Natural Pectin Polysaccharides as Edible Coatings
Abstract
:1. Introduction
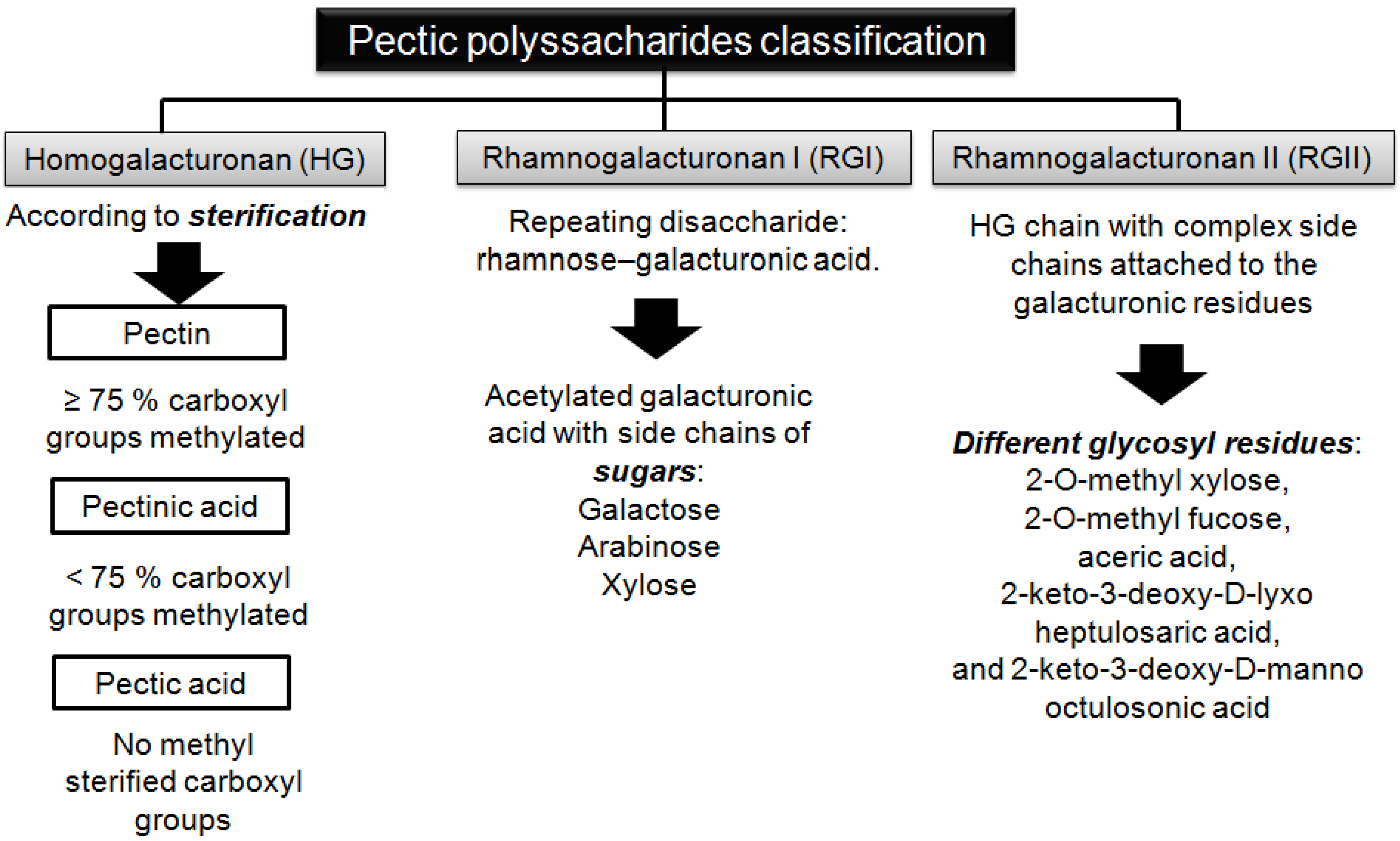
2. Structure and Classification of Pectic Substances
3. Properties and Current Applications in Food Industry
- Pectins with high DM (HM pectins) containing 50% or higher of galacturonic residues. They are used as gelling component in heat-resistant bakery jams, fruit preservatives and juices, confectionaries jellies, milky products, glazing cakes and soft drinks. HM pectins form gels by hydrophobic interactions under acidic conditions in aqueous media and high sugar content.
- Pectins with low DM (LM pectins) are obtained by de-esterification of HM pectins under controlled pH, temperature and time. LM pectins show DM degrees between 20% and 30% [16]. They are used to prepare gels at low pH values in the presence of divalent calcium. Applications in food industry include jams and jellies with low-sugar content, dairy desserts, ice cream with fruit gels, thickening agents of syrups for fruit and vegetable canning and food coatings.

