Spray-on Thin Film PV Solar Cells: Advances, Potentials and Challenges
Abstract
:1. Introduction
2. Recent Advances
2.1. Polymer Solar Cells
2.1.1. Principle of Operation
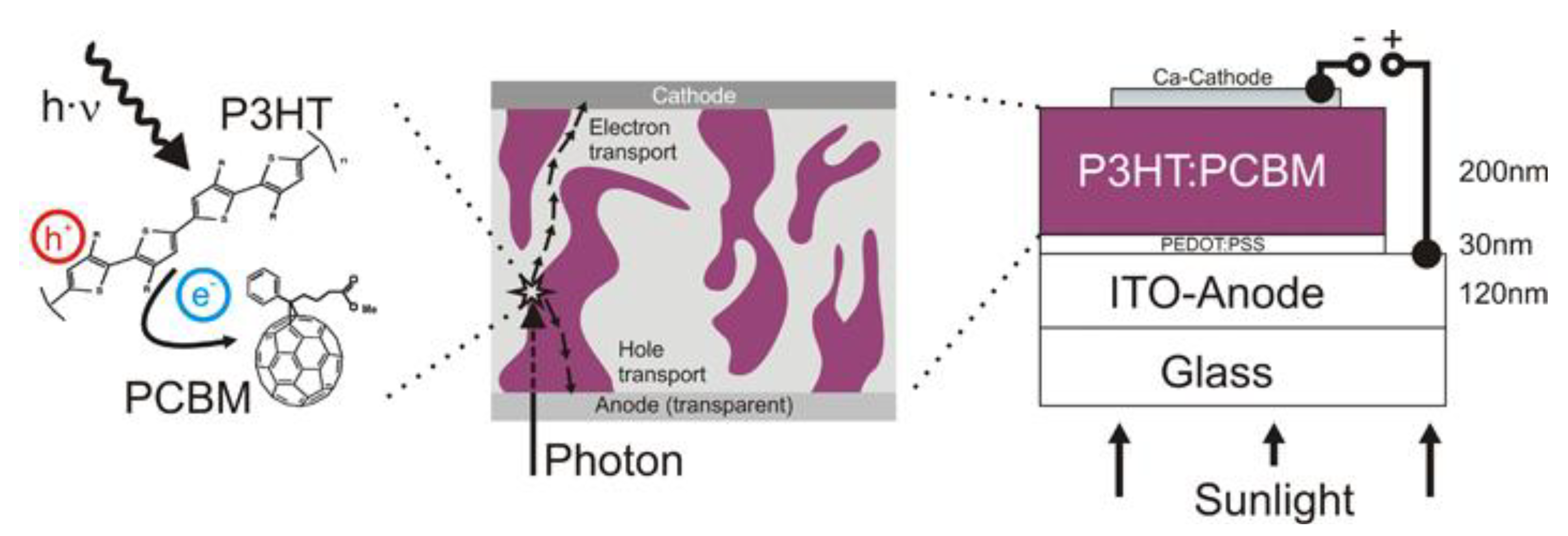
2.1.2. Recent Advances on Spray-on Polymer Solar Cells
2.2. Dye-Sensitized Solar Cells (DSSCs)
2.2.1. Principle of Operation of DSSCs
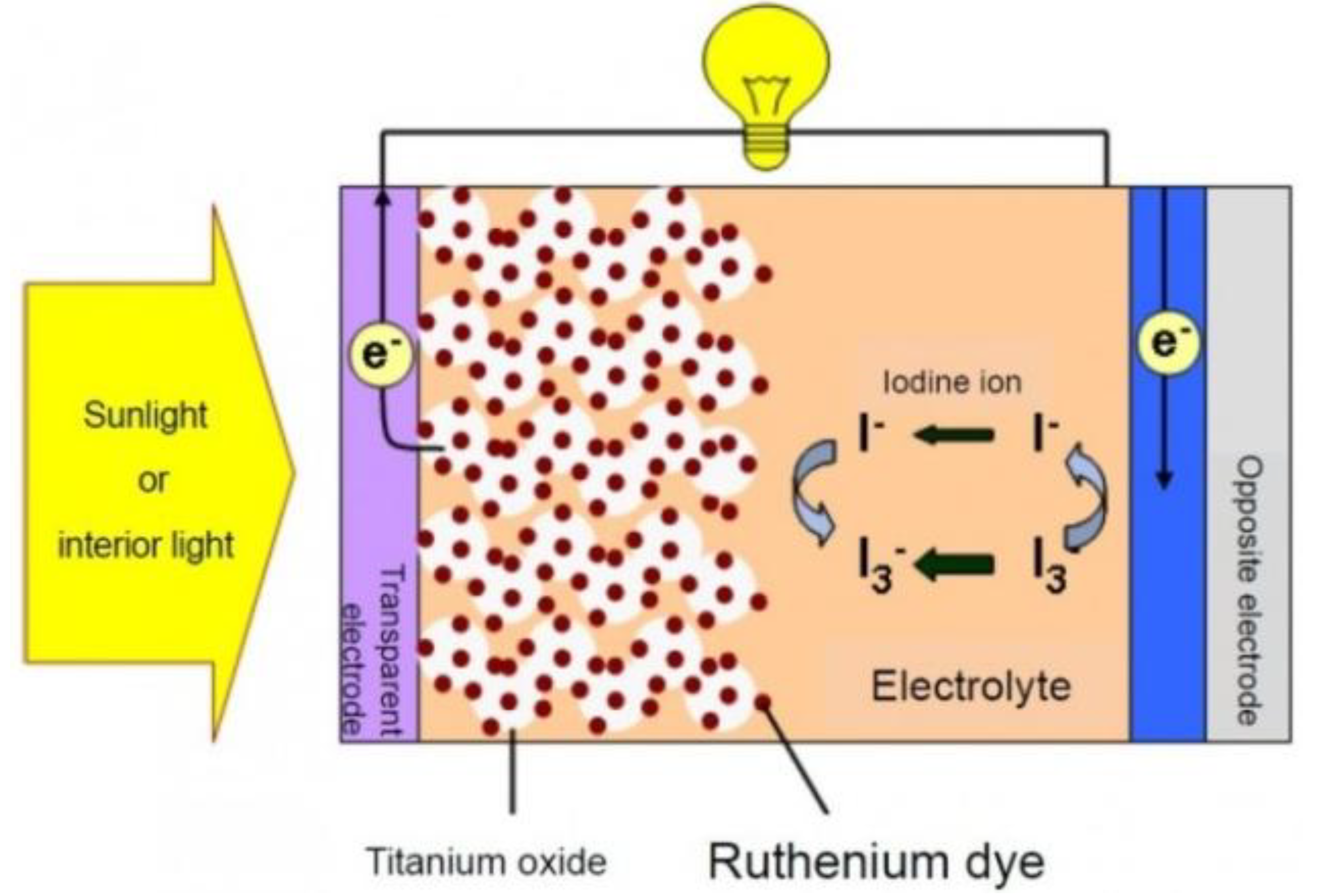
2.2.2. Recent Advances on Spray-on DSSCs
2.3. Quantum Dot Solar Cells (QDSC)
2.3.1. Principle of Operation
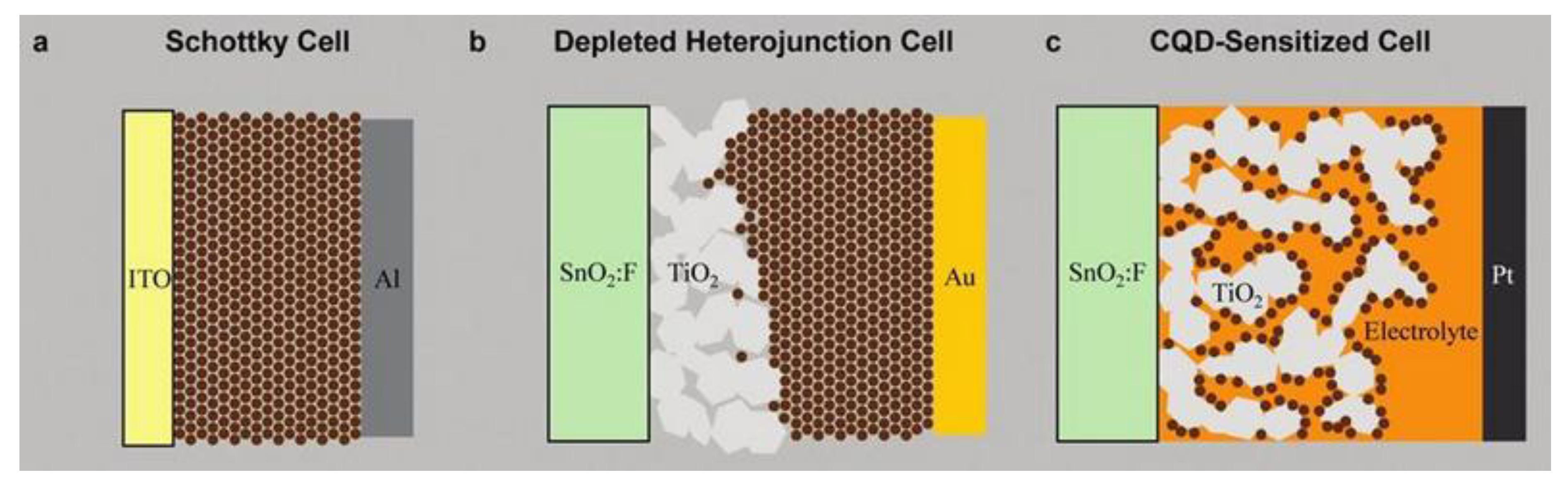
2.3.2. Recent Advances on Spray-on QDSCs
2.4. Other Thin Film Solar Cells
3. Dynamics of Droplet Impact on a Dry Surface
3.1. Droplet Impact on a Cold Surface
3.2. Droplet Impact on a Hot Surface
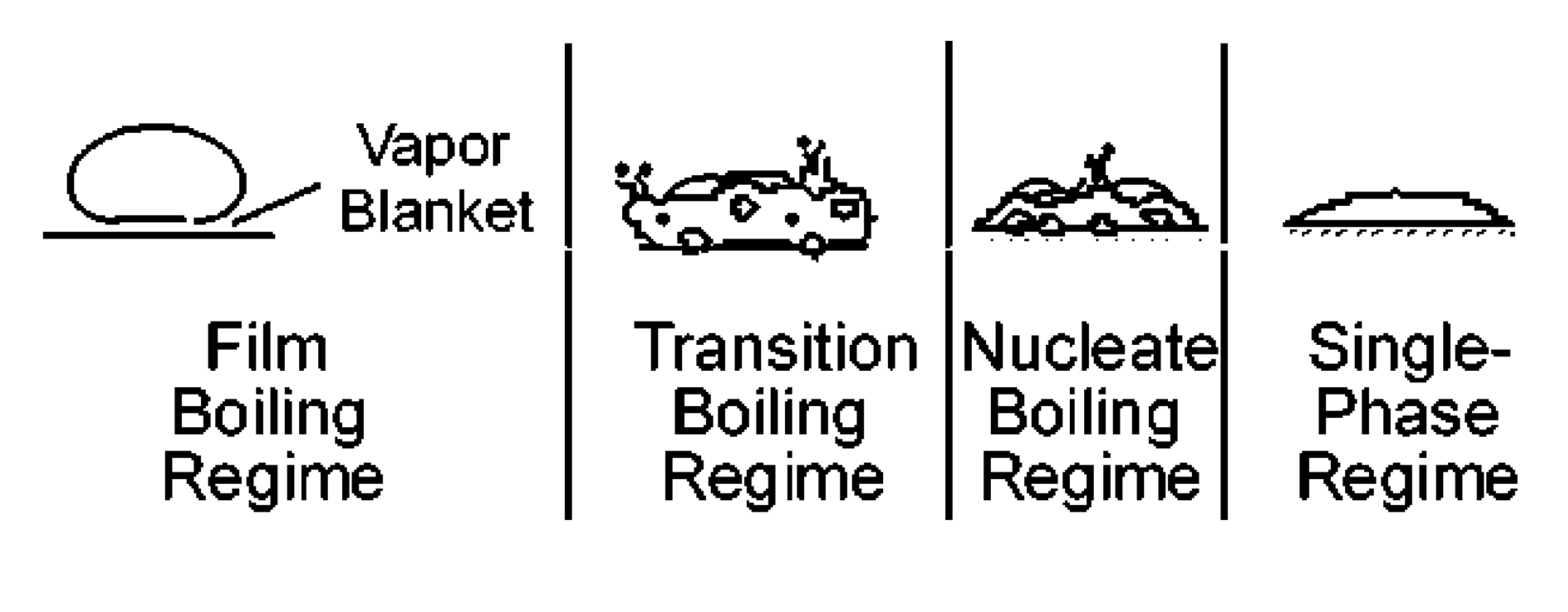
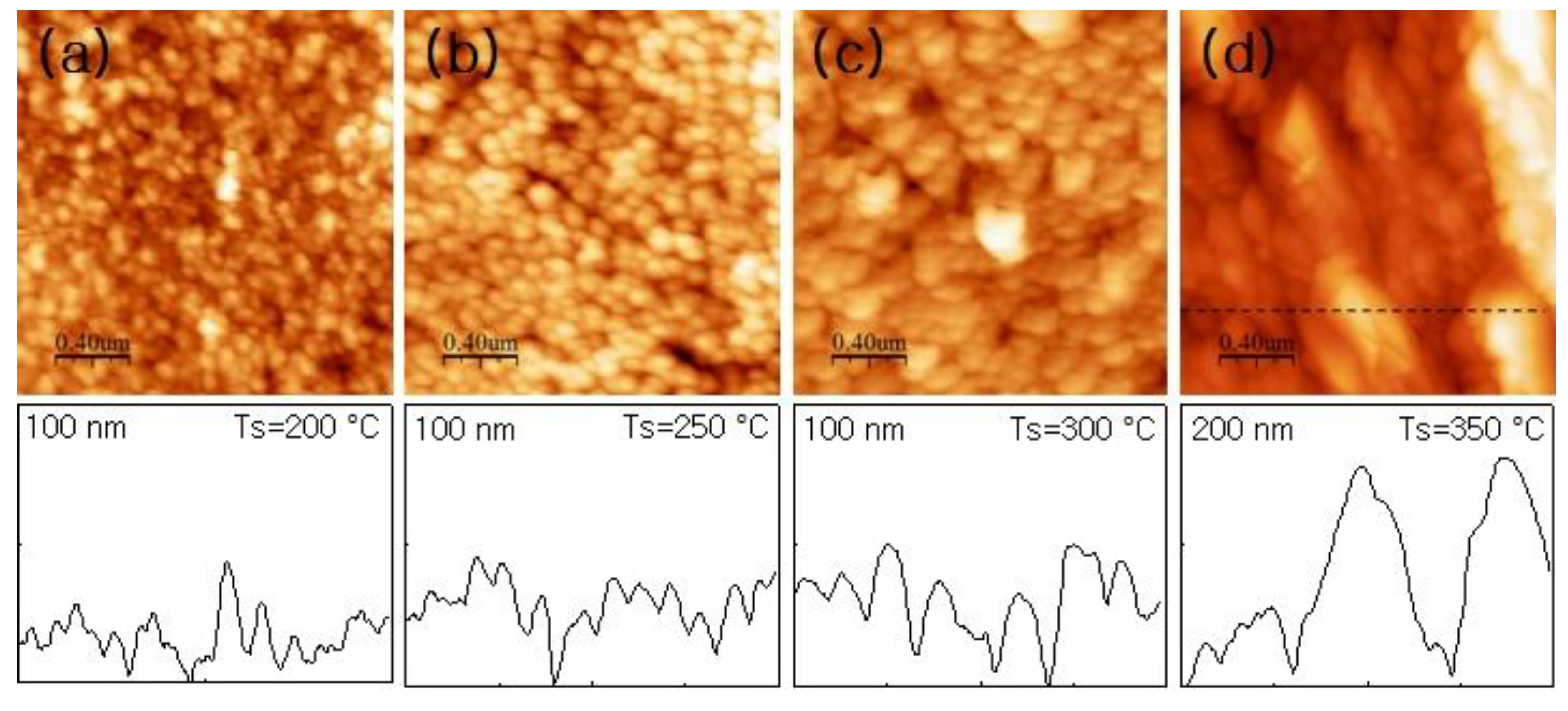

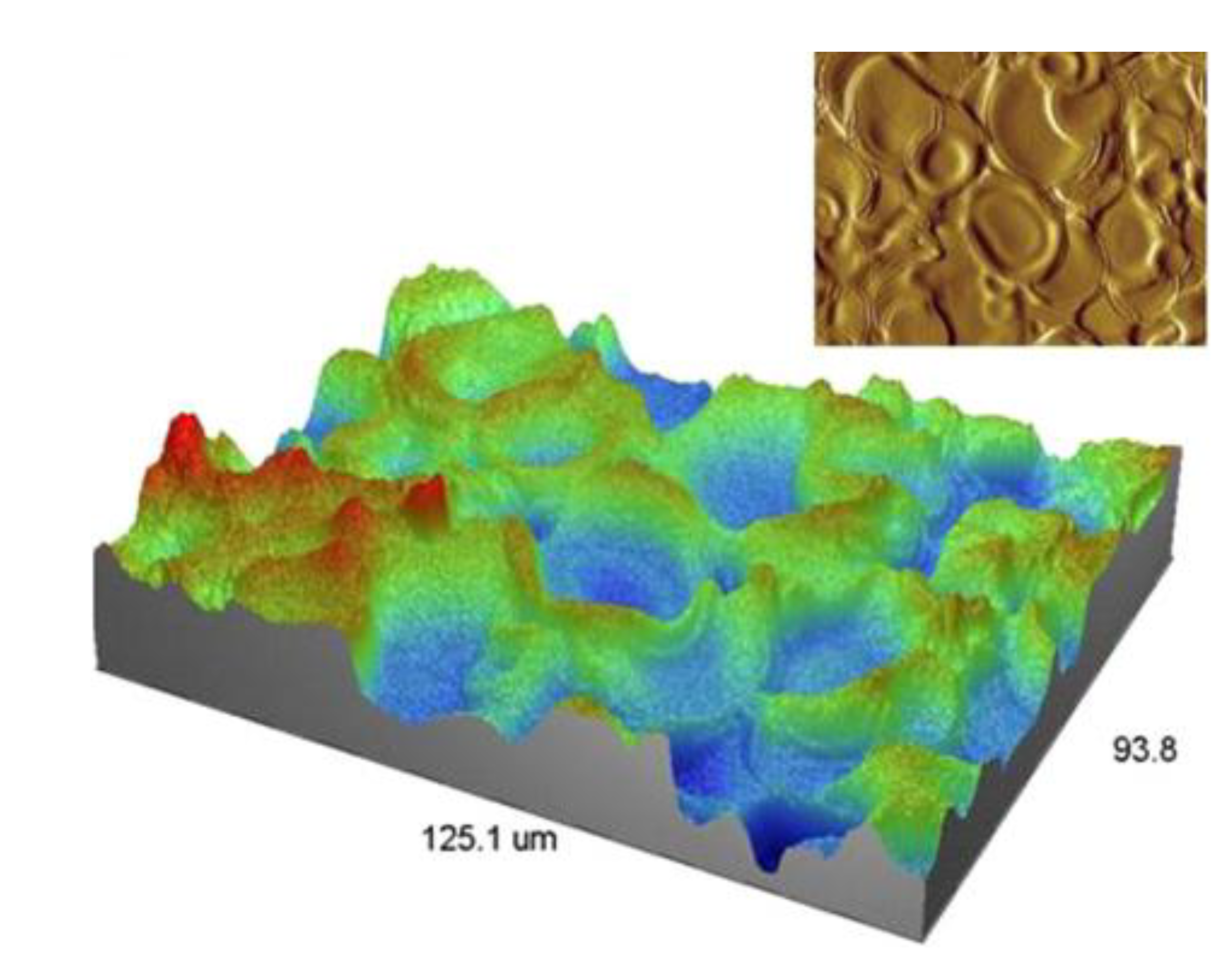
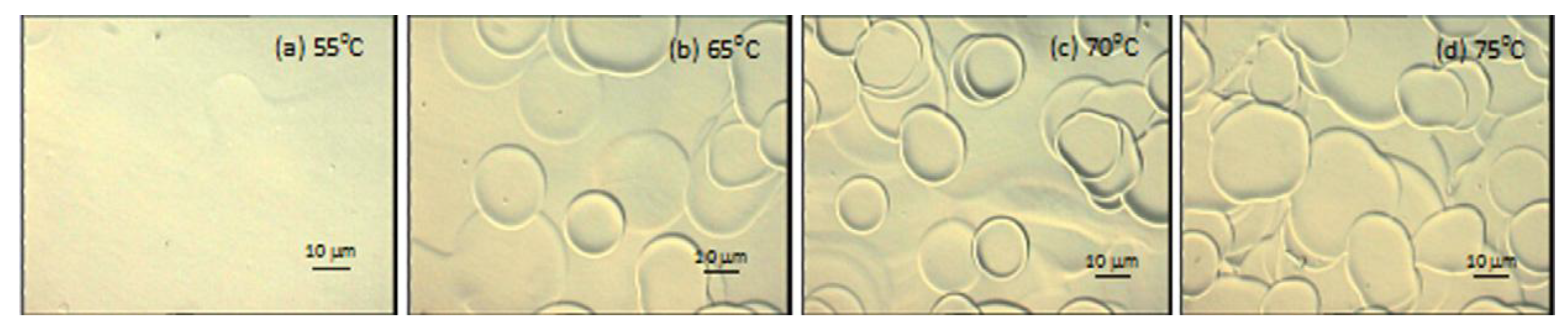
3.3. Droplet Interaction
