The Effect of Sintering Oxygen Partial Pressure on a SmBiO3 Buffer Layer for Coated Conductors via Chemical Solution Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Preparation of the SmBiO3 Buffer Layer
2.2. The Preparation of YBa2Cu3O7−δ Superconducting Layer
2.3. Characterization
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Obradors, X.; Puig, T. Coated conductors for power applications: Materials challenges. Supercond. Sci. Technol. 2014, 27, 98–102. [Google Scholar] [CrossRef]
- Doi, T.J.; Yuasa, T.; Ozawa, T.; Higashiyama, K. Transport critical current densities in uniaxially and biaxially oriented Tl1(Ba0.8Sr0.2)2Ca2Cu3O9 superconducting films on Ag and SrTiO3 substrates prepared by a spray decomposition method. Jpn. J. Appl. Phys. Part 1 1994, 33, 5692–5696. [Google Scholar] [CrossRef]
- Goyal, A.; Lee, D.F.; List, F.A.; Specht, E.D.; Feenstra, R.; Paranthaman, M.; Cui, X.; Lu, S.W.; Martin, P.M.; Kroeger, D.M.; et al. Recent progress in the fabrication of high-Jc tapes by epitaxial deposition of YBCO on RABiTS. Physica C 2001, 357, 903–913. [Google Scholar] [CrossRef]
- Iijima, Y.; Tanabe, N.; Kohno, O.; Ikeno, Y. Biaxially aligned YBa2Cu3O7−δ thin film tapes. Physica C Supercond. 1991, 185, 1959–1960. [Google Scholar] [CrossRef]
- Rupich, M.W.; Li, Q.; Annavarapu, S.; Thieme, C.; Zhang, W.; Prunier, V.; Paranthaman, M.; Goyal, A.; Lee, D.F.; Specht, E.D.; et al. Low cost Y-Ba-Cu-O coated conductors. IEEE Trans. Appl. Supercond. 2001, 11, 2927–2930. [Google Scholar] [CrossRef]
- Iijima, Y.; Tanabe, N.; Kohno, O.; Ikeno, Y. In-plane aligned YBa2Cu3O7−δ thin films deposited on polycrystalline metallic substrates. Appl. Phys. Lett. 1992, 60, 769–771. [Google Scholar] [CrossRef]