4. Extraction Methods for Pectins: New Trends for the Revalorization of Agricultural Residues
| Natural Source | Extraction Parameters | Pectin Yield Extraction (%) | Ref. |
|---|---|---|---|
| Okra pods | Citric and phosphate buffer, pH 2 and 6, 60 min, 80 °C, 1:15 g·mL−1 | 13.3 (pH 2.0); 15.7 (pH 6.0) | [21] |
| Durian rinds | HCl, pH 2.8, 43 min, 86 °C, S:L 1:10 g·mL−1 | 9.1 | [22] |
| Mango peel | Sulfuric acid in water, pH 1.5, 2.5 h, 90 °C | >70 | [23] |
| Banana peel | Citric acid solution, pH 2.0, 160 min, 87 °C, 1:20 g·mL−1 | 13.89 | [24] |
| Citric acid and HCl, pH 1.5, 4 h, 90 °C | 16.54 | [25] | |
| Tropical fruit peels | Citric acid, pH of 2, 3.3 and 4.5, 120 min, 70 °C | 12.56–14.24 | [26] |
| Sugar beet pulp | Citric acid, pH 1, 166 min, 99 °C, 1:20 g·mL−1 | 23.95 | [27] |
| Bagasse and pomace lime fruit | Citric acid, 60 min, 90 °C, 1:20 g·mL−1 | 13.31 (Bagasse); 15.1 (Pomace) | [28] |
| Valencia orange peel | Citric acid extraction, pH 1.5, 75 min, 90 °C | 16.7 | [29] |
| Watermelon seed | HCl, pH 2, 60 min, 85 °C, 1:15 g·mL−1 | 19.75 | [30] |
| Faba bean hulls | HCl, pH 1.5, 80 min, 85 °C, 1:25 g·mL−1 | 15.75 | [31] |
| Honeydew melon seeds and damaged skin | HCl at pH 1, 80 °C for 4 h | 7.9 | [32] |
| Tomato peel | Ammonium oxalate and oxalic acid, 90 °C in two extraction steps (24 and 12 h) | 32.0 | [33] |
| Sunflower head | Sodium citrate, 85 °C, 3.5 h, 1:40 g/mL | 16.90 | [34] |
| Extraction Method | Natural Source | Extraction Parameters | Pectin Yield Extraction (%) | Ref. |
|---|---|---|---|---|
| MAE | Dragon fruit peel | 400 W, 45 °C, 20 min, 24 g·mL−1 | 7.5 | [40] |
| HCl, pH 2.07, 800 W, 65 s, 66.6 g·mL−1 | 18.57 | [41] | ||
| Bagasse and pomace of Mexican lime fruit | Citric acid, 800 W, 120 °C, 5 min, 1:30 g·mL−1 | 16.9 (Bagasse); 8.4 (Pomace) | [28] | |
| Pomelo peel | Tartaric acid solution, pH 1.5, 660 W, 9 min, 1:40 g·mL−1 | 23.83 | [42] | |
| Mango peel | Aqueous solution, pH 2.7, 413 W, 134 s, 1:18 g·mL−1 | 28.86 | [43] | |
| Papaya peel | Aqueous solution, 512 W, pH 1.8, 140 s, 1:15 g·mL−1 | 25.41 | [44] | |
| EMI | Citrange albedos | Aqueous solution, pH 1.2 with H2SO4, 80 °C, 90 min | 29 | [45] |
| UAE | Pomegranate peel | Aqueous solution, pH 1.27, 61.9 °C, 28.31 min, 1:17.52 g·mL−1 | 24.18 | [46] |
| Sisal fiber | Aqueous solution, 61 W, 50 °C, 26 min, 1:28 g·mL−1 | 29.32 | [47] | |
| Sugar beet pulp | Aqueous solution, 10.70 MPa, 120.72 °C, 30.49 min, 44.0 g·mL−1 | 24.63 | [48] | |
| Grapefruit peel | Deionized water, HCl, pH 1.5, 12.56 W/cm2, 66.7 °C, 27.9 min, 1:50 g·mL−1 | 27.34 | [49] | |
| Enzymes | Gold kiwifruit pulp, skin and seed | Purified water, pH 3.7, 25 °C, 30 min, Celluclast 1.5 L enzyme (1.05 mL·kg−1), 1:3 g·mL−1 | 2.14 | [50] |
| Gold kiwifruit pomace | 4.5 | [51] |
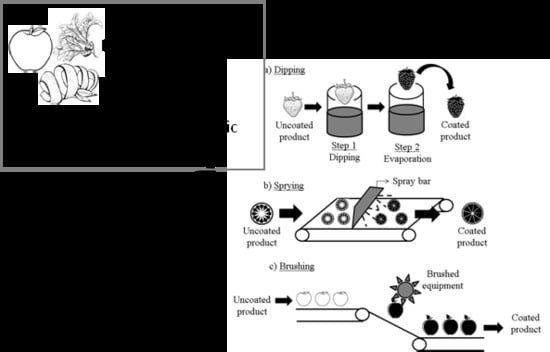
5. Recent Trends in the Use of Pectin Edible Coatings
5.1. Shelf-life Extension of Fresh-cut Fruits and Vegetables
| Coating | Active Agent | Food Matrix | Effect | Ref. |
|---|---|---|---|---|
| Low methoxyl pectin (2%), glycerol, sunflower oil, CaCl2 | – | Melons (Cucumis melon L.) | Antioxidant properties maintaining quality attributes (4 °C, 15 days) | [66] |