4. Photo-Induced Electron Transfer
5. Other Challenges
5.1. Characterization Techniques
5.2. Numerical Simulation of Spray Coating
6. Commercialization of Spray-on Solar Cells
7. Conclusions

Acknowledgments
Conflicts of Interest
References
- Krebs, F.C. Fabrication and processing of polymer solar cells: A review of printing and coating techniques. Sol. Energy Mater. Sol. Cells 2009, 93, 394–412. [Google Scholar] [CrossRef]
- Søndergaard, R.R.; Hosel, M.; Krebs, F.C. Roll-to-roll fabrication of large area functional organic materials. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 16–34. [Google Scholar] [CrossRef]
- Wengeler, L.; Schmitt, M.; Peters, K.; Scharfer, P.; Schabel, W. Comparison of large scale coating techniques for organic and hybrid films in polymer based solar cells. Chem. Eng. Process. 2013, 68, 38–44. [Google Scholar] [CrossRef]
- Smilgies, D.M.; Li, R.; Giri, G.; Chou, K.W.; Diao, Y.; Bao, Z.; Amassian, A. Crystallization of conjugated molecules during solution shearing probed in-situ and in real time by X-ray scattering. Phys. Status Solidi 2013, 7, 177–179. [Google Scholar]
- Galagan, Y.; Coenen, E.W.C.; Sabik, S.; Gorter, H.H.; Barink, M.; Veenstra, S.C.; Kroon, J.M.; Andriessen, R.; Blom, P.W.M. Evaluation of ink-jet printed current collecting grids and busbars for ITO-free organic solar cells. Sol. Energy Mater. Sol. Cells 2012, 104, 32–38. [Google Scholar] [CrossRef]
- Cook, S.; Katoh, R.; Furube, A. Ultrafast studies of charge generation in PCBM:P3HT blend films following excitation of the fullerene PCBM. J. Phys. Chem. C 2009, 113, 2547–2552. [Google Scholar] [CrossRef]
- Structure of a polymer solar cell. Institut für Thermische Verfahrenstechnik, Germany. Available online: https://www.tvt.kit.edu/504_1073.php (accessed on 11 January 2014).
