Surface-Engineered Fire Protective Coatings for Fabrics through Sol-Gel and Layer-by-Layer Methods: An Overview
Abstract
:1. Introduction
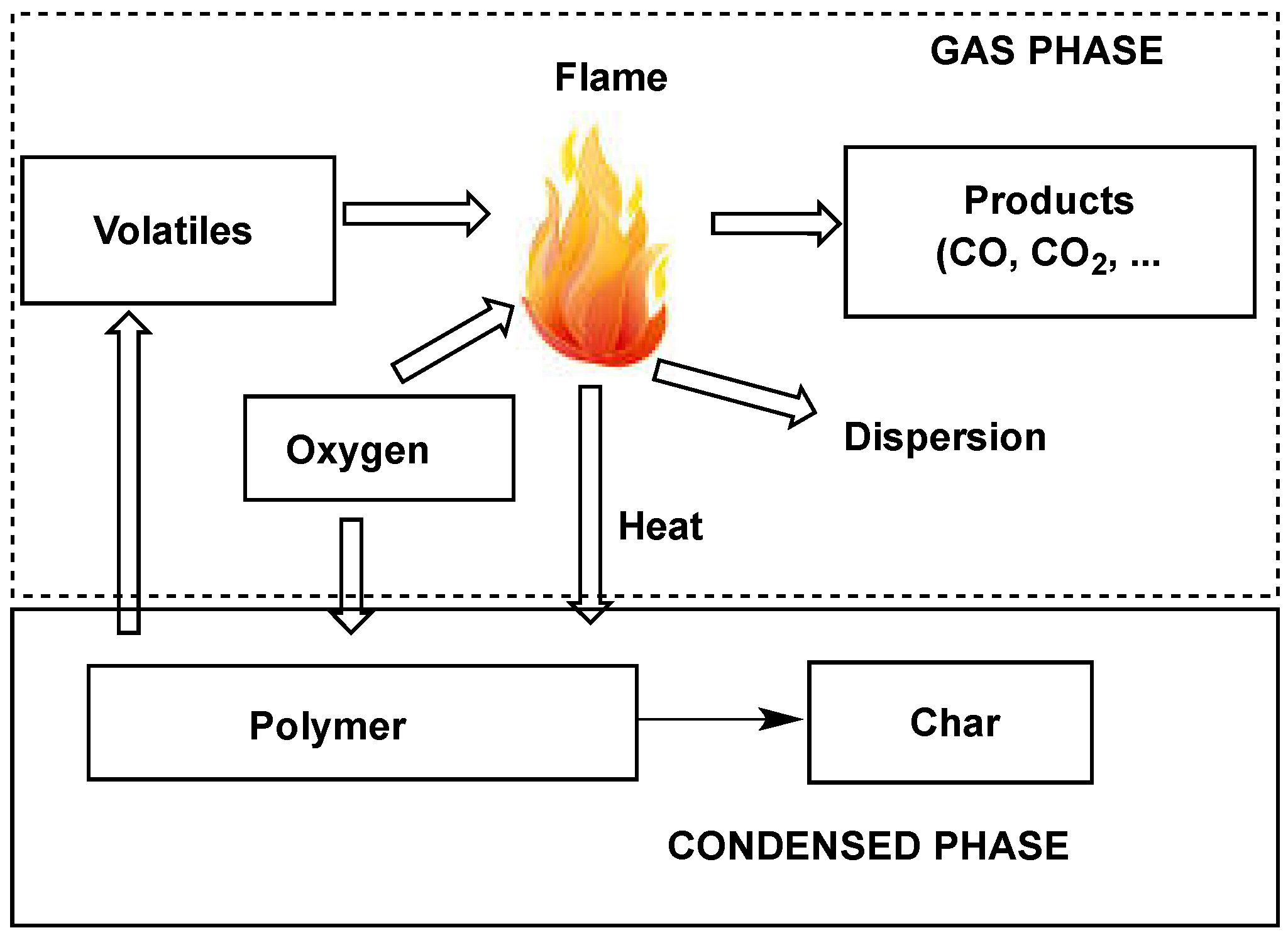
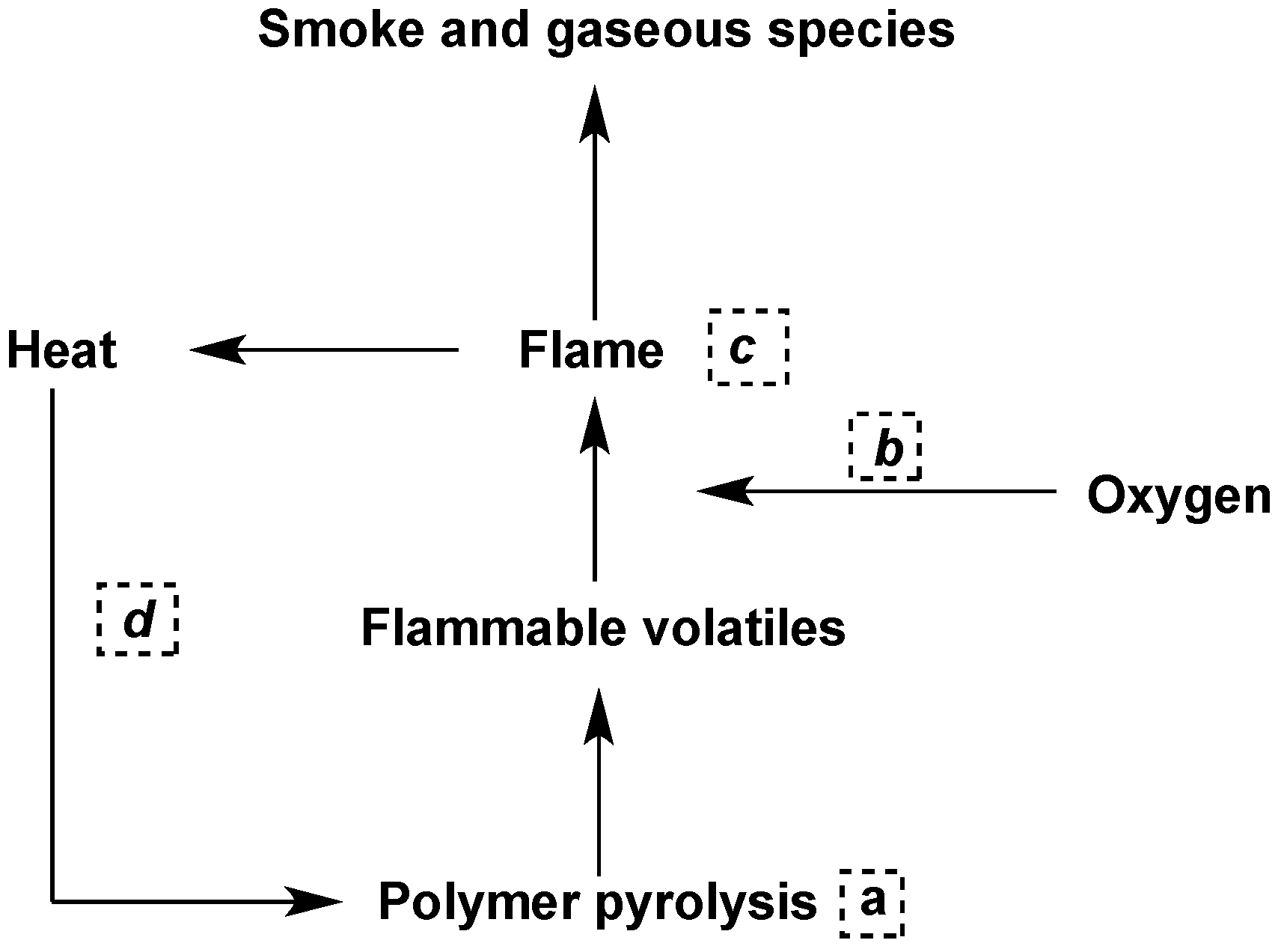
- reduce the heat to below that needed to sustain combustion;
- modify the pyrolysis process to reduce the amount of flammable volatiles in favor of increasing the formation of a less flammable carbonaceous residue (char), which also acts as a barrier between the polymer and the flame (a);
- isolate the flame from the oxygen/air supply (b);
- release chlorine or bromine atoms when the polymer is heated to near the ignition temperature; chlorinated and particularly brominated compounds are very efficient flame inhibitors (c);
- decrease the heat flow back to the polymer to prevent further pyrolysis;
- arrange that a barrier, e.g., char or an intumescent coating, is formed when the polymer is exposed to fire conditions (d).
- the ”golden period” of flame retardant research (1950–1980), characterized by the development of the first patents for organophosphorus-based FRs for cotton together with the production of inherently flame retardant synthetic fibers based on aromatic-structured polymer chains;
- the 1980s–late 1990s period, showing very little novel research in this area;
- the 2000 onward period, during which several attempts were and are being made to develop and improve char-promoting FRs, eventually coupled with phosphorus-containing additives. During this period, the possible replacement of bromine derivatives was also taken into account. Furthermore, this was the period during which nanotechnology was proven to be exploitable for conferring flame retardancy to fabrics, through the creation of self-assembled nanolayer films made of nanoparticles. More specifically, top-down and bottom-up strategies have been successfully utilized: the former uses preformed nanoparticle suspensions, while the bottom-up approach involves the creation of single or aggregates/assemblies of different types of nanoparticles.
2. Sol-Gel Derived Coatings on Fabrics
2.1. Sol-Gel Inorganic Coatings
2.2. Phosphorus-Doped Sol-Gel Coatings
2.3. Smoke Suppressant Effects Provided by Sol-Gel Coatings
2.4. Hybrid Organic–Inorganic Sol-Gel Coatings
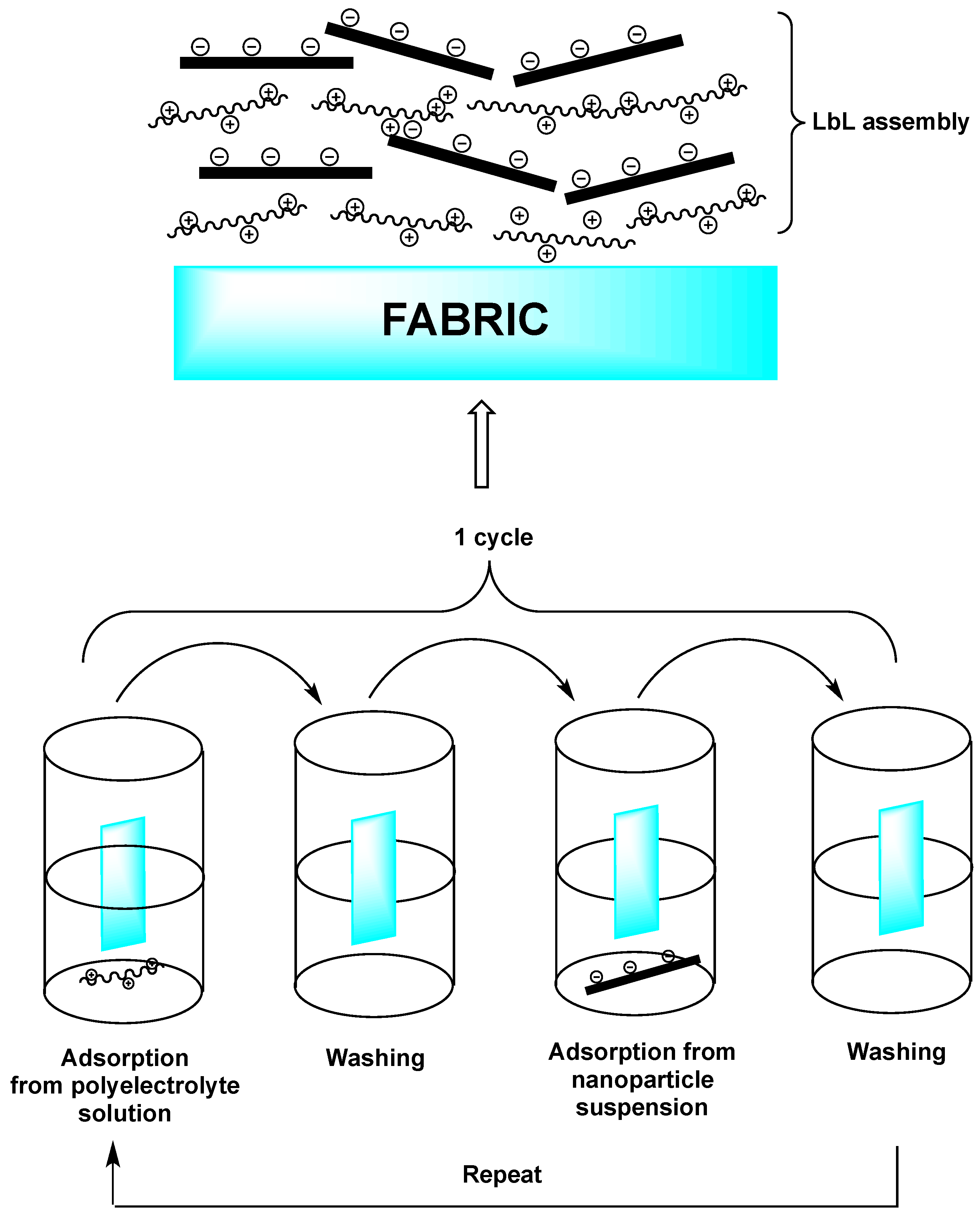
3. Layer-by-Layer Coatings on Fabrics
3.1. Fully Inorganic LbL Coatings
3.2. Intumescent LbL Coatings
3.3. Hybrid Organic–Inorganic LbL Coatings
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Brushlinsky, N.N.; Ahrens, M.; Sokolov, S.V.; Wagner, P. World Fire Statistics; International Association of Fire and Rescue Service (CTIF): Berlin, Germany, 2015. [Google Scholar]
- World Fire Statistics: Information Bulletin of the World Fire Statistics Centre; World Fire Statistics Centre: Geneva, Switzerland, 2014; Volume 29.
- Bourbigot, S. Flame retardancy of textiles e new approaches. In Advances in Fire Retardant Materials; Horrocks, A.R., Price, D., Eds.; Woodhead Publishing: Cambridge, UK, 2008; pp. 9–40. [Google Scholar]
- Weil, E.D.; Levchik, S.V. Flame retardants in commercial use or development for textiles. J. Fire Sci. 2008, 26, 243–281. [Google Scholar] [CrossRef]
- Neisius, M.; Stelzig, T.; Liang, S.; Gaan, S. Flame retardant finishes for textiles. In Functional Finishes for Textiles; Paul, R., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 429–461. [Google Scholar]
- Horrocks, A.R.; Price, D. Fire Retardant Materials; Woodhead Publishing: Cambridge, UK, 2000. [Google Scholar]
- Horrocks, A.R. Flame retardant challenges for textiles and fibers: New chemistry versus innovatory solutions. Polym. Degrad. Stab. 2011, 96, 377–392. [Google Scholar] [CrossRef]