| Pectin (3%), sorbitol, beeswax | – | Mangoes (cv. Ataulfo) | Reduction of physiological changes, respiration rates and weight loss (15 days, room temperature) | [67] |
| Pectin (3%), sorbitol, beeswax | – | Avocados | Reduction of firmness, color, respiration rates and moisture loss (10 °C, 1 month) | [68] |
| Osmotic dehydration (with 0.5% calcium lactate) + pectin coating (1%) | – | Ripe Melons (Cucumis melon cv. inodorus) | Reduction of respiration rate maintaining sensory properties and quality parameters (5 °C, 80% RH, 14 days) | [69] |
| Pectin (2%) + glycerol combined with MAP | – | Nectarines (Prunus persica L. cv. Babygold) | Texture, color and hygienic quality (3 °C, 7 days) | [62] |
| Multi-layered alginate (1%)-β-CD-trans (2%)/pectin (2%) Calcium lactate | trans-cinnamaldehyde encapsulated in β-Cyclodextrins | Watermelon (C. lanatus) | Antimicrobial activity maintaining quality and sensory attributes (4 °C, 12 days) | [70] |
| Multi-layered chitosan (2%)-β-CD-trans (1%)/pectin (1%) CaCl2 | trans-cinnamaldehyde encapsulated in β-Cyclodextrins | Cantaloupe melon | Antimicrobial activity maintaining quality and sensory attributes (4 °C, 9 days) | [71] |
| Multi-layered chitosan (2%)-β-CD-trans (1%)/pectin (2%) CaCl2 | trans-cinnamaldehyde encapsulated in β-Cyclodextrins | Papaya fruits (Carica papaya L. cv Maradol) | Antimicrobial activity maintaining quality and sensory attributes (4 °C, 15 days) | [72] |
| Pectin (2%), glycerol, CaCl2 | N-acetylcysteine Glutathione | Pears (Pyrus communis L.) | Antibrowning, antimicrobial, antioxidant maintaining sensory attributes (4 °C, 14 days) | [73] |
| Pectin (3.5%), glycerol | Sodium benzoate; Potassium sorbate | Strawberries (Fragaria ananassa cv. Albion) | Antimicrobial activity maintaining physico-chemical and sensory attributes (4 °C, 15 days) | [74] |
| Pectin (2%), glycerol, CaCl2 | Apple fiber; Inulin; Ascorbic acid | “Golden delicious” apples | Nutritional value maintaining firmness, color and antioxidant activity (4 °C, 7 days) | [75] |
| Pectin (2%) CaCl2 | Eugenol Citral; Ascorbic acid | Strawberries | Antimicrobial, antioxidant, antibrowning activities maintaining sensory properties (0.5 °C, 7 days) | [76] |
| Pectin (1%) CaCl2 | Eugenol Citral; Ascorbic acid | Raspberries | Antimicrobial, antioxidant, antibrowning activities maintaining sensory properties (0.5 °C, 7 days) | [77] |
| Pectin (3%) + glycerol | Cinnamon leaf essential oil | Peach (Prunus persica) | Antimicrobial, antioxidant activity. Odor acceptability up to 10 days (5 °C) | [78] |
5.2. Pectin Coatings in Pre-frying Treatments
5.3. Pectin Coating as Pre-drying Treatment
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Advancing Sustainable Materials Magement: Facts and Figures 2013. Available online: http://www.epa.gov/wastes/nonhaz/municipal/pubs/2013_advncng_smm_rpt.pdf (accessed on 25 September 2015).
- Collection and transport. Waste Management World Homepage. Available online: http://www.waste-management-world.com/collection-transport (accessed on 25 September 2015).
