Bio-Functional Properties of Bee Pollen: The Case of “Bee Pollen Yoghurt”
Abstract
1. Introduction
2. Materials and Methods
2.1. Bee Pollen Samples
2.2. Collection of Milk and Preparation of Yoghurt Samples
2.3. Reagents and Solutions
2.4. Physicochemical Parameter Analysis
2.5. Extraction of Phenolic Compounds
2.6. Analysis of Bee Pollen Phenolic Compounds Using High Performance Liquid Chromatography Electrospray Ionization Mass Spectrometry (HPLC/ESI-MS)
2.7. Determination of Total Phenolic Content
2.7.1. Preparation of DPPH Free Radical Standard Solution
2.7.2. Preparation of DPPH Free Radical Calibration Curve
2.7.3. Determination of In Vitro Antioxidant Capacity of Bee Pollen Ethanolic Extracts and Pollen Enriched Yoghurts
2.7.4. Sensory Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Parameter Analysis
3.2. Phytochemicals of Bee Pollen
3.3. In Vitro Antioxidant Capacity of Bee Pollen Ethanolic Extracts
3.4. In Vitro Antioxidant Capacity of Bee Pollen Enriched Yoghurts
3.5. Total Phenolic Content of Bee Pollen Ethanolic Extracts
3.6. Total Phenolic Content of Bee Pollen Enriched Yoghurts
3.7. Comparison of the In Vitro Antioxidant Capacity of Bee Pollen Ethanolic Extracts/Bee Pollen Yoghurts with Those of Standard Gallic Acid and Quercetin Solutions and Prospective Healthy Eating Habits
3.8. Sensory Analysis
3.9. Discrimination of Conventional and Bee Pollen Yoghurts According to Milk Type-Quality Control Analysis
3.10. Modeling of Results Using Regression Analysis
4. Conclusions
5. Patent
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roberts, W.A., Jr. Benefiting Beverages. Prepared Foods Website. Available online: https://www.preparedfoods.com/articles/107718-article-benefiting-beverages----august-2009 (accessed on 22 November 2018).
- Basics about Functional Food; US Department of Agriculture, Agricultural Research Service: Washington, DC, USA.
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 Diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, S. Pollen: Production, Nutrition and Health: A Review. Bee Product Science Website. Available online: https://www.bee-hexagon.net (accessed on 22 November 2018).
- Conte, P.; Del Caro, A.; Balestra, F.; Piga, A.; Fadda, C. Bee pollen as a functional ingredient in gluten-free bread: A physical-chemical, technological and sensory approach. LWT Food Sci. Technol. 2018, 90, 1–7. [Google Scholar] [CrossRef]