- Visakh, P.M.; Arao, Y. Flame Retardants. Polymer Blends, Composites and Nanocomposites; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Cai, Y.; Wu, N.; Wei, Q.; Zhang, K.; Xu, Q.; Gao, W.; Song, L.; Hu, Y. Structure, surface morphology, thermal and flammability characterizations of polyamide6/organic-modified Fe-montmorillonite nanocomposite fibers functionalized by sputter coating of silicon. Surf. Coat. Technol. 2008, 203, 264–270. [Google Scholar] [CrossRef]
- Pappas, D. Status and potential of atmospheric plasma processing of materials. J. Vac. Sci. Technol. A Vac. Surf. Films 2011, 29, 020801–020817. [Google Scholar] [CrossRef]
- Dineff, P.; Gospodinova, D.; Kostova, L.; Vladkova, L.; Chen, E. Plasma-aided surface technology for modification of materials referred to fire protection. Probl. At. Sci. Technol. 2008, 6, 198–200. [Google Scholar]
- Tata, J.; Alongi, J.; Frache, A. Optimization of the procedure to burn textile fabrics by cone calorimeter: Part II. Results on nanoparticle-finished polyester. Fire Mater. 2012, 36, 527–536. [Google Scholar] [CrossRef]
- Carosio, F.; Alongi, J.; Frache, A. Influence of surface activation by plasma and nanoparticle adsorption on the morphology, thermal stability and combustion behavior of PET fabrics. Eur. Polym. J. 2011, 47, 893–902. [Google Scholar] [CrossRef]
- Alongi, J.; Tata, J.; Frache, A. Hydrotalcite and nanometric silica as finishing additives to enhance the thermal stability and flame retardancy of cotton. Cellulose 2011, 18, 179–190. [Google Scholar] [CrossRef]
- Horrocks, A.R.; Nazaré, S.; Masood, R.; Kondola, B.; Price, D. Surface modification of fabrics for improved flash-fire resistance using atmospheric pressure plasma in the presence of a functionalized clay and polysiloxane. Polym. Adv. Technol. 2011, 22, 22–29. [Google Scholar] [CrossRef]
- Cireli, A.; Onar, N.; Ebeoglugil, M.F.; Kayatekin, I.; Kutlu, B.; Culha, O.; Celik, E. Development of Flame Retardancy Properties of New Halogen-Free Phosphorous Doped SiO2 Thin Films on Fabrics. J. Appl. Polym. Sci. 2007, 105, 3747–3756. [Google Scholar] [CrossRef]
- Mahltig, B.; Böttcher, H. Modified silica sol coatings for water-repellent textiles. J. Sol-Gel Sci. Technol. 2003, 27, 43–52. [Google Scholar] [CrossRef]
- Yu, M.; Gu, G.; Meng, W.D.; Qing, F.L. Superhydrophobic cotton fabric coating based on a complex layer of silica nanoparticles and perfluorooctylated quaternary ammonium silane coupling agent. Appl. Surf. Sci. 2007, 253, 3669–3673. [Google Scholar] [CrossRef]
- Xue, C.H.; Ji, S.T.; Chen, H.Z.; Wang, M. Superhydrophobic cotton fabrics prepared by sol-gel coating of TiO2 and surface hydrophobization. Sci. Technol. Adv. Mater. 2008, 9, 1–5. [Google Scholar] [CrossRef]
- Mahltig, B.; Fiedler, D.; Böttcher, H. Antimicrobial sol-gel coatings. J. Sol-Gel Sci. Technol. 2004, 32, 219–222. [Google Scholar] [CrossRef]
- Mahltig, B.; Haufe, F.; Böttcher, H. Functionalisation of textiles by inorganic sol-gel coatings. J. Mater. Chem. 2005, 15, 4385–4398. [Google Scholar] [CrossRef]
- Xing, Y.J.; Yang, X.J.; Dai, J.J. Antimicrobial finishing of cotton textile based on water glass by sol-gel method. J. Sol-Gel Sci. Technol. 2007, 43, 187–192. [Google Scholar] [CrossRef]
- Mahltig, B.; Böttcher, H.; Rauch, H.; Dieckman, U.; Nitsche, R.; Fritz, T. Optimized UV protecting coatings by combination of organic and inorganic UV absorbers. Thin Solid Films 2005, 485, 108–114. [Google Scholar] [CrossRef]
- Abidi, N.; Hequet, E.; Tarimala, S.; Dai, L.L. Cotton fabric surface modification for improved UV radiation protection using sol-gel process. J. Appl. Polym. Sci. 2007, 104, 111–117. [Google Scholar] [CrossRef]
- Xing, X.J.; Ding, J. UV photo-stabilization of tetrabutyl titanate for aramid fibers via sol-gel surface modification. J. Appl. Polym. Sci. 2007, 103, 3113–3119. [Google Scholar] [CrossRef]
- Mahltig, B.; Textor, T. Combination of silica sol and dyes on textiles. J. Sol-Gel Sci. Technol. 2006, 39, 111–118. [Google Scholar] [CrossRef]
