Biofilm Formation of a Polymer Brush Coating with Ionic Liquids Compared to a Polymer Brush Coating with a Non-Ionic Liquid
Abstract
1. Introduction
2. Materials and Methods
3. Results
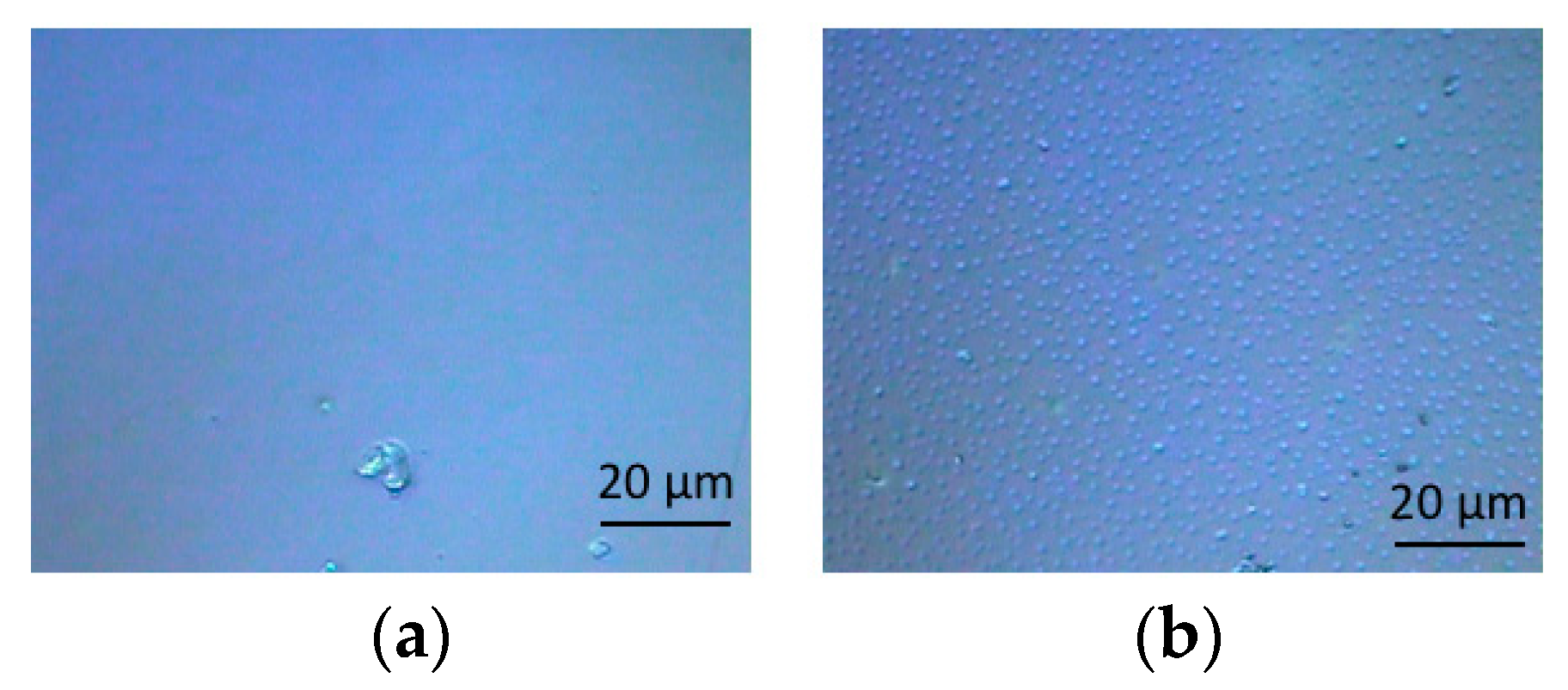
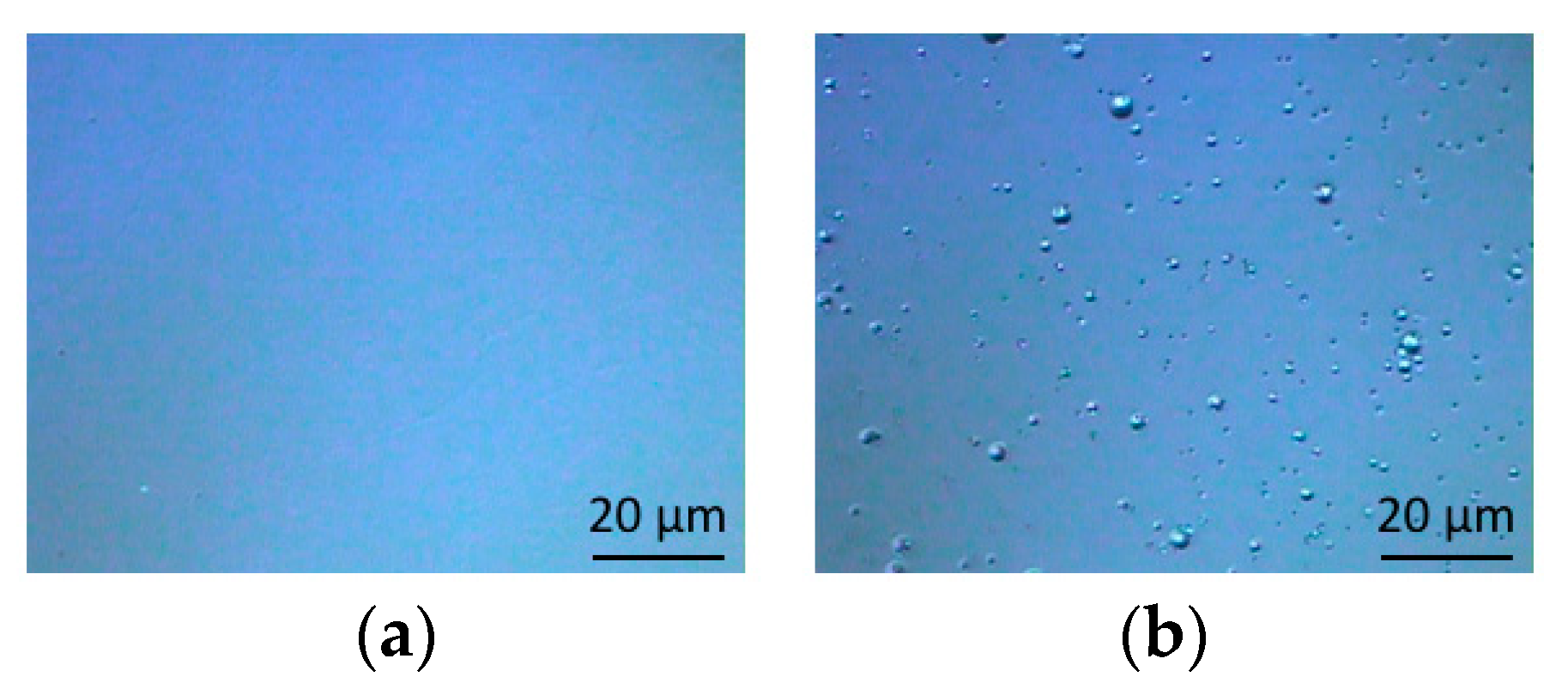
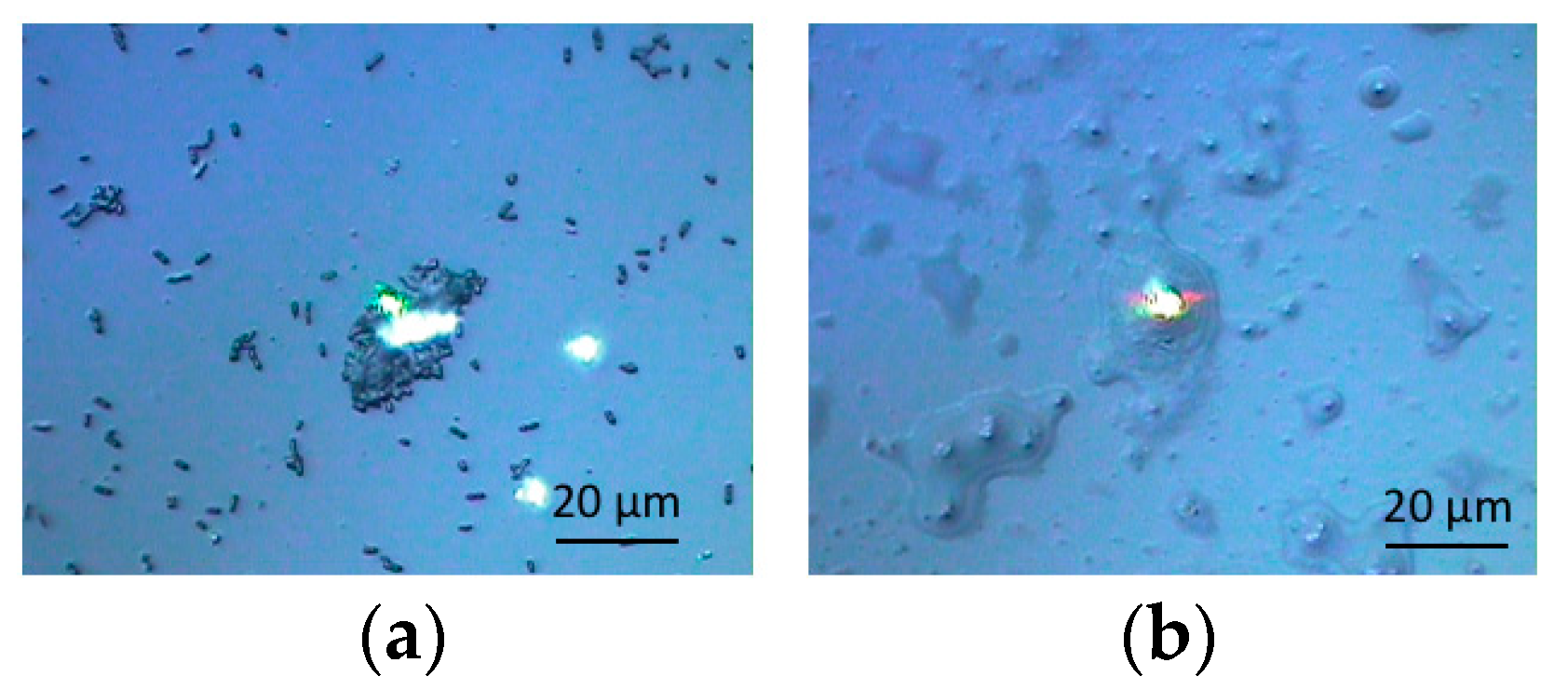
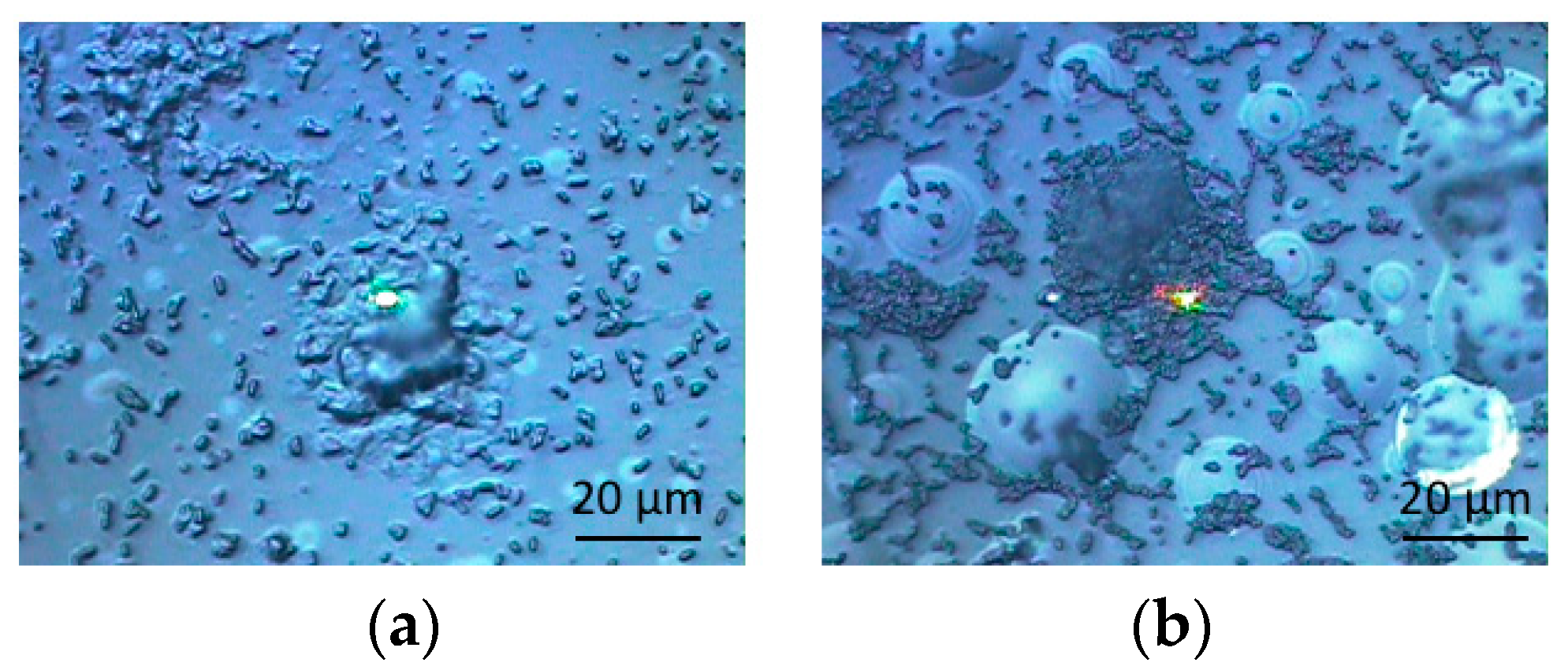
3.1. Optical Microscopic Images
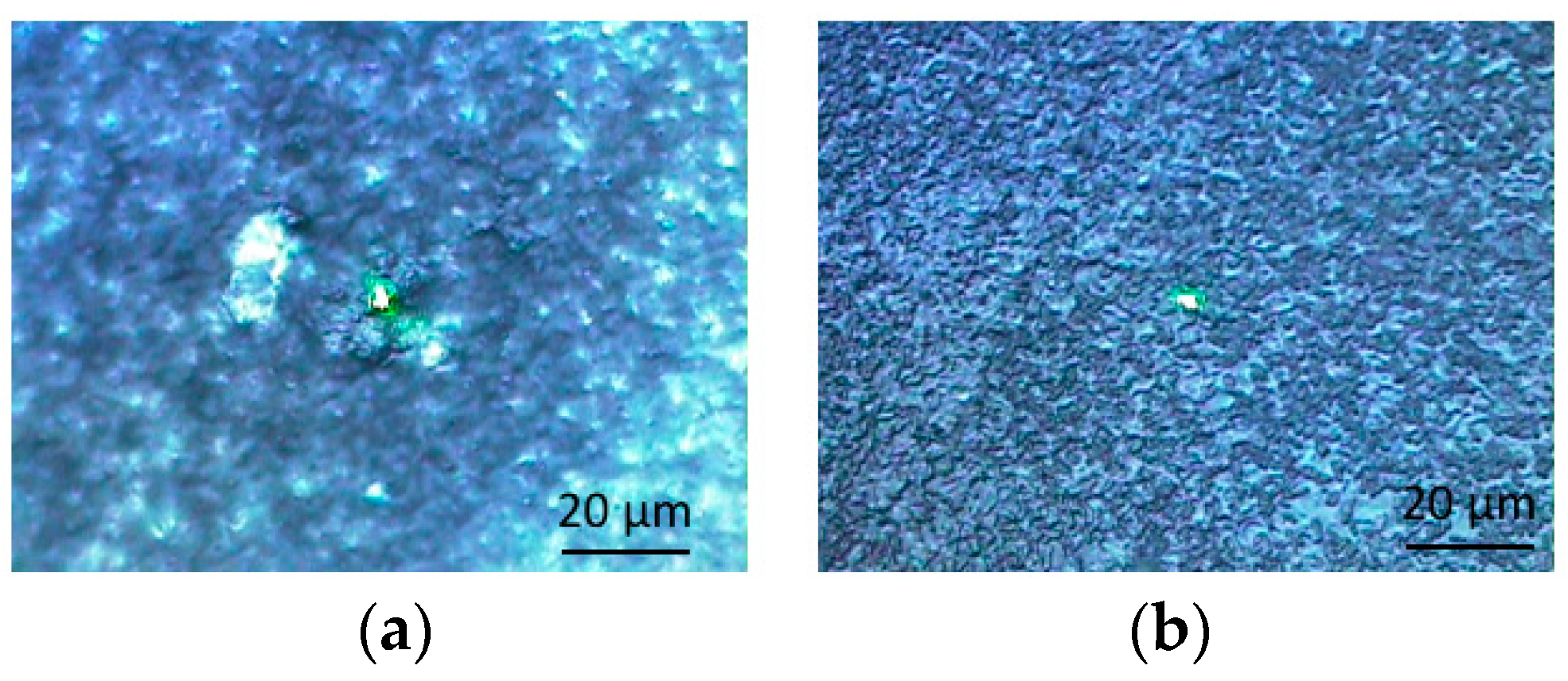
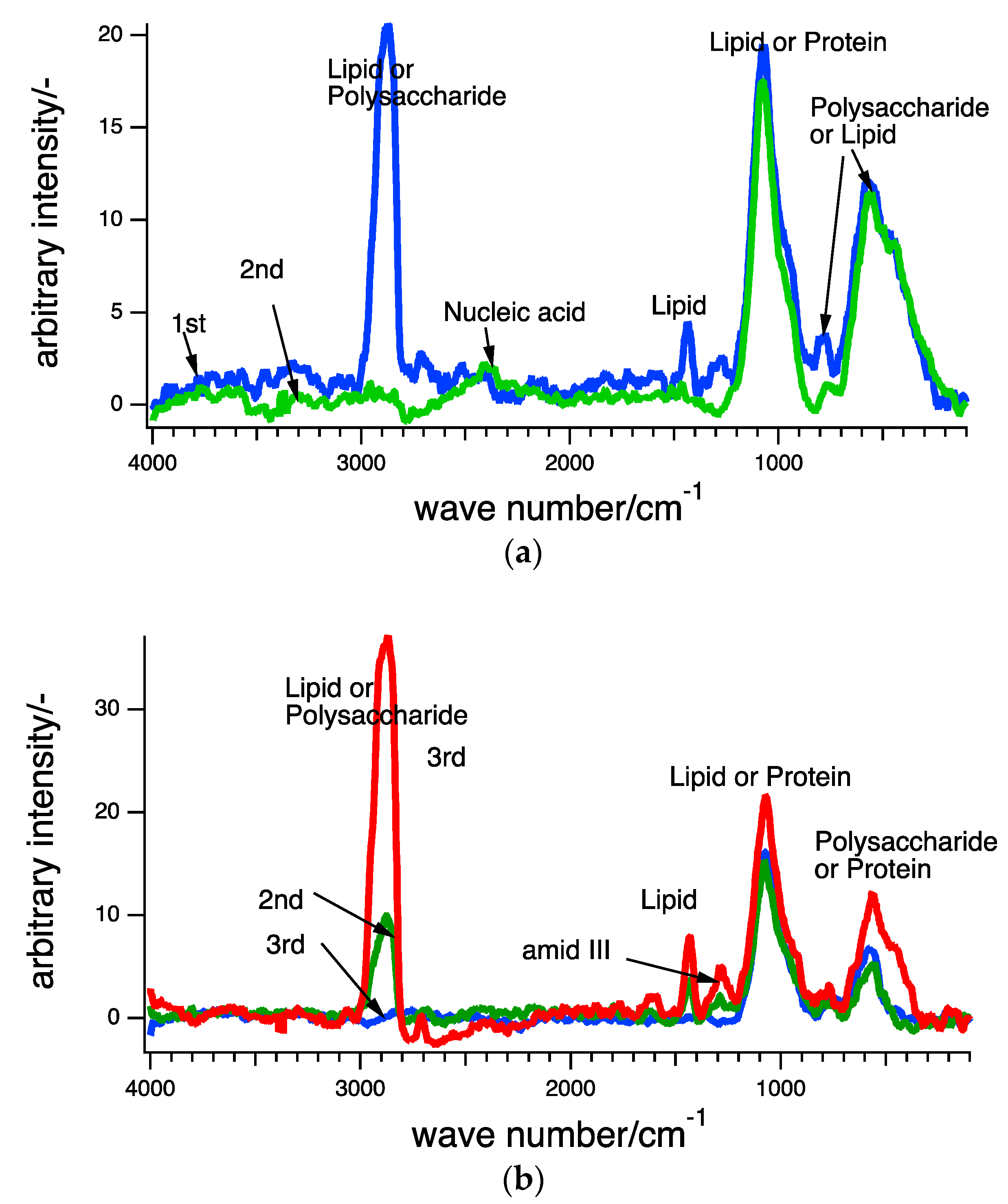
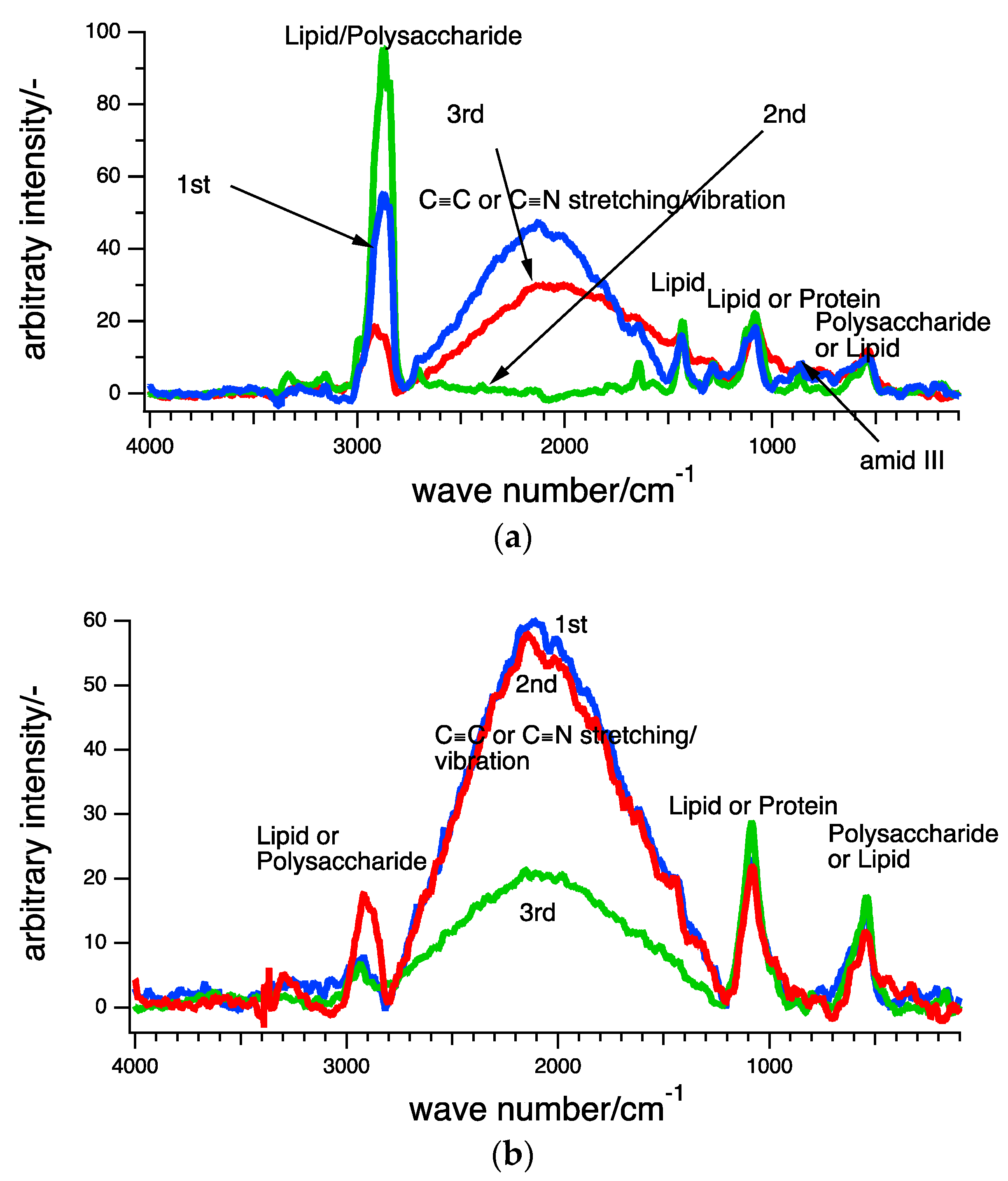
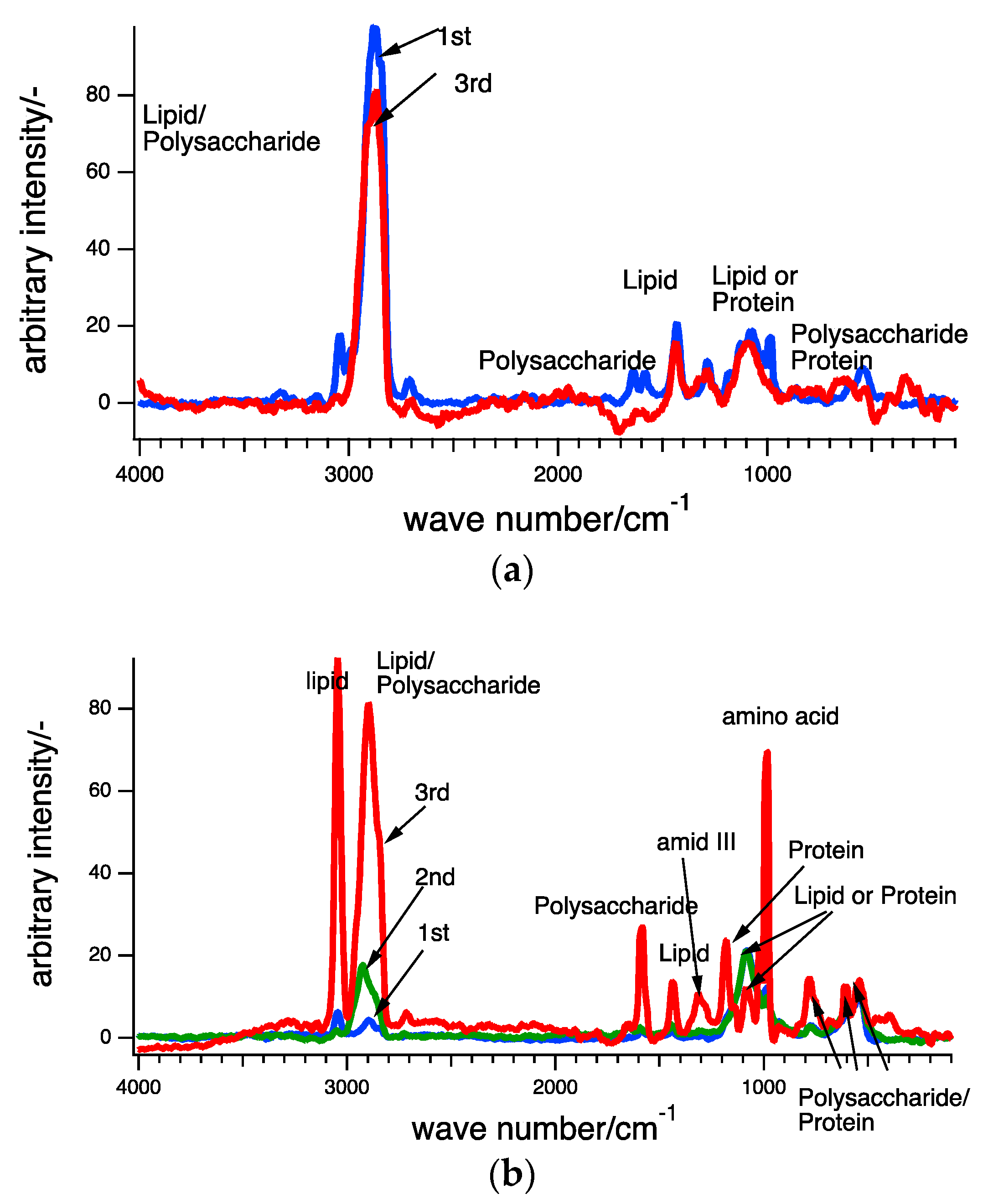
3.2. Raman Scpetroscopy
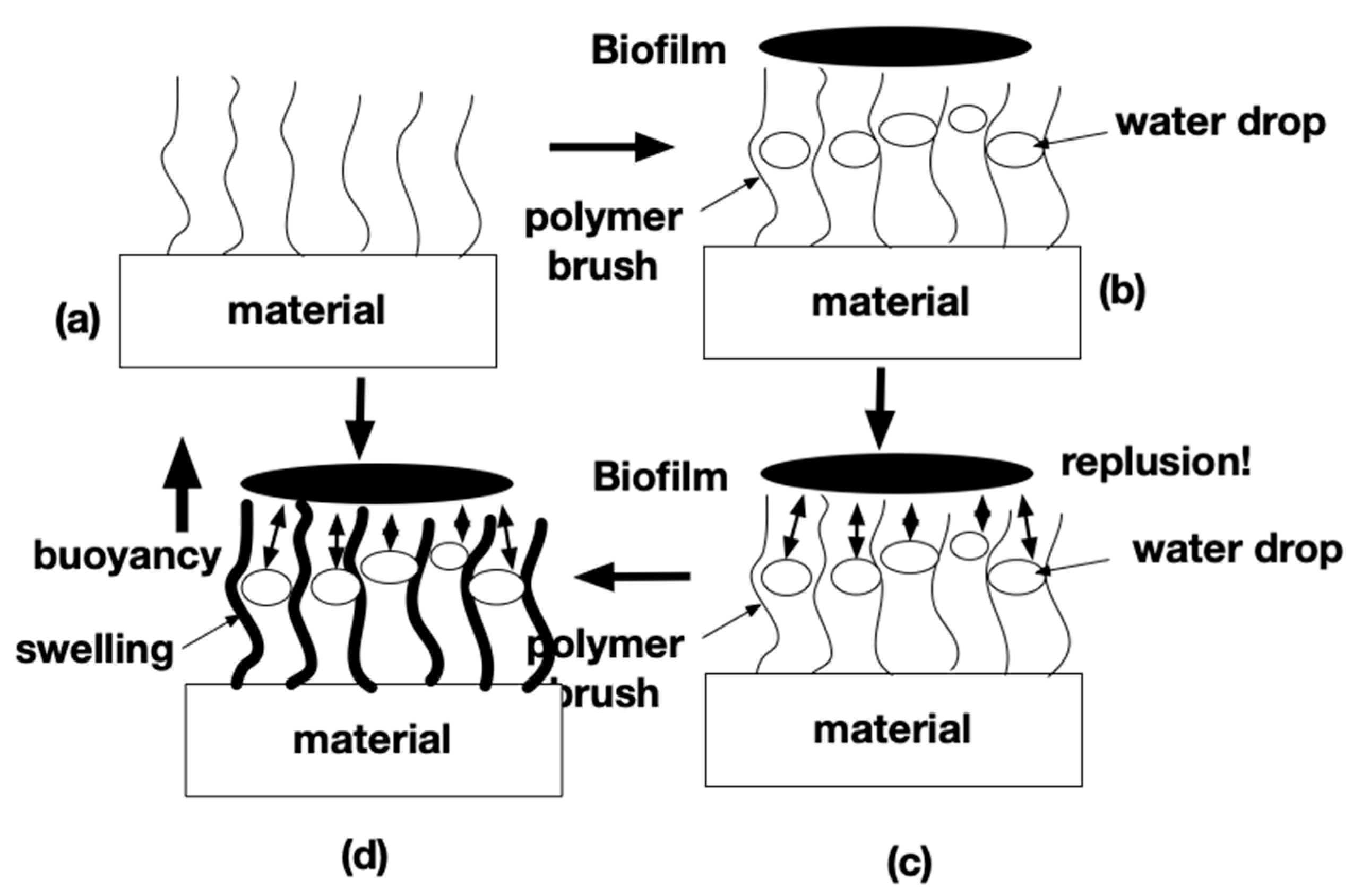
4. Discussion
5. Conclusions
- Optical microscopic observations and Raman spectroscopic analyses confirmed that all specimens could form biofilms on their surfaces (more or less).
- DEMM polymer brush coating tended to form biofilms on the surfaces more than PMMA polymer brush specimens.
- Nucleic acids in biofilms could be confirmed more often for DEMM polymer brush coating specimens.
- These results suggest that the polar characteristics would play an important role in the antifouling effect related to biofilm formation.
- Ionic liquid type polymer brush coating would be beneficial to remove contaminants as a whole, due to the trapping and removing capabilities.
- Ionic liquid type polymer brush coating may change their polar characteristics leading to the antifouling effect of materials’ surfaces in the future.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fusetani, N. Biofouling and antifouling. Nat. Prod. Rep. 2004, 21, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology—Past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 2004, 50, 75–104. [Google Scholar] [CrossRef]
- Chambers, L.D.; Stokes, K.R.; Walsh, F.C.; Wood, R.J. Modern approaches to marine antifouling coatings. Surf. Coat. Technol. 2006, 201, 3642–3652. [Google Scholar] [CrossRef]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling coatings: Recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef] [PubMed]
- Lejars, M.; Margaillan, A.; Bressy, C. Fouling release coatings: A nontoxic alternative to biocidal antifouling coatings. Chem. Rev. 2012, 112, 4347–4390. [Google Scholar] [CrossRef] [PubMed]
- Swain, G.W.; Schultz, M.P. The testing and evaluation of non-toxic antifouling coatings. Biofouling 1996, 10, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Brady, R.F., Jr. A fracture mechanical analysis of fouling release from nontoxic antifouling coatings. Prog. Org. Coat. 2001, 43, 188–192. [Google Scholar] [CrossRef]