- Komosinska-Vassev, K.; Olczyk, P.; Kafmierczak, J.; Mencner, L.; Olczyk, K. Bee Pollen: Chemical composition and therapeutic application. J. Evid. Based Complement. Altern. Med. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, M.; Macchione, N.; Ferrante, C.; Chiavaroli, A.; Recinella, L.; Carradori, S.; Zengin, G.; Cesa, S.; Leporini, L.; Leone, S.; et al. Graminex Pollen: Phenolic pattern, colorimetric analysis and protective effects in immortalized prostate cells (PC3) and rat prostate challenged with LPS. Molecules 2018, 23, 1145. [Google Scholar] [CrossRef] [PubMed]
- Pehrsson, P.R.; Haytowitz, D.B.; Holden, J.M.; Perry, C.R.; Beckler, D.G. USDA’s National Food and Nutrient Analysis Program: Food Sampling. J. Food Compos. Anal. 2000, 13, 379–389. [Google Scholar] [CrossRef]
- Yerlikaya, O. Effect of bee pollen supplement on antimicrobial, chemical, rheological, sensorial properties and probiotic viability of fermented milk beverages. Mijekarstvo 2014, 64, 268–279. [Google Scholar] [CrossRef]
- Lomova, N.; Narizhnyi, S.; Snizhko, O. Yoghurt enrichment with natural bee farming products. Ukr. Food J. 2014, 3, 415–421. [Google Scholar]
- Atallah, A.A. The production of bio-yoghurt with probiotic bacteria, royal jelly and bee pollen grains. J. Nutr. Food Sci. 2016, 6, 510. [Google Scholar]
- Cossu, M.; Juliano, C.; Pisu, R.; Alamanni, M.C. Effects of enrichment with polyphenolic extracts from Sardinian plants on physico-chemical, antioxidant and microbiological properties of yogurt. Ital. J. Food Sci. 2009, 21, 447–459. [Google Scholar]
- Najgebauer-Lejko, D.; Marek Sady, M. Estimation of the antioxidant activity of the commercially available fermented milks. Acta Sci. Pol. Technol. Aliment. 2015, 14, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Graikou, K.; Kapeta, S.; Aligiannis, N.; Sotiroudis, G.; Chondrogianni, N.; Gonos, E.; Chinou, I. Chemical analysis of Greek pollen-Antioxidant, antimicrobial and proteasome activation. Chem. Cent. J. 2011, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Karabagias, I.K.; Koutsoumpou, M.; Liakou, V.; Kontakos, S.; Kontominas, M.G. Characterization and geographical discrimination of saffron from Greece, Spain, Iran, and Morocco based on volatile and bioactivity markers, using chemometrics. Eur. Food Res. Technol. 