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-lactic acid: Production, applications, nanocomposites, and release studies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 552–571. [Google Scholar] [CrossRef]
- Martucci, J.F.; Ruseckaite, R.A. Biodegradable three-layer film derived from bovine gelatin. J. Food Eng. 2010, 99, 377–383. [Google Scholar] [CrossRef]
- Kechichian, V.; Ditchfield, C.; Veiga-Santos, P.; Tadini, C.C. Natural antimicrobial ingredients incorporated in biodegradable films based on cassava starch. LWT-Food Sci. Technol. 2010, 43, 1088–1094. [Google Scholar] [CrossRef]
- Arzu, A.B.; Tulay, O.; Oya, I.S.; Lutfiye, Y.E. The utilisation of microbial poly-hydroxy alkanoates (PHA) in food industry. Res. J. Biotechnol. 2010, 5, 76–79. [Google Scholar]
- Ahmed, J.; Varshney, S.K. Polylactides—Chemistry, properties and green packaging technology: A review. Int. J. Food Prop. 2011, 14, 37–58. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef] [PubMed]
- Anuradha, K.; Padma, P.N.; Venkateshwar, S.; Reddy, G. Fungal isolates from natural pectic substrates for polygalacturonase and multienzyme production. Indian J. Microbiol. 2010, 50, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Lopes da Silva, J.A.; Rao, M.A. Food Polysaccharides and Their Applications, 2nd ed.; Taylor & Francis: Abingdon, UK, 2006. [Google Scholar]
- Kohli, P.; Gupta, R. Alkaline pectinases: A review. Biocatal. Agric. Biotechnol. 2015, 4, 279–285. [Google Scholar] [CrossRef]
- Pedrolli, D.B.; Monteiro, A.C.; Gomes, E.; Carmona, E.C. Pectin and pectinases: Production, characterization and industrial application of microbial pectinolytic enzymes. Open Biotechnol. J. 2009, 3, 9–18. [Google Scholar] [CrossRef]
- Ridley, B.L.; O’Neill, M.A.; Mohnen, D. Pectins: Structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 2001, 57, 929–967. [Google Scholar] [CrossRef]
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef] [PubMed]
- Ovodov, Y.S. Current views on pectin substances. Russ. J. Bioorg. Chem. 2009, 35, 269–284. [Google Scholar] [CrossRef]
- Hua, X.; Wang, K.; Yang, R.; Kang, J.; Yang, H. Edible coatings from sunflower head pectin to reduce lipid uptake in fried potato chips. LWT-Food Sci. Technol. 2015, 62, 1220–1225. [Google Scholar] [CrossRef]
- Ciolacu, L.; Nicolau, A.I.; Hoorfar, J. Global Safety of Fresh Produce. A Handbook of Best Practice, Innovative Commercial Solutions and Case Studies; Woodhead Publishing Limited: Sawston, UK, 2014. [Google Scholar]
- Dhanapal, A.; Sasikala, P.; Rajamani, L.; Kavitha, V.; Yazhini, G. Edible films from polysaccharides. Food Sci. Qual. Manag. 2012, 3, 9–18. [Google Scholar]
- Giovanetti, M.H.; Nogueira, A.; de Oliveira, C.L.; Wosiacki, G. Chromatography—The Most Versatile Method of Chemical Analysis; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Sundari, N. Extraction of pectin from waste peels: A review. Res. J. Pharm. Biol. 2015, 6, 1841–1848. [Google Scholar]
- Alba, K.; Laws, A.P.; Kontogiorgos, V. Isolation and characterization of acetylated lm-pectins extracted from okra pods. Food Hydrocoll. 2015, 43, 726–735. [Google Scholar] [CrossRef]
- Maran, J.P. Statistical optimization of aqueous extraction of pectin from waste durian rinds. Int. J. Biol. Macromol. 2015, 73, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Geerkens, C.H.; Nagel, A.; Just, K.M.; Miller-Rostek, P.; Kammerer, D.R.; Schweiggert, R.M.; Carle, R. Mango pectin quality as influenced by cultivar, ripeness, peel particle size, blanching, drying, and irradiation. Food Hydrocoll. 2015, 51, 241–251. [Google Scholar] [CrossRef]
- Oliveira, T.Í.S.; Rosa, M.F.; Cavalcante, F.L.; Pereira, P.H.F.; Moates, G.K.; Wellner, N.; Mazzetto, S.E.; Waldron, K.W.; Azeredo, H.M.C. Optimization of pectin extraction from banana peels with citric acid by using response surface methodology. Food Chem. 2015, in press. [Google Scholar] [CrossRef]