- Gudipati, C.S.; Greenlief, C.M.; Johnson, J.A.; Prayongpan, P.; Wooley, K.L. Hyperbranched fluoropolymer and linear poly(ethylene glycol) based amphiphilic crosslinked networks as efficient antifouling coatings: An insight into the surface compositions, topographies, and morphologies. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 6193–6208. [Google Scholar] [CrossRef]
- Hellio, C.; Yebra, D. Advances in Marine Antifouling Coatings and Technologies, 1st ed.; Woodhead Publishing: Cambridge, UK, 2009. [Google Scholar]
- Casse, F.; Swain, G.W. The development of microfouling on four commercial antifouling coatings under static and dynamic immersion. Int. Biodeterior. Biodegrad. 2006, 57, 179–185. [Google Scholar] [CrossRef]
- Mittal, K.L. Surface contamination: An overview. In Surface Contamination—Genesis, Detection, and Control, 1st ed.; Springer: Boston, MA, USA, 1979; pp. 3–45. [Google Scholar]
- Dobretsov, S.; Thomason, J.; Williams, D.N. Biofouling Methods, 1st ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2014. [Google Scholar]
- Dürr, S.; Thomason, J.C. Biofouling, 1st ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2009. [Google Scholar]
- Corpe, W.A. Microfouling: The role of primary film forming marine bacteria. In Proceedings of the 3rd International Congress of Marine Corrosion and Fouling, Gaithersburg, MD, USA, 2–6 October 1972; Northwestern University Press: Evanston, IL, USA, 1973. [Google Scholar]
- Claudi, R.; Mackie, G.L. Practical Manual for the Monitoring and Control; Lewis Publishers: Boca Raton, FL, USA, 1994. [Google Scholar]
- Kanematsu, H.; Barry, D.M. Biofilm and Materials Science; Springer: New York, NY, USA, 2015. [Google Scholar]
- Lewandowski, Z.; Beyenal, H. Fundamentals of Biofilm Research, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014; p. 642. [Google Scholar]
- Kalmbach, S.; Manz, W.; Szewzyk, U. Biofilms—Investigative Methods & Applications; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Flemming, H.C.; Murthy, S.P.; Venkatesan, R.; Cooksey, K. Marine and Industrial Biofouling; Springer: Berlin, Germany, 2009. [Google Scholar]
- Kanematsu, H.; Ikigai, H.; Campbell, S.; Beech, I. Sn-Ag alloy plating films mitigating biofilm formation. In Proceedings of the National Association for Surface Finishing Annual Technical Conference 2009, Louisville, KY, USA, 16–17 June 2009; Curran Associates, Inc.: Red Hook, NY, USA, 2009; pp. 406–415. [Google Scholar]
