Discoloration Resistance of Electrolytic Copper Foil Following 1,2,3-Benzotriazole Surface Treatment with Sodium Molybdate
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
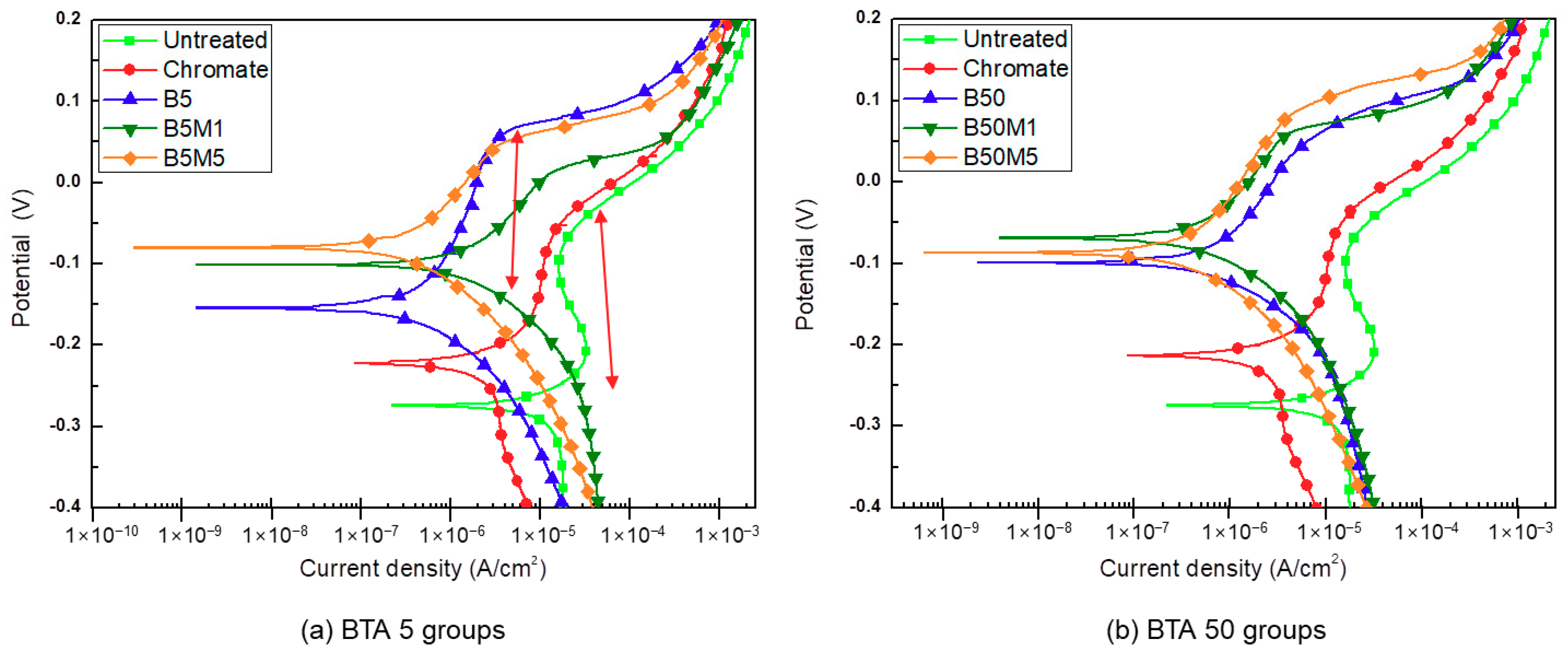
3.1. Characterization of Electro-Chemical Corrosion
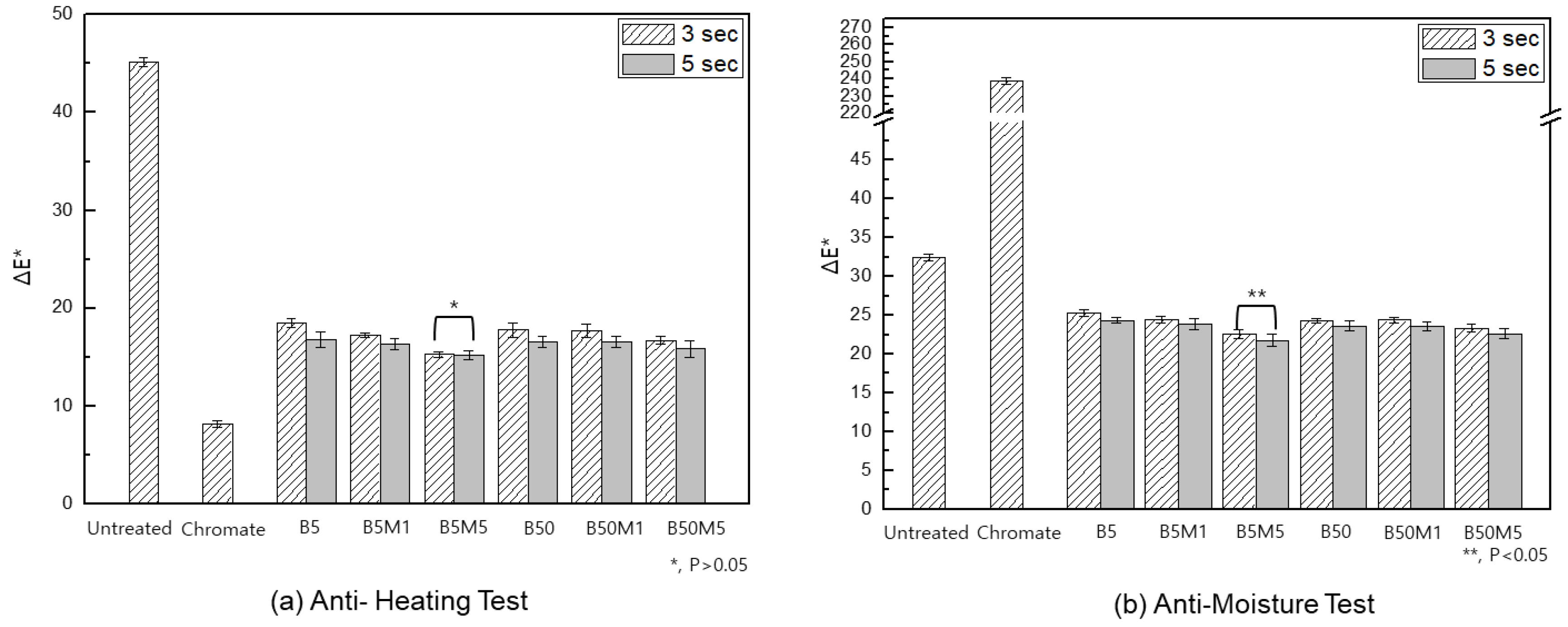
3.2. Anti-Discoloration Characteristic
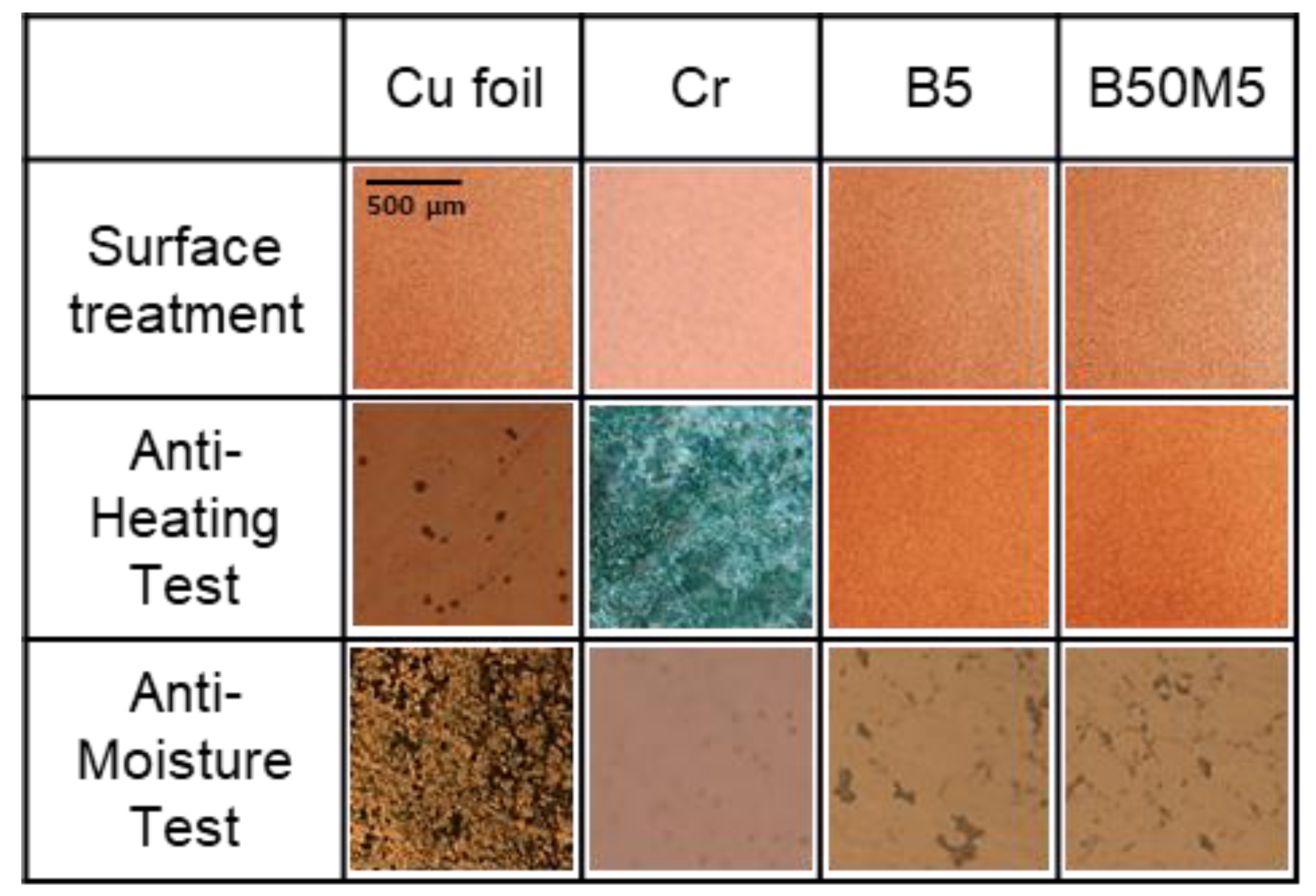
3.3. Surface Morphology Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weiss, B.; Gröger, V.; Khatibi, G.; Kotas, A.; Zimprich, P.; Stickler, R.; Zagar, B. Characterization of mechanical and thermal properties of thin Cu foils and wires. Sens. Actuators A Phys. 2002, 99, 172–182. [Google Scholar] [CrossRef]
- Merchant, H.D.; Minor, M.G.; Liu, Y.L. Mechanical fatigue of thin copper foil. J. Electron. Mater. 1999, 28, 998–1007. [Google Scholar] [CrossRef]
- De Angelis, R.; Knorr, D.; Merchant, H. Through-thickness characterization of copper electrodeposit. J. Electron. Mater. 1995, 24, 927. [Google Scholar] [CrossRef]
- Merchant, H.D.; Liu, W.C.; Giannuzzi, L.A.; Morris, J.G. Grain structure of thin electrodeposited and rolled copper foils. Mater. Charact. 2004, 53, 335–360. [Google Scholar] [CrossRef]
- FitzGerald, K.P.; Nairn, J.; Skennerton, G.; Atrens, A. Atmospheric corrosion of copper and the colour, structure and composition of natural patinas on copper. Corros. Sci. 2006, 48, 2480–2509. [Google Scholar] [CrossRef]
- Rickett, B.I.; Payer, J.H. Composition of copper tarnish products formed in moist air with trace levels of pollutant gas: Sulfur dioxide and sulfur dioxide/nitrogen dioxide. J. Electrochem. Soc. 1995, 142, 3713–3722. [Google Scholar] [CrossRef]