- Cireli, A.; Onar, N. Leaching and fastness behavior of cotton fabrics dyed with different type of dyes using sol-gel process. J. Appl. Polym. Sci. 2008, 109, 97–105. [Google Scholar]
- Li, F.Y.; Xing, Y.J.; Ding, X.; Zu, Y. Immobilization of papain on cotton fabric by sol-gel method. Enzyme Microb. Technol. 2007, 40, 1692–1697. [Google Scholar] [CrossRef]
- Huang, K.S.; Nien, Y.H.; Hsiao, K.C.; Chang, Y.S. Application of DMEU/SiO2 gel solution in the antiwrinkle finishing of cotton fabrics. J. Appl. Polym. Sci. 2006, 102, 4136–4143. [Google Scholar] [CrossRef]
- Caldara, M.; Colleoni, C.; Guido, E.; Re, V.; Rosace, G. Development of a textileoptoelectronic pH meter based on hybrid xerogel doped with methyl red. Sens. Actuators B 2012, 171, 1013–1021. [Google Scholar] [CrossRef]
- Van der Schueren, L.; De Clerck, K.; Brancatelli, G.; Rosace, G.; Van Damme, E.; De Vos, W. Novel cellulose and polyamide halochromic textile sensors based on the encapsulation of methyl red into a sol-gel matrix. Sens. Actuators B 2012, 162, 27–34. [Google Scholar] [CrossRef]
- Moafi, H.F.; Shojaie, A.F.; Zanjanchi, M.A. Flame-retardancy and photocatalytic properties of cellulosic fabric coated by nano-sized titanium dioxide. J. Therm. Anal. Calorim. 2011, 104, 717–724. [Google Scholar] [CrossRef]
- Colleoni, C.; Massafra, M.R.; Rosace, G. Photocatalytic properties and optical characterization of cotton fabric coated via sol-gel with non-crystalline TiO2 modified with poly(ethylene glycol). Surf. Coat. Technol. 2012, 207, 79–88. [Google Scholar] [CrossRef]
- Alongi, J.; Malucelli, G. State of the art and perspectives on sol-gel derived hybrid architectures for flame retardancy of textiles. J. Mater. Chem. 2012, 22, 21805–21809. [Google Scholar] [CrossRef]
- Alongi, J.; Malucelli, G. Thermal degradation of cellulose and cellulosic substrates. In Reactions and Mechanisms in Thermal Analysis of Advanced Materials; Tiwari, A., Raj, B., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 301–332. [Google Scholar]
- Malucelli, G. Hybrid organic/inorganic coatings through dual-cure processes: State of the art and perspectives. Coatings 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Alongi, J.; Ciobanu, M.; Malucelli, G. Cotton fabrics treated with hybrid organic-inorganic coatings obtained through dual-cure processes. Cellulose 2011, 18, 1335–1348. [Google Scholar] [CrossRef]
- Hribernik, S.; Smole, M.S.; Kleinschek, K.S.; Bele, M.; Jamink, J.; Gaberscek, M. Flame retardant activity of SiO2-coated regenerated cellulose fibers. Polym. Degrad. Stab. 2007, 92, 1957–1965. [Google Scholar] [CrossRef]
- Alongi, J.; Ciobanu, M.; Carosio, F.; Tata, J.; Malucelli, G. Thermal stability and flame retardancy of polyester, cotton and relative blend textile fabrics subjected to sol-gel treatments. J. Appl. Polym. Sci. 2011, 119, 1961–1969. [Google Scholar] [CrossRef]
- Alongi, J.; Ciobanu, M.; Malucelli, G. Sol-gel treatments for enhancing flame retardancy and thermal stability of cotton fabrics: Optimization of the process and evaluation of durability. Cellulose 2011, 18, 167–177. [Google Scholar] [CrossRef]
- Alongi, J.; Ciobanu, M.; Malucelli, G. Sol-gel treatments on cotton fabrics for improving thermal and flame stability: Effect of the structure of the alkoxysilane precursor. Carbohydr. Polym. 2012, 87, 627–635. [Google Scholar] [CrossRef]
- Alongi, J.; Ciobanu, M.; Malucelli, G. Thermal stability, flame retardancy and mechanical properties of cotton fabrics treated with inorganic coatings synthesized through sol-gel processes. Carbohydr. Polym. 2012, 87, 2093–2099. [Google Scholar] [CrossRef]
- Alongi, J.; Malucelli, G. Thermal stability, flame retardancy and abrasion resistance of cotton and cotton-linen blends treated by sol-gel silica coatings containing alumina micro- or nano-particles. Polym. Degrad. Stab. 2013, 98, 1428–1438. [Google Scholar] [CrossRef]