- Kanematsu, H.; Ikigai, H.; Yoshitake, M. Evaluation of various metallic coatings on steel to mitigate biofilm formation. Int. J. Mol. Sci. 2009, 10, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Kanematsu, H.; Ikegai, H.; Kuroda, D. Biofilm and metallic materials. Rust Prev. Control Jpn. 2011, 55, 369–377. [Google Scholar]
- Kanematsu, H.; Kougo, T.; Kuroda, D.; Itoh, H.; Noorani, R. Prevention coating against biofilm formation. In Proceedings of the Sur/Fin Asia-Pacific 2012, Singapore, 4–7 December 2012; pp. 52–56. [Google Scholar]
- Kanematsu, H.; Kuroda, D.; Itoh, H.; Ikigai, H. Biofilm formation of a closed loop system and its visualization. CAMP-ISIJ 2012, 25, 753–754. [Google Scholar]
- Kanematsu, H.; Kuroda, D.; Itoh, H.; Kobayashi, T.; Noorani, R.; Yamamoto, M.; Tsukuda, I. Chemical surface treatment for prevention against biofilm formation. In Proceedings of the Sur/Fin Asia-Pacific 2012, Singapore, 4–7 December 2012; pp. 57–62. [Google Scholar]
- Kanematsu, H.; Kuroda, D.; Koya, S.; Itoh, H. Development of production process on labo scale for biofilm formation by immersion into closed circulation water system. J. Surf. Finish. Soc. Jpn. 2012, 63, 459–461. [Google Scholar] [CrossRef]
- Kanematsu, H.; Miura, Y.; Itoh, H.; Ikigai, H.; Tanaka, M. Condensation behavior of silicon into biofilm formed by germs in ambient atmosphere in a laboratory and its observation. CAMP-ISIJ 2012, 25, 810–811. (In Japanese) [Google Scholar]
- Kanematsu, H.; Hihara, T.; Ishihara, T.; Imura, K.; Kogo, T. Evaluation for corrosion resistance of nano-cluster layer and biofilm formation. Int. J. Eng. Sci. Res. Technol. 2013, 2, 2424–2432. [Google Scholar]
- Kanematsu, H.; Hirai, N.; Miura, Y.; Itoh, H.; Kuroda, D.; Umeki, S. Biofilm leading to corrosion on material surface and the moderation by alternative electro-magnetic field. In Proceedings of the Materials Science and Technology Conference and Exhibition 2013 (MS&T 2013), Monatreal, QC, Canada, 27–31 October 2013; pp. 2761–2767. [Google Scholar]
- Kanematsu, H.; Hirai, N.; Miura, Y.; Itoh, H.; Masuda, T.; Kuroda, D. Evaluation technique for biofilm formed on biomaterials. In Proceedings of the 4th International Symposium on Advanced Materials Development and Integration of Novel Structured Metallic and Inorganic Materials, Nagoya, Japan, 13–15 December 2013; Nagoya University: Nagoya, Japan, 2013. [Google Scholar]