- Obradors, X.; Puig, T.; Pomar, A.; Sandiumenge, F.; Mestres, N.; Coll, M. Progress towards all-chemical superconducting YBa2Cu3O7-coated conductors. Sci. Technol. 2006, 19, S13–S26. [Google Scholar] [CrossRef]
- Wang, B.B.; Liu, L.F.; Wu, X.; Yao, Y.J.; Wang, M.L.; Lu, S.D. Surface morphology of cerium oxide layer and its effect on the performance of superconducting layer. J. Supercond. Nov. Magn. 2016, 29, 1–8. [Google Scholar] [CrossRef]
- Schwartz, R.W.; Schneller, T.; Waser, R. Chemical solution deposition of electronic oxide films. C. R. Chim. 2004, 7, 433–461. [Google Scholar] [CrossRef]
- Ghasemi, A.; Ashrafizadeh, F.; Golozar, M.A. Optimisation of processing parameters on formation of silica coating on nickel substrates by sol–gel processing. Surf. Eng. 2013, 21, 131–138. [Google Scholar] [CrossRef]
- Zhao, Y.; Pu, M.H.; Li, G.; Du, X.H.; Zhou, H.M.; Zhang, Y.B.; Yang, X.S.; Wang, Y.; Sun, R.P.; Cheng, C.H. Development of a new series of buffer layers for REBCO coated conductors. Physica C 2007, 463, 574–579. [Google Scholar] [CrossRef]
- Bellakki, M.B.; Prakash, A.S.; Shivakumara, C.; Hegde, M.S.; Shukla, A.K. Solution-combustion synthesis of Bi1−xLnxO1.5 (Ln = Y and La-Yb) oxide ion conductors. Bull. Mater. Sci. 2006, 29, 339–345. [Google Scholar] [CrossRef]
- Pollefeyt, G.; Rottiers, S.; Vermeir, P.; Lommens, P.; Hühne, R.; Buysser, K.D.; Driessche, I.V. Feasibility study of the synthesis of YBiO3 thin films by aqueous chemical solution deposition as an alternative for CeO2 buffer layers in coated conductors. J. Mater. Chem. A 2013, 1, 3613–3619. [Google Scholar] [CrossRef]
