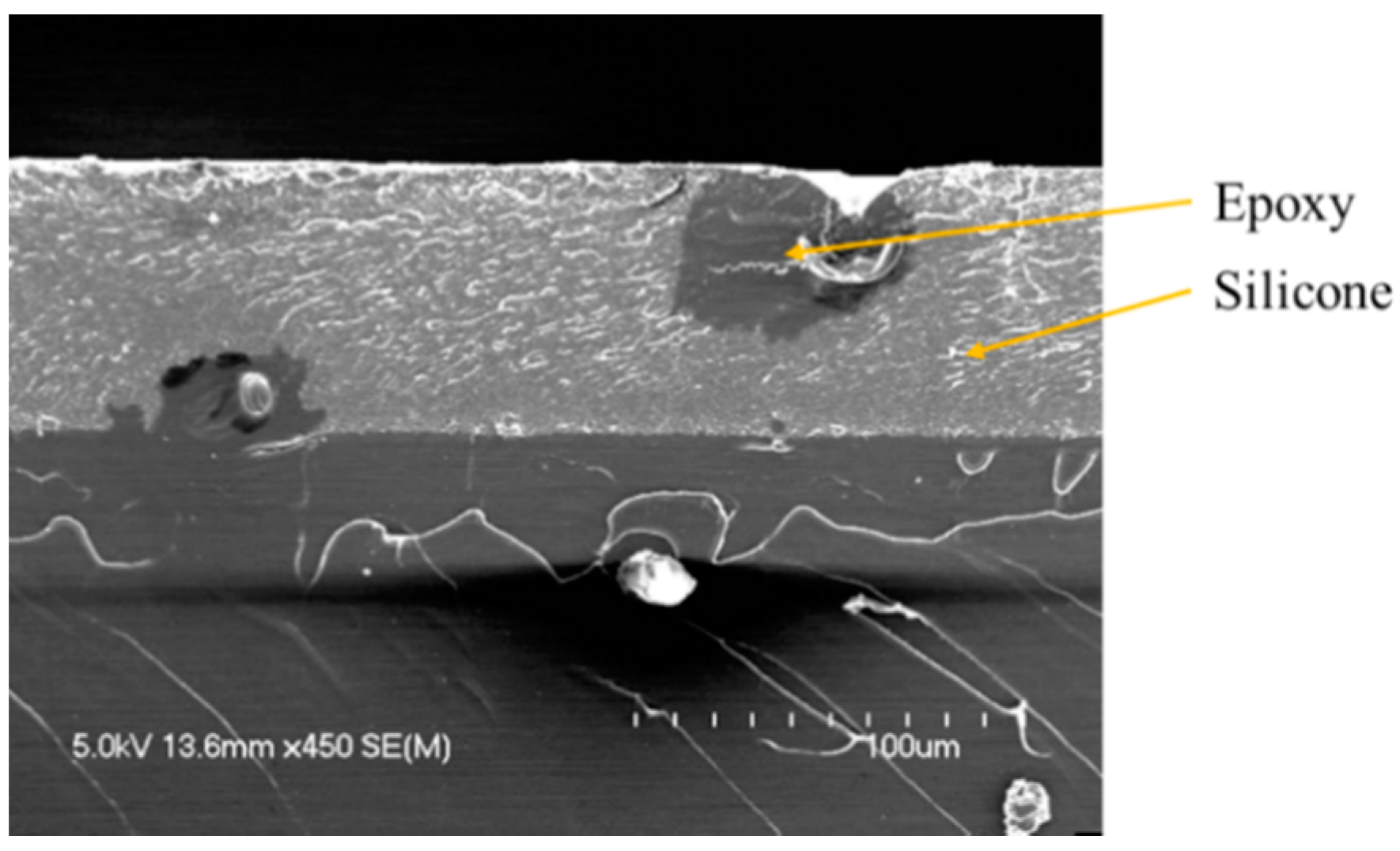
When a smaller amount of phosphate additives is introduced in the formulation (2.5 wt.%), evidences of phase separation are found with a concentration gradient of silicone through the thickness. Nevertheless, some inhomogeneities in the matrix are observed (
Figure 3), consisting in isolated spherical particles composed of epoxy resin dispersed in the continuous silicon matrix. Furthermore, the phosphorus element is found in the silicone phase whereas the stratification is not complete in those areas. This corroborates its higher affinity with silicone compare to that of epoxy. The phosphate additive destabilizes the interface between the two phases and, in that case, influences the preferential orientation of the phases in the course of film forming process. The dispersion of the filler is portioned in the silicone medium, however interacting in some way in the course of crosslinking reactions. A minimum amount of filler may be needed to allow a homogeneous dispersion into the silicone medium and this would need further investigation to the forces questioned in this process. This morphology corresponds to a type II/type III stratification pattern. However, this incomplete phase separation has no influence on the adhesion properties of the coatings as similar adhesion ratings are obtained with 2.5 wt.% and 5 wt.% of fillers, for both RDP and BDP [
24].