- Kanematsu, H.; Hirai, N.; Miura, Y.; Tanaka, M.; Kogo, T.; Itoh, H. Various metals from water by biofilm from an ambient germs in a reaction container. In Proceedings of the Materials Science and Technology Conference and Exhibition 2013 (MS&T 2013), Montreal, QC, Canada, 27–31 October 2013; pp. 2154–2161. [Google Scholar]
- Kanematsu, H.; Itoh, H.; Miura, Y.; Masuda, T.; Kuroda, D.; Hirai, N.; Barry, D.M.; McGrath, P.B. Biofilm formation on polymer materials by a laboratory acceleration reactor. In Proceedings of the 4th International Symposium on Advanced Materials Development and Integration of Novel Structured Metallic and Inorganic Materials, Nagoya, Japan, 13–15 December 2013; Nagoya University: Nagoya, Japan, 2013. [Google Scholar]
- Kougo, T.; Yamamoto, Y.; Naiki, A.; Ogino, Y.; Kanzaki, T.; Kanematsu, H.; Ikigai, H.; Itoh, H. Proposal for Evaluation to Biofilm Formation on Metal Oxides in Atmospheric Exposure Type Circulation Water System. Mem. Suzuka Natl. Coll. Technol. 2013, 46, 81–84. [Google Scholar]
- Kanematsu, H.; Kogo, T.; Itoh, H.; Wada, N.; Yoshitake, M. Fogged glass by biofilm formation and its evaluation. In Proceedings of the Materials Science and Technology Conference and Exhibition 2013 (MS&T 2013), Montreal, QC, Canada, 27–31 October 2013; pp. 2427–2433. [Google Scholar]
- Kanematsu, H.; Kogo, T.; Kuroda, D.; Itoh, H.; Kirihara, S. Biofilm formation and evaluation for spray coated metal films on laboratory scale. In Proceedings of the Thermal Spray 2013—Innovative Coating Solutions for the Global Economy, Busan, Korea, 13–15 May 2013; pp. 520–525. [Google Scholar]
- Kanematsu, H.; Kougo, T.; Kuroda, D.; Ogino, Y.; Yamamoto, Y. Biofilm formation derived from ambient air and the characteristics of apparatus. J. Phys. Conf. Ser. 2013, 433, 012031. [Google Scholar] [CrossRef]
- Kanematsu, H.; Okura, Y.; Hirai, N.; Miura, Y.; Itoh, H.; Tanaka, M. Condensation of some metal elements in biofilm. CAMP-ISIJ 2013, 26, 417. (In Japanese) [Google Scholar]
- Ikegai, H.; Kobayashi, M.; Ken-ichi Iimura; Hosokawa, A.; Uesugi, K.; Korda, D.; Kanematsu, H.; Toda, H. X-ray computed tomography for observing microbiologically influenced corrosion of carbon steel caused by Pseudomonas aeruginosa biofilm sig synchrotron radiation in Spring-8. Bact. Adherence Biofilm 2014, 28, 67–70. (In Japanese) [Google Scholar]
- Kanematsu, H.; Kogo, T.; Noda, M.; Hirai, N.; Ogawa, A.; Miura, Y.; Itoh, H.; Yoshitake, M. Composite coating to control biofilm formation and MIC. In Proceedings of the 17th International Congress on Marine Corrosion and Fouling (ICMCF), National University of Singapore, Singapore, 6–10 July 2014; p. 101. [Google Scholar]