- Watanabe, M.; Tomita, M.; Ichino, T. Analysis of tarnish films on copper exposed in hot spring area. J. Electrochem. Soc. 2002, 149, B97–B102. [Google Scholar] [CrossRef]
- Kajiwara, T.; Tanii, Y.; Hashimoto, K. Method of Making Thin Copper Foil for Printed Wiring Board. U.S. Patent 5,114,543 A, 19 May 1992. [Google Scholar]
- Yamanishi, K.; Oshima, H.; Sakaguchi, K. Copper Foil for Printed Circuits and Process for Producing the Same. U.S. Patent 5,366,814 A, 22 November 1994. [Google Scholar]
- Morisaki, S.; Mase, K. Copper Foil Having Bond Strength. U.S. Patent 4,010,005 A, 1 March 1977. [Google Scholar]
- Hua, L.; Chan, Y.C.; Wu, Y.P.; Wu, B.Y. The determination of hexavalent chromium (Cr6+) in electronic and electrical components and products to comply with RoHS regulations. J. Hazard. Mater. 2009, 163, 1360–1368. [Google Scholar] [CrossRef]
- Zucchi, F.; Trabanelli, G.; Monticelli, C. The inhibition of copper corrosion in 0.1 M NaCl under heat exchange conditions. Corros. Sci. 1996, 38, 147–154. [Google Scholar] [CrossRef]
- Finšgar, M.; Milošev, I. Inhibition of copper corrosion by 1,2,3-benzotriazole: A review. Corros. Sci. 2010, 52, 2737–2749. [Google Scholar] [CrossRef]
- Ishida, H.; Johnson, R. The inhibition of copper corrosion by azole compounds in humid environments. Corros. Sci. 1986, 26, 657–667. [Google Scholar] [CrossRef]
- Xu, Z.; Lau, S.; Bohn, P.W. The role of benzotriazole in corrosion inhibition: Formation of an oriented monolayer on Cu2O. Surf. Sci. 1993, 296, 57–66. [Google Scholar] [CrossRef]
- Shams El Din, A.M.; Mohammed, R.A.; Haggag, H.H. Corrosion inhibition by molybdate/polymaliate mixtures. Desalination 1997, 114, 85–95. [Google Scholar] [CrossRef]
- Ramesh, S.; Rajeswari, S.; Maruthamuthu, S. Corrosion inhibition of copper by new triazole phosphonate derivatives. Appl. Surf. Sci. 2004, 229, 214–225. [Google Scholar] [CrossRef]