- Chilvery, A.K.; Batra, A.K.; Guggilla, P.; Lal, R.B.; Surabhi, R. A versatile technique for the fabrication of PEDOT:PSS films for organic solar cells. Energy Sci. Technol. 2012, 4, 6–11. [Google Scholar]
- Kim, K.-J.; Kim, Y.-S.; Kang, W.-S.; Kang, B.-H.; Yeom, S.-H.; Kim, D.-E.; Kim, J.-H.; Kang, S.-W. Inspection of substrate-heated modified PEDOT:PSS morphology for all spray deposited organic photovoltaics. Sol. Energy Mater. Sol. Cells 2010, 94, 1303–1306. [Google Scholar] [CrossRef]
- Weickert, J.; Sun, H.; Palumbiny, C.; Hesse, H.C.; Schmidt-Mende, L. Spray-deposited PEDOT:PSS for inverted organic solar cells. Sol. Energy Mater. Sol. Cells 2010, 94, 2371–2374. [Google Scholar] [CrossRef]
- Steirer, K.X.; Berry, J.J.; Reese, M.O.; van Hest, M.F.A.M.; Miedaner, A.; Liberatore, M.W.; Collins, R.T.; Ginley, D.S. Ultrasonically sprayed and inkjet printed thin film electrodes for organic solar cells. Thin Solid Films 2009, 517, 2781–2786. [Google Scholar] [CrossRef]
- Lee, J.-H.; Sagawa, T.; Yoshikawa, S. Morphological and topographical characterizations in spray coated organic solar cells using an additional solvent spray deposition. Org. Electron. 2011, 12, 2165–2173. [Google Scholar] [CrossRef]
- Lee, J.-H.; Sagawa, T.; Yoshikawa, S. Thickness dependence of photovoltaic performance of additional spray coated solar cells. Thin Solid Films 2013, 529, 464–469. [Google Scholar] [CrossRef]
- Lewis, J.E.; Lafalce, E.; Toglia, P.; Jiang, X. Over 30% transparency large area inverted organic solar array by spray. Sol. Energy Mater. Sol. Cells 2011, 95, 2816–2822. [Google Scholar] [CrossRef]
- Girotto, C.; Moia1, D.; Rand, B.P.; Aernouts, T.; Heremans, P. Efficient Polymer Solar Cells via an All-Spray-Coated Deposition. In Proceedings of the 35th IEEE Photovoltaic Specialists Conference (PVSC), Honolulu, HI, USA, 20–25 June 2010; pp. 001068–001072.
