Influence of Grit-Blasting and Hydrofluoric Acid Etching Treatment on Surface Characteristics and Biofilm Formation on Zirconia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
- (a)
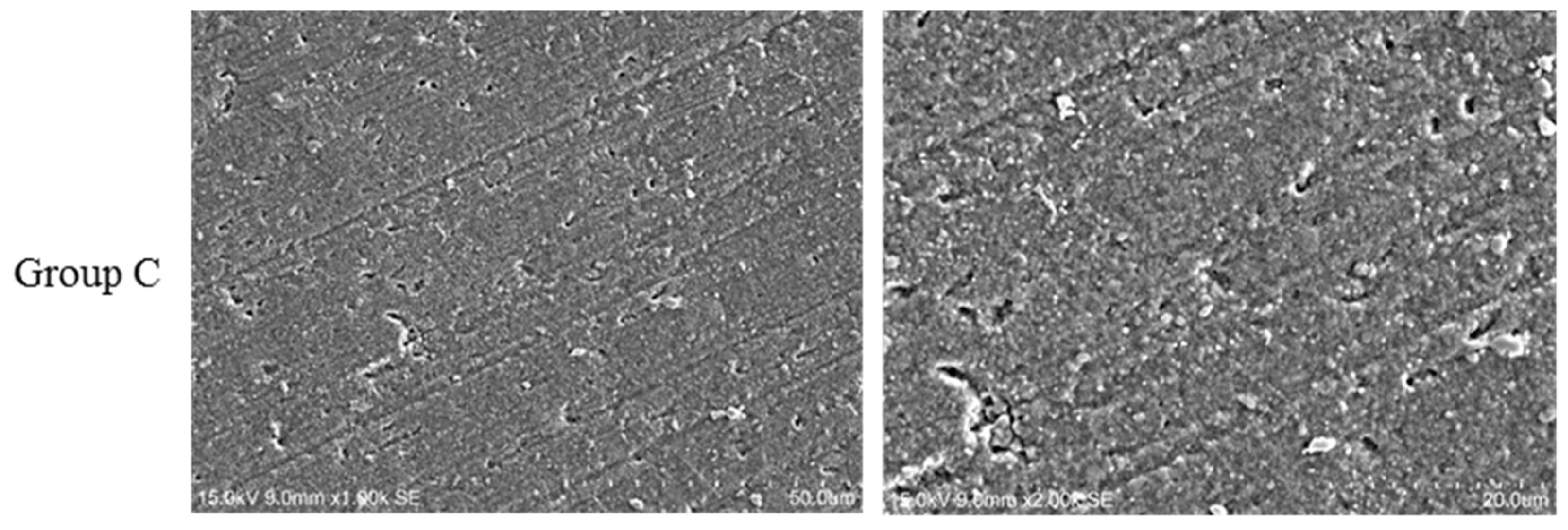
- Group C (control group): No further modification treatment;
- (b)
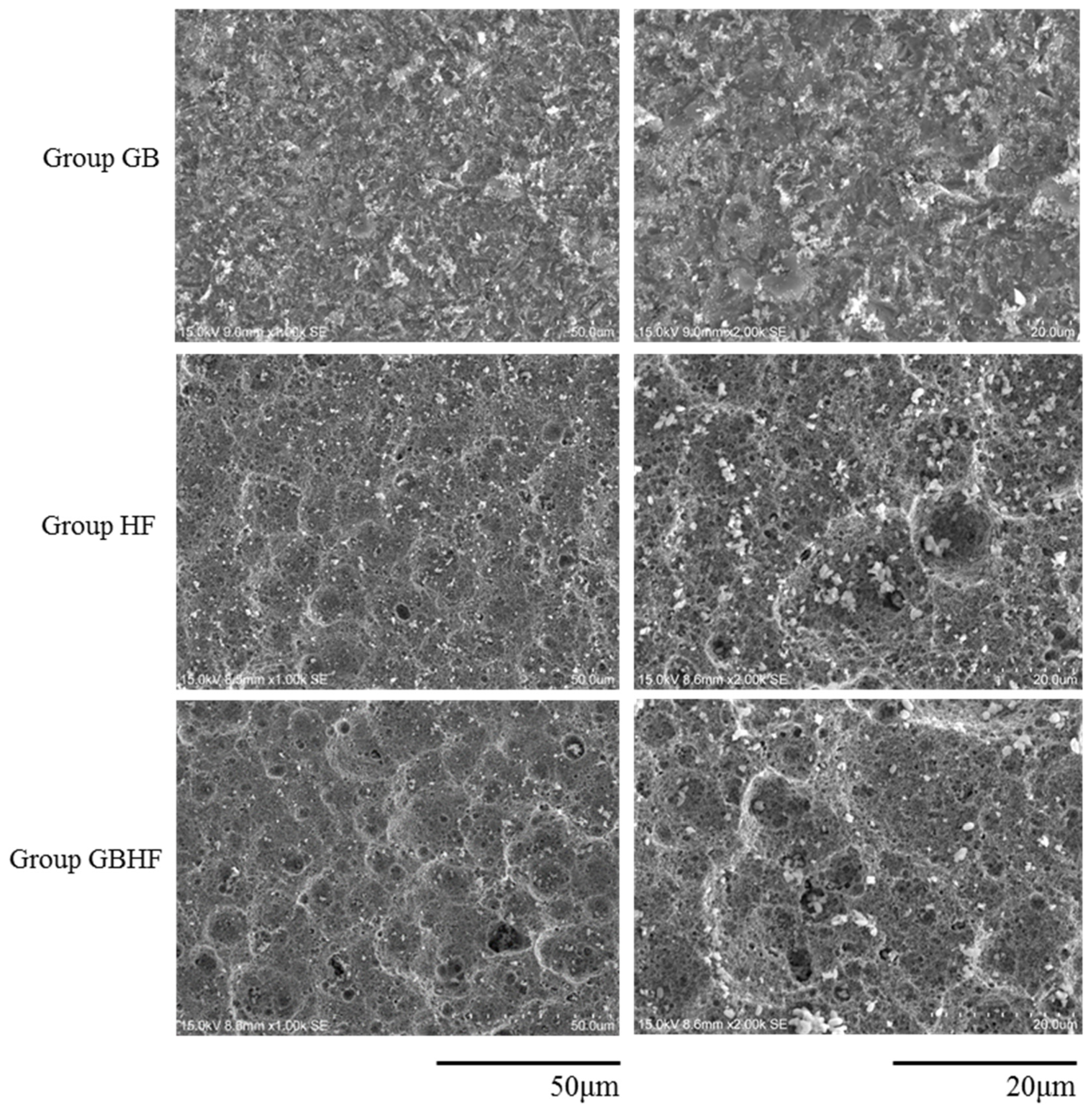
- Group GB: The zirconia samples were grit-blasting with 110 μm silica-coated alumina particles (Rocatecs, 3M ESPE, Seefeld, Germany) at a constant pressure of 3.0 bar for 15 s;
- (c)
- Group HF: The zirconia samples were then treated with 40% hydrofluoric acid (FARCO, Hong Kong, China) for 25 min at 100 °C, and then rinsed with deionized water for 60 s and air dried; and
- (d)
- Group GBHF: Zirconia surface were treated with grit-blasting with 110 μm silica-coated alumina particles (Rocatecs) at a constant pressure of 3.0 bar for 15 s, then treated with 40% hydrofluoric acid for 25 min at 100 °C, and rinsed with deionized water for 60 s and air dried.