- Gelman, D.; Starosvetsky, D.; Ein-Eli, Y. Copper corrosion mitigation by binary inhibitor compositions of potassium sorbate and benzotriazole. Corros. Sci. 2014, 82, 271–279. [Google Scholar] [CrossRef]
- Villamil, R.; Corio, P.; Rubim, J.; Agostinho, S. Sodium dodecylsulfate–benzotriazole synergistic effect as an inhibitor of processes on copper| chloridric acid interfaces. J. Electroanal. Chem. 2002, 535, 75–83. [Google Scholar] [CrossRef]
- Saremi, M.; Dehghanian, C.; Sabet, M.M. The effect of molybdate concentration and hydrodynamic effect on mild steel corrosion inhibition in simulated cooling water. Corros. Sci. 2006, 48, 1404–1412. [Google Scholar] [CrossRef]
- Vukasovich, M.S.; Farr, J.P.G. Molybdate in corrosion inhibition—A review. Polyhedron 1986, 5, 551–559. [Google Scholar] [CrossRef]
- Ramesh, S.; Rajeswari, S. Evaluation of inhibitors and biocide on the corrosion control of copper in neutral aqueous environment. Corros. Sci. 2005, 47, 151–169. [Google Scholar] [CrossRef]
- Mustafa, C.; Shahinoor Islam Dulal, S. Molybdate and nitrite as corrosion inhibitors for copper-coupled steel in simulated cooling water. Corrosion 1996, 52, 16–22. [Google Scholar] [CrossRef]
- Deyab, M.A.; Abd El-Rehim, S.S. Inhibitory effect of tungstate, molybdate and nitrite ions on the carbon steel pitting corrosion in alkaline formation water containing Cl− ion. Electrochim. Acta 2007, 53, 1754–1760. [Google Scholar] [CrossRef]
- Zhang, D.-Q.; Joo, H.G.; Lee, K.Y. Investigation of molybdate–benzotriazole surface treatment against copper tarnishing. Surf. Interface Anal. 2009, 41, 164–169. [Google Scholar] [CrossRef]