- Girotto, C.; Moia, D.; Rand, B.P.; Heremans, P. High-performance organic solar cells with spray-coated hole-transport and active layers. Adv. Funct. Mater. 2011, 21, 64–72. [Google Scholar] [CrossRef]
- Girotto, C.; Rand, B.P.; Genoe, J.; Heremans, P. Exploring spray coating as a deposition technique for the fabrication of solution-processed solar cells. Sol. Energy Mater. Sol. Cells 2009, 93, 454–458. [Google Scholar] [CrossRef]
- Tait, J.G.; Rand, B.P.; Heremans, P. Concurrently pumped ultrasonic spray coating for donor:acceptor and thickness optimization of organic solar cells. Org. Electron. 2013, 14, 1002–1008. [Google Scholar] [CrossRef]
- Chen, L.-M.; Hong, Z.; Kwan, W.L.; Lu, C.-H.; Lai, Y.-F.; Lei, B.; Liu, C.-P.; Yang, Y. Multi-source/component spray coating for polymer solar cells. ACS Nano 2010, 4, 4744–4752. [Google Scholar] [CrossRef]
- Peh, R.J.; Lu, Y.; Zhao, F.; Lee, C.-L.K.; Kwan, W.L. Vacuum-free processed transparent inverted organic solar cells with spray-coated PEDOT:PSS anode. Sol. Energy Mater. Sol. Cells 2011, 95, 3579–3584. [Google Scholar] [CrossRef]
- Ali, M.; Abbas, M.; Shah, S.K.; Tuerhong, R.; Generosi, A.; Paci, B.; Hirsch, L.; Gunnella, R. Realization of solution processed multi-layer bulk heterojunction organic solar cells by electro-spray deposition. Org. Electron. 2012, 13, 2130–2137. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, J.; Kang, H.; Kim, G.; Kim, N.; Lee, K. Controlled electro-spray deposition of highly conductive PEDOT:PSS films. Sol. Energy Mater. Sol. Cells 2012, 98, 39–45. [Google Scholar] [CrossRef]
- Duraisamy, N.; Muhammad, N.M.; Ali, A.; Jo, J.; Choi, K.-H. Characterization of poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) thin film deposited through electrohydrodynamic atomization technique. Mater. Lett. 2012, 83, 80–83. [Google Scholar] [CrossRef]
- Muhammad, N.M.; Naeem, A.M.; Duraisamy, N.; Kim, D.-S.; Choi, K.-H. Fabrication of high quality zinc-oxide layers through electrohydrodynamic atomization. Thin Solid Films 2012, 520, 1751–1756. [Google Scholar] [CrossRef]
- Choi, K.-H.; Duraisamy, N.; Muhammad, N.M.; Kim, I.; Choi, H.; Jo, J. Structural and optical properties of electrohydrodynamically atomized TiO2 nanostructured thin films. Appl. Phys. A 2012, 107, 715–722. [Google Scholar] [CrossRef]
- Hau, S.K.; Yip, H.-L.; Leong, K.; Jen, A.K.-Y. Spray coating of silver nanoparticle electrodes for inverted polymer solar cells. Org. Electron. 2009, 10, 719–723. [Google Scholar] [CrossRef]
- Po, R.; Carbonera, C.; Bernardi, A.; Tinti, F.; Camaioni, N. Polymer and carbon-based electrodes for polymer solar cells: Toward low-cost, continuous fabrication over large area. Sol. Energy Mater. Sol. Cells 2012, 100, 97–114. [Google Scholar] [CrossRef]
- Kim, S.; Yim, J.; Wang, X.; Bradley, D.D.C.; Lee, S.; deMello, J.C. Spin- and spray-deposited single-walled carbon-nanotube electrodes for organic solar cells. Adv. Funct. Mater. 2010, 20, 2310–2316. [Google Scholar] [CrossRef]
- Akter, T.; Kim, W.S. Reversibly stretchable transparent conductive coatings of spray-deposited silver nanowires. ACS Appl. Mater. Interfaces 2012, 4, 1855–1859. [Google Scholar]
- Perse, L.S.; Bizjak, A.; Orel, B. The role of rheological properties and spraying parameters on the spectral selectivity of Thickness Insensitive Spectrally Selective (TISS) paint coating. Sol. Energy Mater. Sol. Cells 2013, 110, 115–125. [Google Scholar] [CrossRef]
- Eslamian, M. A model for the fabrication of polymer solar cells by spray coating. Dry. Technol. 2013, 31, 405–413. [Google Scholar] [CrossRef]
- Yu, B.-K.; Vak, D.; Jo, J.; Na, S.-I.; Kim, S.-S.; Kim, M.-K.; Kim, D.-Y. Factors to be considered in bulk heterojunction polymer solar cells fabricated by the spray process. IEEE J. Sel. Top. Quantum Electron. 2010, 16, 1838–1846. [Google Scholar] [CrossRef]
- Lonakar, G.S.; Mahajan, M.S.; Ghosh, S.S.; Sali, J.V. Modeling thin film formation by Ultrasonic Spray method: A case of PEDOT:PSS thin films. Org. Electron. 2012, 13, 2575–2581. [Google Scholar] [CrossRef]
- Park, S.-Y.; Kang, Y.-J.; Lee, S.; Kim, D.-G.; Kim, J.-K.; Kim, J.-H.; Kang, J.-W. Spray-coated organic solar cells with large-area of 12.25 cm2. Sol. Energy Mater. Sol. Cells 2011, 95, 852–855. [Google Scholar] [CrossRef]
- Etxebarria, I.; Tait, J.G.; Gehlhaar, R.; Pacios, R.; Cheyns, D. Surface treatment patterning of organic photovoltaic films for low-cost modules. Org. Electron. 2013, 14, 430–435. [Google Scholar] [CrossRef]
- Susanna, G.; Salamandra, L.; Brown, T.M.; di Carlo, A.; Brunetti, F.; Reale, A. Airbrush spray-coating of polymer bulk-heterojunction solar cells. Sol. Energy Mater. Sol. Cells 2011, 95, 1775–1778. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, R.; Shi, W.; Guan, Z.; Yu, J. Effect of in situ annealing on the performance of spray coated polymer solar cells. Sol. Energy Mater. Sol. Cells 2013, 111, 200–205. [Google Scholar] [CrossRef]