- Castillo-Israel, K.A.T.; Baguio, S.F.; Diasanta, M.D.B.; Lizardo, R.C.M.; Dizon, E.I.; Mejico, M.I.F. Extraction and characterization of pectin from Saba banana [musa ‘saba’(musa acuminata x musa balbisiana)] peel wastes: A preliminary study. Int. Food Res. J. 2015, 22, 202–207. [Google Scholar]
- Liew, S.Q.; Chin, N.L.; Yusof, Y.A.; Cheok, C.Y. Citric acid extraction of pectin from tropical fruit peels of passion fruit, dragon fruit and soursop. J. Food Agric. Environ. 2015, 13, 45–51. [Google Scholar]
- Li, D.Q.; Du, G.M.; Jing, W.W.; Li, J.F.; Yan, J.Y.; Liu, Z.Y. Combined effects of independent variables on yield and protein content of pectin extracted from sugar beet pulp by citric acid. Carbohyd. Polym. 2015, 129, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Aldana, D.; Contreras-Esquivel, J.C.; Nevárez-Moorillón, G.V.; Aguilar, C.N. Characterization of edible films from pectic extracts and essential oil from mexican lime. CyTA J. Food. 2014, 13, 17–25. [Google Scholar] [CrossRef]
- Casas-Orozco, D.; Villa, A.L.; Bustamante, F.; González, L.M. Process development and simulation of pectin extraction from orange peels. Food Bioprod. Process. 2015, 96, 86–98. [Google Scholar] [CrossRef]
- Korish, M. Potential utilization of citrullus lanatus var. Colocynthoides waste as a novel source of pectin. J. Food Sci. Technol. 2015, 52, 2401–2407. [Google Scholar] [CrossRef] [PubMed]
- Korish, M. Faba bean hulls as a potential source of pectin. J. Food Sci. Technol. 2015, 52, 6061–6066. [Google Scholar] [CrossRef] [PubMed]
- Denman, L.J.; Morris, G.A. An experimental design approach to the chemical characterisation ofpectin polysaccharides extracted from cucumis melo inodorus. Carbohydr. Polym. 2015, 117, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Grassino, A.N.; Halambek, J.; Djaković, S.; Rimac Brnčić, S.; Dent, M.; Grabarić, Z. Utilization of tomato peel waste from canning factory as a potential source for pectin production and application as tin corrosion inhibitor. Food Hydrocoll. 2016, 52, 265–274. [Google Scholar] [CrossRef]
- Kang, J.; Hua, X.; Yang, R.; Chen, Y.; Yang, H. Characterization of natural low-methoxyl pectin from sunflower head extracted by sodium citrate and purified by ultrafiltration. Food Chem. 2015, 180, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Giosafatto, C.V.L.; di Pierro, P.; Gunning, P.; Mackie, A.; Porta, R.; Mariniello, L. Characterization of citrus pectin edible films containing transglutaminase-modified phaseolin. Carbohydr. Polym. 2014, 106, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Giosafatto, C.V.L.; Di Pierro, P.; Gunning, A.P.; Mackie, A.; Porta, R.; Mariniello, L. Trehalose-containing hydrocolloid edible films prepared in the presence of transglutaminase. Biopolymers 2014, 101, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Porta, R.; Mariniello, L.; di Pierro, P.; Sorrentino, A.; Giosafatto, C.V.L. Transglutaminase crosslinked pectin- and chitosan-based edible films: A review. Crit. Rev. Food Sci. Nutr. 2011, 51, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, P.; Sorrentino, A.; Mariniello, L.; Giosafatto, C.V.L.; Porta, R. Chitosan/whey protein film as active coating to extend ricotta cheese shelf-life. LWT Food Sci. Technol. 2011, 44, 2324–2327. [Google Scholar] [CrossRef]
- Rossi Marquez, G.; di Pierro, P.; Esposito, M.; Mariniello, L.; Porta, R. Application of transglutaminase-crosslinked whey protein/pectin films as water barrier coatings in fried and baked foods. Food Bioprocess Technol. 2014, 7, 447–455. [Google Scholar] [CrossRef]
- Thirugnanasambandham, K.; Sivakumar, V.; Prakash Maran, J. Process optimization and analysis of microwave assisted extraction of pectin from dragon fruit peel. Carbohyd. Polym. 2014, 112, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, S.; Abdullah, A.; Momeny, E.; Kang, O.L. Optimization studies on microwave assisted extraction of dragon fruit (Hylocereus polyrhizus) peel pectin using response surface methodology. Int. Food Res. J. 2015, 22, 233–239. [Google Scholar]