2017, 243, 1577–1591. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Vavoura, M.V.; Badeka, A.; Kontakos, S.; Kontominas, M.G. Differentiation of Greek thyme honeys according to geographical origin based on the combination of phenolic compounds and conventional quality parameters using chemometrics. Food Anal. Methods 2014, 7, 2113–2121. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Locatelli, M.; Gindro, R.; Travaglia, F.; Coïsson, J.-D.; Rinaldi, M.; Arlorio, M. Study of the DPPH•-scavenging activity: Development of a free software for the correct interpretation of data. Food Chem. 2009, 114, 889–897. [Google Scholar] [CrossRef]
- Miller, J.N.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry, 6th ed.; Prentice Hall: Harlow, UK, 2010. [Google Scholar]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remón, A.; M’Hiri, N.; García-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef] [PubMed]
- Markham, K.R.; Campos, M.G. 7- and 8-O-methylherbacetin-3-O-sophorosides from bee pollen and some structure/activity observations. Phytochemistry 1996, 43, 763–767. [Google Scholar] [CrossRef]
- Buenger, J.; Ackermann, H.; Jentzsch, A.; Mehling, A.; Pfitzner, I.; Reiffen, K.A.; Schroeder, K.R.; Wollenweber, U. An interlaboratory comparison of methods used to assess antioxidant potentials. Int. J. Cosmet. Sci. 2006, 28, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, B.W.; Davis, O.K.; Boue, S.; Delucca, A.; Deeby, T. Antioxidant activity of Sonoran Desert bee pollen. Food Chem. 2009, 115, 1299–1305. [Google Scholar] [CrossRef]
- Rebiai, A.; Lanez, T. Chemical composition and antioxidant activity of Apis mellifera bee pollen from Northwest Algeria. J. Fundam. Appl. Sci. 2012, 4, 26–35. [Google Scholar] [CrossRef]
- Menghini, L.; Leporini, L.; Vecchiotti, G.; Locatelli, M.; Carradori, S.; Ferrante, C.; Zengin, G.; Recinella, L.; Chiavarolia, A.; Leone, S.; et al. Crocus sativus L. stigmas and byproducts: Qualitative fingerprint, antioxidant potentials and enzyme inhibitory activities. Food Res. Int. 2018, 108, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, T.R.; Satter, L.D.; Pariza, M.W.; Galli, M.P.; Albright, K.; Tolosa, M.X. Conjugated Linoleic Acid (CLA) content of milk from cows offered diets rich in linoleic and linolenic acid. J. Dairy Sci. 2000, 83, 1016–1027. [Google Scholar] [CrossRef]