- No, I.-J.; Shin, P.-K.; Kannappan, S.; Kumar, P.; Ochiai, S. Fabrication and characteristics of organic thin-film solar cells with active layer of interpenetrated hetero-junction structure. Appl. Phys. Res. 2012, 4, 83–90. [Google Scholar]
- Nie, W.; Coffin, R.C.; Liu, J.; Li, Y.; Peterson, E.D.; MacNeill, C.M.; Noftle, R.E.; Carroll, D.L. High efficiency organic solar cells with spray coated active layers comprised of a low band gap conjugated polymer. Appl. Phys. Lett. 2012, 100, 083301:1–083301:4. [Google Scholar]
- Kim, Y.; Kim, G.; Lee, J.; Lee, K. Morphology controlled bulk-heterojunction layers of fully electro-spray coated organic solar cells. Sol. Energy Mater. Sol. Cells 2012, 105, 272–279. [Google Scholar] [CrossRef]
- Eslamian, M.; Newton, J.E. Spray-on PEDOT:PSS and P3HT:PCBM thin films for polymer solar cells. Coatings 2014, 1. in press. [Google Scholar]
- Abdellah, A.; Virdi, K.S.; Meier, R.; Döblinger, M.; Müller-Buschbaum, P.; Scheu, C.; Lugli, P.; Scarpa, G. Successive spray deposition of P3HT/PCBM organic photoactive layers: Material composition and device characteristics. Adv. Funct. Mater. 2012, 22, 4078–4086. [Google Scholar] [CrossRef]
- Park, S.-E.; Hwang, J.-Y.; Kim, K.; Jung, B.; Kim, W.; Hwangn, J. Spray deposition of electrohydrodynamically atomized polymer mixture for active layer fabrication in organic photovoltaics. Sol. Energy Mater. Sol. Cells 2011, 95, 352–356. [Google Scholar] [CrossRef]
- Hoth, C.N.; Steim, R.; Schilinsky, P.; Choulis, S.A.; Tedde, S.F.; Hayden, O.; Brabec, C.J. Topographical and morphological aspects of spray coated organic photovoltaics. Org. Electron. 2009, 10, 587–593. [Google Scholar] [CrossRef]
- Steirer, K.X.; Reese, M.O.; Rupert, B.L.; Kopidakis, N.; Olson, D.C.; Collins, R.T.; Ginley, D.S. Ultrasonic spray deposition for production of organic solar cells. Sol. Energy Mater. Sol. Cells 2009, 93, 447–453. [Google Scholar] [CrossRef]
- Fan, S.-Q.; Li, C.-J.; Yang, G.-J.; Zhang, L.-Z.; Gao, J.-C.; Xi, Y.-X. Fabrication of nano-TiO2 coating for dye-sensitized solar cell by vacuum cold spraying at room temperature. J. Therm. Spray Technol. 2007, 16, 893–897. [Google Scholar] [CrossRef]
- Halme, J.; Saarinen, J.; Lund, P. Spray deposition and compression of TiO2 nanoparticle films for dye-sensitized solar cells on plastic substrates. Sol. Energy Mater. Sol. Cells 2006, 90, 887–899. [Google Scholar] [CrossRef]
- Hwang, D.; Lee, H.; Jang, S.-Y.; Jo, S.M.; Kim, D.; Seo, Y.; Kim, D.Y. Electrospray preparation of hierarchically-structured mesoporous TiO2 spheres for use in highly Efficient dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2011, 3, 2719–2725. [Google Scholar] [CrossRef]
- Lee, H.; Hwang, D.; Jo, S.M.; Kim, D.; Seo, Y.; Kim, D.Y. Low-temperature fabrication of TiO2 electrodes for flexible dye-sensitized solar cells using an electrospray process. ACS Appl. Mater. Interfaces 2012, 4, 3308–3315. [Google Scholar]
- Hong, J.-T.; Seo, H.; Lee, D.-G.; Jang, J.-J.; An, T.-P.; Kim, H.-J. A nano-porous TiO2 thin film coating method for dye sensitized solar cells (DSSCs) using electrostatic spraying with dye solution. J. Electrost. 2010, 68, 205–211. [Google Scholar] [CrossRef]
- Okuya, M.; Nakade, K.; Kaneko, S. Porous TiO2 thin films synthesized by a spray pyrolysis deposition (SPD) technique and their application to dye-sensitized solar cells. Sol. Energy Mater. Sol. Cells 2002, 70, 425–435. [Google Scholar] [CrossRef]
- Kovendhan, M.; Joseph, D.P.; Manimuthu, P.; Ganesan, S.; Sambasivam, S.; Maruthamuthu, P.; Suthanthiraraj, S.A.; Venkateswaran, C.; Mohan, R. Spray deposited Nb2O5 thin film electrodes for fabrication of dye sensitized solar cells. Trans. Indian Inst. Met. 2011, 64, 185–188. [Google Scholar] [CrossRef]
- Joseph, D.P.; Saravanan, M.; Muthuraaman, B.; Renugambal, P.; Sambasivam, S.; Raja, S.P.; Maruthamuthu, P.; Venkateswaran, C. Spray deposition and characterization of nanostructured Li doped NiO thin films for application in dye-sensitized solar cells. Nanotechnology 2008, 19. [Google Scholar] [CrossRef]
- Han, J.; Kim, H.; Kim, D.Y.; Jo, S.M.; Jang, S.-Y. Water-soluble polyelectrolyte-grafted multiwalled carbon nanotube thin films for efficient counter electrode of dye-sensitized solar cells. ACS Nano 2010, 4, 3503–3509. [Google Scholar] [CrossRef]
- Sargent, E.H. Colloidal quantum dot solar cells. Nat. Photonics 2012, 6, 133–135. [Google Scholar] [CrossRef]
- Quantum dot solar cell architecture. Sargent’s group, University of Toronto. Available online: http://light.utoronto.ca/ (accessed on 14 January 2014).