- Quoc, L.P.T.; Huyen, V.T.N.; Hue, L.T.N.; Hue, N.T.H.; Thuan, N.H.D.; Tam, N.T.T.; Thuan, N.N.; Duy, T.H. Extraction of pectin from pomelo (Citrus maxima) peels with the assistance of microwave and tartaric acid. Int. Food Res. J. 2015, 22, 1637–1641. [Google Scholar]
- Maran, J.P.; Swathi, K.; Jeevitha, P.; Jayalakshmi, J.; Ashvini, G. Microwave-assisted extraction of pectic polysaccharide from waste mango peel. Carbohyd. Polym. 2015, 123, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Maran, J.P.; Prakash, K.A. Process variables influence on microwave assisted extraction of pectin from waste Carcia papaya L. peel. Int. J. Biol. Macromol. 2015, 73, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Zouambia, Y.; Youcef Ettoumi, K.; Krea, M.; Moulai-Mostefa, N. A new approach for pectin extraction: Electromagnetic induction heating. Arabian J. Chem. 2014. [Google Scholar] [CrossRef]
- Moorthy, I.G.; Maran, J.P.; Surya, S.M.; Naganyashree, S.; Shivamathi, C.S. Response surface optimization of ultrasound assisted extraction of pectin from pomegranate peel. Int. J. Biol. Macromol. 2015, 72, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Maran, J.P.; Priya, B. Ultrasound-assisted extraction of pectin from sisal waste. Carbohyd. Polym. 2015, 115, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Fu, X.; Luo, Z.G. Properties and extraction of pectin-enriched materials from sugar beet pulp by ultrasonic-assisted treatment combined with subcritical water. Food Chem. 2015, 168, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ma, X.; Xu, Y.; Cao, Y.; Jiang, Z.; Ding, T.; Ye, X.; Liu, D. Ultrasound-assisted heating extraction of pectin from grapefruit peel: Optimization and comparison with the conventional method. Food Chem. 2015, 178, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Yuliarti, O.; Matia-Merino, L.; Goh, K.K.T.; Mawson, J.; Williams, M.A.K.; Brennan, C. Characterization of gold kiwifruit pectin from fruit of different maturities and extraction methods. Food Chem. 2015, 166, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Yuliarti, O.; Goh, K.K.T.; Matia-Merino, L.; Mawson, J.; Brennan, C. Extraction and characterisation of pomace pectin from gold kiwifruit (actinidia chinensis). Food Chem. 2015, 187, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Valdés, A.; Vidal, L.; Beltrán, A.; Canals, A.; Garrigós, M.C. Microwave-assisted extraction of phenolic compounds from almond skin byproducts (prunus amygdalus): A multivariate analysis approach. J. Agric. Food Chem. 2015, 63, 5395–5402. [Google Scholar] [CrossRef] [PubMed]
- Ballard, T.S.; Mallikarjunan, P.; Zhou, K.; O’Keefe, S. Microwave-assisted extraction of phenolic antioxidant compounds from peanut skins. Food Chem. 2010, 120, 1185–1192. [Google Scholar] [CrossRef]
- Song, J.; Li, D.; Liu, C.; Zhang, Y. Optimized microwave-assisted extraction of total phenolics (TP) from ipomoea batatas leaves and its antioxidant activity. Innov. Food Sci. Emerg. Technol. 2011, 12, 282–287. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Falguera, V.; Quintero, J.P.; Jiménez, A.; Muñoz, J.A.; Ibarz, A. Edible films and coatings: Structures, active functions and trends in their use. Trends Food Sci. Technol. 2011, 22, 292–303. [Google Scholar] [CrossRef]
- Sánchez-Ortega, I.; García-Almendárez, B.E.; Santos-López, E.M.; Amaro-Reyes, A.; Barboza-Corona, J.E.; Regalado, C. Antimicrobial edible films and coatings for meat and meat products preservation. Sci. World J. 2014. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Graü, M.A.; Soliva-Fortuny, R.; Martín-Belloso, O. Edible coatings to incorporate active ingredients to fresh-cut fruits: A review. Trends Food Sci. Technol. 2009, 20, 438–447. [Google Scholar] [CrossRef]