In conclusion, when a low amount of additive is added (2.5 wt.%), self-layering is slightly compromised in some areas of the film. The filler destabilizes the equilibrium between the two solutions, and impairs the formation of two distinct and homogeneous layers. On the contrary, the phosphorus-based fillers tested do not affect the formation of oriented heterophase structures when they are introduced at 5 wt.%: they mostly migrate, with a concentration gradient more or less pronounced, towards the air interface with the silicone resin. In that case, some disparities in the concentration gradient can be observed, which may be due to a difference in viscosity between the two phases. Finally, coating’s composition provided nice visual appearance and a high adhesion onto the polycarbonate substrate. It was proven that it is possible to add a substantial amount (5 wt.%) of liquid functional filler in a self-layering formulation without affecting the self-stratification process, the coating aspect and the adhesion. In these formulations, the phosphorus FR additives migrate into the upper silicone layer, which can potentially lead to an effective FR effect of the coating when exposed to fire.
3.2. Flame Retardant Properties
Virgin PC and PC coated with the unfilled and filled self-stratified compositions were then tested for fire performances (MLC) and flammability (UL-94 and LOI).
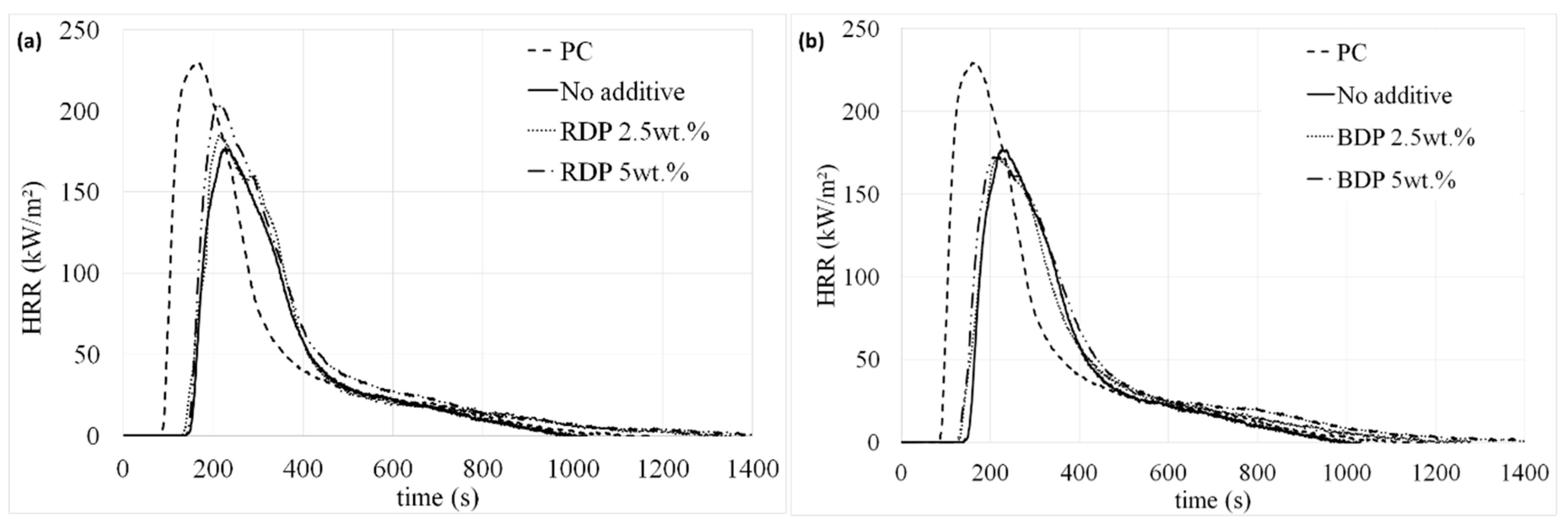
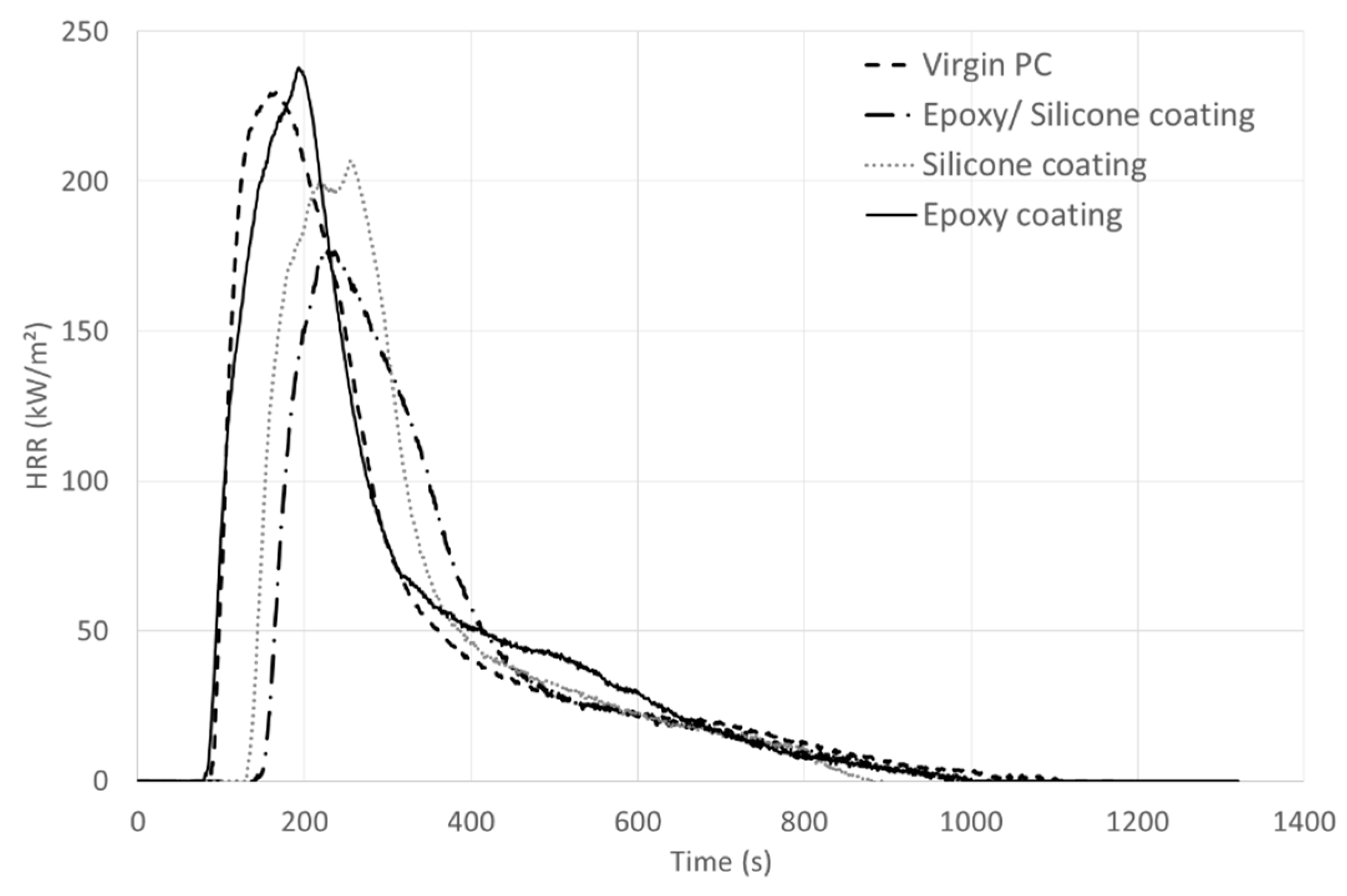
HRR curves and characteristic MLC parameters of the systems are given in
Figure 4 and
Table 2. It is noteworthy that no ignition of the coated samples (filled and unfilled) occurs at 35 kW/m² (mild fire scenario), even after 30 min of exposure; whereas the raw PC ignites after 319 s and releases a total heat of 35 MJ/m² (with a pHRR of 202 kW/m², see
Supplementary Materials Figure S1). PC is a char-forming polymer. At 50 kW/m², after its ignition, it melts and forms a char which swells until a height of about eight centimeters is reached. The char then degrades and only ashes remain at the end of the test.