- Yang, J.-S.; Lim, S.-L.; Kim, S.-B.; Kim, K.-J. Electrolyte Solution for Manufacturing Electrolytic Copper Foil and Electrolytic Copper Foil Manufacturing Method Using the Same. U.S. Patent 20040104117A1, 3 June 2004. [Google Scholar]
- Stansbury, E.E.; Buchanan, R.A. Kinetics of Single Half-Cell Reactions. In Fundamentals of Electrochemical Corrosion; Stansbury, E.E., Buchanan, R.A., Eds.; ASM International: Novelty, OH, USA, 2000. [Google Scholar]
- Bard, A.J.; Faulkner, L.R.; Leddy, J.; Zoski, C.G. Electrochemical methods: Fundamentals and Applications; Wiley: Hoboken, NJ, USA, 1980. [Google Scholar]
- Domínguez-Crespo, M.A.; Onofre-Bustamante, E.; Torres-Huerta, A.M.; Rodríguez-Gómez, F.J. Morphology and corrosion performance of chromate conversion coatings on different substrates. J. Mex. Chem. Soc. 2008, 52, 241–248. [Google Scholar]
- Jiang, L.; He, Y.; Niu, X.; Li, Y.; Luo, J. Synergetic effect of benzotriazole and non-ionic surfactant on copper chemical mechanical polishing in KIO4-based slurries. Thin Solid Films 2014, 558, 272–278. [Google Scholar] [CrossRef]
- Finšgar, M.; Lesar, A.; Kokalj, A.; Milošev, I. A comparative electrochemical and quantum chemical calculation study of BTAH and BTAOH as copper corrosion inhibitors in near neutral chloride solution. Electrochim. Acta 2008, 53, 8287–8297. [Google Scholar] [CrossRef]
- Breslin, C.; Treacy, G.; Carroll, W. Studies on the passivation of aluminium in chromate and molybdate solutions. Corros. Sci. 1994, 36, 1143–1154. [Google Scholar] [CrossRef]
- Moshier, W.; Davis, G. Interaction of molybdate anions with the passive film on aluminum. Corrosion 1990, 46, 43–50. [Google Scholar] [CrossRef]