- Rojas, M.A. Use of edible coatings for fresh-cut fruits and vegetables. In Advances in Fresh-cut Fruits and Vegetables Processing; CRC Press: Boca Raton, FL, USA, 2010; pp. 285–311. [Google Scholar]
- Espitia, P.J.P.; Du, W.-X.; Avena-Bustillos, R.d.J.; Soares, N.d.F.F.; McHugh, T.H. Edible films from pectin: Physical-mechanical and antimicrobial properties—A review. Food Hydrocolloid 2014, 35, 287–296. [Google Scholar] [CrossRef]
- Martín-Diana, A.B.; Rico, D.; Frías, J.M.; Barat, J.M.; Henehan, G.T.M.; Barry-Ryan, C. Calcium for extending the shelf life of fresh whole and minimally processed fruits and vegetables: A review. Trends Food Sci. Technol. 2007, 18, 210–218. [Google Scholar] [CrossRef]
- Ramirez, M.E.; Timón, M.L.; Petrón, M.J.; Andrés, A.I. Effect of chitosan, pectin and sodium caseinate edible coatings on shelf life of fresh-cut Prunus persica var. Nectarine. J. Food Process. Preserv. 2015. [Google Scholar] [CrossRef]
- Rico, D.; Martín-Diana, A.B.; Barat, J.M.; Barry-Ryan, C. Extending and measuring the quality of fresh-cut fruit and vegetables: A review. Trends Food Sci. Technol. 2007, 18, 373–386. [Google Scholar] [CrossRef]
- Zhang, Y.; Rempel, C.; McLaren, D. Edible coating and film materials: Carbohydrates. In Innovations in Food Packaging, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 305–323. [Google Scholar]
- Ciolacu, L.; Nicolau, A.I.; Hoorfar, J. Edible coatings for fresh and minimally processed fruits and vegetables. In Global Safety of Fresh Produce; Hoorfar, J., Ed.; Woodhead Publishing: Sawston, UK, 2014; pp. 233–244. [Google Scholar]
- Oms-Oliu, G.; Soliva-Fortuny, R.; Martín-Belloso, O. Using polysaccharide-based edible coatings to enhance quality and antioxidant properties of fresh-cut melon. LWT Food Sci. Technol. 2008, 41, 1862–1870. [Google Scholar] [CrossRef]
- Moalemiyan, M.; Ramaswamy, H.S.; Maftoonazad, N. Pectin-based edible coating for shelf-life extension of ataulfo mango. J. Food Process Eng. 2012, 35, 572–600. [Google Scholar] [CrossRef]
- Maftoonazad, N.; Ramaswamy, H.S. Effect of pectin-based coating on the kinetics of quality change associated with stored avocados. J. Food Process. Preserv. 2008, 32, 621–643. [Google Scholar] [CrossRef]
- Ferrari, C.C.; Sarantópoulos, C.I.G.L.; Carmello-Guerreiro, S.M.; Hubinger, M.D. Effect of osmotic dehydration and pectin edible coatings on quality and shelf life of fresh-cut melon. Food Bioprocess Technol. 2013, 6, 80–91. [Google Scholar] [CrossRef]
- Sipahi, R.E.; Castell-Perez, M.E.; Moreira, R.G.; Gomes, C.; Castillo, A. Improved multilayered antimicrobial alginate-based edible coating extends the shelf life of fresh-cut watermelon (citrullus lanatus). LWT Food Sci. Technol. 2013, 51, 9–15. [Google Scholar] [CrossRef]
- Martiñon, M.E.; Moreira, R.G.; Castell-Perez, M.E.; Gomes, C. Development of a multilayered antimicrobial edible coating for shelf-life extension of fresh-cut cantaloupe (Cucumis melo L.) stored at 4 °C. LWT-Food Sci. Technol. 2014, 56, 341–350. [Google Scholar] [CrossRef]
- Brasil, I.M.; Gomes, C.; Puerta-Gomez, A.; Castell-Perez, M.E.; Moreira, R.G. Polysaccharide-based multilayered antimicrobial edible coating enhances quality of fresh-cut papaya. LWT-Food Sci. Technol. 2012, 47, 39–45. [Google Scholar] [CrossRef]
- Oms-Oliu, G.; Soliva-Fortuny, R.; Martín-Belloso, O. Edible coatings with antibrowning agents to maintain sensory quality and antioxidant properties of fresh-cut pears. Postharvest Biol. Technol. 2008, 50, 87–94. [Google Scholar] [CrossRef]
- Treviño-Garza, M.Z.; García, S.; Flores-González, M.S.; Arévalo-Niño, K. Edible active coatings based on pectin, pullulan, and chitosan increase quality and shelf life of strawberries (Fragaria ananassa). J. Food Sci. 2015, 80, M1823–M1830. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.R.; Cassani, L.; Martín-Belloso, O.; Soliva-Fortuny, R. Effects of polysaccharide-based edible coatings enriched with dietary fiber on quality attributes of fresh-cut apples. J. Food Sci. Technol. 2015. [Google Scholar] [CrossRef]