Even at a higher heat flux (50 kW/m²), the coating allows protecting temporarily the PC. MLC results obtained for the coating with the unfilled epoxy/silicone coating evidence an improvement of the fire behavior, as both the pHRR and THR are reduced, respectively by 24% and 21% compared to raw PC. Ignition time is also delayed more than 50 s, which is remarkable with such thickness (55 µm). Ignition occurs only because PC swells under the coating, which thus progressively delaminates from the edges of the plate due to the uprising of the PC char. Once ignited, PC forms a char which rapidly swells up to 8 cm. The char is then consumed and collapses before the flame out takes place. At the end of the test, PC is completely consumed, which also explains the pHRR and THR values obtained.
MLC experiments on PC samples coated with only one resin, whether the epoxy resin or the silicone one, have been carried out to try to explain the behavior of PC coated with the unfilled epoxy/silicone blend (
Figure 5,
Table 3). When the epoxy resin is applied on PC, the behavior during the MLC test is similar to that of pure PC: no delay of the TTI and similar pHRR and THR. Thus, the epoxy resin does not bring any fire retardant effect, which is not surprising as it is composed of Bisphenol A (similarly to PC). On the contrary, the application of a silicone coating on PC leads to a fire retardant effect: slight decrease of pHRR and THR and TTI increase of about 40 s. Thus, the presence of silicone is responsible for the improvement of the fire behavior when the epoxy/silicone coating is applied to PC. However, the epoxy resin contributes to bringing better adherence between the self-stratifying coating and PC. In fact, the silicone coating gives a 3B rating on PC compared to a 5B rating with the self-stratifying coating. Moreover, there is no interlayer adhesion failure between the epoxy and the silicone layers thanks to the use of the self-stratifying coating.
Similar trends are observed when PC is coated with the epoxy/ silicone/ filler mixture, whatever the amount of additive incorporated. However, although the best TTI is obtained when RDP/BDP are incorporated at 2.5 wt.%, fire behavior are similar compared to that of the binary composition. The impact of RDP and BDP addition in terms of flame retardant behavior is thus negligible compared to the FR effect of the silicone coating itself. Finally, BDP seems slightly more efficient than RDP, which is not surprising as at a same ratio, BDP contains more phosphorus than RDP. Indeed, there is 10.7% of P in RDP whereas 8.9% are present in BDP. All the systems were consumed at the end of the test at 50 kW/m²: only residue of silica and ashes remained. The slight thickness difference between the two samples (
Table 1) is not responsible for this effect since any correlation is noted between the thickness, the TTI and THR: close THRs are reached by both filled coatings at equal weight percentage whereas a reverse effect is observed on the time before ignition.
LOI and UL-94 tests were also performed on uncoated and coated PC formulations (
Table 4). The epoxy/silicone reference coating does not improve the intrinsic flame retardant properties of PC in the case of vertical burning tests: close LOI values (27 vol.% and 28 vol.% respectively for the raw and coated PC) and a NC rating are obtained for both samples. Up to 28 vol.%, the extinction of virgin PC occurs mainly because of the flaming drops or of the rapid combustion of the material. The unfilled coating allows reducing the combustion speed of the material and prevents the dripping of PC. At UL-94 test, virgin PC is not classified at 3 mm, but close to meet the requirements of V-2 classification: short combustion time (<32 s) and dripping (
Table 5).