- Richard, D.; Kefi, K.; Barbe, U.; Bausero, P.; Visioli, F. Polyunsaturated fatty acids as antioxidants. Pharmacol. Res. 2008, 57, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.F. Advanced Dairy Chemistry, 2nd ed.; Chapman and Hall: London, UK, 1995; Volume 3. [Google Scholar]
- Ikawa, M.; Schaper, T.D.; Dollard, C.A.; Sasner, J.J. Utilization of Folin–Ciocalteu phenol reagent for the detection of certain nitrogen compounds. J. Agric. Food Chem. 2003, 51, 1811–1815. [Google Scholar] [CrossRef] [PubMed]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough study of reactivity of various compound classes toward the Folin−Ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, S. Quality and standards of pollen and beeswax. APIACTA 2004, 38, 334–341. [Google Scholar]
- Cerdá, B.; Tomás-Barberán, F.A.; Espín, J.C. Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak-aged wine in humans: Identification of biomarkers and individual variability. J. Agric. Food Chem. 2005, 53, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Bialonska, D.; Kasimsetty, S.G.; Khan, S.I.; Ferreira, D. Urolithins, intestinal microbial metabolites of pomegranate ellagitannins, exhibit potent antioxidant activity in a cell-based assay. J. Agric. Food Chem. 2009, 57, 10181–10186. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Simonoff, J. Handbook of Regression Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Durbin, J.; Watson, G.S. Testing for serial correlation in least squares regression III. Biometrika 1971, 58, 1–19. [Google Scholar] [CrossRef]
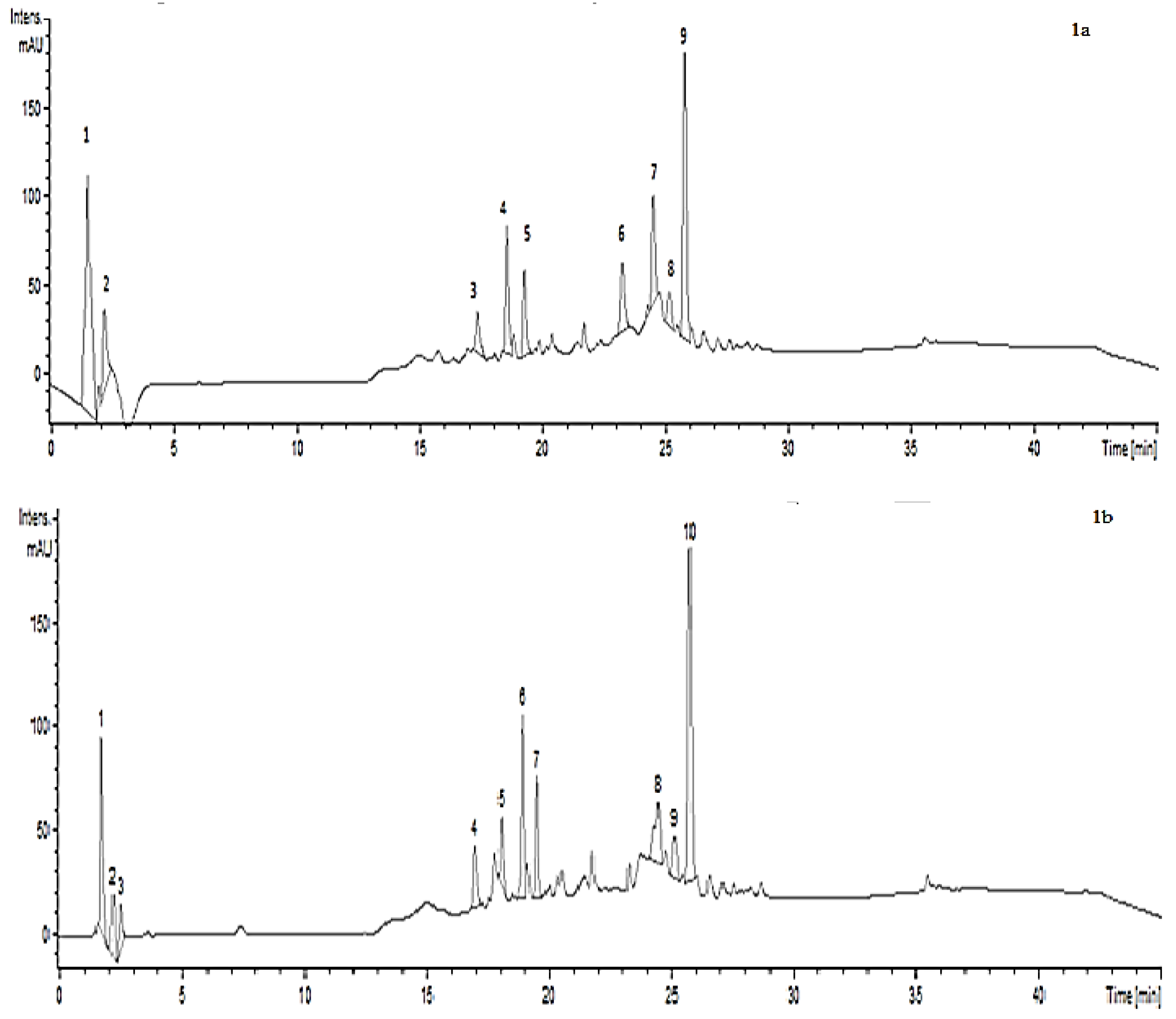

| Compound | Rt (min) | [M − H]+ (m/z) | Compound | Rt (min) | [M − H]− (m/z) |
|---|---|---|---|---|---|
| 4,9-dimethoxyfuro[3,2-g]chromen-7-One (C13H10O5) (Isopimpinellin) | 1.6 | 247 | Hydroxycaffeic acid (C9H8O5) | 1.8 | 195 |
| Unknown | 2.3 | 235 | Urolithin B (C13H8O3) | 2.5 | 211 |
| Quercetin 3-O-xylosyl-glucuronide (C26H26O17) | 17.4 | 145 | p-Coumaroyl tyrosine | 2.6 | 326 |
| Unknown | 18.5 | 438 | Quercetin 3-O-rhamnosyl-galactoside (C27H30O16) | 17.0 | 609 |
| Unknown | 19.3 | 438 | Quercetin 3-O-xylosyl-glucuronide (C26H26O17) | 18.1 | 609 |
| Unknown | 23.1 | 616 | Isorhamnetin-3-O-glucoside 7-O rhamnoside (C28H32O16) | 18.9 | 623 |