- Brzezinski, S.; Kowalczyk, D.; Borak, B.; Jasiorski, M.; Tracz, A. Applying the sol-gel method to the deposition of nanocoats on textiles to improve their abrasion resistance. J. Appl. Polym. Sci. 2012, 125, 3058–3067. [Google Scholar] [CrossRef]
- Alongi, J.; Colleoni, C.; Malucelli, G.; Rosace, G. Hybrid phosphorus-doped silica architectures derived from a multistep sol-gel process for improving thermal stability and flame retardancy of cotton fabrics. Polym. Degrad. Stab. 2012, 97, 1334–1344. [Google Scholar] [CrossRef]
- Alongi, J.; Colleoni, C.; Rosace, G.; Malucelli, G. The role of pre-hydrolysis on multistep sol-gel processes for enhancing the flame retardancy of cotton. Cellulose 2013, 20, 525–535. [Google Scholar] [CrossRef]
- Brancatelli, G.; Colleoni, C.; Massafra, M.R.; Rosace, G. Effect of hybrid phosphorus-doped silica thin films produced by sol-gel method on the thermal behavior of cotton fabrics. Polym. Degrad. Stab. 2011, 96, 483–490. [Google Scholar] [CrossRef]
- Alongi, J.; Colleoni, C.; Rosace, G.; Malucelli, G. Thermal and fire stability of cotton fabrics coated with hybrid phosphorus-doped silica films. J. Therm. Anal. Calorim. 2012, 110, 1207–1216. [Google Scholar] [CrossRef]
- Lewin, M. Synergism and catalysis in flame retardancy of polymers. Polym. Adv. Technol. 2001, 12, 215–222. [Google Scholar] [CrossRef]
- Alongi, J.; Colleoni, C.; Rosace, G.; Malucelli, G. Phosphorus- and nitrogen-doped silica coatings for enhancing the flame retardancy of cotton: Synergisms or additive effects? Polym. Degrad. Stab. 2013, 98, 579–589. [Google Scholar] [CrossRef]
- Alongi, J.; Ciobanu, M.; Malucelli, G. Novel flame retardant finishing systems for cotton fabrics based on phosphorus-containing compounds and silica derived from sol-gel processes. Carbohydr. Polym. 2011, 85, 599–608. [Google Scholar] [CrossRef]
- Yaman, N. Preparation and flammability properties of hybrid materials containing phosphorous compounds via sol-gel process. Fibers Polym. 2009, 10, 413–418. [Google Scholar] [CrossRef]
- Kappes, R.S.; Urbainczyk, T.; Artz, U.; Textor, T.; Gutmann, J.S. Flame retardants based on amino silanes and phenylphosphonic acid. Polym. Degrad. Stab. 2016, 129, 168–179. [Google Scholar] [CrossRef]
- Dasari, A.; Yu, Z.Z.; Cai, G.P.; Mai, Y.W. Recent developments in the fire retardancy of polymeric materials. Prog. Polym. Sci. 2013, 38, 1357–1387. [Google Scholar] [CrossRef]
- Alongi, J.; Malucelli, G. Cotton fabrics treated with novel oxidic phases acting as effective smoke suppressants. Carbohydr. Polym. 2012, 90, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Jie, G.; Song, L.; Hu, S.; Lv, X.; Wang, X. Flame retardancy and thermal degradation of cotton textiles based on UV-curable flame retardant coatings. Thermochim. Acta 2011, 513, 75–82. [Google Scholar] [CrossRef]
- Przybylak, M.; Maciejewski, H.; Dutkiewicz, A.; Wesołek, D.; Władyka-Przybylak, M. Multifunctional, strongly hydrophobic and flame-retarded cotton fabrics modified with flame retardant agents and silicon compounds. Polym. Degrad. Stab. 2016, 128, 55–64. [Google Scholar] [CrossRef]
- Sehic, A.; Tomsic, B.; Jerman, I.; Vasiljevic, J.; Medved, J.; Simoncic, B. Synergistic inhibitory action of P- and Si-containing precursors in sol-gel coatings on the thermal degradation of polyamide 6. Polym. Degrad. Stab. 2016, 128, 245–252. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, Y.T.; Wang, X.; Acuña, P.; Zhu, P.; Wagenknecht, U.; Heinrich, G.; Zhang, X.Q.; Wang, R.; Wang, D.-Y. Effect of phosphorus-containing inorganic–organic hybrid coating on the flammability of cotton fabrics: Synthesis, characterization and flammability. Chem. Eng. J. 2016, 294, 167–175. [Google Scholar] [CrossRef]
- Iler, R.K. Multilayers of colloidal particles. J. Colloid Interface Sci. 1966, 21, 569–594. [Google Scholar] [CrossRef]