- Kanematsu, H.; Kogo, T.; Sano, K.; Noda, M.; Wada, N.; Yoshitake, M. Nano-composite coating on glasses for biofilm control. J. Mater. Sci. Surf. Eng. 2014, 1, 58–63. [Google Scholar]
- Kanematsu, H.; Noda, M.; Hirai, N.; Ogawa, A.; Kogo, T.; Miura, Y.; Ito, H. A trial for MIC study using a circulation-type laboratory biofilm reactor. In Proceedings of the 17th International Congress on Marine Corrosion and Fouling (ICMCF), National University of Singapore, Singapore, 6–10 July 2014; p. 75. [Google Scholar]
- Kanematsu, H.; Tanaka, M. Biofilm analyses and their importance in materials science and engineering. Bunseki Kagaku 2014, 63, 569–580. (In Japanese) [Google Scholar] [CrossRef]
- Ikegai, H.; Hirai, N.; Kobayashi, M.; Toda, H.; Moroboshi, T.; Ikeda, T.; Ikeda, S.; Uesugi, K.; Kuroda, D.; Kanematsu, H. Microbiologically influenced corrosion to carbon steel and biomineralization caused by pseudomonas aeruginosa biofilm. Bact. Adherence Biofilm 2015, 29, 93–96. (In Japanese) [Google Scholar]
- Kanematsu, H.; Sasaki, S.; Miura, Y.; Kogo, T.; Sano, K.; Wada, N.; Yoshitake, M.; Tanaka, T. Composite coating to control biofilm formation and effect of alternate electro-magnetic field. Mater. Technol. 2015, 30, 21–26. [Google Scholar] [CrossRef]
- Ogawa, A.; Noda, M.; Kanematsu, H.; Sano, K. Application of bacterial16S rRNAgene analysis to a comparison of the degree of biofilm formation on the surface of metal coated glasses. Mater. Technol. 2015, 30, 61–65. [Google Scholar] [CrossRef]
- Kanematsu, H.; Kudara, H.; Kanesaki, S.; Kogo, T.; Ikegai, H.; Ogawa, A.; Hirai, N. Application of a loop-type laboratory biofilm reactor to the evaluation of biofilm for some metallic materials and polymers such as urinary stents and catheters. Materials 2016, 9, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Kanematsu, H.; Umeki, S.; Hirai, N.; Miura, Y.; Wada, N.; Kogo, T.; Tohji, K.; Otani, H.; Okita, K.; Ono, T. Verification of effect of alternative electromagnetic treatment on control of biofilm and scale formation by a new laboratory biofilm reactor. Ceram. Trans. 2016, 259, 199–212. [Google Scholar] [CrossRef]
- Kanematsu, H.; Umeki, S.; Ogawa, A.; Hirai, N.; Kogo, T.; Tohji, K. The cleaning effect on metallic materials under a weak alsternating electromagnetic field and biofilm. In Proceedings of the Ninth Pacific Rim International Conference on Advanced Materials and Processing (PRICM9), Kyoto, Japan, 1 August 2016; pp. 1–4. [Google Scholar]