- Li, G.; Pu, M.H.; Du, X.H.; Zhou, H.M.; Zhao, Y. A new single buffer layer for YBCO coated conductors prepared by chemical solution deposition. Physica C 2007, 452, 43–47. [Google Scholar] [CrossRef]
- Li, G.; Pu, M.H.; Zhou, H.M.; Du, X.H.; Zhao, Y. Possible new single-buffer layers for YBa2Cu3O7−y coated conductors prepared by chemical solution deposition. J. Mater. Res. 2007, 22, 2398–2403. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Pu, M.H.; Mu, L.Y.; Sun, R.P.; Li, G.; Wang, W.T.; Bai, Y.Q.; Zhao, Y. Effect of annealing condition growth of GdBiO3 film prepared by chemical solution deposition. Cryo. Supercond. 2008, 36, 36–39. [Google Scholar]
- Sun, R.P.; Li, G.; Pu, M.H.; Wang, W.T.; Zhang, Y. Low temperature processing technology for preparation of potential DyBiO3 buffer layers of coated conductors. Rare. Metal. Mat. Eng. 2009, 38, 901–904. [Google Scholar]
- Wang, W.T.; Pu, M.H.; Wang, W.; Zhang, H.; Cheng, C.H.; Zhao, Y. Influence of partial melting temperature on structure and superconducting properties of YBCO film by non-fluorine MOD. J. Supercond. Nov. Magn. 2010, 23, 989–993. [Google Scholar] [CrossRef]
- Gyorgy, E.M.; Van Dover, R.B.; Jackson, K.A.; Schneemeyer, L.F.; Waszczak, J.V. Anisotropic critical currents in Ba2YCu3O7 analyzed using an extended Bean model. Appl. Phys. Lett. 1989, 55, 283–285. [Google Scholar] [CrossRef]
- High_purity-gas/Ar. Available online: http://www.kodi.cn/GetKnowledge/zh-CN/High_purity_gas/Ar.aspx (accessed on 17 October 2016).
- Patterson, A.L. The scherrer formula for X-ray particle size determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
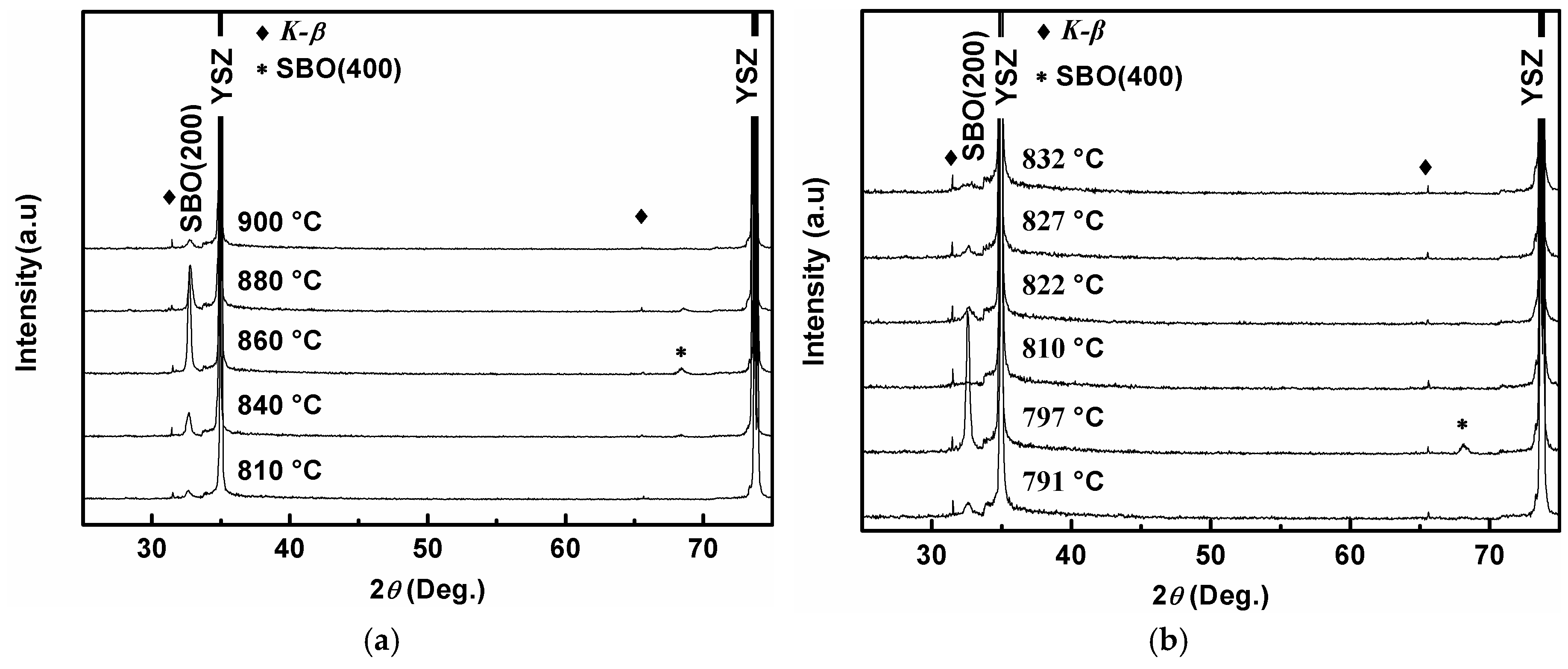