2.2. Surface Roughness
2.3. Contact Angle
2.4. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Spectroscopy (EDX)
2.5. Bacterial Strains and Biofilm Formation
2.6. Quantification of Biofilm Formation
2.7. Statistical Analysis
3. Results
3.1. Surface Roughness
3.2. Contact Angle
3.3. Scanning Electron Microscopy (SEM)
3.4. Energy-Dispersive X-ray Spectroscopy (EDX)
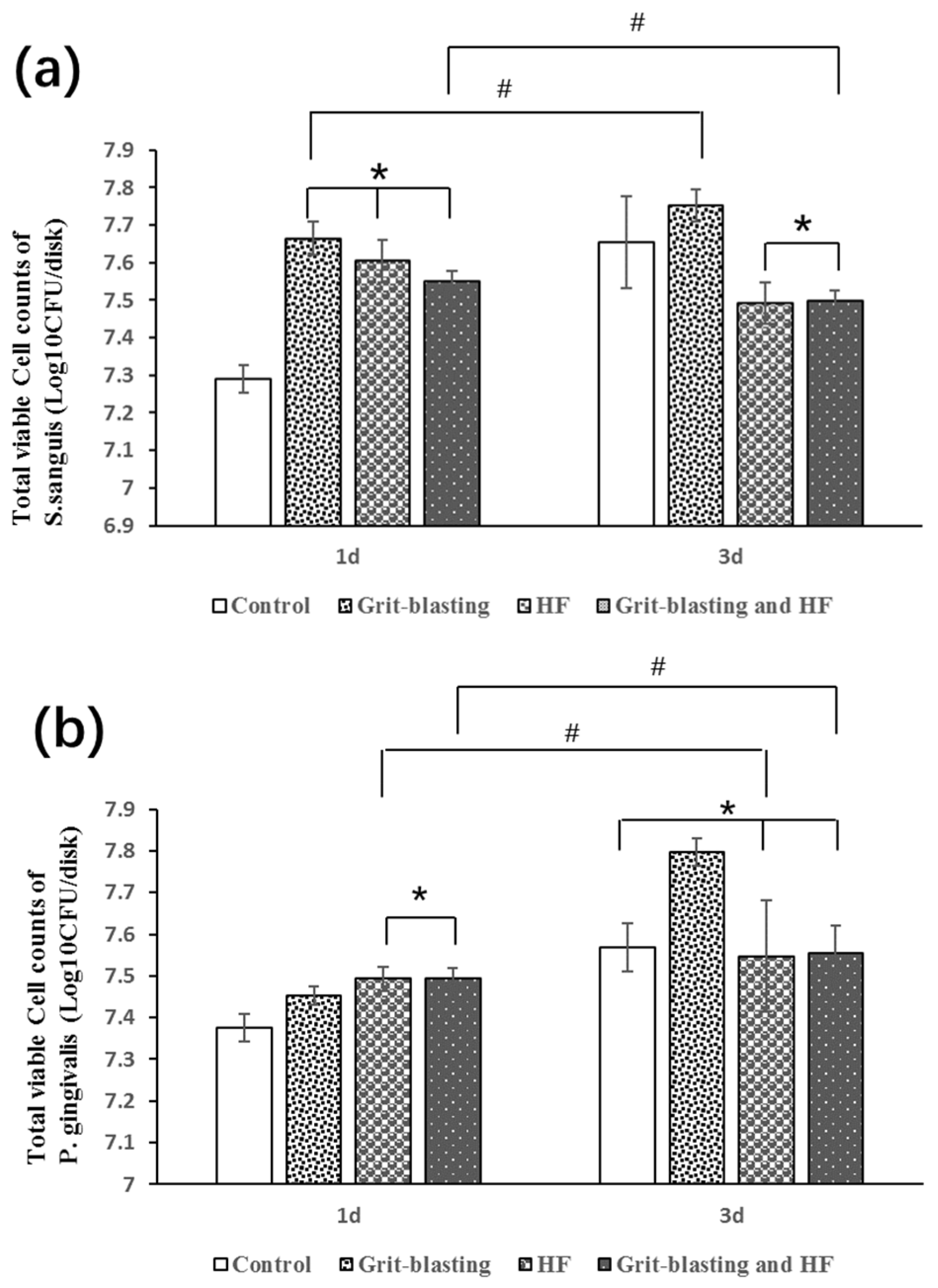
3.5. Biofilm Formation
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vichi, A.; Louca, C.; Corciolani, G.; Ferrari, M. Color related to ceramic and zirconia restorations: A review. Dent. Mater. 2011, 27, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Linkevicius, T.; Vaitelis, J. The effect of zirconia or titanium as abutment material on soft peri-implant tissues: A systematic review and meta-analysis. Clin. Oral Implants Res. 2015, 26, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Van Eric Dooren, D.; Calamita, M.; Calgaro, M.; Coachman, C.; Ferencz, J.L.; Silva, N.R. Mechanical, biological and clinical aspects of zirconia implants. Eur. J. Esthet. Dent. 2012, 7, 396–417. [Google Scholar]
- Hashim, D.; Cionca, N.; Courvoisier, D.S.; Mombelli, A. A systematic review of the clinical survival of zirconia implants. Clin. Oral Investig. 2016, 20, 1403–1417. [Google Scholar] [CrossRef] [PubMed]
- Siwen, L.; Shishi, L.; Yanhong, W.; Hongmei, M. Effects of different surface modifications on micro-structure and adhesion of zirconia ceramic: An in vitro study. West China J. Stomatol. 2017, 35, 43–50. [Google Scholar]
- Ito, Y.; Okawa, T.; Fukumoto, T.; Tsurumi, A.; Tatsuta, M.; Fujii, T.; Tanaka, J.; Tanaka, M. Influence of atmospheric pressure low-temperature plasma treatment on the shear bond strength between zirconia and resin cement. J. Prosthodont. Res. 2016, 60, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz-Savas, T.; Demir, N.; Ozturk, A.N.; Kilic, H.S. Effect of different surface treatments on the bond strength of lithium disilicate ceramic to the zirconia core. Photomed. Laser Surg. 2016, 34, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.C.K.; Tsoi, J.K.H.; Zwahlen, R.A.; Matinlinna, J.P. Effects of silica-coating and a zirconate coupling agent on shear bond strength of flowable resin-zirconia bonding. Int. J. Adhes. Adhes. 2014, 50, 11–16. [Google Scholar] [CrossRef]
- Ho, B.J.; Tsoi, J.K.H.; Liu, D.; Lung, C.Y.K.; Wong, H.M.; Matinlinna, J.P. Effects of sandblasting distance and angles on resin cement bonding to zirconia and titanium. Int. J. Adhes. Adhes. 2015, 62, 25–31. [Google Scholar] [CrossRef]