When the polymer is coated with the filled mixture, the fire behavior is strongly dependent on the amount of fillers used in the coating, whatever the type of additive (BDP or RDP). The addition of 2.5 wt.% of additives does not improve the fire behavior compared to the unfilled system: similar LOI value and same rating at UL-94 test. For these materials, the amount of additives is too low to allow the decrease of the flame spread. With 5 wt.% of additives, although the rating at UL-94 test is the same (NC), the behavior is much different: the combustion time is very short (≤2 s) after the first ignition and remains shorter than the unfilled system after the second ignition (
Table 5). The classification is very close to V-1, as with both additives= only one specimen out of the five tested has a t
2 afterflame time higher than 30 s (in bold,
Table 5). In addition, LOI values are significantly improved: 34 vol.% and 32 vol.% respectively with RDP and BDP fillers. The charring is more pronounced (fast expansion of the foamed structure) and no dripping occurs. Last but not least, samples were more prone for burning during the second ignition with a lower concentration of additive in the coating.
Thus, similar behavior between RDP and BDP can be registered: they are both more efficient when they are introduced at a higher amount (5 wt.%). As with MLC, the slight difference observed between RDP and BDP may be due to the higher amount of phosphorus in the RDP formulation.
To conclude, formulations containing 5 wt.% of RDP and BDP exhibit the most promising results: charring is more pronounced and dripping is avoided. At MLC test, fire performances of coated formulations are mainly enhanced through the shift of the TTI toward higher values due to the silicone coating. The influence of the two phosphorus-based additives on the fire properties is not significant compared to the improvements already reached by the unfilled epoxy/silicone system.
Occurrence and efficiency of the systems depend not only on the FR itself, but also on the interactions with the degradation products of the different components of the materials (resins and substrate). To go deeper in the understanding of the mode of action of the phosphorus compounds, TG analyses and difference weight loss calculations were carried out on the silicone resin, containing phosphorus additives or without. During a fire scenario, the silicone will be the resin in contact with the open flame or exposed to the heat source as it is located in the upper layer of the film. Consequently, only the silicone resin was considered.