| Unknown | 24.6 | 600 | Quercetin 3-O-rutinoside (C27H30O16) | 19.4 | 609 |
| Unknown | 25.2 | 584 | Unknown | 24.6 | 598 |
| Unknown | 25.7 | 584 | Unknown | 25.1 | 582 |
| Unknown | 25.7 | 582 |
| Ethanolic Extracts (mg∙L−1) | (AC) (%) | [DPPH•]0 (mg∙L−1) | [DPPH•]t (mg∙L−1) | Δ[DPPH•] (%) | [DPPH•]REM (%) |
|---|---|---|---|---|---|
| 5000 | 75.70 ± 0.01 a | 27.60 | 12.78 ± 0.01 e | 53.70 ± 0.02 i | 46.30 ± 0.02 m |
| 10000 | 81.44 ± 0.01 b | 27.60 | 11.22 ± 0.01 f | 59.35 ± 0.02 j | 40.65 ± 0.02 n |
| 25000 | 85.71 ± 0.03 c | 27.60 | 8.31 ± 0.01 g | 69.89 ± 0.04 k | 30.11 ± 0.04 o |
| 30000 | 86.81 ± 0.01 d | 27.60 | 7.82 ± 0.01 h | 71.67 ± 0.04 l | 28.33 ± 0.04 p |
| Type of Milk | Control | 0.5% (w/v) | 1.0% (w/v) | 2.5% (w/v) | 3.0% (w/v) |
|---|---|---|---|---|---|
| Cow milk (N = 10) | |||||
| TPC (mgGAE/L) | 2882.5 ± 1.32 a | 4131.5 ± 0.50 d | 4935.83 ± 1.04 g | 7180 ± 1.80 j | 7771.5 ± 2.29 m |
| AC (%) | 71.90 ± 0.02 aa | 82.06 ± 0.01 ad | 90.33 ± 0.02 ag | 98.69 ± 0.01 aj | 98.79 ± 0.01 am |
| Goat milk (N = 10) | |||||
| TPC (mgGAE/L) | 2198.3 ± 1.53 b | 4107.17 ± 2.0 e | 4877.5 ± 0.50 h | 7094.33 ± 3.75 k | 7490.5 ± 0.50 n |
| AC(%) | 71.50 ± 0.01 ab | 81.46 ± 0.05 ae | 88.53 ± 0.04 ah | 95.88 ± 0.01 ak | 95.91 ± 0.02 an |
| Sheep milk (N = 10) | |||||
| TPC (mgGAE/L) | 2900.3 ± 2.25 c | 4315.33 ± 0.76 f | 5093 ± 0.50 i | 7546.2 ± 0.7 l | 8780 ± 2.25 o |
| AC (%) | 74.65 ± 0.01 ac | 86.79 ± 0.03 af | 94.40 ± 0.08 ai | 99.40 ± 0.02 al | 99.69 ± 0.01 ao |
| Milk Type | Control | 0.5% (w/v) | 1.0% (w/v) | 2.5% (w/v) | 3.0% (w/v) |
|---|---|---|---|---|---|
| Cow milk (N = 10) | |||||
| Taste | 4.65 ± 0.30 a | 5.00 ± 0.00 b | 5.00 ± 0.00 b | 3.67 ± 0.00 c | 3.00 ± 0.29 d |
| Odour | 5.00 ± 0.00 e | 4.80 ± 0.10 f | 4.90 ± 0.10 f | 4.00 ± 0.00 g | 3.67 ± 0.58 g |
| Appearance | 4.70 ± 0.30 h | 5.00 ± 0.00 h | 5.00 ± 0.00 h | 4.33 ± 0.58 h | 4.20 ± 0.00 h |
| Cohesion | 4.42 ± 0.58 i | 5.00 ± 0.00 i | 5.00 ± 0.00 i | 4.72 ± 0.18 j | 4.67 ± 0.15 j |
| Goat milk (N = 10) | |||||
| Taste | 4.45 ± 0.55 k | 4.40 ± 0.60 l | 4.10 ± 0.80 l | 3.66 ± 0.65 m | 3.10 ± 0.65 m |
| Odour | 4.16 ± 0.84 n | 4.80 ± 0.20 o | 4.3 ± 0.70 o | 3.50 ± 0.50 o | 3.30 ± 0.27 o |
| Appearance | 5.00 ± 0.00 p | 4.75 ± 0.20 p | 4.70 ± 0.25 p | 4.50 ± 0.50 p | 4.20 ± 0.75 p |