- Sano, K.; Kanematsu, H.; Kogo, T.; Hirai, N.; Tanaka, T. Corrosion and biofilm for a composite coated iron observed by FTIR-ATR and Raman spectroscopy. Trans. Inst. Mater. Finish. 2016, 94, 139–145. [Google Scholar] [CrossRef]
- Kanematsu, H. Evaluation and countermeasure for biofilms on inorganic surface. In Biofilm Structure and Formation for Biocontrol and Countermeasure of Biofilms and Its Growth; Matsumura, Y., Ed.; CMC Publisher: Osaka, Japan, 2017; pp. 162–189. [Google Scholar]
- Kanematsu, H.; Barry, D.M.; Ikegai, H.; Yoshitake, M.; Mizunoe, Y. Biofilm evaluation methods outside body to inside—Problem presentations for the future. Med. Res. Arch. 2017, 5, 1469. [Google Scholar]
- Kanematsu, H.; Saito, T.; Barry, D.M.; Hirai, N.; Kogo, T.; Ogawa, A.; Tsunashima, K. Effects of ionic liquids on biofilm formation in a loop-type laboratory biofilm reactor. ECS Trans. 2017, 80, 1147–1155. [Google Scholar] [CrossRef]
- Kanematsu, H.; Satoh, M.; Shindo, K.; Barry, D.M.; Hirai, N.; Ogawa, A.; Kogo, T.; Utsumi, Y.; Yamaguchi, A.; Ikegai, H.; et al. Biofilm formation behaviors on graphene by E. coli and S. epidermidis. ECS Trans. 2017, 80, 1167–1175. [Google Scholar] [CrossRef]
- Sano, K.; Masuda, T.; Kanematsu, H.; Yokoyama, S.; Hirai, N.; Ogawa, A.; Kougo, T.; Yamazaki, K.; Tanaka, T. Biofouling on mortar mixed with steel slags in a laboratory biofilm reactor. In Proceedings of the Irago Conference 2016, Irago, Aichi, Japan, 1–2 November 2016; p. 02004. [Google Scholar]
- Kanematsu, H. A new international standard for testing antibacterial effects. Adv. Mater. Process. 2017, 175, 26–29. [Google Scholar]
- Kanematsu, H.; Maeda, S.; Barry, D.M.; Umeki, S.; Tohji, K.; Hirai, N.; Ogawa, A.; Kogo, T.; Ikegai, H.; Mizunoe, Y. Effects of elastic waves at several frequencies on biofilm formation in circulating laboratory biofilm reactors. Ceram. Trans. 2018, 265, 43–51. [Google Scholar]
- Kanematsu, H.; Oizumi, A.; Sato, T.; Kamijo, T.; Honma, S.; Barry, D.M.; Hirai, N.; Ogawa, A.; Kogo, T.; Kuroda, D.; et al. Polymer brush made by ionic liquids and the inhibition effects for biofilm formation. ECS Trans. 2018, 85, 1089–1095. [Google Scholar] [CrossRef]
- Kanematsu, H.; Shindo, K.; Barry, D.M.; Hirai, N.; Ogawa, A.; Kuroda, D.; Kogo, T.; Sano, K.; Ikegai, H.; Mizunoe, Y. Electrochemical responses of graphene with biofilm formation on various metallic substrates by using laboratory biofilm reactors. ECS Trans. 2018, 85, 491–498. [Google Scholar] [CrossRef]