3.3. Thermal Stability
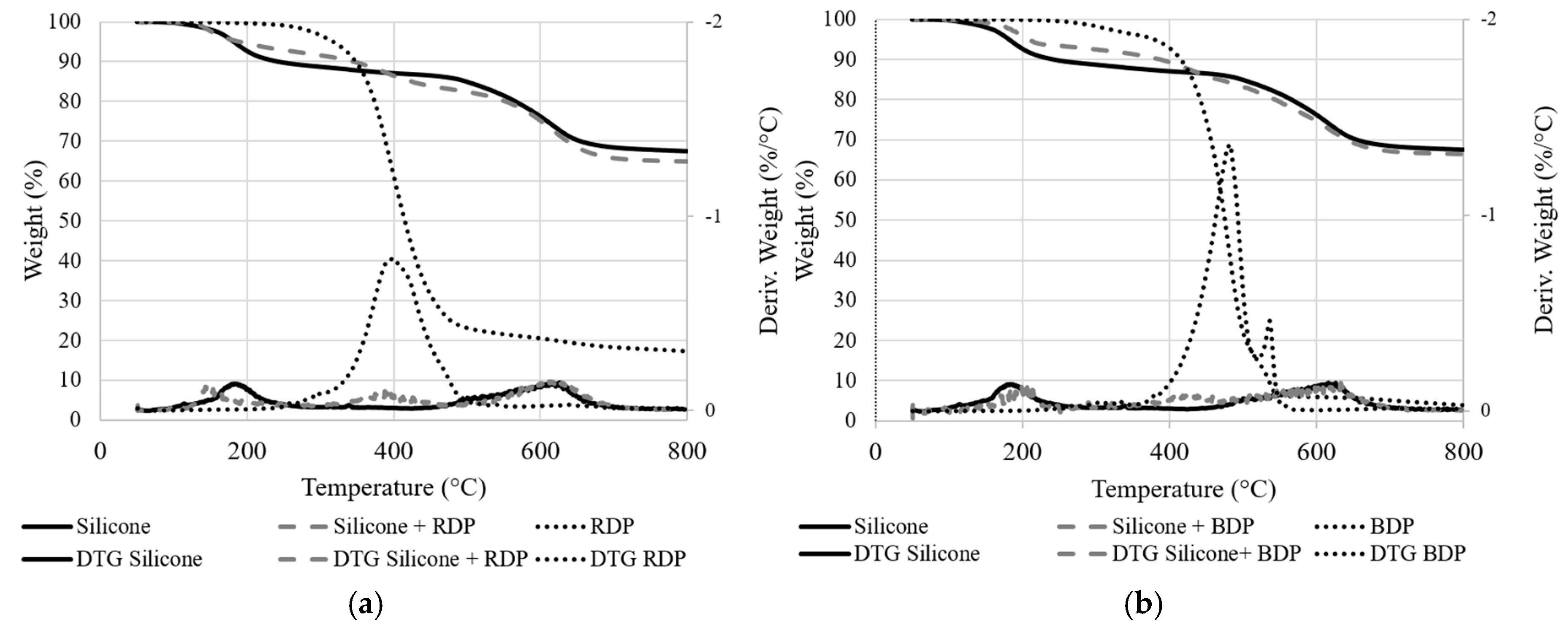
The thermal stability of the unfilled system and systems filled with RDP and BDP were investigated using TG analyses (
Figure 6 and
Figure 7. Weight difference curves between experimental and calculated TG curves when RDP or BDP is added to the resin (10 wt.%) under N
2 conditions). The percentage of fillers introduced in the silicone medium was kept constant compared to the epoxy/silicone system as the major part of the filler migrates to the silicone layer (i.e., 10 wt.% of filler in the silicone medium).
The decomposition of the pure silicone film involves a three-step process with a 68 wt.% residual weight at 800 °C. The first degradation step may be coupled with the release of the remaining solvents from the resin’s preparation and the release of silicone oligomers (11 wt.%) and the second and third steps (overlapped) are correlated with to the release of aromatic compounds (such as benzene and bisphenyl, 21 wt.%) [
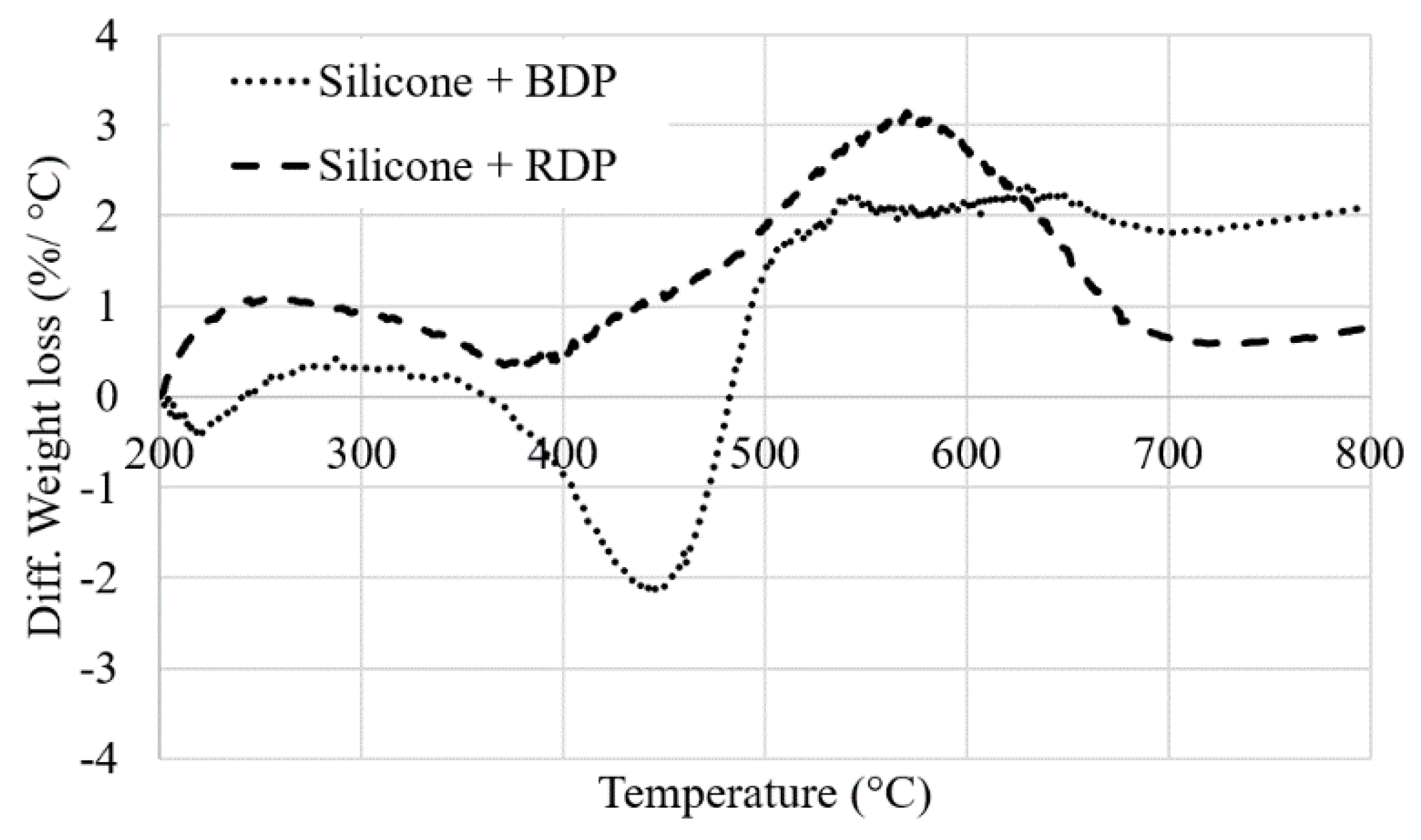
25]. The total weight loss is only 32% at 800 °C, demonstrating the excellent thermal stability of the silicone resin (under TGA conditions). When FR liquid additives are incorporated, an additional degradation step occurs close to the maximum degradation temperature of RDP and BDP (at respectively 398 and 420 °C). This leads to a slight destabilization of the system, particularly with BDP between 370 and 480 °C (−2 %/°C,
Figure 7). Weight difference curves between experimental and calculated TG curves when RDP or BDP is added to the resin (10 wt.%) under N
2 conditions). The residual weights of the additives are low (respectively 4 wt.% and 17 wt.% at 800 °C with BDP and RDP), although it does not neither influence significantly the thermal stability of the resins nor favor the formation of a charring structure at high temperature.
TG experiments enable us to define characteristic temperatures of degradation. Accordingly, heat treatments were performed at 300 °C in a tubular furnace and under a nitrogen flow on the silicone and the silicone/fillers systems (