- Liu, D.; Pow, E.H.N.; Tsoi, J.K.H.; Matinlinna, J.P. Evaluation of four surface coating treatments for resin to zirconia bonding. J. Mech. Behav. Biomed. 2014, 32, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Tsoi, J.K.-H.; Matinlinna, J.P.; Wong, H.M. Effects of some chemical surface modifications on resin zirconia adhesion. J. Mech. Behav. Biomed. 2015, 46, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Matinlinna, J.P.; Choi, A.H.; Tsoi, J.K.H. Bonding promotion of resin composite to silica-coated zirconia implant surface using a novel silane system. Clin. Oral Implants Res. 2013, 24, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Matinlinna, J.P.; Tsoi, J.K.H.; Pow, E.H.N.; Miyazaki, T.; Shibata, Y.; Kan, C.W. A new modified laser pretreatment for porcelain zirconia bonding. Dent. Mater. 2013, 29, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Kohal, R.J.; Bächle, M.; Att, W.; Chaar, S.; Altmann, B.; Renz, A.; Butz, F. Osteoblast and bone tissue response to surface modified zirconia and titanium implant materials. Dent. Mater. 2013, 29, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Al-Radha, A.S.D.; Dymock, D.; Younes, C.; O’Sullivan, D. Surface properties of titanium and zirconia dental implant materials and their effect on bacterial adhesion. J. Dent. 2012, 40, 146–153. [Google Scholar] [CrossRef] [PubMed]
- De Avila, E.D.; Avila-Campos, M.J.; Vergani, C.E.; Spolidório, D.M.P.; de Assis Mollo, F., Jr. Structural and quantitative analysis of a mature anaerobic biofilm on different implant abutment surfaces. J. Prosthet. Dent. 2016, 115, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Villard, N.; Seneviratne, C.; Tsoi, J.K.H.; Heinonen, M.; Matinlinna, J. Candida albicans aspects of novel silane system-coated titanium and zirconia implant surfaces. Clin. Oral Implants Res. 2015, 26, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Smielak, B.; Klimek, L. Effect of hydrofluoric acid concentration and etching duration on select surface roughness parameters for zirconia. J. Prosthet. Dent. 2015, 113, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.W.; Matinlinna, J.P. Evaluation of the microtensile bond strength between resin composite and hydrofluoric acid etched ceramic in different storage media. J. Adhes. Sci. Technol. 2011, 25, 2671–2685. [Google Scholar] [CrossRef]
- Xie, H.; Chen, C.; Dai, W.; Chen, G.; Zhang, F. In vitro short-term bonding performance of zirconia treated with hot acid etching and primer conditioning. Dent. Mater. J. 2013, 32, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Karande, A.M.; Vergeese, C.S.; Khandeparkar, R.; Mahajan, T. Inter relationship between the implant and biofilm based on their microbial linkage. J. Adv. Med. Dent. Sci. Res. 2017, 5, 46–53. [Google Scholar]
- Han, A.F.; Tsoi, J.K.H.; Rodrigues, F.P.; Leprince, J.G.; Palin, W.M. Bacterial adhesion mechanisms on dental implant surfaces and the influencing factors. Int. J. Adhes. Adhes. 2016, 69, 58–71. [Google Scholar] [CrossRef]
- Kumar, Y.; Jain, V.; Chauhan, S.S.; Bharate, V.; Koli, D.; Kumar, M. Influence of different forms and materials (zirconia or titanium) of abutments in periimplant soft-tissue healing using matrix metalloproteinase-8: A randomized pilot study. J. Prosthet. Dent. 2017. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, C.; Pita, M.S.; de Souza Santos, E.; Monesi, N.; Pedrazzi, V.; de Albuquerque Junior, R.F.; Ribeiro, R.F. Microbiome of titanium and zirconia dental implants abutments. Dent. Mater. 2016, 32, 93–101. [Google Scholar] [CrossRef] [PubMed]
- De Avila, E.D.; Vergani, C.E.; Junior, F.A.M.; Junior, M.J.; Shi, W.; Lux, R. Effect of titanium and zirconia dental implant abutments on a cultivable polymicrobial saliva community. J. Prosthet. Dent. 2017. [Google Scholar] [CrossRef] [PubMed]
- Hiroshi, I.; Sasaki, H.; Saito, K.; Honma, S.; Yajima, Y.; Yoshinari, M. Response of osteoblast-like cells to zirconia with different surface topography. Dent. Mater. J. 2013, 32, 122–129. [Google Scholar]
- Ruiz-Cabello, F.M.; Rodríguez-Valverde, M.; Cabrerizo-Vílchez, M. Equilibrium contact angle or the most-stable contact angle? Adv. Colloid Interface Sci. 2014, 206, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Shen, S.; Qian, M.; Zhang, F.; Chen, C.; Tay, F.R. Effects of acid treatment on dental zirconia: An in vitro study. PLoS ONE 2015, 10, e0136263. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Koo, H.; Ren, D. Effects of material properties on bacterial adhesion and biofilm formation. J. Dent. Res. 2015, 94, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implants Res. 2006, 17, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Chaparro, P.J.; Duarte, P.M.; Shibli, J.A.; Montenegro, S.; Lacerda Heluy, S.; Figueiredo, L.C.; Faveri, M.; Feres, M. The current weight of evidence of the microbiologic profile associated with peri-implantitis: A systematic review. J. Periodontol. 2016, 87, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmad, A.; Wiedmann-Al-Ahmad, M.; Fackler, A.; Follo, M.; Hellwig, E.; Bächle, M.; Hannig, C.; Han, J.-S.; Wolkewitz, M.; Kohal, R. In vivo study of the initial bacterial adhesion on different implant materials. Arch. Oral Biol. 2013, 58, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Sharma, P.K.; Busscher, H.J.; van der Mei, H.C.; Krom, B.P. Role of extracellular DNA in initial bacterial adhesion and surface aggregation. Appl. Environ. Microbiol. 2010, 76, 3405–3408. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cheung, G.S.; Watson, G.S.; Watson, J.A.; Lin, S.; Schwarzkopf, L.; Green, D.W. The nanotipped hairs of gecko skin and biotemplated replicas impair and/or kill pathogenic bacteria with high efficiency. Nanoscale 2016, 8, 18860–18869. [Google Scholar] [CrossRef] [PubMed]