- Guerreiro, A.C.; Gago, C.M.L.; Faleiro, M.L.; Miguel, M.G.C.; Antunes, M.D.C. The use of polysaccharide-based edible coatings enriched with essential oils to improve shelf-life of strawberries. Postharvest Biol. Technol. 2015, 110, 51–60. [Google Scholar] [CrossRef]
- Guerreiro, A.C.; Gago, C.M.L.; Faleiro, M.L.; Miguel, M.G.C.; Antunes, M.D.C. Raspberry fresh fruit quality as affected by pectin- and alginate-based edible coatings enriched with essential oils. Sci. Hortic. 2015, 194, 138–146. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Silva-Espinoza, B.A.; Cruz-Valenzuela, M.R.; Leyva, J.M.; Ortega-Ramírez, L.A.; Carrazco-Lugo, D.K.; Pérez-Carlón, J.J.; Melgarejo-Flores, B.G.; González-Aguilar, G.A.; Miranda, M.R.A. Pectin–cinnamon leaf oil coatings add antioxidant and antibacterial properties to fresh-cut peach. Flavour Fragr. J. 2013, 28, 39–45. [Google Scholar] [CrossRef]
- Valdes, A.; Mellinas, A.C.; Ramos, M.; Burgos, N.; Jimenez, A.; Garrigos, M.C. Use of herbs, spices and their bioactive compounds in active food packaging. RSC Adv. 2015, 5, 40324–40335. [Google Scholar] [CrossRef]
- Mellinas, C.; Valdés, A.; Ramos, M.; Burgos, N.; del Carmen Garrigós, M.; Jiménez, A. Active edible films: Current state and future trends. J. Appl. Polym. Sci. 2015. [Google Scholar] [CrossRef]
- Daraei Garmakhany, A.; Mirzaei, H.O.; Maghsudlo, Y.; Kashaninejad, M.; Jafari, S.M. Production of low fat french-fries with single and multi-layer hydrocolloid coatings. J. Food Sci. Technol. 2014, 51, 1334–1341. [Google Scholar] [CrossRef] [PubMed]
- Suyatma, N.E.; Ulfah, K.; Prangdimurti, E.; Ishikawa, Y. Effect of blanching and pectin coating as pre-frying treatments to reduce acrylamide formation in banana chips. Int. Food Res. J. 2015, 22, 936–942. [Google Scholar]
- Oliveira, S.M.; Brandão, T.R.S.; Silva, C.L.M. Influence of drying processes and pretreatments on nutritional and bioactive characteristics of dried vegetables: A review. Food Eng. Rev. 2015. [Google Scholar] [CrossRef]
- Garcia, C.C.; Caetano, L.C.; de Souza Silva, K.; Mauro, M.A. Influence of edible coating on the drying and quality of papaya (Carica papaya). Food Bioprocess Technol. 2014, 7, 2828–2839. [Google Scholar] [CrossRef]
- Silva, K.S.; Garcia, C.C.; Amado, L.R.; Mauro, M.A. Effects of edible coatings on convective drying and characteristics of the dried pineapple. Food Bioprocess Technol. 2015, 8, 1465–1475. [Google Scholar] [CrossRef]
- Canizares, D.; Mauro, M.A. Enhancement of quality and stability of dried papaya by pectin-based coatings as air-drying pretreatment. Food Bioprocess Technol. 2015, 8, 1187–1197. [Google Scholar] [CrossRef]
- Akbarian, M.; Ghanbarzadeh, B.; Sowti, M.; Dehghannya, J. Effects of pectin-CMC-based coating and osmotic dehydration pretreatments on microstructure and texture of the hot-air dried quince slices. J. Food Process. Preserv. 2015, 39, 260–269. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdés, A.; Burgos, N.; Jiménez, A.; Garrigós, M.C. Natural Pectin Polysaccharides as Edible Coatings. Coatings 2015, 5, 865-886. https://doi.org/10.3390/coatings5040865
Valdés A, Burgos N, Jiménez A, Garrigós MC. Natural Pectin Polysaccharides as Edible Coatings. Coatings. 2015; 5(4):865-886. https://doi.org/10.3390/coatings5040865
Chicago/Turabian StyleValdés, Arantzazu, Nuria Burgos, Alfonso Jiménez, and María Carmen Garrigós. 2015. "Natural Pectin Polysaccharides as Edible Coatings" Coatings 5, no. 4: 865-886. https://doi.org/10.3390/coatings5040865
APA StyleValdés, A., Burgos, N., Jiménez, A., & Garrigós, M. C. (2015). Natural Pectin Polysaccharides as Edible Coatings. Coatings, 5(4), 865-886. https://doi.org/10.3390/coatings5040865