- Zhou, Y.; Zuo, Y.; Lin, B. The compounded inhibition of sodium molybdate and benzotriazole on pitting corrosion of Q235 steel in NaCl + NaHCO3 solution. Mater. Chem. Phys. 2017, 192, 86–93. [Google Scholar] [CrossRef]
- Jones, D.A. Principles and Prevention of Corrosion, 2nd ed.; Prentice Hall: Upper Saddle River, NY, USA, 1996; pp. 168–198. [Google Scholar]
- Chandrasekaran, M. Metals for Biomedical Devices. In Forging of Metals and Alloys for Biomedical Applications; Niinomi, M., Ed.; Woodhead Publishing: Cambridge, UK, 2010; pp. 235–250. [Google Scholar]
- Kim, S.W.; Lee, C.T. Environment-friendly trivalent chromate treatment for Zn electroplating. J. Korean Ind. Eng. Chem. 2006, 17, 433. [Google Scholar]
- Youda, R.; Nishihara, H.; Aramaki, K. SERS and impedance study of the equilibrium between complex formation and adsorption of benzotriazole and 4-hydroxybenzotriazole on a copper electrode in sulphate solutions. Electrochim. Acta 1990, 35, 1011–1017. [Google Scholar] [CrossRef]
- Dorward, R.C.; Hasse, K.R. Incubation effects in precracked stress corrosion specimens from Al-Zn-Mg-Cu alloy 7075. Corros. Sci. 1979, 19, 131–140. [Google Scholar] [CrossRef]
- Laget, V.; Jeffcoate, C.; Isaacs, H.; Buchheit, R. Dehydration-induced loss of corrosion protection properties in chromate conversion coatings on aluminum alloy 2024-T3. J. Electrochem. Soc. 2003, 150, B425–B432. [Google Scholar] [CrossRef]
- Lopez-Garrity, O.; Frankel, G. Corrosion inhibition of aluminum alloy 2024-T3 by sodium molybdate. J. Electrochem. Soc. 2014, 161, C95–C106. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, J.; Peng, D.; Li, X.; Huang, Y. In-Situ Scanning Micro-Electrochemical Characterization of Corrosion Inhibitors on Copper. Available online: https://dr.ntu.edu.sg/handle/10220/40846 (accessed on 26 October 2018).