- Decher, G.; Hong, J.D. Buildup of ultrathin multilayer films by a self-assembly process: II. Consecutive adsorption of anionic and cationic bipolar amphiphiles on charged surfaces. Makromol. Chem. Macromol. Symp. 1991, 46, 321–327. [Google Scholar] [CrossRef]
- Shimazaki, Y.; Mitsuishi, M.; Ito, S.; Yamamoto, M. Preparation and Characterization of the Layer-by-Layer Deposited Ultrathin Film Based on the Charge-Transfer Interaction in Organic Solvents. Langmuir 1998, 14, 2768–2773. [Google Scholar] [CrossRef]
- Stockton, W.B.; Rubner, M.F. Molecular-Level Processing of Conjugated Polymers. 4. Layer-by-Layer Manipulation of Polyaniline via Hydrogen-Bonding Interactions. Macromolecules 1997, 30, 2717–2725. [Google Scholar] [CrossRef]
- Fang, M.M.; Kaschak, D.M.; Sutorik, A.C.; Mallouk, T.E. A “Mix and Match” Ionic−Covalent Strategy for Self-Assembly of Inorganic Multilayer Films. J. Am. Chem. Soc. 1997, 119, 12184–12191. [Google Scholar] [CrossRef]
- Ichinose, I.; Kawakami, T.; Kunitake, T. Alternate Molecular Layers of Metal Oxides and Hydroxyl Polymers Prepared by the Surface Sol-Gel Process. Adv. Mater. 1998, 10, 535–539. [Google Scholar] [CrossRef]
- Wang, X.; Naka, K.; Wang, C.; Itoh, H.; Uemura, T.; Chujo, Y. Layer-by-Layer Films Based on Charge Transfer Interaction of π-Conjugated Poly(dithiafulvene) and Incorporation of Gold Nanoparticles into the Films. J. Appl. Polym. Sci. 2007, 103, 1608–1615. [Google Scholar] [CrossRef]
- Serizawa, T.; Hamada, K.; Kitayama, T.; Katsukawa, K.; Hatada, K.; Akashi, M. Stepwise Assembly of Isotactic Poly(Methyl Methacrylate) and Syndiotactic Poly(Methacrylic Acid) on a Substrate. Langmuir 2000, 16, 7112–7115. [Google Scholar] [CrossRef]
- Serizawa, T.; Yamashita, H.; Fujiwara, T.; Kimura, Y.; Akashi, M. Stepwise Assembly of Enantiomeric Poly(lactide)s on Surfaces. Macromolecules 2001, 34, 1996–2001. [Google Scholar] [CrossRef]
- Malucelli, G. Layer-by-Layer nanostructured assemblies for the fire protection of fabrics. Mater. Lett. 2016, 166, 339–342. [Google Scholar] [CrossRef]
- Malucelli, G.; Carosio, F.; Alongi, J.; Fina, A.; Frache, A.; Camino, G. Materials Engineering For Surface-Confined Flame Retardancy. Mater. Sci. Eng. R Rep. 2014, 84, 1–20. [Google Scholar] [CrossRef]
- Jang, W.S.; Rawson, I.; Grunlan, J.C. Layer-by-layer assembly of thin film oxygen barrier. Thin Solid Films 2008, 516, 4819–4825. [Google Scholar] [CrossRef]
- Grunlan, J.C.; Jang, W.-S. Layer-by-layer assembly of nano brick walls: Tailoring film growth and gas permeability. ACS Polym. Prepr. 2008, 49, 342. [Google Scholar]
- Priolo, M.A.; Gamboa, D.; Grunlan, J.C. Transparent clay-polymer nano brick wall assemblies with tailorable oxygen barrier. ACS Appl. Mater. Interfaces 2010, 2, 312–320. [Google Scholar] [CrossRef]
- Podsiadlo, P.; Sui, L.; Elkasabi, Y.; Burgardt, P.; Lee, J.; Miryala, A.; Kusumaatmaja, W.; Carman, M.R.; Shtein, M.; Kieffer, J.; et al. Layer-by-layer assembled films of cellulose nanowires with antireflective properties. Langmuir 2007, 23, 7901–7906. [Google Scholar] [CrossRef] [PubMed]
- Rivadulla, F.; Mateo Mateo, C.; Correa-Duarte, M.A. Layer-by-Layer Polymer Coating of Carbon Nanotube: Tuning of Electrical Conductivity in Random Networks. J. Am. Chem. Soc. 2010, 132, 3751–3755. [Google Scholar] [CrossRef] [PubMed]
- Dvoracek, C.M.; Sukhonosova, G.; Benedik, M.J.; Grunlan, J.C. Antimicrobial behavior of polyelectrolyte–surfactant thin film assemblies. Langmuir 2009, 25, 10322–10328. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Frongia, M.E.; Cardellach, M.; Miller, C.A.; Stafford, G.P.; Leggett, G.J.; Hatton, P.V. Functionalised nanoscale coatings using layer-by-layer assembly for imparting antibacterial properties to polylactide-co-glycolide surfaces. Acta Biomater. 2015, 21, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Schulz, J.; Grunlan, J.C. Polyelectrolyte/Nanosilicate Thin-Film Assemblies: Influence of pH on Growth, Mechanical Behavior, and Flammability. Appl. Mater. Interfaces 2009, 1, 2338–2340. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Schulz, J.; Mannen, S.; Delhom, C.; Condon, B.; Chang, S.; Zammarano, M.; Grunlan, J.C. Flame retardant behavior of polyelectrolyte-clay thin film assemblies on cotton fabric. ACS Nano 2010, 4, 3325–3330. [Google Scholar] [CrossRef] [PubMed]
- Laufer, G.; Carosio, F.; Martinez, R.; Camino, G.; Grunlan, J.C. Growth and fire resistance of colloidal silica-polyelectrolyte thin film assemblies. J. Colloid Interface Sci. 2011, 35669–35677. [Google Scholar] [CrossRef] [PubMed]
- Carosio, F.; Laufer, G.; Alongi, J.; Camino, G.; Grunlan, J.C. Layer-by-layer assembly of silica-based flame retardant thin film on PET fabric. Polym. Degrad. Stab. 2011, 96, 745–754. [Google Scholar] [CrossRef]
- Alongi, J.; Carosio, F.; Frache, A.; Malucelli, G. Layer by Layer coatings assembled through dipping, vertical or horizontal spray for cotton flame retardancy. Carbohydr. Polym. 2013, 92, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Carosio, F.; Di Blasio, A.; Cuttica, F.; Alongi, J.; Frache, A.; Malucelli, G. Flame Retardancy of Polyester Fabrics Treated by Spray-Assisted Layer-by-Layer Silica Architectures. Ind. Eng. Chem. Res. 2013, 52, 9544–9550. [Google Scholar] [CrossRef]
- Li, Y.C.; Mannen, S.; Schulz, J.; Grunlan, J.C. Growth and fire protection behavior of POSS-based multilayer thin films. J. Mater. Chem. 2011, 21, 3060–3069. [Google Scholar] [CrossRef]
- Carosio, F.; Alongi, J.; Malucelli, G. α-Zirconium phosphate-based nanoarchitectures on polyester fabrics through layer-by-layer assembly. J. Mater. Chem. 2011, 21, 10370–10376. [Google Scholar] [CrossRef]
- Li, Y.C.; Mannen, S.; Morgan, A.B.; Chang, S.C.; Yang, Y.H.; Condon, B.; Grunlan, J.C. Intumescent All-Multilayer Nanocoating Capable of Extinguishing Flame on Fabric. Adv. Mater. 2011, 23, 3926–3930. [Google Scholar] [CrossRef] [PubMed]
- Apaydin, K.; Laachachi, A.; Ball, V.; Jimenez, M.; Bourbigot, S.; Toniazzo, V.; Ruch, D. Polyallylamine–montmorillonite as super flame retardant coating assemblies by layer-by layer deposition on polyamide. Polym. Degrad. Stab. 2013, 98, 627–634. [Google Scholar] [CrossRef]
- Carosio, F.; Alongi, J.; Malucelli, G. Layer by Layer ammonium polyphosphate-based coatings for flame retardancy of polyester-cotton blends. Carbohydr. Polym. 2012, 88, 1460–1470. [Google Scholar] [CrossRef]
- Alongi, J.; Carosio, F.; Malucelli, G. Influence of ammonium polyphosphate-/poly(acrylic acid)- based Layer by Layer architectures on the char formation in cotton, polyester and their blends. Polym. Degrad. Stab. 2012, 97, 1644–1653. [Google Scholar] [CrossRef]
- Carosio, F.; Alongi, J.; Malucelli, G. Flammability and combustion properties of ammonium polyphosphate-/poly(acrylic acid)- based Layer by Layer architectures deposited on cotton, polyester and their blends. Polym. Degrad. Stab. 2013, 98, 1626–1630. [Google Scholar] [CrossRef]
- Zhang, T.; Yan, H.; Wang, L.; Fang, Z. Controlled formation of self-extinguishing intumescent coating on ramie fabric via layer-by-layer assembly. Ind. Eng. Chem. Res. 2013, 52, 6138–6140. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, T.; Yan, H.Q.; Peng, M.; Fang, Z.; Li, Y.; Wang, H. Flame-retardant Coating by Alternate Assembly of Poly(vinylphosphonic acid) and Polyethylenimine for Ramie Fabrics. Chin. J. Polym. Sci. 2014, 32, 305–314. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, T.; Yan, H.; Peng, M.; Fang, Z. Modification of ramie fabric with a metal-ion-doped flame-retardant coating. J. Appl. Polym. Sci. 2013, 129, 2986–2997. [Google Scholar] [CrossRef]
- Zhang, T.; Yan, H.; Peng, M.; Wang, L.; Fang, Z. Construction of flame retardant nanocoating on ramie fabric via layer-by-layer assembly of carbon nanotube and ammonium polyphosphate. Nanoscale 2013, 5, 3013–3020. [Google Scholar] [CrossRef] [PubMed]