- Tachibana, Y.; Umekita, K.; Otsuka, Y.; Kuwabata, S. Performance improvement of CdS quantum dots sensitized TiO2 solar cells by introducing a dense TiO2 blocking layer. J. Phys. D Appl. Phys. 2008, 41. [Google Scholar] [CrossRef]
- Dedigamuwa, G.S. Fabrication and Characterization of Surfactant-Free PbSe Quantum Dot Films and PbSe-Polymer Hybrid Structures. Ph.D. Dissertation, University of South Florida, Tampa, FL, USA, 2010. [Google Scholar]
- Wienke, J.; van der Zanden, B.; Tijssen, M.; Zeman, M. Performance of spray-deposited ZnO:In layers as front electrodes in thin-film silicon solar cells. Sol. Energy Mater. Sol. Cells 2008, 92, 884–890. [Google Scholar] [CrossRef]
- Ren, Y.; Zhao, G.; Chen, Y. Fabrication of textured SnO2:F thin films by spray pyrolysis. Appl. Surf. Sci. 2011, 258, 914–918. [Google Scholar] [CrossRef]
- Goyal, D.J.; Agashe, C.; Takwale, M.G.; Marathe, B.R.; Bhide, V.G. Development of transparent and conductive ZnO films by spray pyrolysis. J. Mater. Sci. 1992, 27, 4705–4708. [Google Scholar] [CrossRef]
- Chantarat, N.; Hsu, S.-H.; Lin, C.-C.; Chiang, M.-C.; Chen, S.-Y. Mechanism of an AZO-coated FTO film in improving the hydrogen plasma durability of transparent conducting oxide thin films for amorphous-silicon based tandem solar cells. J. Mater. Chem. 2012, 22, 8005–8012. [Google Scholar]
- Chu, T.L.; Chu, S.S. Recent progress in thin-film cadmium telluride solar cells. Prog. Photovolt. Res. Appl. 1993, 1, 31–42. [Google Scholar] [CrossRef]
- Ahn, S.-J.; Kim, K.-H.; Yoon, K.-H. Nanoparticle derived Cu(In,Ga)Se2 absorber layer for thin film solar cells. Colloids Surf. A Physicochem. Eng. Asp. 2008, 313–314, 171–174. [Google Scholar] [CrossRef]
- Hibberd, C.J.; Chassaing, E.; Liu, W.; Mitzi, D.B.; Lincot, D.; Tiwari, A.N. Non-vacuum methods for formation of Cu(In,Ga)(Se,S)2 thin film photovoltaic absorbers. Prog. Photovolt. Res. Appl. 2010, 18, 434–452. [Google Scholar] [CrossRef]
- Schulz, D.L.; Curtis, C.J.; Flitton, R.A.; Weisner, H.; Keane, J.; Matson, R.J.; Jones, K.M.; Parilla, P.A.; Noufi, R.; Ginley, D.S. Cu-In-Ga-Se nanoparticle colloids as spray deposition precursors for Cu(In,Ga)Se2 solar cell materials. J. Electron. Mater. 1998, 27, 433–437. [Google Scholar] [CrossRef]
- Prathap, P.; Reddy, A.S.; Reddy, G.R.; Miles, R.W.; Reddy, K.T.R. Characterization of novel sprayed Zn1−xMgxO films for photovoltaic application. Sol. Energy Mater. Sol. Cells 2010, 94, 1434–1436. [Google Scholar] [CrossRef]
- Ernits, K.; Brémaud, D.; Buecheler, S.; Hibberd, C.J.; Kaelin, M.; Khrypunov, G.; Müller, U.; Mellikov, E.; Tiwari, A.N. Characterisation of ultrasonically sprayed InxSy buffer layers for Cu(In,Ga)Se2 solar cells. Thin Solid Films 2007, 515, 6051–6054. [Google Scholar] [CrossRef]
- Naghavi, N.; Abou-Ras, D.; Allsop, N.; Barreau, N.; Bucheler, S.; Ennaoui, A.; Fischer, C.-H.; Guillen, C.; Hariskos, D.; Herrero, J.; et al. Buffer layers and transparent conducting oxides for chalcopyrite Cu(In,Ga)(S,Se)2 based thin film photovoltaics: Present status and current developments. Prog. Photovolt. Res. Appl. 2010, 18, 411–433. [Google Scholar] [CrossRef]
- Buecheler, S.; Pianezzi, F.; Fella, C.; Chirila, A.; Decock, K.; Burgelman, M.; Tiwari, A.N. Interface formation between CuIn1−xGaxSe2 absorber and In2S3 buffer layer deposited by ultrasonic spray pyrolysis. Thin Solid Films 2011, 519, 7560–7563. [Google Scholar] [CrossRef]
- Akhavan, V.A.; Goodfellow, B.W.; Panthani, M.G.; Steinhagen, C.; Harvey, T.B.; Stolle, C.J.; Korgel, B.A. Colloidal CIGS and CZTS nanocrystals: A precursor route to printed photovoltaics. J. Solid State Chem. 2012, 189, 2–12. [Google Scholar] [CrossRef]
- Buecheler, S.; Corica, D.; Guettler, D.; Chirila, A.; Verma, R.; Müller, U.; Niesen, T.P.; Palmc, J.; Tiwari, A.N. Ultrasonically sprayed indium sulfide buffer layers for Cu(In,Ga)(S,Se)2 thin-film solar cells. Thin Solid Films 2009, 517, 2312–2315. [Google Scholar] [CrossRef]
- Prathap, P.; Revathi, N.; Reddy, A.S.; Subbaiah, Y.P.V.; Reddy, K.T.R. Synthesis of conducting Zn1−xMgxO: Al layers by spray pyrolysis for photovoltaic application. Thin Solid Films 2011, 519, 7592–7595. [Google Scholar] [CrossRef]
- Yarin, A.L. Drop impact dynamics: Splashing, spreading, receding, bouncing. Annu. Rev. Fluid Mech. 2006, 38, 159–192. [Google Scholar] [CrossRef]
- Rioboo, R.; Tropea, C.; