Emerging Corrosion Inhibitors for Interfacial Coating
Abstract
:1. Introduction
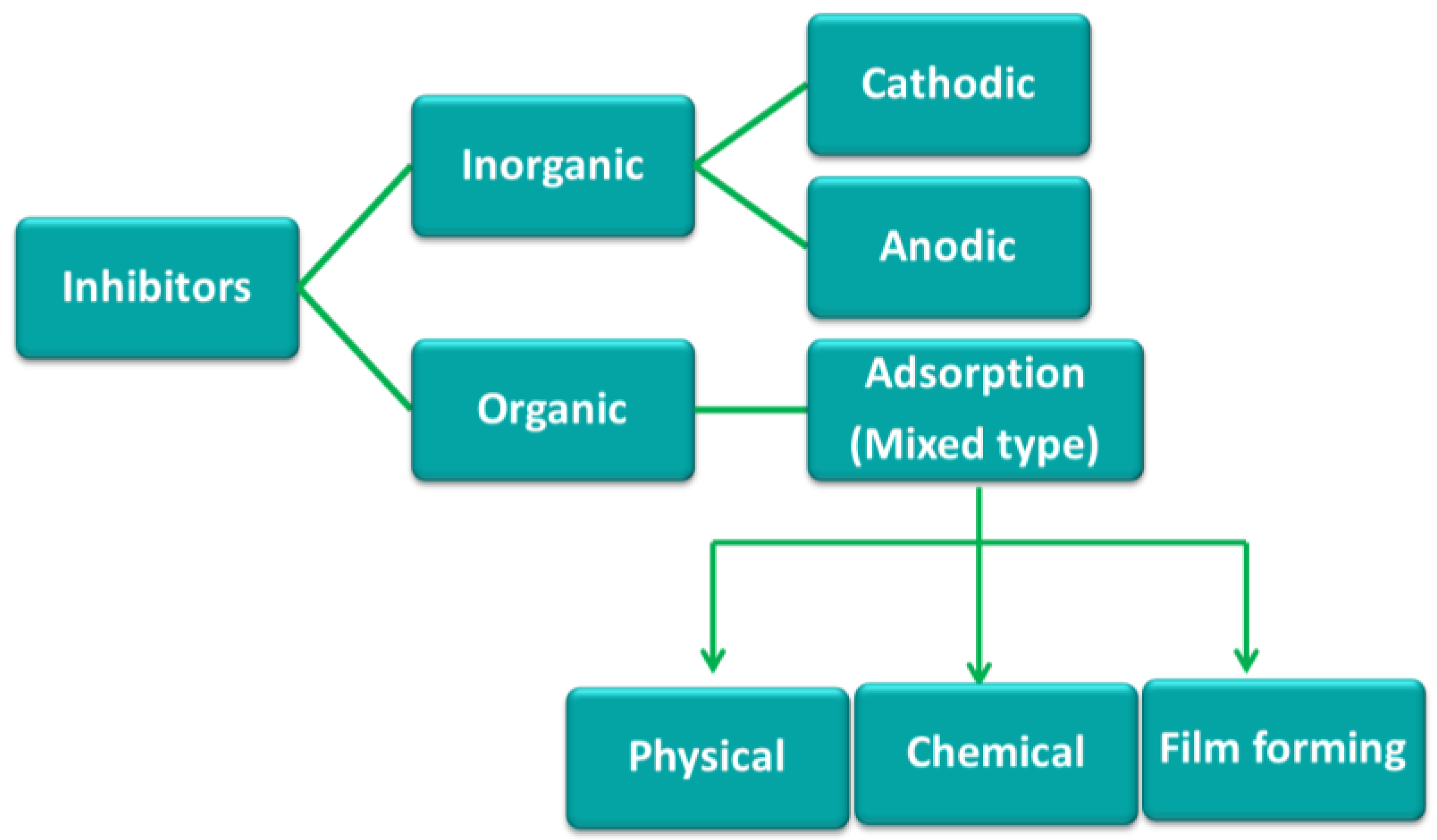
- Adsorption: the inhibitor is chemically adsorbed on the surface of the metal and forms a protective thin film with inhibitor effect.
- Surface layer: formation of an oxide film for protection of the metal surface.
- Passivation: the inhibitor reacts with corrosive elements of aqueous media, forming protective precipitates.
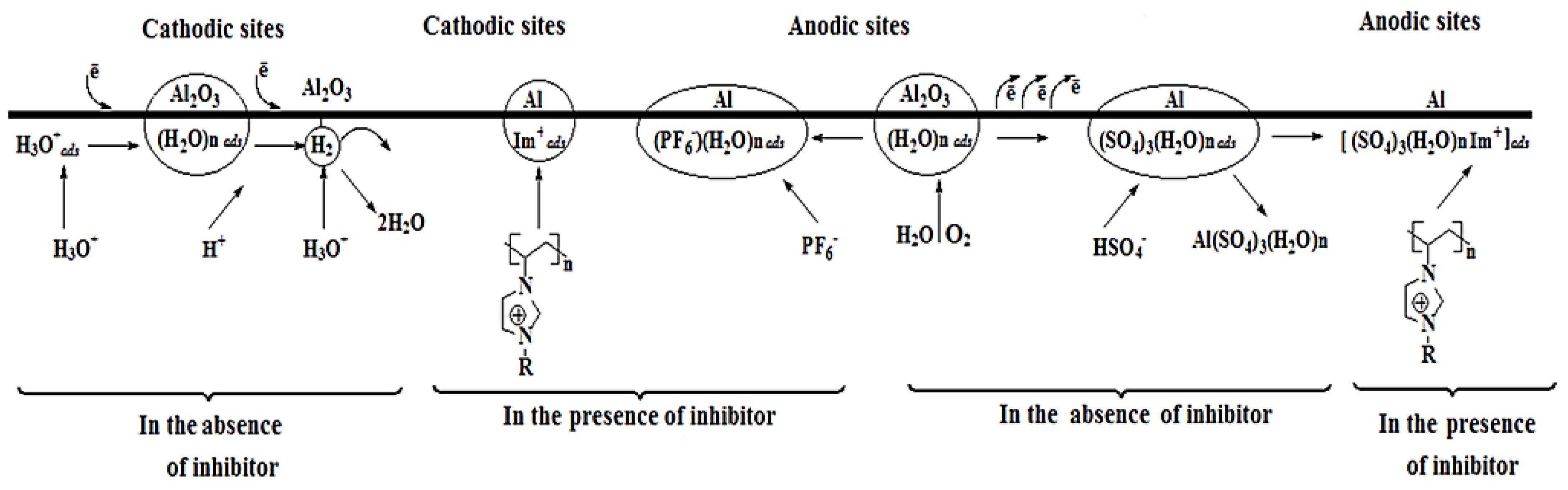
2. Ionic Liquid (IL) Based Corrosion Inhibitors
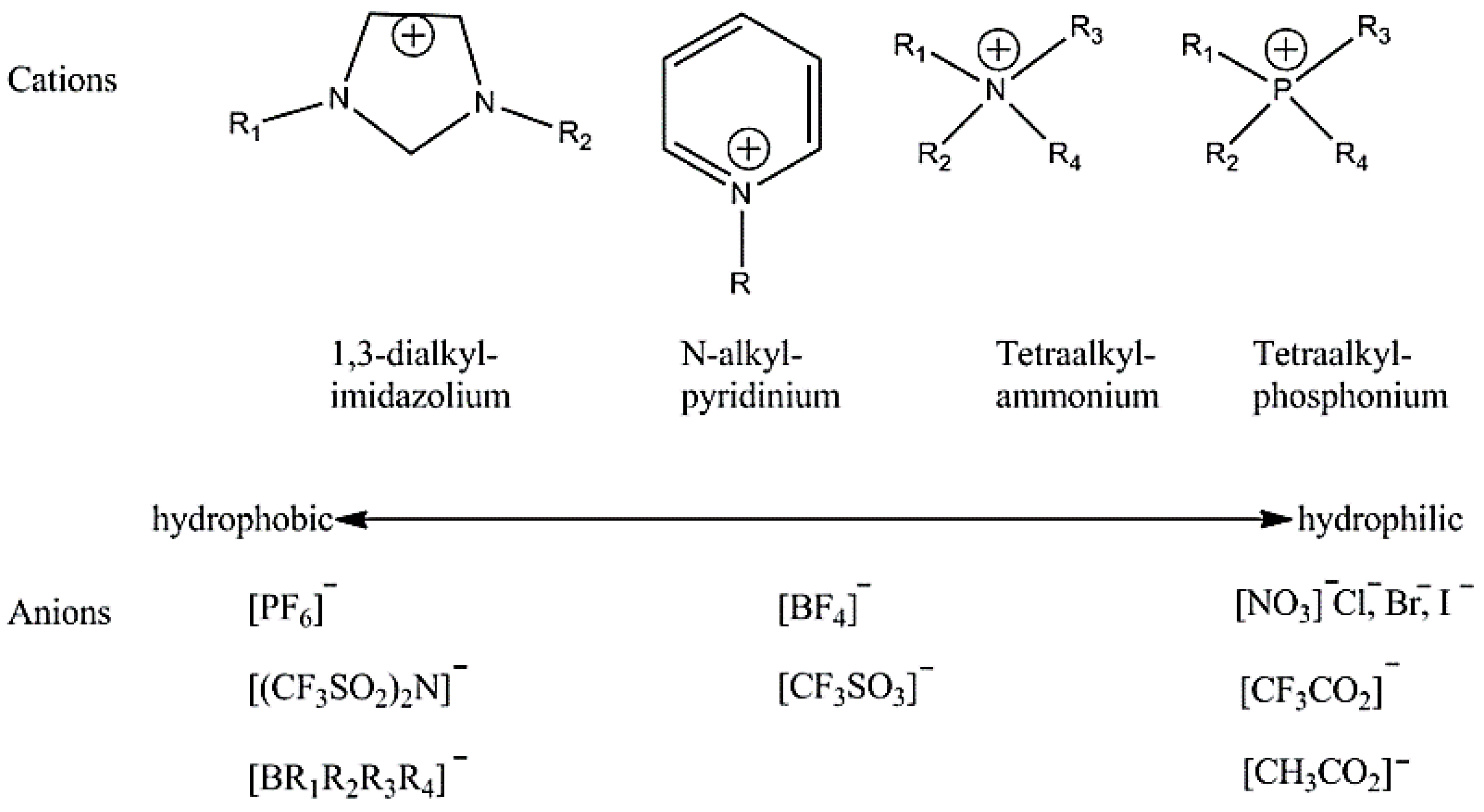
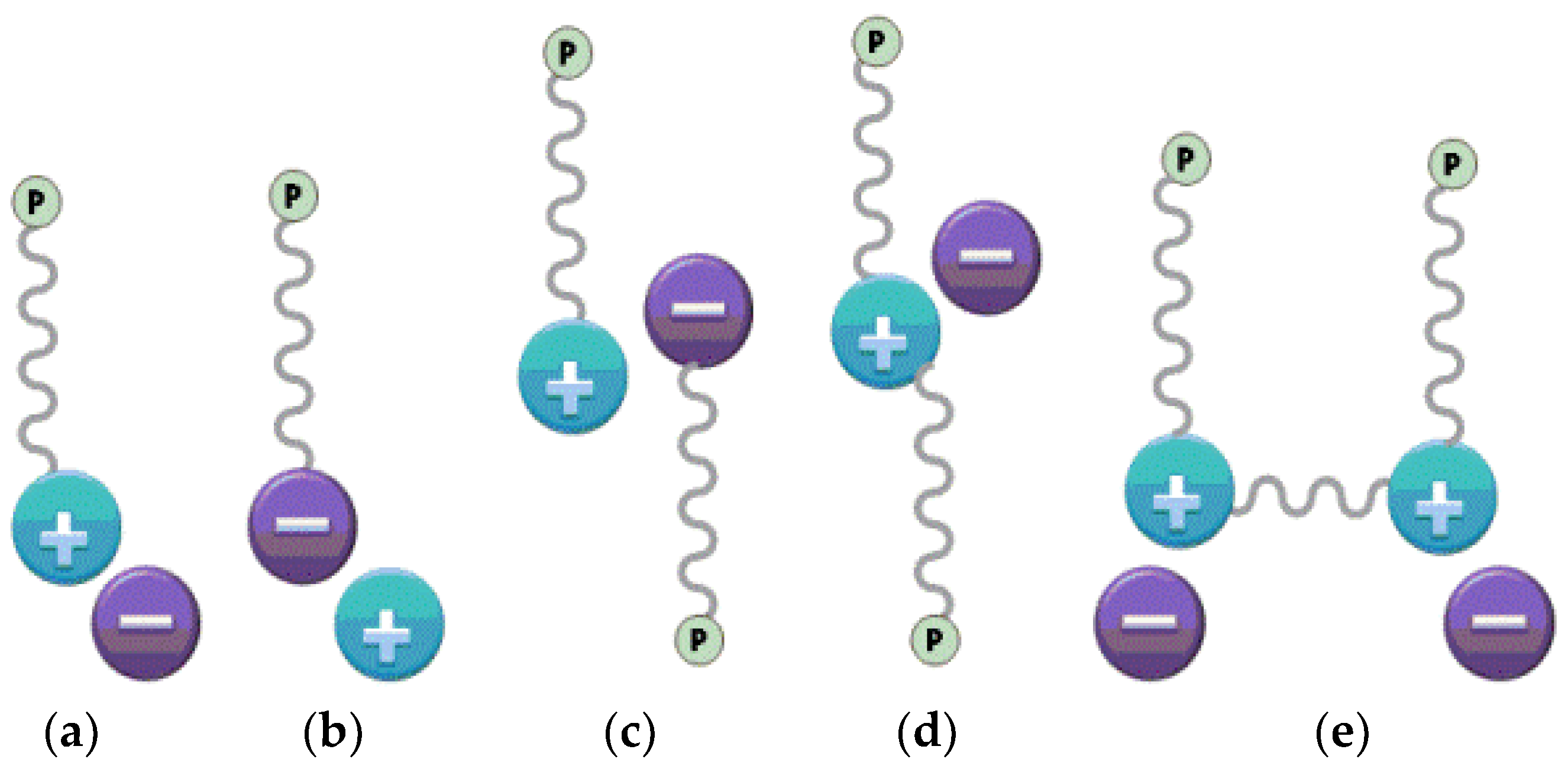
2.1. Effect of IL Structure on Corrosion Inhibition
2.1.1. Cation Effect
2.1.2. Anion Effect
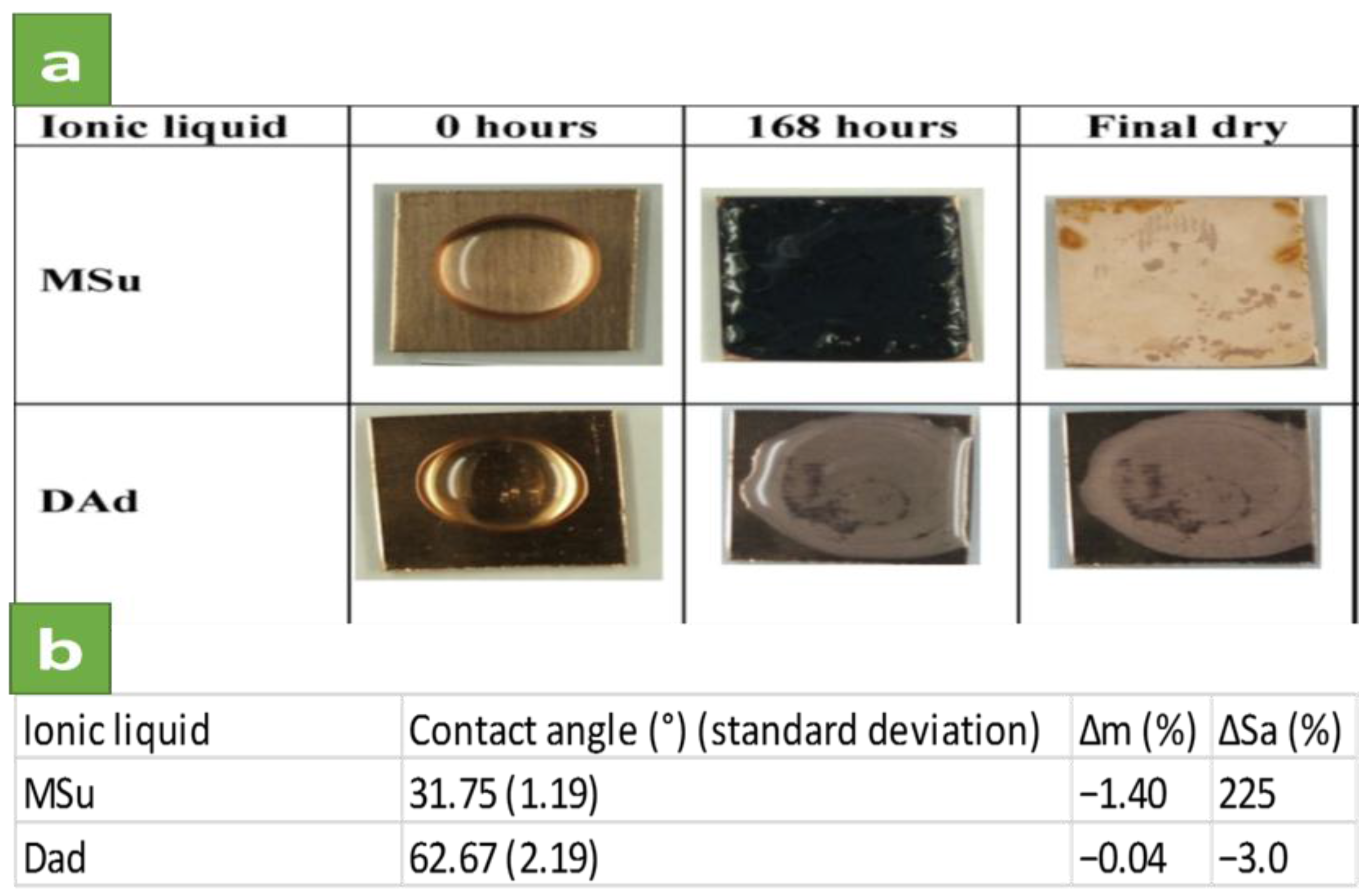
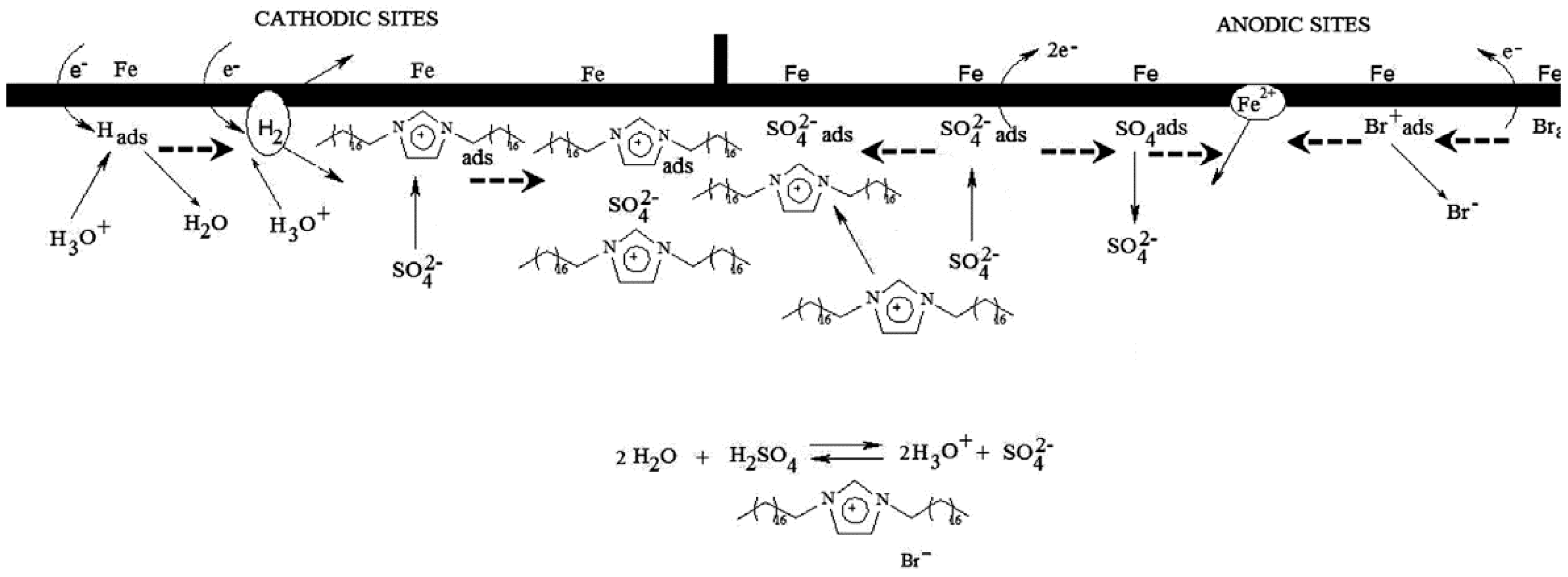
2.2. Synergistic Corrosion Inhibition Using ILs
3. Poly Ionic Liquid (PIL) Based Corrosion Inhibitor
3.1. PIL Structure Diversity
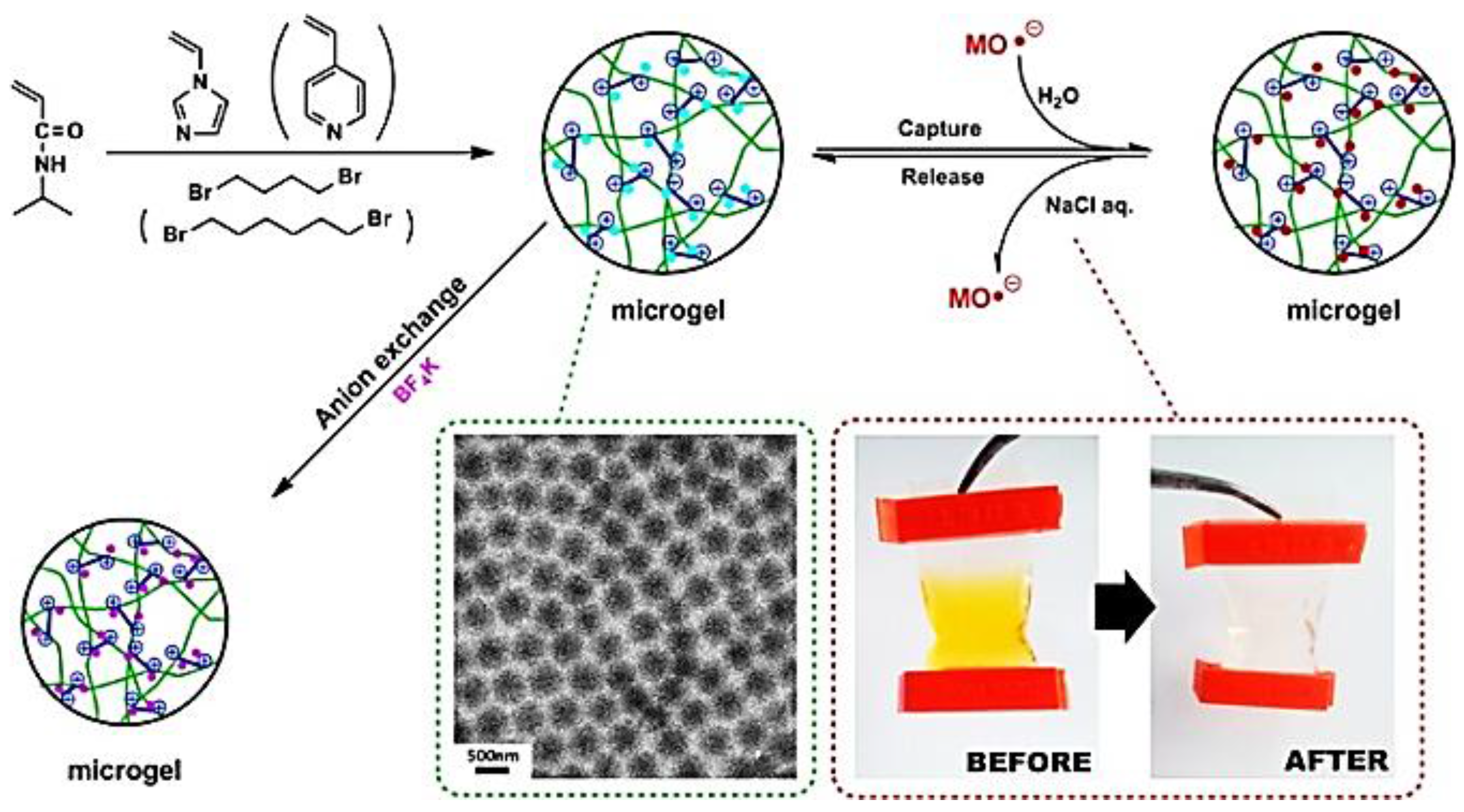
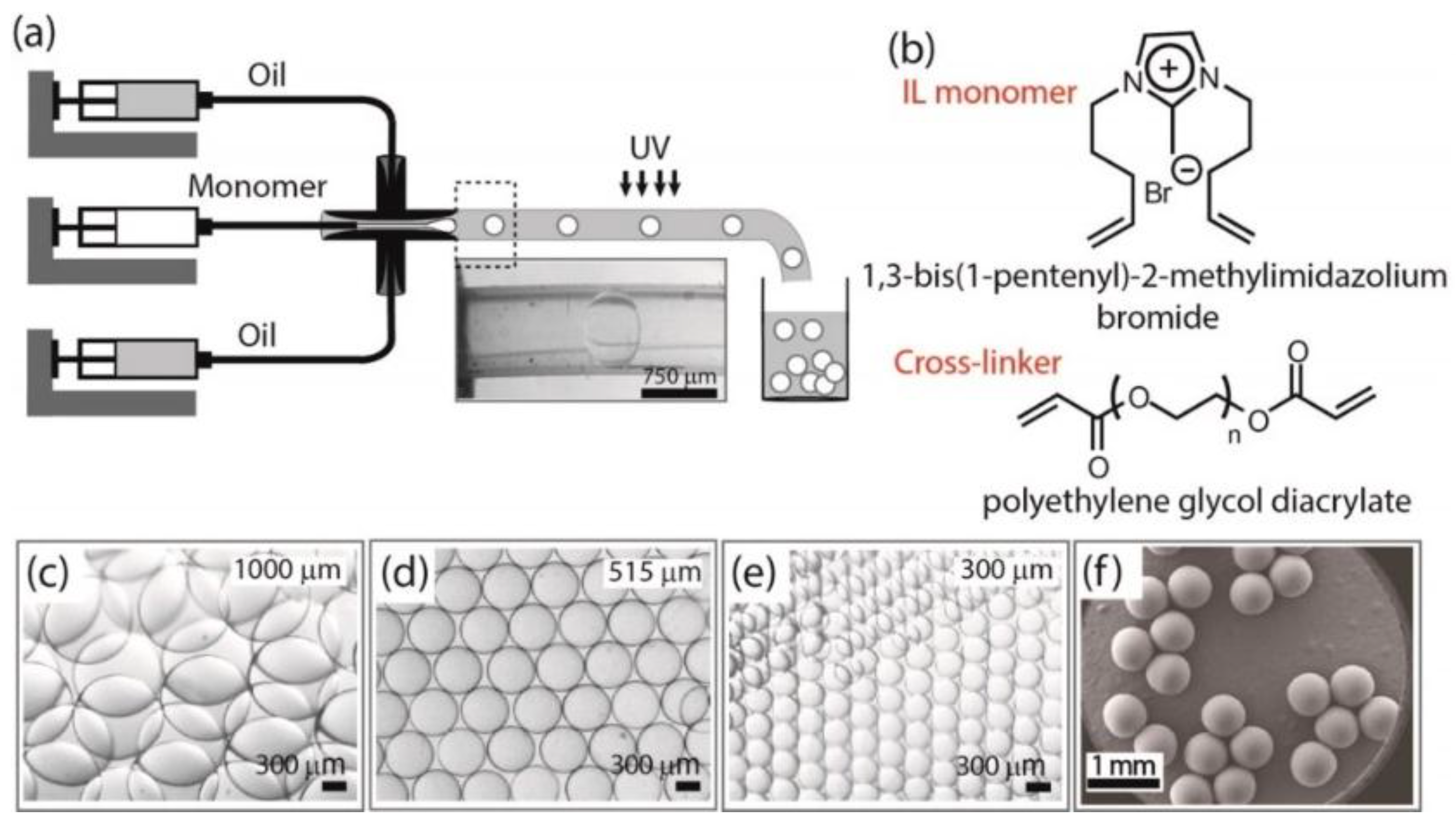
3.1.1. PIL Colloidal Particles
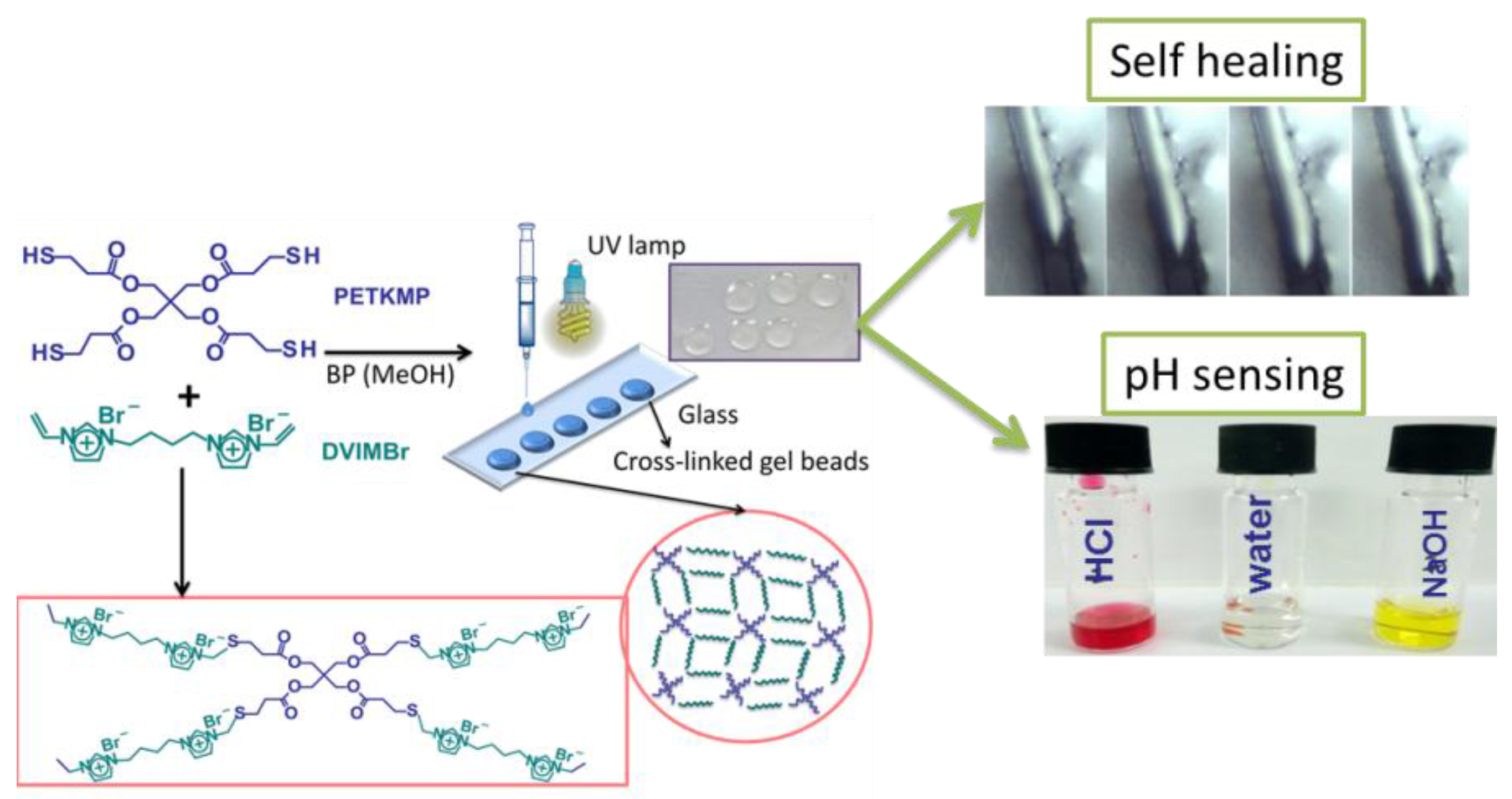
3.1.2. PIL Gel
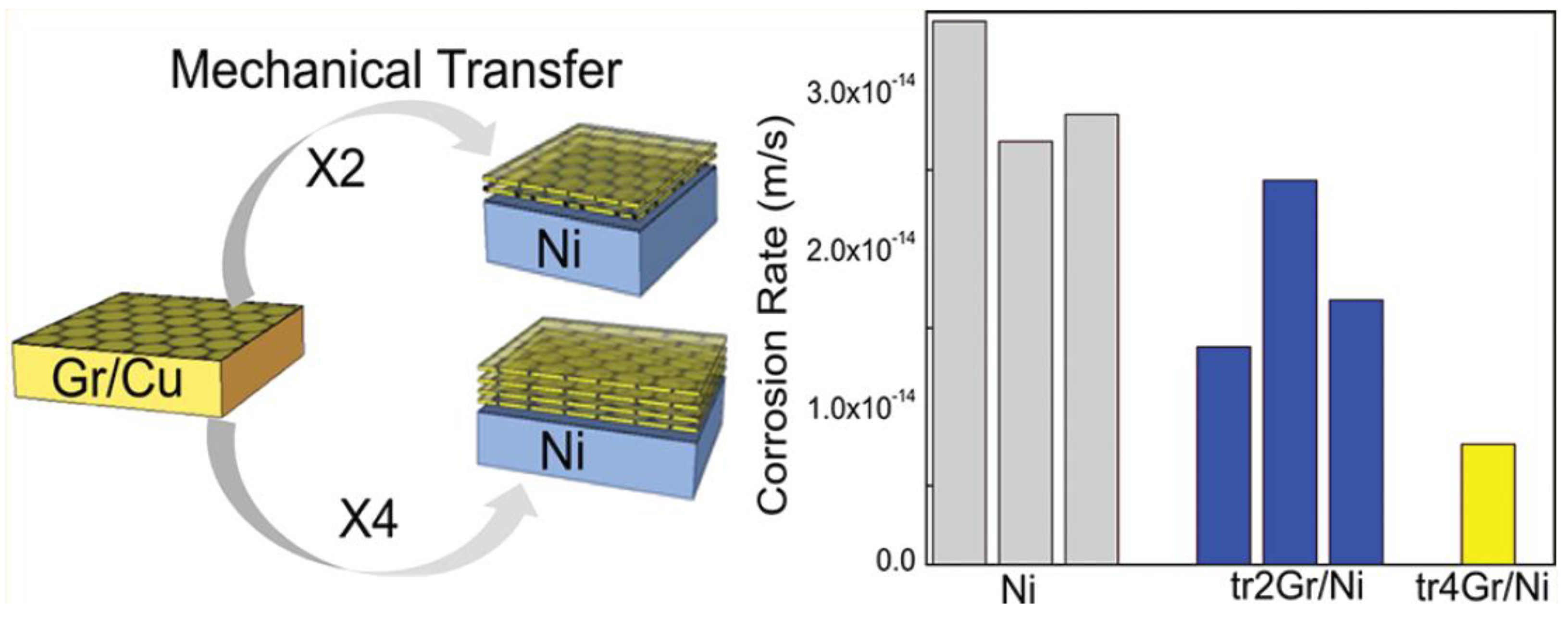
4. Graphene as Green Corrosion Inhibitor in Anticorrosion Coating
- Missing bonds;
- Pentagonal and hexagonal lattices;
- Lattice distortion;
- Local thickness variations;
- Presence of impurities.
Quantum Chemical Methods as Efficient Tools to Study Corrosion Inhibitors
5. Emerging Embedment Methods of Corrosion Inhibitors
5.1. Self-Healing Coating
5.1.1. Encapsulated Type Self-Healing
5.1.2. Effective Parameters and Challenges of Microcapsule Embedment for Corrosion Inhibition
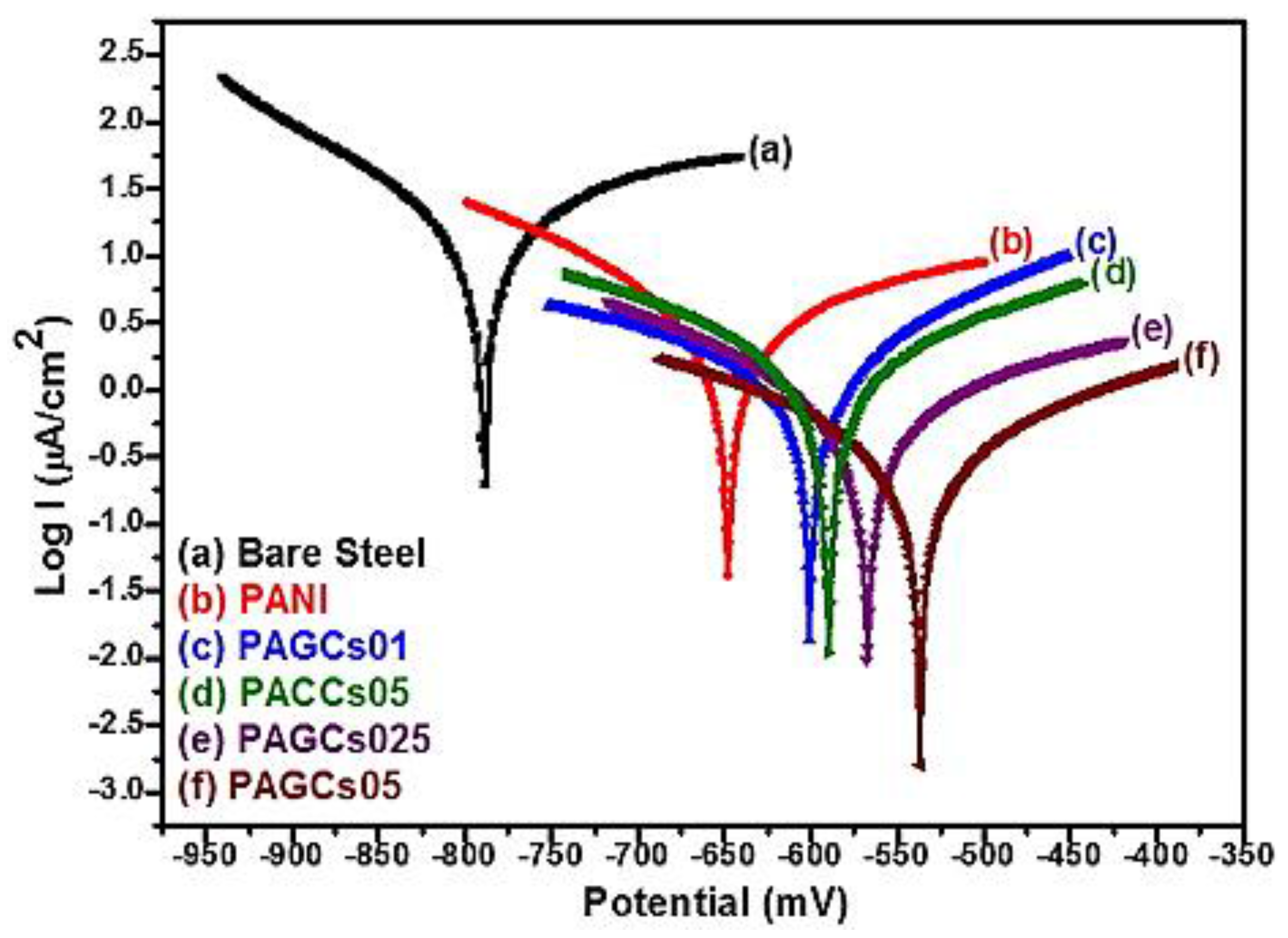
6. Evaluation of Corrosion Inhibitors Using Advanced Characterization Techniques
7. Conclusions and Future Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wazarkar, K.; Patil, D.; Rane, A.; Balgude, D.; Kathalewar, M.; Sabnis, A. Microencapsulation: An emerging technique in the modern coating industry. RSC Adv. 2016, 6, 106964–106979. [Google Scholar] [CrossRef]
- Dariva, C.G.; Galio, A.F. Corrosion inhibitors–principles, mechanisms and applications. In Developments in Corrosion Protection; Aliofkhazraei, M., Ed.; INTECH: Winchester, UK, 2014; pp. 365–379. [Google Scholar]
- Benali, O.; Cherkaoui, O.; Lallam, A. Adsorption and corrosion inhibition of new synthesized pyridazinium-based ionic liquid on carbon steel in 0.5MH2SO4. J. Mater. Environ. Sci. 2015, 6, 598–606. [Google Scholar]
- Saji, V.S. A review on recent patents in corrosion inhibitors. Recent Pat. Corros. Sci. 2010, 2, 6–12. [Google Scholar] [CrossRef]
- Bardal, E. Corrosion and Protection; Springer Science & Business Media: London, UK, 2007. [Google Scholar]
- Yaro, A.S.; Khadom, A.A.; Wael, R.K. Apricot juice as green corrosion inhibitor of mild steel in phosphoric acid. Alex. Eng. J. 2013, 52, 129–135. [Google Scholar] [CrossRef]
- Sherif, E.-S.M. Effects of 2-amino-5-(ethylthio)-1,3,4-thiadiazole on copper corrosion as a corrosion inhibitor in 3% NaCl solutions. Appl. Surf. Sci. 2006, 252, 8615–8623. [Google Scholar] [CrossRef]
- Revie, R.W. Corrosion and Corrosion Control, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Olivares, O.; Likhanova, N.; Gomez, B.; Navarrete, J.; Llanos-Serrano, M.; Arce, E.; Hallen, J. Electrochemical and XPS studies of decylamides of α-amino acids adsorption on carbon steel in acidic environment. Appl. Surf. Sci. 2006, 252, 2894–2909. [Google Scholar] [CrossRef]
- Olivares-Xometl, O.; Likhanova, N.; Domínguez-Aguilar, M.; Arce, E.; Dorantes, H.; Arellanes-Lozada, P. Synthesis and corrosion inhibition of α-amino acids alkylamides for mild steel in acidic environment. Mater. Chem. Phys. 2008, 110, 344–351. [Google Scholar] [CrossRef]
- El-Maksoud, S.A.; Fouda, A. Some pyridine derivatives as corrosion inhibitors for carbon steel in acidic medium. Mater. Chem. Phys. 2005, 93, 84–90. [Google Scholar] [CrossRef]
- Noor, E.A. Evaluation of inhibitive action of some quaternary N-heterocyclic compounds on the corrosion of Al–Cu alloy in hydrochloric acid. Mater. Chem. Phys. 2009, 114, 533–541. [Google Scholar] [CrossRef]
- Martínez-Palou, R.; Rivera, J.; Zepeda, L.; Rodríguez, A.; Hernández, M.; Marín-Cruz, J.; Estrada, A. Evaluation of corrosion inhibitors synthesized from fatty acids and fatty alcohols isolated from sugar cane wax. Corrosion 2004, 60, 465–470. [Google Scholar] [CrossRef]
- Olivares-Xometl, O.; Likhanova, N.; Martínez-Palou, R.; Domínguez-Aguilar, M. Electrochemistry and XPS study of an imidazoline as corrosion inhibitor of mild steel in an acidic environment. Mater. Corros. 2009, 60, 14–21. [Google Scholar] [CrossRef]
- Popova, A.; Christov, M.; Zwetanova, A. Effect of the molecular structure on the inhibitor properties of azoles on mild steel corrosion in 1 m hydrochloric acid. Corros. Sci. 2007, 49, 2131–2143. [Google Scholar] [CrossRef]
- Antonijević, M.M.; Milić, S.M.; Petrović, M.B. Films formed on copper surface in chloride media in the presence of azoles. Corros. Sci. 2009, 51, 1228–1237. [Google Scholar] [CrossRef]
- Tallman, D.E.; Spinks, G.; Dominis, A.; Wallace, G.G. Electroactive conducting polymers for corrosion control. J. Solid State Electrochem. 2002, 6, 73–84. [Google Scholar] [CrossRef]
- Hu, J.; Ji, Y.; Shi, Y.; Hui, F.; Duan, H.; Lanza, M. A review on the use of graphene as a protective coating against corrosion. Ann. Mater. Sci. Eng. 2014, 1, 16. [Google Scholar]
- Martínez-Palou, R.; Sánche, P.F. Perspectives of Ionic Liquids Applications for Clean Oilfield Technologies; INTECH Open Access Publisher: Rijeka, Croatia, 2011. [Google Scholar]
- Zhang, Q.; Hua, Y. Corrosion inhibition of mild steel by alkylimidazolium ionic liquids in hydrochloric acid. Electrochim. Acta 2009, 54, 1881–1887. [Google Scholar] [CrossRef]
- Yuan, J.; Antonietti, M. Poly(ionic liquid)s: Polymers expanding classical property profiles. Polymer 2011, 52, 1469–1482. [Google Scholar] [CrossRef]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Olivier-Bourbigou, H.; Magna, L.; Morvan, D. Ionic liquids and catalysis: Recent progress from knowledge to applications. Appl. Catal. A General 2010, 373, 1–56. [Google Scholar] [CrossRef]
- Han, X.; Armstrong, D.W. Ionic liquids in separations. Acc. Chem. Res. 2007, 40, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Bara, J.E.; Carlisle, T.K.; Gabriel, C.J.; Camper, D.; Finotello, A.; Gin, D.L.; Noble, R.D. Guide to CO2 separations in imidazolium-based room-temperature ionic liquids. Ind. Eng. Chem. Res. 2009, 48, 2739–2751. [Google Scholar] [CrossRef]
- Anderson, J.L.; Armstrong, D.W.; Wei, G.-T. Ionic liquids in analytical chemistry. Anal. Chem. 2006, 78, 2892–2902. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, Q.; Ye, C.; Liu, W.; Cui, Z. Friction and wear behaviors of ionic liquid of alkylimidazolium hexafluorophosphates as lubricants for steel/steel contact. Wear 2004, 256, 44–48. [Google Scholar] [CrossRef]
- Kuang, D.; Wang, P.; Ito, S.; Zakeeruddin, S.M.; Grätzel, M. Stable mesoscopic dye-sensitized solar cells based on tetracyanoborate ionic liquid electrolyte. J. Am. Chem. Soc. 2006, 128, 7732–7733. [Google Scholar] [CrossRef] [PubMed]
- Likhanova, N.V.; Domínguez-Aguilar, M.A.; Olivares-Xometl, O.; Nava-Entzana, N.; Arce, E.; Dorantes, H. The effect of ionic liquids with imidazolium and pyridinium cations on the corrosion inhibition of mild steel in acidic environment. Corros. Sci. 2010, 52, 2088–2097. [Google Scholar] [CrossRef]
- Łuczak, J.; Hupka, J.; Thöming, J.; Jungnickel, C. Self-organization of imidazolium ionic liquids in aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2008, 329, 125–133. [Google Scholar] [CrossRef]
- Espinosa, T.; Sanes, J.; Jiménez, A.-E.; Bermúdez, M.-D. Surface interactions, corrosion processes and lubricating performance of protic and aprotic ionic liquids with ofhc copper. Appl. Surf. Sci. 2013, 273, 578–597. [Google Scholar] [CrossRef]
- Elachouri, M.; Hajji, M.; Kertit, S.; Essassi, E.; Salem, M.; Coudert, R. Some surfactants in the series of 2-(alkyldimethylammonio) alkanol bromides as inhibitors of the corrosion of iron in acid chloride solution. Corros. Sci. 1995, 37, 381–389. [Google Scholar] [CrossRef]
- Quraishi, M.A.; Rafiquee, M.Z.A.; Khan, S.; Saxena, N. Corrosion inhibition of aluminium in acid solutions by some imidazoline derivatives. J. Appl. Electrochem. 2007, 37, 1153–1162. [Google Scholar] [CrossRef]
- Gasparac, R.; Martin, C.; Stupnisek-Lisac, E. In situ studies of imidazole and its derivatives as copper corrosion inhibitors. I. Activation energies and thermodynamics of adsorption. J. Electrochem. Soc. 2000, 147, 548–551. [Google Scholar] [CrossRef]
- Zhang, D.-Q.; Gao, L.-X.; Zhou, G.-D. Inhibition of copper corrosion by bis-(1,1′-benzotriazoly)-α,ω-diamide compounds in aerated sulfuric acid solution. Appl. Surf. Sci. 2006, 252, 4975–4981. [Google Scholar] [CrossRef]
- Zhang, Q.; Hua, Y. Corrosion inhibition of aluminum in hydrochloric acid solution by alkylimidazolium ionic liquids. Mater. Chem. Phys. 2010, 119, 57–64. [Google Scholar] [CrossRef]
- Shuncun, S.; Pinggui, Y.; Chenzhong, C.; Xueye, W.; Jieshu, S.; Junxi, L. Synthesis of new ionic liquids and corrosion inhibition performance of its cationic imidazoline group. J. Chem. Ind. Eng. China 2005, 56, 1112–1119. [Google Scholar]
- Ford, F.; Burstein, G.; Hoar, T. Bare surface reaction rates and their relation to environment controlled cracking of aluminum alloys I. Bare surface reaction rates on aluminum-7 weight percent magnesium in aqueous solutions. J. Electrochem. Soc. 1980, 127, 1325–1331. [Google Scholar] [CrossRef]
- Uerdingen, M.; Treber, C.; Balser, M.; Schmitt, G.; Werner, C. Corrosion behaviour of ionic liquids. Green Chem. 2005, 7, 321–325. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Es’haghi, M. Corrosion inhibition of mild steel in acidic media by [BMIm]Br ionic liquid. Mater. Chem. Phys. 2009, 114, 267–271. [Google Scholar] [CrossRef]
- Chong, A.L.; Mardel, J.I.; MacFarlane, D.R.; Forsyth, M.; Somers, A.E. Synergistic corrosion inhibition of mild steel in aqueous chloride solutions by an imidazolinium carboxylate salt. ACS Sustain. Chem. Eng. 2016, 4, 1746–1755. [Google Scholar] [CrossRef]
- Zhang, H.; Hong, K.; Mays, J.W. Synthesis of block copolymers of styrene and methyl methacrylate by conventional free radical polymerization in room temperature ionic liquids. Macromolecules 2002, 35, 5738–5741. [Google Scholar] [CrossRef]
- Woecht, I.; Schmidt-Naake, G.; Beuermann, S.; Buback, M.; García, N. Propagation kinetics of free-radical polymerizations in ionic liquids. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 1460–1469. [Google Scholar] [CrossRef]
- Ricks-Laskoski, H.L.; Snow, A.W. Synthesis and electric field actuation of an ionic liquid polymer. J. Am. Chem. Soc. 2006, 128, 12402–12403. [Google Scholar] [CrossRef] [PubMed]
- Green, O.; Grubjesic, S.; Lee, S.; Firestone, M.A. The design of polymeric ionic liquids for the preparation of functional materials. Polym. Rev. 2009, 49, 339–360. [Google Scholar] [CrossRef]
- Anderson, E.B.; Long, T.E. Imidazole- and imidazolium-containing polymers for biology and material science applications. Polymer 2010, 51, 2447–2454. [Google Scholar] [CrossRef]
- Green, M.D.; Long, T.E. Designing imidazole-based ionic liquids and ionic liquid monomers for emerging technologies. Polym. Rev. 2009, 49, 291–314. [Google Scholar] [CrossRef]
- Shaplov, A.S.; Vlasov, P.S.; Lozinskaya, E.I.; Shishkan, O.A.; Ponkratov, D.O.; Malyshkina, I.A.; Vidal, F.; Wandrey, C.; Godovikov, I.A.; Vygodskii, Y.S. Thiol-ene click chemistry as a tool for a novel family of polymeric ionic liquids. Macromol. Chem. Phys. 2012, 213, 1359–1369. [Google Scholar] [CrossRef]
- Shaplov, A.S.; Goujon, L.; Vidal, F.; Lozinskaya, E.I.; Meyer, F.; Malyshkina, I.A.; Chevrot, C.; Teyssie, D.; Odinets, I.L.; Vygodskii, Y.S. Ionic IPNs as novel candidates for highly conductive solid polymer electrolytes. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 4245–4266. [Google Scholar] [CrossRef]
- Allen, M.H.; Green, M.D.; Getaneh, H.K.; Miller, K.M.; Long, T.E. Tailoring charge density and hydrogen bonding of imidazolium copolymers for efficient gene delivery. Biomacromolecules 2011, 12, 2243–2250. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Lin, B.; Si, Z.; Qiu, L.; Chu, F.; Zhao, J.; Yan, F. Bis-imidazolium-based anion-exchange membranes for alkaline fuel cells. J. Power Sources 2012, 217, 329–335. [Google Scholar] [CrossRef]
- Mecerreyes, D. Polymeric ionic liquids: Broadening the properties and applications of polyelectrolytes. Prog. Polym. Sci. 2011, 36, 1629–1648. [Google Scholar] [CrossRef]
- Arellanes-Lozada, P.; Olivares-Xometl, O.; Guzmán-Lucero, D.; Likhanova, N.V.; Domínguez-Aguilar, M.A.; Lijanova, I.V.; Arce-Estrada, E. The inhibition of aluminum corrosion in sulfuric acid by poly (1-vinyl-3-alkyl-imidazolium hexafluorophosphate). Materials 2014, 7, 5711–5734. [Google Scholar] [CrossRef] [PubMed]
- Atta, A.M.; El-Mahdy, G.A.; Allohedan, H.A.; Abdullah, M.M. Poly (ionic liquid) based on modified ionic polyacrylamide for inhibition steel corrosion in acid solution. Int. J. Electrochem. Sci. 2015, 10, 10389–10401. [Google Scholar]
- Borisova, D.; Akçakayıran, D.; Schenderlein, M.; Möhwald, H.; Shchukin, D.G. Nanocontainer-based anticorrosive coatings: Effect of the container size on the self-healing performance. Adv. Funct. Mater. 2013, 23, 3799–3812. [Google Scholar] [CrossRef]
- Abdelhedi-Miladi, I.; Obadia, M.M.; Allaoua, I.; Serghei, A.; Romdhane, H.B.; Drockenmuller, E. 1,2,3-triazolium-based poly(ionic liquid)s obtained through click chemistry polyaddition. Macromol. Chem. Phys. 2014, 215, 2229–2236. [Google Scholar] [CrossRef]
- Stepto Robert, F.T. Dispersity in polymer science (IUPAC recommendations 2009). Pure Appl. Chem. 2009, 81, 351–353. [Google Scholar] [CrossRef]
- Muldoon, M.J.; Gordon, C.M. Synthesis of gel-type polymer beads from ionic liquid monomers. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 3865–3869. [Google Scholar] [CrossRef]
- Marcilla, R.; Sanchez-Paniagua, M.; Lopez-Ruiz, B.; Lopez-Cabarcos, E.; Ochoteco, E.; Grande, H.; Mecerreyes, D. Synthesis and characterization of new polymeric ionic liquid microgels. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 3958–3965. [Google Scholar] [CrossRef]
- Yuan, J.; Antonietti, M. Poly(ionic liquid) latexes prepared by dispersion polymerization of ionic liquid monomers. Macromolecules 2011, 44, 744–750. [Google Scholar] [CrossRef]
- Yuan, J.; Soll, S.; Drechsler, M.; Müller, A.H.E.; Antonietti, M. Self-assembly of poly(ionic liquid)s: Polymerization, mesostructure formation, and directional alignment in one step. J. Am. Chem. Soc. 2011, 133, 17556–17559. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.L.; Ye, Y.; Schmitt, A.L.; Banik, S.M.; Elabd, Y.A.; Mahanthappa, M.K. Effect of nanoscale morphology on the conductivity of polymerized ionic liquid block copolymers. Macromolecules 2011, 44, 5727–5735. [Google Scholar] [CrossRef]
- Yang, J.; Qiu, L.; Liu, B.; Peng, Y.; Yan, F.; Shang, S. Synthesis of polymeric ionic liquid microsphere/Pt nanoparticle hybrids for electrocatalytic oxidation of methanol and catalytic oxidation of benzyl alcohol. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 4531–4538. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, Y.; Nie, J.; Ji, Z.; Xu, J.; Zhang, X.; Du, B. Thermosensitive ionic microgels via surfactant-free emulsion copolymerization and in situ quaternization cross-linking. ACS Appl. Mater. Interfaces 2014, 6, 4498–4513. [Google Scholar] [CrossRef] [PubMed]
- Amato, D.V.; Amato, D.N.; Flynt, A.S.; Patton, D.L. Functional, sub-100 nm polymer nanoparticles via thiol-ene miniemulsion photopolymerization. Polym. Chem. 2015, 6, 5625–5632. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Lee, T.Y.; Roper, T. Thiol–enes: Chemistry of the past with promise for the future. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 5301–5338. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Lowe, A.B.; Bowman, C.N. Thiol-click chemistry: A multifaceted toolbox for small molecule and polymer synthesis. Chem. Soc. Rev. 2010, 39, 1355–1387. [Google Scholar] [CrossRef] [PubMed]
- Jasinski, F.; Lobry, E.; Tarablsi, B.; Chemtob, A.; Croutxé-Barghorn, C.; Le Nouen, D.; Criqui, A. Light-mediated thiol–ene polymerization in miniemulsion: A fast route to semicrystalline polysulfide nanoparticles. ACS Macro Lett. 2014, 3, 958–962. [Google Scholar] [CrossRef]
- Taghavikish, M.; Subianto, S.; Dutta, N.K.; de Campo, L.; Mata, J.P.; Rehm, C.; Choudhury, N.R. Polymeric ionic liquid nanoparticle emulsions as a corrosion inhibitor in anticorrosion coatings. ACS Omega 2016, 1, 29–40. [Google Scholar] [CrossRef]
- Raghavan, P.; Manuel, J.; Zhao, X.; Kim, D.-S.; Ahn, J.-H.; Nah, C. Preparation and electrochemical characterization of gel polymer electrolyte based on electrospun polyacrylonitrile nonwoven membranes for lithium batteries. J. Power Sources 2011, 196, 6742–6749. [Google Scholar] [CrossRef]
- Murata, Y.; Sasaki, N.; Miyamoto, E.; Kawashima, S. Use of floating alginate gel beads for stomach-specific drug delivery. Eur. J. Pharm. Biopharm. 2000, 50, 221–226. [Google Scholar] [CrossRef]
- Kashyap, N.; Kumar, N.; Kumar, M.N.V.R. Hydrogels for pharmaceutical and biomedical applications. Crit. Rev. Ther. Drug Carr. Syst. 2005, 22, 107–149. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, H.; Wang, R.; Yan, Y.; Zheng, B.; Wang, Y. A facile one-step synthesis to cross-linked polymeric nanoparticles as highly active and selective catalysts for cycloaddition of CO2 to epoxides. Chem. Commun. 2010, 46, 3399–3401. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.T.; Barikbin, Z.; Badruddoza, A.Z.M.; Doyle, P.S.; Khan, S.A. Monodisperse polymeric ionic liquid microgel beads with multiple chemically switchable functionalities. Langmuir 2013, 29, 9535–9543. [Google Scholar] [CrossRef] [PubMed]
- Taghavikish, M.; Subianto, S.; Dutta, N.K.; Choudhury, N.R. Facile fabrication of polymerizable ionic liquid based-gel beads via thiol–ene chemistry. ACS Appl. Mater. Interfaces 2015, 7, 17298–17306. [Google Scholar] [CrossRef] [PubMed]
- Graphene. Available online: https://www.graphene-info.com/introduction (accessed on 24 October 2017).
- Prasai, D.; Tuberquia, J.C.; Harl, R.R.; Jennings, G.K.; Bolotin, K.I. Graphene: Corrosion-inhibiting coating. ACS Nano 2012, 6, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Wang, S. Enhancing polymer performance through graphene sheets. J. Appl. Polym. Sci. 2011, 119, 3670–3674. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhang, G.; Du, J.; Tang, L.; Xu, J.; Li, J. New role of graphene oxide as active hydrogen donor in the recyclable palladium nanoparticles catalyzed ullmann reaction in environmental friendly ionic liquid/supercritical carbon dioxide system. J. Mater. Chem. 2011, 21, 3485–3494. [Google Scholar] [CrossRef]
- Song, H.; Zhang, L.; He, C.; Qu, Y.; Tian, Y.; Lv, Y. Graphene sheets decorated with SnO2 nanoparticles: In situ synthesis and highly efficient materials for cataluminescence gas sensors. J. Mater. Chem. 2011, 21, 5972–5977. [Google Scholar] [CrossRef]
- Zhao, J.; Pei, S.; Ren, W.; Gao, L.; Cheng, H.-M. Efficient preparation of large-area graphene oxide sheets for transparent conductive films. ACS Nano 2010, 4, 5245–5252. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Zhi, L. Graphene-based electrode materials for rechargeable lithium batteries. J. Mater. Chem. 2009, 19, 5871–5878. [Google Scholar] [CrossRef]
- Reddy, A.L.M.; Srivastava, A.; Gowda, S.R.; Gullapalli, H.; Dubey, M.; Ajayan, P.M. Synthesis of nitrogen-doped graphene films for lithium battery application. ACS Nano 2010, 4, 6337–6342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, L.L.; Zhao, X.S.; Wu, J. Graphene/polyaniline nanofiber composites as supercapacitor electrodes. Chem. Mater. 2010, 22, 1392–1401. [Google Scholar] [CrossRef]
- Kalita, G.; Matsushima, M.; Uchida, H.; Wakita, K.; Umeno, M. Graphene constructed carbon thin films as transparent electrodes for solar cell applications. J. Mater. Chem. 2010, 20, 9713–9717. [Google Scholar] [CrossRef]
- Ramanathan, T.; Abdala, A.A.; Stankovich, S.; Dikin, D.A.; Herrera Alonso, M.; Piner, R.D.; Adamson, D.H.; Schniepp, H.C.; Chen, X.; Ruoff, R.S.; et al. Functionalized graphene sheets for polymer nanocomposites. Nat. Nanotechnol. 2008, 3, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Salavagione, H.J.; Martinez, G.; Gomez, M.A. Synthesis of poly(vinyl alcohol)/reduced graphite oxide nanocomposites with improved thermal and electrical properties. J. Mater. Chem. 2009, 19, 5027–5032. [Google Scholar] [CrossRef]
- Cai, D.; Song, M. Recent advance in functionalized graphene/polymer nanocomposites. J. Mater. Chem. 2010, 20, 7906–7915. [Google Scholar] [CrossRef]
- Zhu, J. Graphene production: New solutions to a new problem. Nat Nano 2008, 3, 528–529. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Huang, T.-C.; Peng, C.-W.; Yeh, T.-C.; Lu, H.-I.; Hung, W.-I.; Weng, C.-J.; Yang, T.-I.; Yeh, J.-M. Novel anticorrosion coatings prepared from polyaniline/graphene composites. Carbon 2012, 50, 5044–5051. [Google Scholar] [CrossRef]
- Yasmin, A.; Daniel, I.M. Mechanical and thermal properties of graphite platelet/epoxy composites. Polymer 2004, 45, 8211–8219. [Google Scholar] [CrossRef]
- Rafiee, M.A.; Rafiee, J.; Wang, Z.; Song, H.; Yu, Z.Z.; Koratkar, N. Enhanced mechanical properties of nanocomposites at low graphene content. ACS Nano 2009, 3, 3884–3890. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.G.; Bauer, J.L.; Maryanski, M.J.; Heimann, P.J.; Barlow, J.P.; Gosau, J.M.; Allred, R.E. Characterization of epoxy functionalized graphite nanoparticles and the physical properties of epoxy matrix nanocomposites. Compos. Sci. Technol. 2010, 70, 1120–1125. [Google Scholar] [CrossRef]
- Chiang, C.L.; Hsu, S.W. Synthesis, characterization and thermal properties of novel epoxy/expandable graphite composites. Polym. Int. 2010, 59, 119–126. [Google Scholar] [CrossRef]
- Martin-Gallego, M.; Verdejo, R.; Lopez-Manchado, M.A.; Sangermano, M. Epoxy-graphene UV-cured nanocomposites. Polymer 2011, 52, 4664–4669. [Google Scholar] [CrossRef]
- Sørensen, P.A.; Kiil, S.; Dam-Johansen, K.; Weinell, C.E. Anticorrosive coatings: A review. J. Coat. Technol. Res. 2009, 6, 135–176. [Google Scholar] [CrossRef]
- Bao, C.; Guo, Y.; Song, L.; Kan, Y.; Qian, X.; Hu, Y. In situ preparation of functionalized graphene oxide/epoxy nanocomposites with effective reinforcements. J. Mater. Chem. 2011, 21, 13290–13298. [Google Scholar] [CrossRef]
- Borisova, D.; Möhwald, H.; Shchukin, D.G. Mesoporous silica nanoparticles for active corrosion protection. ACS Nano 2011, 5, 1939–1946. [Google Scholar] [CrossRef] [PubMed]
- Shchukin, D.G.; Grigoriev, D.O.; Mohwald, H. Application of smart organic nanocontainers in feedback active coatings. Soft Matter 2010, 6, 720–725. [Google Scholar] [CrossRef]
- Dry, C. Procedures developed for self-repair of polymer matrix composite materials. Compos. Struct. 1996, 35, 263–269. [Google Scholar] [CrossRef]
- Dry, C.M.; Sottos, N.R. Passive smart self-repair in polymer matrix composite materials. Proc. SPIE Int. Soc. Opt. Eng. 1993, 1916, 438–444. [Google Scholar]
- Zhang, M.Q.; Rong, M.Z. Intrinsic self-healing of covalent polymers through bond reconnection towards strength restoration. Polym. Chem. 2013, 4, 4878–4884. [Google Scholar] [CrossRef]
- Chen, Y.; Kushner, A.M.; Williams, G.A.; Guan, Z. Multiphase design of autonomic self-healing thermoplastic elastomers. Nat. Chem. 2012, 4, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Kessler, M.R. Self-healing: A new paradigm in materials design. Proc. Inst. Mech. Eng. Part G J. Aerosp. Eng. 2007, 221, 479–495. [Google Scholar] [CrossRef]
- Lvov, Y.M.; Shchukin, D.G.; Möhwald, H.; Price, R.R. Halloysite clay nanotubes for controlled release of protective agents. ACS Nano 2008, 2, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, N.; Mishra, R.; Goda, C.; Arora, M. Microencapsulation—A novel approach in drug delivery: A review. Indo Glob. J. Pharm. Sci. 2012, 2, 1–20. [Google Scholar]
- Liu, X.; Zhang, H.; Wang, J.; Wang, Z.; Wang, S. Preparation of epoxy microcapsule based self-healing coatings and their behavior. Surf. Coat. Technol. 2012, 206, 4976–4980. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, H.; Yang, J. Synthesis of organic silane microcapsules for self-healing corrosion resistant polymer coatings. Corros. Sci. 2012, 65, 561–566. [Google Scholar] [CrossRef]
- Brown, E.N.; Kessler, M.R.; Sottos, N.R.; White, S.R. In situ poly(urea-formaldehyde) microencapsulation of dicyclopentadiene. J. Microencapsul. 2003, 20, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; White, S.R.; Braun, P.V. Self-healing polymer coatings. Adv. Mater. 2009, 21, 645–649. [Google Scholar] [CrossRef]
- Samadzadeh, M.; Boura, S.H.; Peikari, M.; Kasiriha, S.M.; Ashrafi, A. A review on self-healing coatings based on micro/nanocapsules. Prog. Org. Coat. 2010, 68, 159–164. [Google Scholar] [CrossRef]
- Wilson, G.O.; Andersson, H.M.; White, S.R.; Sottos, N.R.; Moore, J.S.; Braun, P.V. Self-healing polymers. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Inc.: New York, NY, USA, 2002. [Google Scholar]
- Bandeira, P.; Monteiro, J.; Baptista, A.M.; Magalhães, F.D. Tribological performance of PTFE-based coating modified with microencapsulated [HMIM][NTf2] ionic liquid. Tribol. Lett. 2015, 59, 1–15. [Google Scholar] [CrossRef]
- García, S.J.; Fischer, H.R.; White, P.A.; Mardel, J.; González-García, Y.; Mol, J.M.C.; Hughes, A.E. Self-healing anticorrosive organic coating based on an encapsulated water reactive silyl ester: Synthesis and proof of concept. Prog. Org. Coat. 2011, 70, 142–149. [Google Scholar] [CrossRef]
- Minami, H.; Fukaumi, H.; Okubo, M.; Suzuki, T. Preparation of ionic liquid-encapsulated polymer particles. Colloid Polym. Sci. 2013, 291, 45–51. [Google Scholar] [CrossRef]