- Milner, S.T.; Witten, T.A.; Cates, M.E. Theory of the grafted polymer brush. Macromolecules 1988, 21, 2610–2619. [Google Scholar] [CrossRef]
- Milner, S.T. Polymer brushes. Science 1991, 251, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F. Antifouling Surfaces and Materials—From Land to Marine Environment, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Sato, T.; Morinaga, T.; Marukane, S.; Narutomi, T.; Igarashi, T.; Kawano, Y.; Ohno, K.; Fukuda, T.; Tsuji, Y. Novel solid-state polymer electrolyte of colloidal crystal decorated with ionic-liquid polymer brush. Adv. Mater. 2011, 23, 4868–4872. [Google Scholar] [CrossRef] [PubMed]
- Arafune, H.; Kamijo, T.; Morinaga, T.; Honma, S.; Sato, T.; Tsuji, Y. A robust lubrication system using an ionic liquid polymer brush. Adv. Mater. Interfaces 2015, 2, 1500187. [Google Scholar] [CrossRef]
- Morinaga, T.; Honma, S.; Ishizuka, T.; Kamijo, T.; Sato, T.; Tsuji, Y. Synthesis of monodisperse silica particles grafted with concentrated ionic liquid-type polymer brushes by surface-initiated atom transfer radical polymerization for use as a solid state polymer electrolyte. Polymers 2016, 8, 146–159. [Google Scholar] [CrossRef]
- Hideyuiki, K.; Barry, D.M.; Ikegai, H.; Yoshitake, M.; Mizunoe, Y. Nanofibers and biofilm in materials science. In Handbook of Nanofibers. Vol. I: Fundamental Aspects, Experimental Setup, Synthesis, Properties and Physicochemical Characterization; Barhoum, A., Bechelany, M., HamdyMakhlouf, A.S., Eds.; Springer Nature Switzerland AG: Basel, Switzerland, 2018; pp. 1–21. [Google Scholar]
- Yamamoto, S.; Ejaz, M.; Tsujii, Y.; Fukuda, T. Surface interaction forces of well-defined, high-density polymer brushes studies by atomic force microscopy. 2. Effect of graft density. Macromolecules 2000, 33, 5608–5612. [Google Scholar] [CrossRef]
- Tsujii, Y.; Ohno, K.; Yamamoto, S.; Goto, A.; Fukuda, T. Structure and properties of high-density polymer brushes prepared by surface-initiated living radical polymerization. In Surface-Initiated Polymerization; Jordan, R., Ed.; Springer Nature: Heidelberg, Germany, 2006; pp. 1–45. [Google Scholar]
- Nomura, A.; Ohno, K.; Fukuda, T.; Sato, T.; Tsujii, Y. Lubrication mechanisms of concentrated polymer brushes in solvents: Effect of solvent viscosity. Polym. Chem. 2012, 3, 148–153. [Google Scholar] [CrossRef]
- Zhu, B.; Edmondson, S. ARGET ATRP: Procedure for PMMA polymer brush growth. Available online: https:www.sigmaaldrich.com/technical-documents/articles/crp-guide/arget-atrp-procedure-for-pmma-polymer-brush-growth.html (accessed on 7 November 2018).