- Perera-Costa, D.; Bruque, J.M.; González-Martín, M.L.; Gómez-García, A.C.; Vadillo-Rodríguez, V. Studying the influence of surface topography on bacterial adhesion using spatially organized microtopographic surface patterns. Langmuir 2014, 30, 4633–4641. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.A.; Turkyilmaz, I. Evaluation of the sealing capability of implants to titanium and zirconia abutments against porphyromonas gingivalis, prevotella intermedia, and fusobacterium nucleatum under different screw torque values. J. Prosthet. Dent. 2014, 112, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Pita, P.P.C.; Rodrigues, J.A.; Ota-Tsuzuki, C.; Miato, T.F.; Zenobio, E.G.; Giro, G.; Figueiredo, L.C.; Gonçalves, C.; Gehrke, S.A.; Cassoni, A. Oral streptococci biofilm formation on different implant surface topographies. BioMed Res. Int. 2015, 2015, 159625. [Google Scholar] [CrossRef] [PubMed]
| Group | Surface Treatment | Surface Roughness (μm) | Water Contact Angle (θ) |
|---|---|---|---|
| Group C | Control | 0.17 ± 0.03 a | 100.24 ± 2.49 d |
| Group GB | Grit-blasting | 0.56 ± 0.05 b | 109.87 ± 8.22 d |
| Group HF | Etching with hydrofluoric acid | 1.47 ± 0.04 c | 79.13 ± 5.20 e |
| Group GBHF | Grit-blasting and then etching with hydrofluoric acid | 1.48 ± 0.05 c | 81.35 ± 1.15 e |
| Element Conc (wt %) | Group C | Group GB | Group HF | Group GBHF |
|---|---|---|---|---|
| O | 10.606 | 16.363 | 10.098 | 10.675 |
| Al | 1.591 | 3.768 | 1.603 | 1.605 |
| Si | 0.199 | 4.674 | 0.057 | 0.236 |
| Y | 8.686 | 6.014 | 8.955 | 8.297 |
| Zr | 78.918 | 69.182 | 79.287 | 79.189 |
| Total | 100.000 | 100.000 | 100.000 | 100.000 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, A.; Tsoi, J.K.-H.; Matinlinna, J.P.; Chen, Z. Influence of Grit-Blasting and Hydrofluoric Acid Etching Treatment on Surface Characteristics and Biofilm Formation on Zirconia. Coatings 2017, 7, 130. https://doi.org/10.3390/coatings7080130
Han A, Tsoi JK-H, Matinlinna JP, Chen Z. Influence of Grit-Blasting and Hydrofluoric Acid Etching Treatment on Surface Characteristics and Biofilm Formation on Zirconia. Coatings. 2017; 7(8):130. https://doi.org/10.3390/coatings7080130
Chicago/Turabian StyleHan, Aifang, James K.-H. Tsoi, Jukka P. Matinlinna, and Zhuofan Chen. 2017. "Influence of Grit-Blasting and Hydrofluoric Acid Etching Treatment on Surface Characteristics and Biofilm Formation on Zirconia" Coatings 7, no. 8: 130. https://doi.org/10.3390/coatings7080130
APA StyleHan, A., Tsoi, J. K.-H., Matinlinna, J. P., & Chen, Z. (2017). Influence of Grit-Blasting and Hydrofluoric Acid Etching Treatment on Surface Characteristics and Biofilm Formation on Zirconia. Coatings, 7(8), 130. https://doi.org/10.3390/coatings7080130