- Minami, H.; Okubo, M.; Oshima, Y. Preparation of cured epoxy resin particles having one hollow by polyaddition reaction. Polymer 2005, 46, 1051–1056. [Google Scholar] [CrossRef]
- Nesterova, T.; Dam-Johansen, K.; Pedersen, L.T.; Kiil, S. Microcapsule-based self-healing anticorrosive coatings: Capsule size, coating formulation, and exposure testing. Prog. Org. Coat. 2012, 75, 309–318. [Google Scholar] [CrossRef]
- Mehta, N.K.; Bogere, M.N. Environmental studies of smart/self-healing coating system for steel. Prog. Org. Coat. 2009, 64, 419–428. [Google Scholar] [CrossRef]
- Kumar, A.; Mathur, N. Photocatalytic degradation of aniline at the interface of TiO2 suspensions containing carbonate ions. J. Colloid Interface Sci. 2006, 300, 244–252. [Google Scholar] [CrossRef] [PubMed]
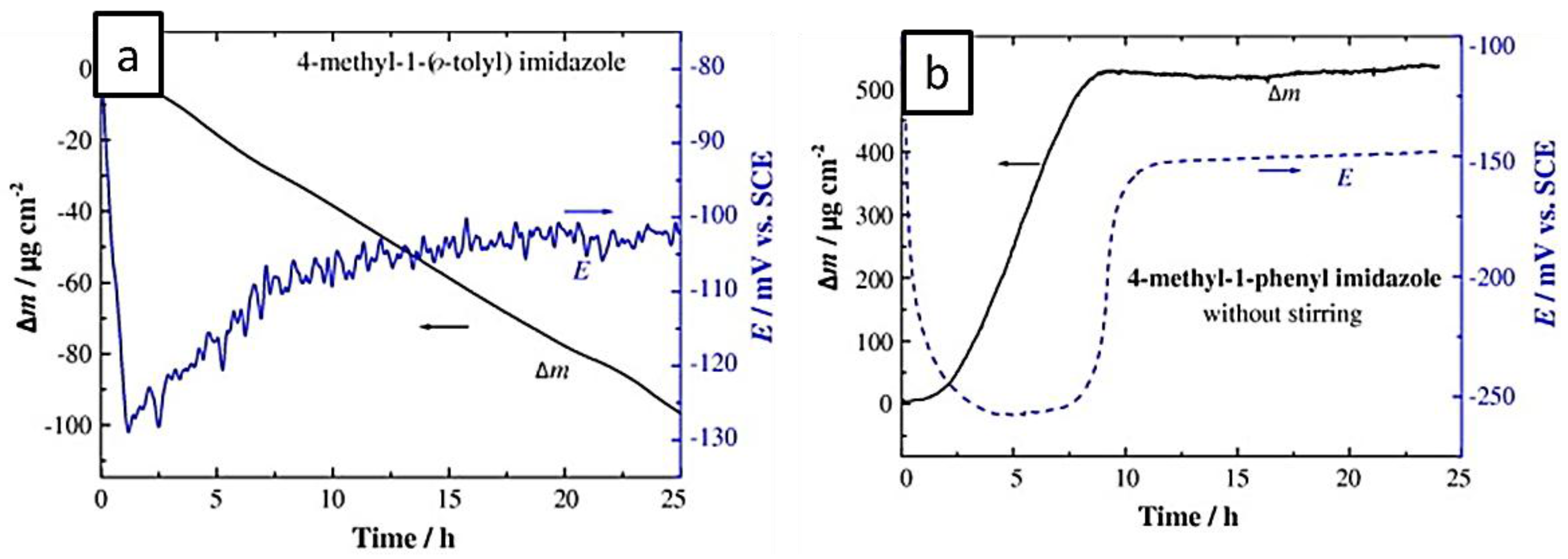
- Otmacic Curkovic, H.; Stupnisek-Lisac, E.; Takenouti, H. Electrochemical quartz crystal microbalance and electrochemical impedance spectroscopy study of copper corrosion inhibition by imidazoles. Corros. Sci. 2009, 51, 2342–2348. [Google Scholar] [CrossRef]
- Dornbusch, M. The use of modern electrochemical methods in the development of corrosion protective coatings. Prog. Org. Coat. 2008, 61, 240–244. [Google Scholar] [CrossRef]
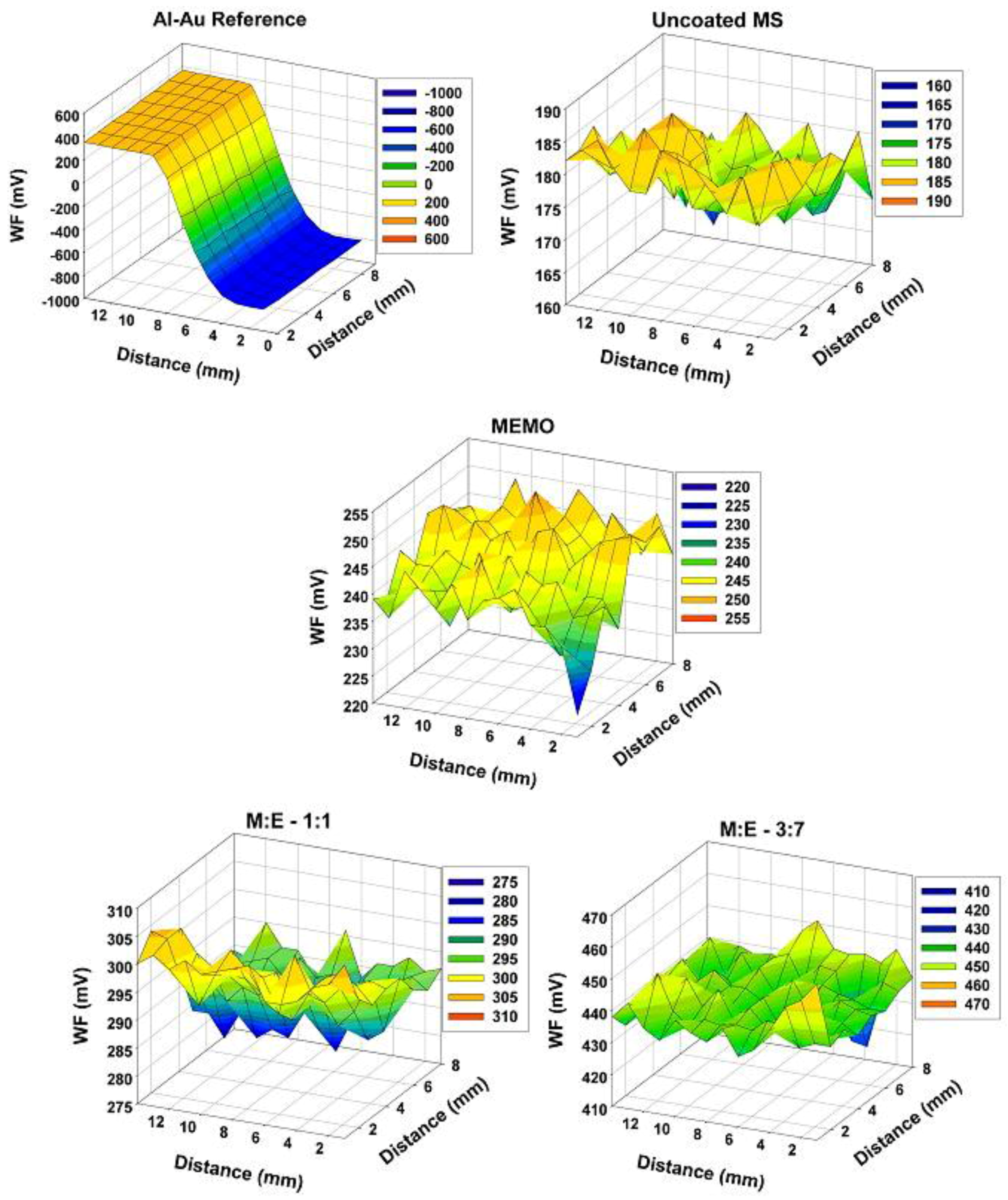
- Nazarov, A.; Prosek, T.; Thierry, D. Application of EIS and SKP methods for the study of the zinc/polymer interface. Electrochim. Acta 2008, 53, 7531–7538. [Google Scholar] [CrossRef]
- Wapner, K.; Stratmann, M.; Grundmeier, G. Application of the scanning kelvin probe for the study of the corrosion resistance of interfacial thin organosilane films at adhesive/metal interfaces. Silicon Chem. 2005, 2, 235–245. [Google Scholar] [CrossRef]
- Kannan, A.G.; Choudhury, N.R.; Dutta, N.K. Electrochemical performance of sol-gel derived phospho-silicate-methacrylate hybrid coatings. J. Electroanal. Chem. 2010, 641, 28–34. [Google Scholar] [CrossRef]
- Kannan, A.G.; Choudhury, N.R.; Dutta, N.K. Synthesis and characterization of methacrylate phospho-silicate hybrid for thin film applications. Polymer 2007, 48, 7078–7086. [Google Scholar] [CrossRef]
- Stratmann, M. The investigation of the corrosion properties of metals, covered with adsorbed electrolyte layers—A new experimental technique. Corros. Sci. 1987, 27, 869–872. [Google Scholar] [CrossRef]
- Pepe, A.; Galliano, P.; Aparicio, M.; Durán, A.; Ceré, S. Sol-gel coatings on carbon steel: Electrochemical evaluation. Surf. Coat. Technol. 2006, 200, 3486–3491. [Google Scholar] [CrossRef]
- Sherif, E.-S.M.; Erasmus, R.M.; Comins, J.D. In situ raman spectroscopy and electrochemical techniques for studying corrosion and corrosion inhibition of iron in sodium chloride solutions. Electrochim. Acta 2010, 55, 3657–3663. [Google Scholar] [CrossRef]
- Shibayama, M. Small-angle neutron scattering on polymer gels: Phase behavior, inhomogeneities and deformation mechanisms. Polym. J. 2011, 43, 18–34. [Google Scholar] [CrossRef]
- Matsunaga, T.; Sakai, T.; Akagi, Y.; Chung, U.-I.; Shibayama, M. SANS and SLS studies on Tetra-Arm PEG gels in as-prepared and swollen states. Macromolecules 2009, 42, 6245–6252. [Google Scholar] [CrossRef]
- Saffer, E.M.; Lackey, M.A.; Griffin, D.M.; Kishore, S.; Tew, G.N.; Bhatia, S.R. Sans study of highly resilient poly (ethylene glycol) hydrogels. Soft Matter 2014, 10, 1905–1916. [Google Scholar] [CrossRef] [PubMed]
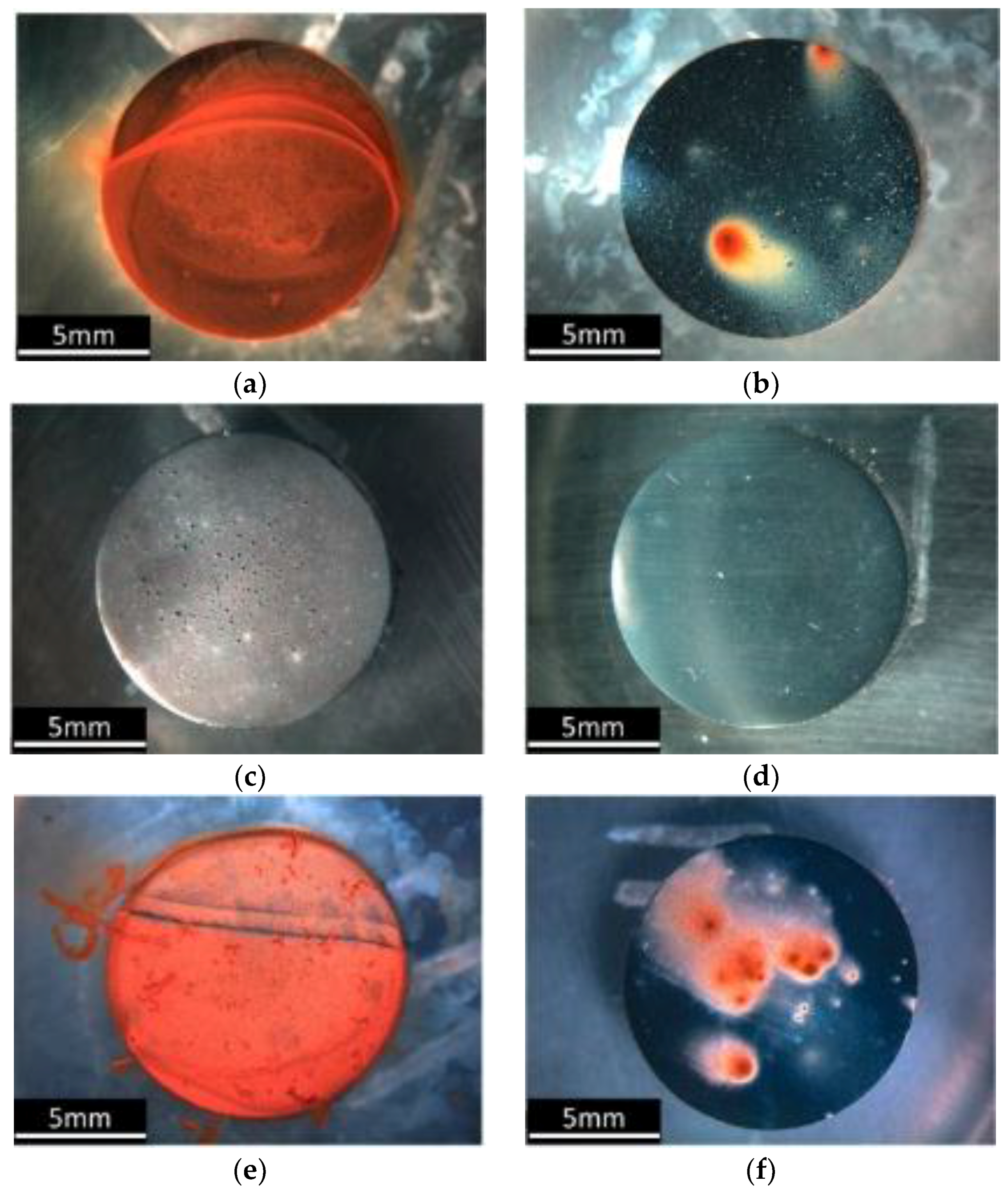
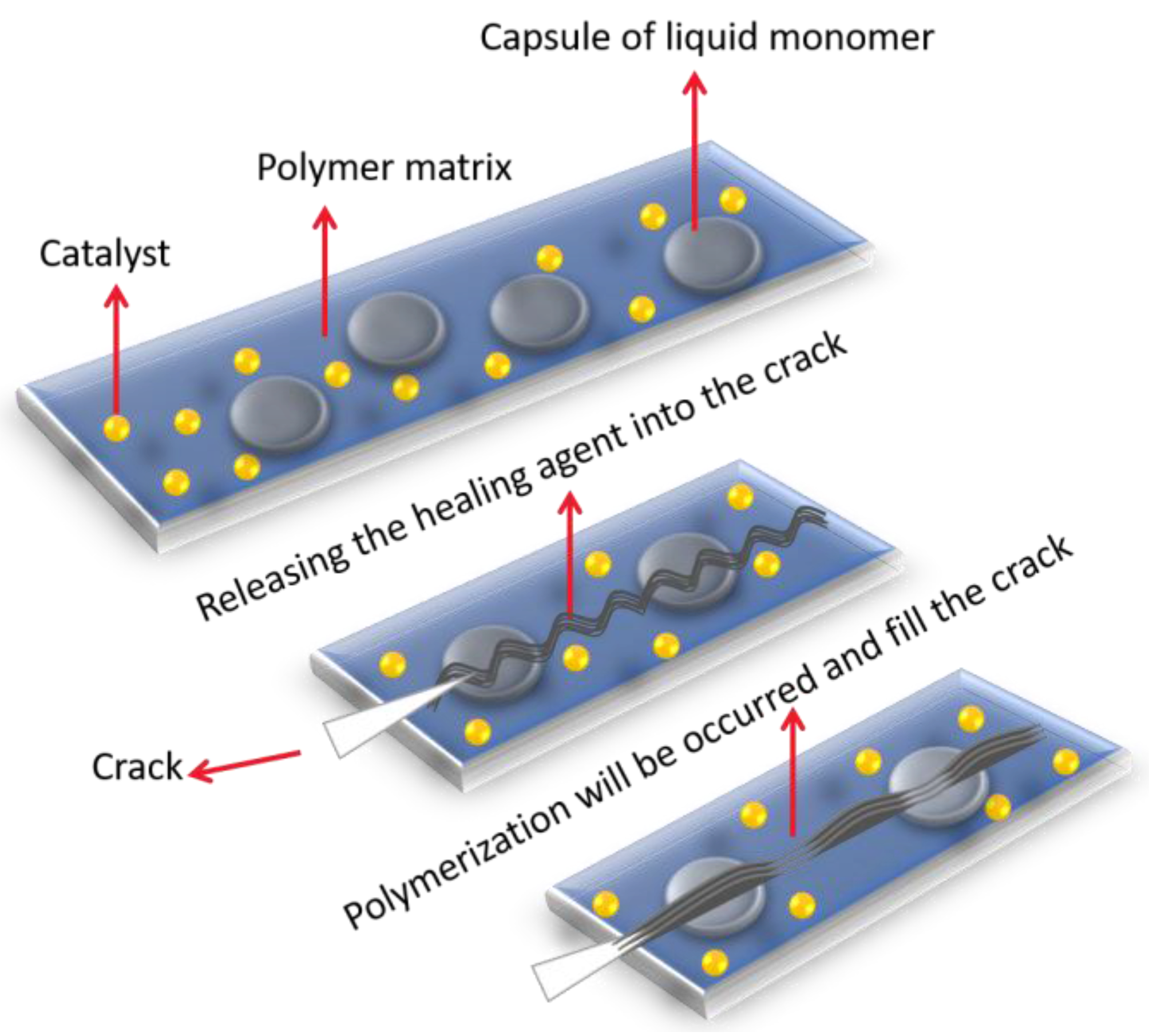
| Microcapsule Components (Core and Shell) | Chemistry | Specific Feature | Size of Capsule | Ref. |
|---|---|---|---|---|
| Shell: polysulphone Core: 1-hexyl-3-methylimidazolium bis(trifluoromethylsulphonyl) imide [HMIM][NTf2] ionic liquid | Solvent evaporation. | Chemically stable within the high-temperature curing conditions necessary for the coating system (up to approximately 380 °C). | Below 10 µm | Magalhães et al. [116] |
| Shell: epoxy–amine(ethylenediamine (EDA)) Core: epoxy | Interfacial polymerization | improved compatibility and adhesion with the coating matrix especially if the coating is alkaline | 100 μm | Liu et al. [110] |
| Shell: poly(urea–formaldehyde) Core: 1H,1H,2H,2H-perfluorooctyl triethoxysilane (POTS) | In situ polymerization | good corrosion protection ability to steel; self-healing behaviour was realised under ambient condition without any manual intervention | 100 μm | Huang et al. [111] |
| Shell: poly(urea-formaldehyde) Core: octyldimethylsilyloleate | In situ polymerization | great potential of the silyl esters as healing agents and good results in corrosion protection | 50 and 100 μm | García et al. [117] |
| Shell: ethylene glycol dimethacrylate (EGDM) Core: ionic liquid, 1-hexyl-3-methylimidazolium bis(trifluoromethane sulfonyl)amide | Self-assembling of phase separated polymer (SaPSeP method) | ionic conductivity; good results in corrosion protection | Multi hollow structure | Okubo et al. [118,119] |
| Component | Characteristics |
|---|---|
| Corrosion inhibitor | Stability and shelf-life |
| Deliverability | |
| Reactivity | |
| Shrinkage | |
| Physical and mechanical properties | |
| Thermal stability | |
| Microcapsule shell wall | Chemical compatibility |
| Mechanical properties | |
| Dispersion | |
| Thermal stability | |
| Catalyst, curing agent, or reaction initiator | Solubility |
| Chemical compatibility | |
| Reactivity | |
| Dispersion | |
| Thermal stability |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taghavikish, M.; Dutta, N.K.; Roy Choudhury, N. Emerging Corrosion Inhibitors for Interfacial Coating. Coatings 2017, 7, 217. https://doi.org/10.3390/coatings7120217
Taghavikish M, Dutta NK, Roy Choudhury N. Emerging Corrosion Inhibitors for Interfacial Coating. Coatings. 2017; 7(12):217. https://doi.org/10.3390/coatings7120217
Chicago/Turabian StyleTaghavikish, Mona, Naba Kumar Dutta, and Namita Roy Choudhury. 2017. "Emerging Corrosion Inhibitors for Interfacial Coating" Coatings 7, no. 12: 217. https://doi.org/10.3390/coatings7120217
APA StyleTaghavikish, M., Dutta, N. K., & Roy Choudhury, N. (2017). Emerging Corrosion Inhibitors for Interfacial Coating. Coatings, 7(12), 217. https://doi.org/10.3390/coatings7120217