Figure 8). From the numerical pictures, the formation of an expanded foamed structure with small cells, whatever the phosphorus compound used is evidenced: the fillers do not modify the cellular structure. Finally, RDP and BDP have low influence on the thermal stability of the silicone resin; the amount of the remaining residue at 800 °C is almost the same, and the degradation process remains unchanged compared to the pure silicone. Based on those observations, the formation of an expanded coating during the test explains, at least partially, the fire protection brought by the paint and the silicone-based coating containing the fillers. The silicone and silicone/filler compositions allow the formation of a protective barrier, thus reducing heat transfers from the external heating source to the substrate.
Visual observations during the horizontal fire tests confirm the higher char yield obtained when phosphorus compounds are incorporated into the systems, even if thermal analyses did not show major improvements in terms of stability. Based on the literature review, phosphorus compounds accumulate in the condensed phase in the char layer, at the surface of the burning specimens in a blend of PC-ABS + RDP [
26]. Results suggested that PC undergoes photo-fries rearrangement upon thermal decomposition, and RDP would react with the formed phenolic groups through a trans-esterification mechanism. Kinetic analysis of the thermal decomposition of PC containing RDP supported the proposed mechanism.
In conclusion, the high thermal stability of the silicone resin under inert atmosphere at elevated temperatures has been demonstrated. As this component is at the interface with the air in the self-stratifying coating composition, it will consequently be the first in contact with either the heat source or the flame during the fire tests. According to the results, the silicone resin provides some thermal stability to the system, which may explain the higher time to ignition obtained during MLC experiments. Under elevated temperatures, the silicone coating protects the underlying substrate (and the epoxy resin) from decomposition and creates a barrier between the heat source and the substrate. Moreover, the similar behavior in terms of thermal stability for the silicone resin with and without fillers could explain the close results at MLC test for all the epoxy/silicone coatings.