| Cohesion | 4.70 ± 0.30 p | 4.60 ± 0.35 p | 4.60 ± 0.40 p | 4.40 ± 0.60 p | 4.30 ± 0.67 p |
| Sheep milk (N = 10) | |||||
| Taste | 4.67 ± 0.33 p | 5.00 ± 0.00 p | 4.67 ± 0.33 p | 3.83 ± 1.04 p | 3.17 ± 0.76 p |
| Odour | 5.00 ± 0.00 q | 5.00 ± 0.00 q | 5.00 ± 0.00 q | 4.67 ± 0.33 q | 3.77 ± 0.59 r |
| Appearance | 5.00 ± 0.00 s | 5.00 ± 0.00 s | 5.00 ± 0.00 s | 4.33 ± 0.58 s | 4.33 ± 0.58 s |
| Cohesion | 5.00 ± 0.00 s | 5.00 ± 0.00 s | 5.00 ± 0.00 s | 5.00 ± 0.00 s | 5.00 ± 0.00 s |
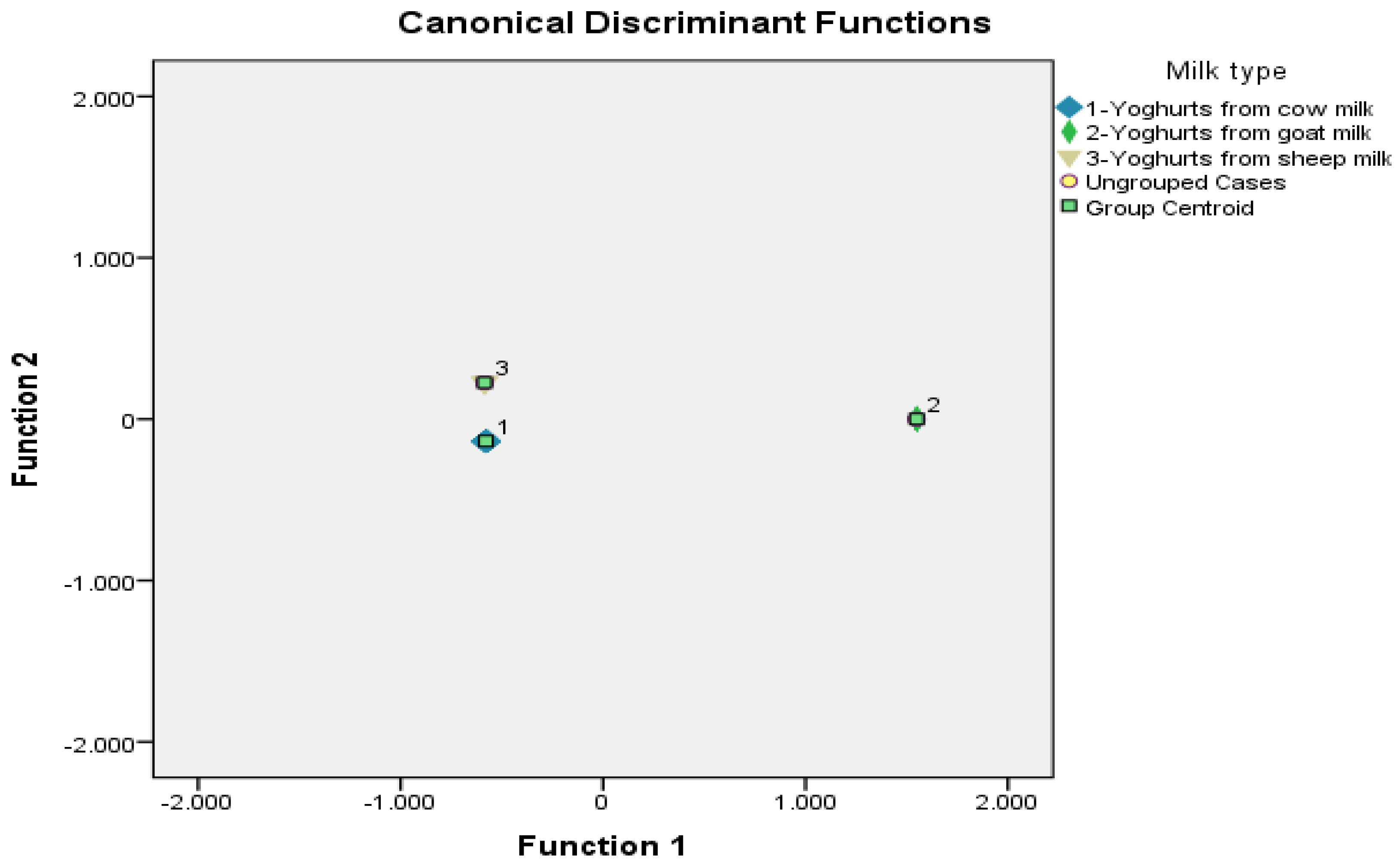
| Discriminatory Parameters of Conventional and Bee Pollen Enriched Yoghurts | Discriminant Function | Fisher’s Coefficient | Probability | |
|---|---|---|---|---|
| 1 | 2 | F | p | |
| Total phenolic content of conventional yoghurts (control samples) | −4.190 | −0.145 | 494,107.133 | <0.001 |
| Total phenolic content of yoghurts supplemented with 0.5% (w/v) of bee pollen | 6.233 | 1.466 | 74,090.195 | <0.001 |
| Total phenolic content of yoghurts supplemented with 1.0% (w/v) of bee pollen | −4.486 | 0.645 | 170,809.528 | <0.001 |
| In Vitro antioxidant capacity of yoghurts supplemented with 2.5% (w/v) of bee pollen | 0.584 | −1.318 | 42,385.052 | <0.001 |
| Taste scores of yoghurts supplemented with 1.0% (w/v) of bee pollen | 8.957 | 2.257 | 10.787 | 0.001 |
| Appearance scores of yoghurts supplemented with 3.0% (w/v) of bee pollen | 0.006 | −0.282 | 3.631 | 0.046 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karabagias, I.K.; Karabagias, V.K.; Gatzias, I.; Riganakos, K.A. Bio-Functional Properties of Bee Pollen: The Case of “Bee Pollen Yoghurt”. Coatings 2018, 8, 423. https://doi.org/10.3390/coatings8120423
Karabagias IK, Karabagias VK, Gatzias I, Riganakos KA. Bio-Functional Properties of Bee Pollen: The Case of “Bee Pollen Yoghurt”. Coatings. 2018; 8(12):423. https://doi.org/10.3390/coatings8120423
Chicago/Turabian StyleKarabagias, Ioannis K., Vassilios K. Karabagias, Ilias Gatzias, and Kyriakos A. Riganakos. 2018. "Bio-Functional Properties of Bee Pollen: The Case of “Bee Pollen Yoghurt”" Coatings 8, no. 12: 423. https://doi.org/10.3390/coatings8120423
APA StyleKarabagias, I. K., Karabagias, V. K., Gatzias, I., & Riganakos, K. A. (2018). Bio-Functional Properties of Bee Pollen: The Case of “Bee Pollen Yoghurt”. Coatings, 8(12), 423. https://doi.org/10.3390/coatings8120423