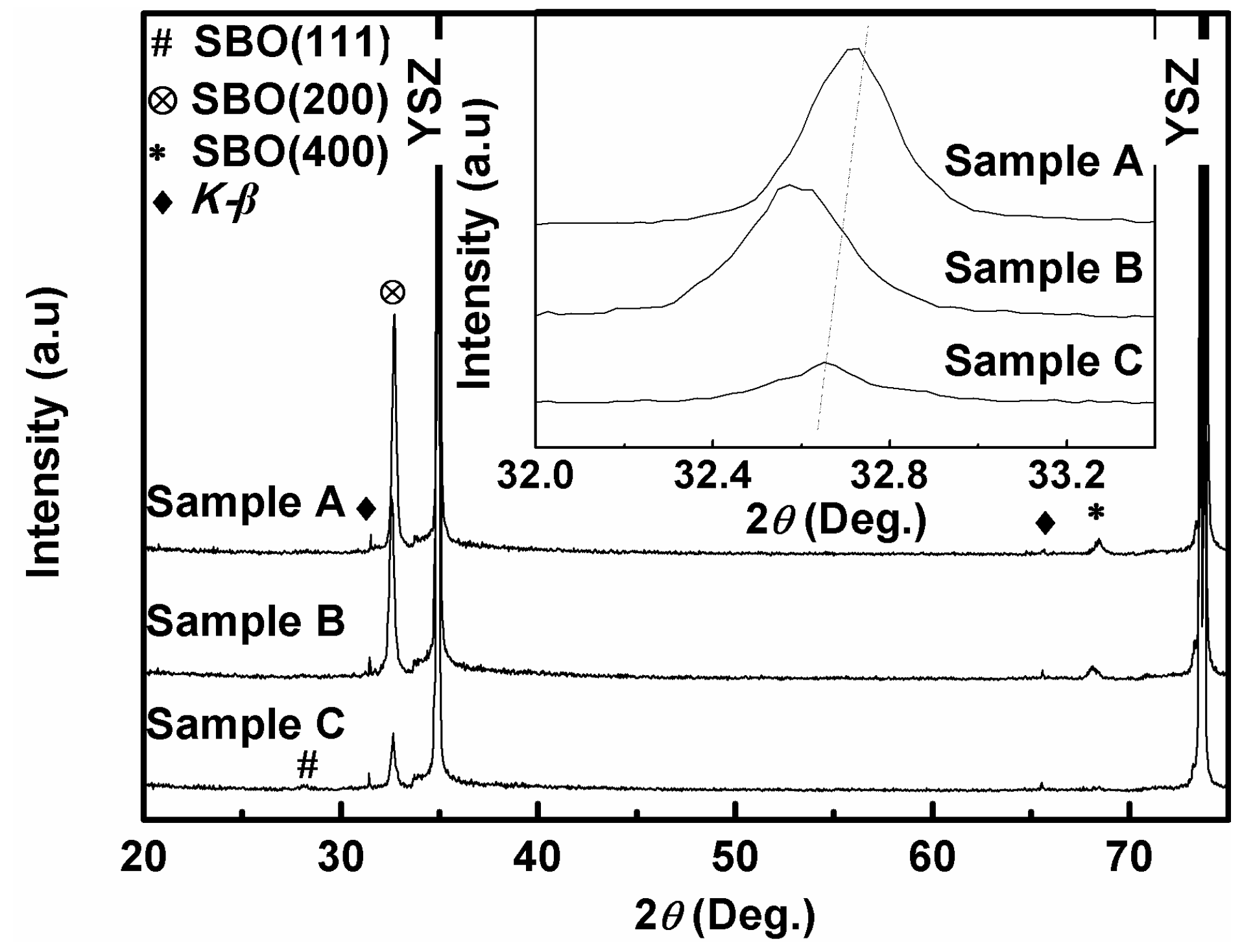
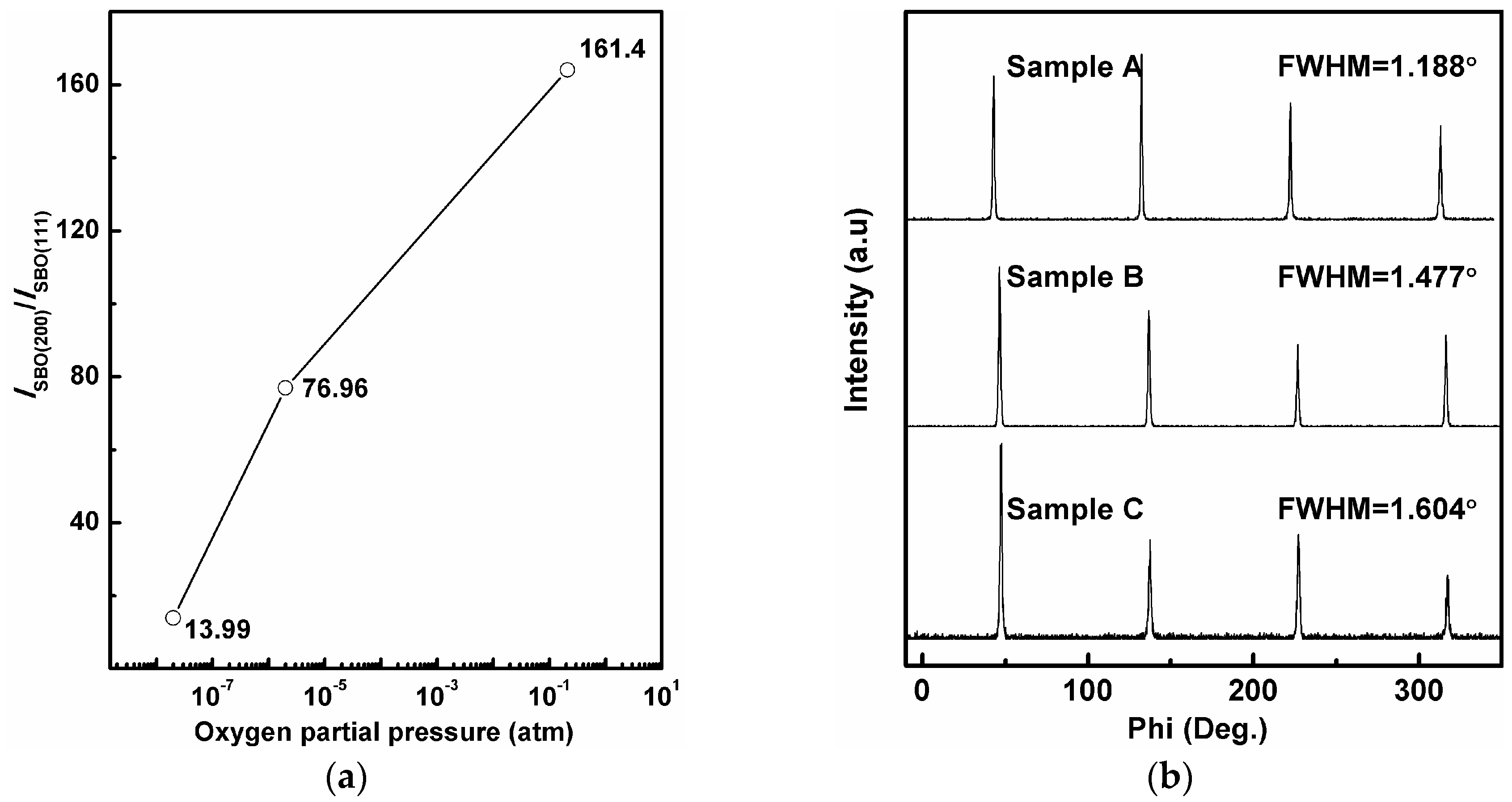
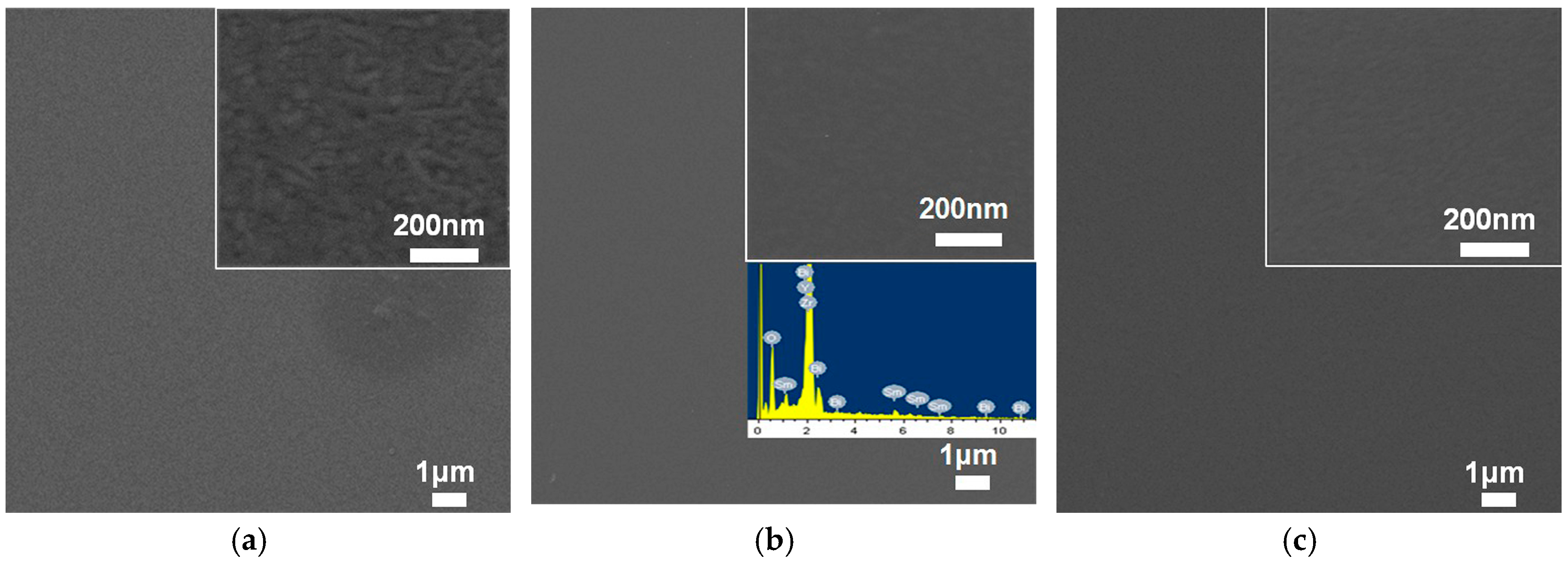
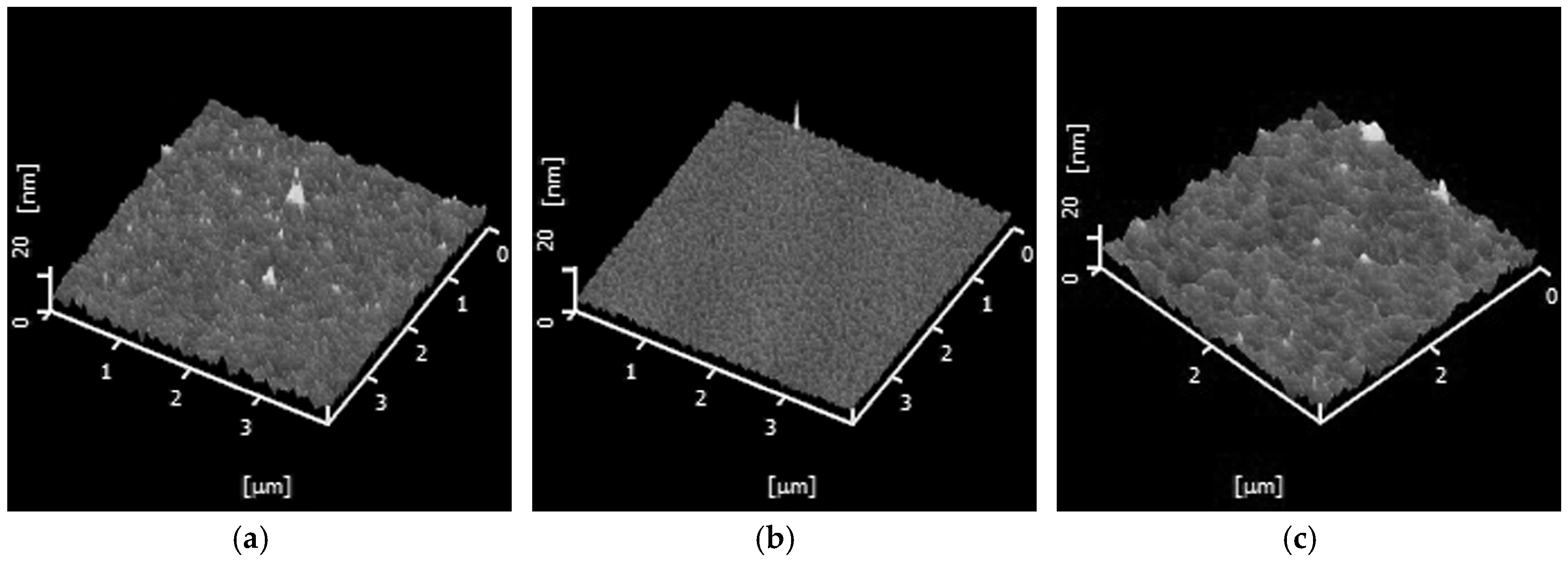
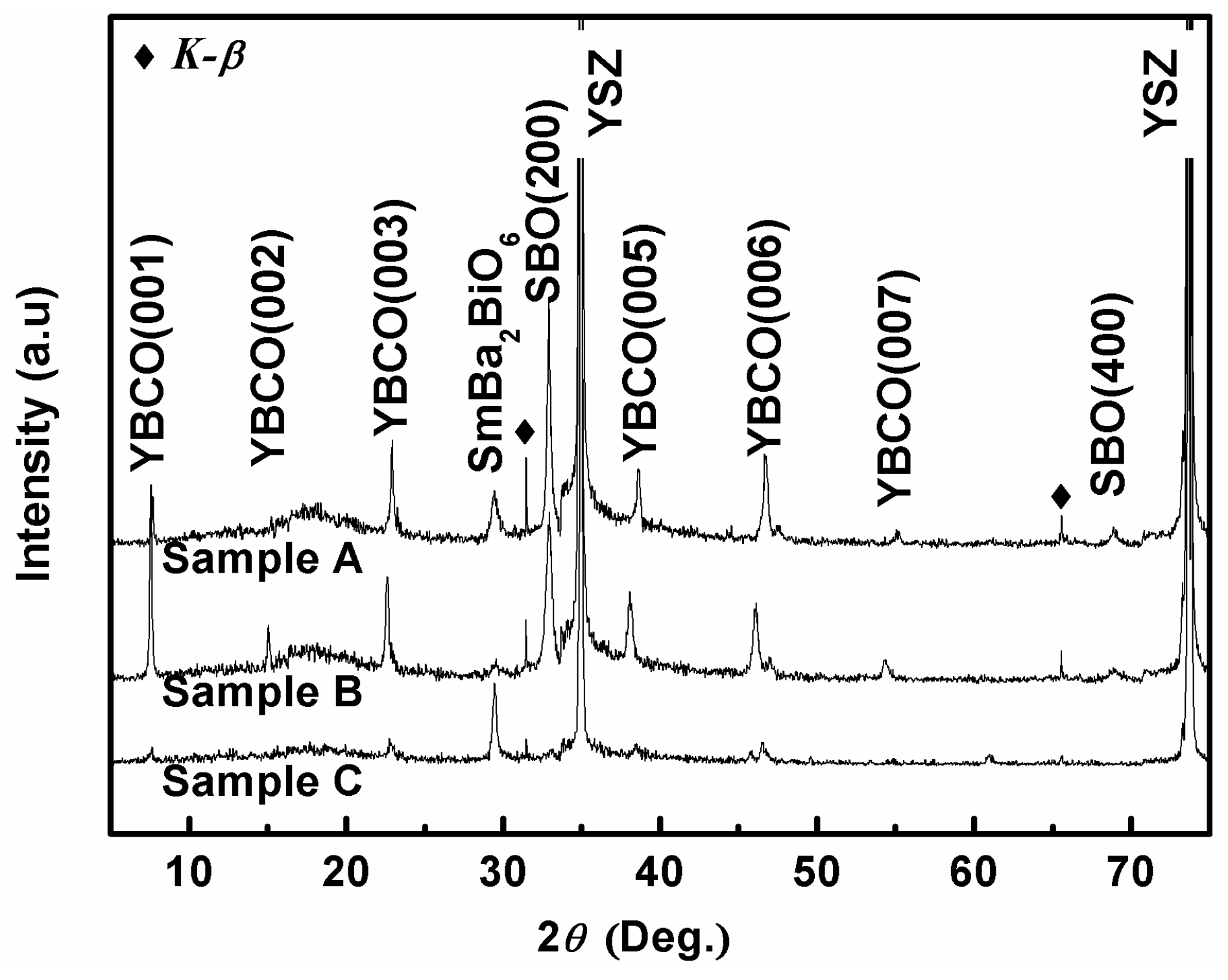
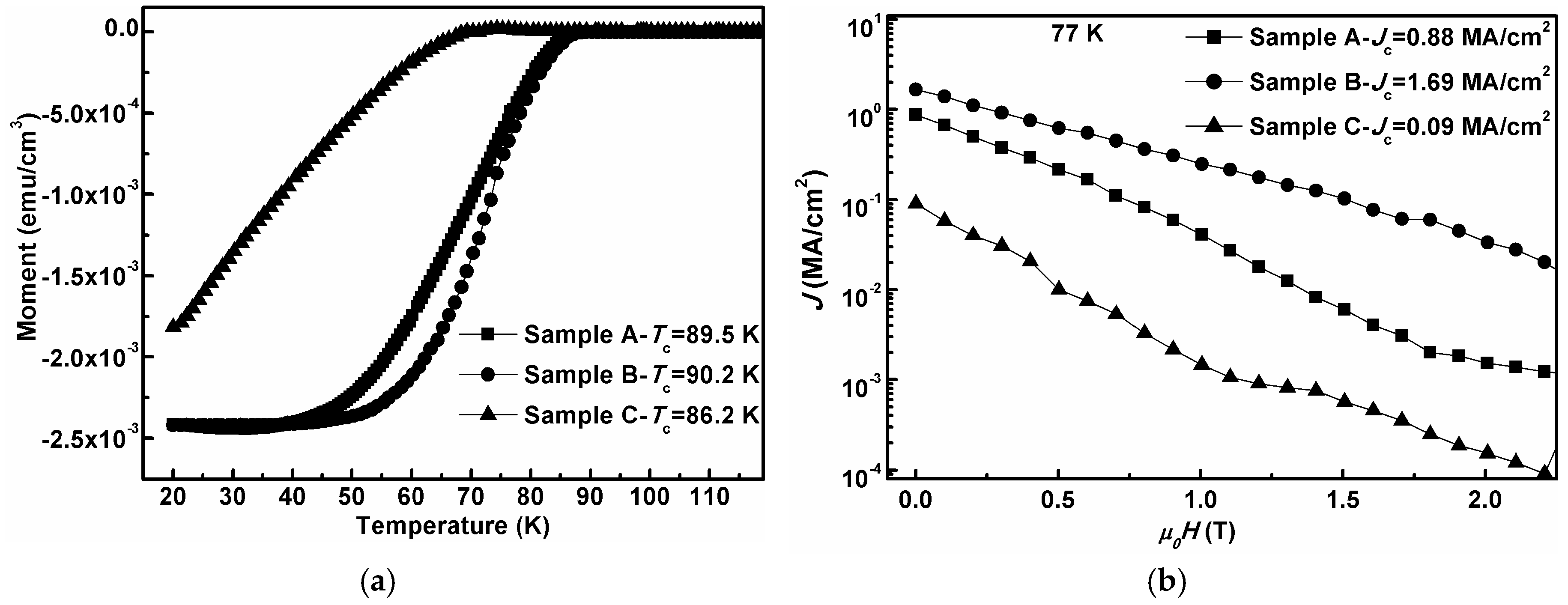
| Oxygen Partial Pressure (atm) | Temperature and Time (°C, min) | 2θ Values of SBO(200) Peak (°) | Grain Size (nm) |
|---|---|---|---|
| 0.21 (Sample A) | 860 °C-30 min | 32.729° | 38.77 |
| 2 × 10−6 (Sample B) | 797 °C-60 min | 32.589° | 37.16 |
| 0.2 × 10−6 (Sample C) | 780 °C-80 min | 32.664° | 38.74 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Zhao, Y.; Pu, M.; Zhang, Y.; Zhang, H.; Cheng, C. The Effect of Sintering Oxygen Partial Pressure on a SmBiO3 Buffer Layer for Coated Conductors via Chemical Solution Deposition. Coatings 2016, 6, 50. https://doi.org/10.3390/coatings6040050
Zhu X, Zhao Y, Pu M, Zhang Y, Zhang H, Cheng C. The Effect of Sintering Oxygen Partial Pressure on a SmBiO3 Buffer Layer for Coated Conductors via Chemical Solution Deposition. Coatings. 2016; 6(4):50. https://doi.org/10.3390/coatings6040050
Chicago/Turabian StyleZhu, Xiaolei, Yong Zhao, Minghua Pu, Yong Zhang, Hong Zhang, and Cuihua Cheng. 2016. "The Effect of Sintering Oxygen Partial Pressure on a SmBiO3 Buffer Layer for Coated Conductors via Chemical Solution Deposition" Coatings 6, no. 4: 50. https://doi.org/10.3390/coatings6040050
APA StyleZhu, X., Zhao, Y., Pu, M., Zhang, Y., Zhang, H., & Cheng, C. (2016). The Effect of Sintering Oxygen Partial Pressure on a SmBiO3 Buffer Layer for Coated Conductors via Chemical Solution Deposition. Coatings, 6(4), 50. https://doi.org/10.3390/coatings6040050