- Guirao, C.; Carosio, F.; Boutevin, B.; Cottet, H.; Loubat, C. Phosphonated Oligoallylamine: Synthesis, Characterization in Water, and Development of Layer by Layer Assembly. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 1244–1250. [Google Scholar] [CrossRef]
- Carosio, F.; Negrell-Guirao, C.; Di Blasio, A.; Alongi, J.; David, G.; Camino, G. Tunable thermal and flame response of phosphonated oligoallylamines layer by layer assemblies on cotton. Carbohydr. Polym. 2015, 115, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Yang, J.; Gao, J.; Wang, X. Thin films of intumescent flame retardant-polyacrylamide and exfoliated graphene oxide fabricated via layer-by-layer assembly for improving flame retardant properties of cotton fabric. Ind. Eng. Chem. Res. 2012, 51, 12355–12366. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Li, Y.; Sun, J. Intumescent Flame-Retardant and Self-Healing Superhydrophobic Coatings on Cotton Fabric. ACS Nano 2015, 9, 4070–4076. [Google Scholar] [CrossRef] [PubMed]
- Narkhede, M.; Thota, S.; Mosurkal, R.; Muller, W.S.; Kumar, J. Layer-by-layer assembly of halogen-free polymeric materials on nylon/cotton blend for flame retardant applications. Fire Mater. 2016, 40, 206–218. [Google Scholar] [CrossRef]
- Carosio, F.; Alongi, J. Influence of layer by layer coatings containing octapropylammonium polyhedral oligomeric silsesquioxane and ammonium polyphosphate on the thermal stability and flammability of acrylic fabrics. J. Anal. Appl. Pyrolysis 2016, 119, 114–123. [Google Scholar] [CrossRef]
- Apaydin, K.; Laachachi, A.; Ball, V.; Jimenez, M.; Bourbigot, S.; Ruch, D. Layer-by-layer deposition of a TiO2-filled intumescent coating and its effect on the flame retardancy of polyamide and polyester fabrics. Colloids Surf. A Physicochem. Eng. Asp. 2015, 469, 1–10. [Google Scholar] [CrossRef]
- Laufer, G.; Kirkland, C.; Morgan, A.; Grunlan, J.C. Intumescent Multilayer Nanocoating, Made with Renewable Polyelectrolytes, for Flame-Retardant Cotton. Biomacromolecules 2012, 13, 2843–2848. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yan, H.; Fang, Z.; Wang, J.; Wang, H. On the flameproof treatment of ramie fabrics using a spray-assisted layer-by-layer technique. Polym. Degrad. Stab. 2015, 121, 11–17. [Google Scholar] [CrossRef]
- Leistner, M.; Abu-Odeh, A.A.; Rohmer, S.C.; Grunlan, J.C. Water-based chitosan/melamine polyphosphate multilayer nanocoating that extinguishes fire on polyester-cotton fabric. Carbohydr. Polym. 2015, 130, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Zhang, X.; Meng, Y.; Gu, Z.; Bao, C.; Ding, X.; Li, X.; Chen, X.; Tian, X. Intumescent flame retardant coatings on cotton fabric of chitosan and ammonium polyphosphate via layer-by-layer assembly. Surf. Coat. Technol. 2015, 262, 9–14. [Google Scholar] [CrossRef]
- Fang, F.; Xiao, D.; Zhang, X.; Meng, Y.; Cheng, C.; Bao, C.; Ding, X.; Cao, H.; Tian, X. Construction of intumescent flame retardant and antimicrobial coating on cotton fabric via layer-by-layer assembly technology. Surf. Coat. Technol. 2015, 276, 726–734. [Google Scholar] [CrossRef]
- Fang, F.; Chen, X.; Zhang, X.; Cheng, C.; Xiao, D.; Meng, Y.; Ding, X.; Zhang, H.; Tian, X. Environmentally friendly assembly multilayer coating for flame retardant and antimicrobial cotton fabric. Prog. Org. Coat. 2016, 90, 258–266. [Google Scholar] [CrossRef]
- Carosio, F.; Fontaine, G.; Alongi, J.; Bourbigot, S. Starch-Based Layer by Layer Assembly: Efficient and Sustainable Approach to Cotton Fire Protection. ACS Appl. Mater. Interfaces 2015, 7, 12158–12167. [Google Scholar] [CrossRef] [PubMed]
- Alongi, J.; Carletto, R.A.; Di Blasio, A.; Carosio, F.; Bosco, F.; Malucelli, G. DNA: A novel, green, natural flame retardant and suppressant for cotton. J. Mater. Chem. A 2013, 1, 4779–4785. [Google Scholar] [CrossRef]