- Mert, B.D.; Mert, M.E.; Kardaş, G.; Yazıcı, B. Experimental and theoretical investigation of 3-amino-1,2,4-triazole-5-thiol as a corrosion inhibitor for carbon steel in HCl medium. Corros. Sci. 2011, 53, 4265–4272. [Google Scholar] [CrossRef]



| Sample Code | BTA (mM) | Sodium-Molybdate (mM) | Surface Treatment Time (s) | |
|---|---|---|---|---|
| Comparison Groups | Untreated | – | – | – |
| Chromate | – | – | – | |
| Modified Groups | B5 | 5 | – | 3/5 |
| B5M1 | 5 | 1 | ||
| B5M5 | 5 | 5 | ||
| B50 | 50 | – | ||
| B50M1 | 50 | 1 | ||
| B50M5 | 50 | 5 | ||
| Sample Code | Ev (V) | Standard Deviation | Icorr (A/cm2) | Standard Deviation (10−6) |
|---|---|---|---|---|
| Untreated | −0.278 | ±0.012 | 1.152 × 10−5 | ±1.984 |
| Chromate | −0.227 | ±0.008 | 1.906 × 10−6 | ±0.331 |
| B5(3 s) | −0.152 | ±0.007 | 2.392 × 10−6 | ±2.616 |
| B5M1(3 s) | −0.115 | ±0.007 | 6.880 × 10−7 | ±0.149 |
| B5M5(3 s) | −0.096 | ±0.005 | 7.249 × 10−7 | ±0.271 |
| B50(3 s) | −0.158 | ±0.007 | 5.336 × 10−7 | ±0.099 |
| B50M1(3 s) | −0.120 | ±0.006 | 6.469 × 10−7 | ±0.151 |
| B50M5(3 s) | −0.104 | ±0.011 | 7.223 × 10−7 | ±0.143 |
| B5(5 s) | −0.155 | ±0.004 | 3.708 × 10−7 | ±0.090 |
| B5M1(5 s) | −0.098 | ±0.007 | 1.623 × 10−6 | ±0.551 |
| B5M5(5 s) | −0.081 | ±0.005 | 3.749 × 10−7 | ±0.115 |
| B50(5 s) | −0.101 | ±0.005 | 8.213 × 10−7 | ±0.245 |
| B50M1(5 s) | −0.074 | ±0.009 | 6.505 × 10−7 | ±0.203 |
| B50M5(5 s) | −0.070 | ±0.036 | 4.357 × 10−7 | ±0.135 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, D.-J.; Kim, Y.-K.; Yoon, J.-M.; Park, I.-S. Discoloration Resistance of Electrolytic Copper Foil Following 1,2,3-Benzotriazole Surface Treatment with Sodium Molybdate. Coatings 2018, 8, 427. https://doi.org/10.3390/coatings8120427
Shin D-J, Kim Y-K, Yoon J-M, Park I-S. Discoloration Resistance of Electrolytic Copper Foil Following 1,2,3-Benzotriazole Surface Treatment with Sodium Molybdate. Coatings. 2018; 8(12):427. https://doi.org/10.3390/coatings8120427
Chicago/Turabian StyleShin, Dong-Jun, Yu-Kyoung Kim, Jeong-Mo Yoon, and Il-Song Park. 2018. "Discoloration Resistance of Electrolytic Copper Foil Following 1,2,3-Benzotriazole Surface Treatment with Sodium Molybdate" Coatings 8, no. 12: 427. https://doi.org/10.3390/coatings8120427
APA StyleShin, D.-J., Kim, Y.-K., Yoon, J.-M., & Park, I.-S. (2018). Discoloration Resistance of Electrolytic Copper Foil Following 1,2,3-Benzotriazole Surface Treatment with Sodium Molybdate. Coatings, 8(12), 427. https://doi.org/10.3390/coatings8120427