- Zhu, B.; Edmondson, S. Polydopamine-melanin initiators for surface-initiated ATRP. Polymer 2011, 52, 2141–2149. [Google Scholar] [CrossRef]
- Chao, Y.; Zhang, T. Surface-enhanced Raman scattering (SERS) revealing chemical variation during biofilm formation: From initial attachment to mature biofilm. Anal. Bioanal. Chem. 2012, 404, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Lakin, P. General Outline and Strategies for IR and Raman Spectral Interpretation. In Infrared and Raman Spectroscopy–Principles and Spectral Interpretation; Elsevier: Burlington, MA, USA, 2011; pp. 117–133. [Google Scholar]
- George, J.; Thomas, J. Raman spectroscopy of protein and nucleic acid assmeblies. Annu. Rev. Biophys. Biomol. Struct. 1999, 28, 1–27. [Google Scholar]
- Yuen, S.N.; Choi, S.M.; Phillips, D.L.; Ma, C.Y. Raman and FTIR spectroscopic study of carboxymethylated non-starch polysaccharides. Food Chem. 2009, 114, 1091–1098. [Google Scholar] [CrossRef]
- Czamara, K.; Majzner, K.; Pacia, M.Z.; Kochan, K.; Kaczor, A.; Baranska, M. Raman spectroscopy of lipids: A review. J. Raman Spectrosc. 2015, 46, 4–20. [Google Scholar] [CrossRef]
- Lakin, P. IR and Raman spectra-structure correlations: Characteristic group frequencies. In Infrared and Raman Spectroscopy–Principles and Spectral Interpretation; Elsevier: Amsterdam, The Netherland, 2011; pp. 73–115. [Google Scholar]
- Cao, B.; Shi, L.; Brown, N.R.; Xiong, Y.; Fredrickson, K.J.; Fomine, F.M.; Marshall, M.J.; Lipton, M.S.; Beyenal, H. Extracellular polymeric substances from Shewanella sp. HRCR-1 biofilms: Characterization by infrared spectroscopy and proteomics. Environ. Microbiol. 2011, 13, 1018–1031. [Google Scholar] [CrossRef] [PubMed]
- Sano, K.; Kanematsu, H.; Hirai, N.; Tanaka, T. Preparation and its anti-biofouling effect observation of organic metal dispersed silane based composite coating. J. Surf. Finish. Soc. Jpn. 2016, 67, 268–273. [Google Scholar] [CrossRef]
- Ogawa, A.; Kiyohara, T.; Kobayashi, Y.H.; Sano, K.; Kanematsu, H. Nickel, moluybdenum, and tungsten nanoparticle-dispersed alkylalkoxysilane polymer for biomaterial coating: evaluation of effects on bacterial biofilm formation and biosafety. Biomed. Res. Clin. Pract. 2017, 2, 1–7. [Google Scholar] [CrossRef]
- Nyquist, R.A. Interpreting Infrared, Raman, and Nuclear Magnetic Resonance Spectra; Academic Press: San Diego, CA, USA, 2001. [Google Scholar]
- Hamasha, K.M. Raman Spectroscopy for the Microbiological Characterization and Identification of Medically Relevant Bacteria. Ph.D. Thesis, Wayne State University, Detroit, MI, USA, 2011. [Google Scholar]
- Rzhepishevska, O.; Hakobyan, S.; Ruhal, R.; Gautrot, J.; Barbero, D.; Ramstedt, M. The surface charge of anti-bacterial coatings alters motility and biofilm architecture. Biomater. Sci. 2013, 1, 589–602. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanematsu, H.; Oizumi, A.; Sato, T.; Kamijo, T.; Honma, S.; Barry, D.M.; Hirai, N.; Ogawa, A.; Kogo, T.; Kuroda, D.; et al. Biofilm Formation of a Polymer Brush Coating with Ionic Liquids Compared to a Polymer Brush Coating with a Non-Ionic Liquid. Coatings 2018, 8, 398. https://doi.org/10.3390/coatings8110398
Kanematsu H, Oizumi A, Sato T, Kamijo T, Honma S, Barry DM, Hirai N, Ogawa A, Kogo T, Kuroda D, et al. Biofilm Formation of a Polymer Brush Coating with Ionic Liquids Compared to a Polymer Brush Coating with a Non-Ionic Liquid. Coatings. 2018; 8(11):398. https://doi.org/10.3390/coatings8110398
Chicago/Turabian StyleKanematsu, Hideyuki, Atsuya Oizumi, Takaya Sato, Toshio Kamijo, Saika Honma, Dana M. Barry, Nobumitsu Hirai, Akiko Ogawa, Takeshi Kogo, Daisuke Kuroda, and et al. 2018. "Biofilm Formation of a Polymer Brush Coating with Ionic Liquids Compared to a Polymer Brush Coating with a Non-Ionic Liquid" Coatings 8, no. 11: 398. https://doi.org/10.3390/coatings8110398
APA StyleKanematsu, H., Oizumi, A., Sato, T., Kamijo, T., Honma, S., Barry, D. M., Hirai, N., Ogawa, A., Kogo, T., Kuroda, D., Sano, K., Tsunashima, K., Lee, S.-H., & Lee, M.-H. (2018). Biofilm Formation of a Polymer Brush Coating with Ionic Liquids Compared to a Polymer Brush Coating with a Non-Ionic Liquid. Coatings, 8(11), 398. https://doi.org/10.3390/coatings8110398