- Alongi, J.; Carletto, R.A.; Di Blasio, A.; Carosio, F.; Bosco, F.; Malucelli, G. Intrinsic intumescent-like flame retardant properties of DNA-treated cotton fabrics. Carbohydr. Polym. 2013, 96, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Carosio, F.; Di Blasio, A.; Alongi, J.; Malucelli, G. Green DNA-based flame retardant coatings assembled through Layer by Layer. Polymer 2013, 54, 5148–5153. [Google Scholar] [CrossRef]
- Alongi, J.; Carosio, F.; Malucelli, G. Layer by layer complex architectures based on ammonium polyphosphate, chitosan and silica on polyester-cotton blends: Flammability and combustion behavior. Cellulose 2012, 19, 1041–1050. [Google Scholar] [CrossRef]
- Huang, G.; Liang, H.; Wang, X.; Gao, J. Poly(acrylic acid)/clay thin films assembled by layer-by-layer deposition for improving the flame retardancy properties of cotton. Ind. Eng. Chem. Res. 2012, 51, 12299–12309. [Google Scholar] [CrossRef]
- Gao, D.; Li, R.; Lv, B.; Ma, J.; Tian, F.; Zhang, J. Flammability, thermal and physical-mechanical properties of cationic polymer/montmorillonite composite on cotton fabric. Compos. Part B 2015, 77, 329–337. [Google Scholar] [CrossRef]
- Pan, H.; Song, L.; Hu, Y.; Liew, K.M. An Eco-friendly Way to improve flame retardancy of cotton fabrics: Layer-by-Layer Assembly of semi-biobased Substance. Energy Proc. 2015, 75, 174–179. [Google Scholar] [CrossRef]
- New, J.; Zope, I.S.; Abdul Rahman, S.N.; LiWendy Yap, X.; Dasari, A. Physiological comfort and flame retardancy of fabrics with electrostatic self-assembled coatings. Mater. Des. 2016, 89, 413–420. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, L.; Zhong, Y.; Mao, Z. Fire retardancy and durability of poly(N-benzyloxycarbonyl-3,4-dihydroxyphenylalanine)-montmorillonite composite film coated polyimide fabric. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Pappas, S.P. Radiation Curing. Science and Technology; Springer: New York, NY, USA, 1992. [Google Scholar]
- Carosio, F.; Alongi, J. Few durable layers suppress cotton combustion due to the joint combination of layer by layer assembly and UV-curing. RSC Adv. 2015, 5, 71482–71490. [Google Scholar] [CrossRef]
- Chang, S.; Slopek, R.P.; Condon, B.; Grunlan, J.C. Surface Coating for Flame-Retardant Behavior of Cotton Fabric Using a Continuous Layer-by-Layer Process. Ind. Eng. Chem. Res. 2014, 53, 3805–3812. [Google Scholar] [CrossRef]

| Feature | Sol-Gel | Layer-by-Layer |
|---|---|---|
| Main approach |
|
|
| ||
| Process sustainability |
|
|
| Durability (washing fastness) |
|
|
| Compatibility with existing textile finishing processes |
|
|
| Comfort of the treated textiles (in terms of stiffness and touch) |
|
|
| Multifunctionality of the treatment |
|
|
| Sample | TSR (m2/m2) | CO (ppm) | CO2 (%) | |
|---|---|---|---|---|
| 1st Peak | 2nd Peak | Peak | ||
| Untreated cotton | 24 | 0.0011 | 0.0018 | 0.146 |
| Cotton treated with silica | 11 | 0.0005 | 0.0045 | 0.112 |
| Cotton treated with silica + Zinc oxide | 9 | 0.0002 | 0.0032 | 0.095 |
| Cotton treated with silica + Zinc acetate dihydrate | 18 | 0.0004 | 0.0040 | 0.110 |
| Cotton treated with Zinc borate | 17 | 0.0004 | 0.0040 | 0.108 |
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malucelli, G. Surface-Engineered Fire Protective Coatings for Fabrics through Sol-Gel and Layer-by-Layer Methods: An Overview. Coatings 2016, 6, 33. https://doi.org/10.3390/coatings6030033
Malucelli G. Surface-Engineered Fire Protective Coatings for Fabrics through Sol-Gel and Layer-by-Layer Methods: An Overview. Coatings. 2016; 6(3):33. https://doi.org/10.3390/coatings6030033
Chicago/Turabian StyleMalucelli, Giulio. 2016. "Surface-Engineered Fire Protective Coatings for Fabrics through Sol-Gel and Layer-by-Layer Methods: An Overview" Coatings 6, no. 3: 33. https://doi.org/10.3390/coatings6030033
APA StyleMalucelli, G. (2016). Surface-Engineered Fire Protective Coatings for Fabrics through Sol-Gel and Layer-by-Layer Methods: An Overview. Coatings, 6(3), 33. https://doi.org/10.3390/coatings6030033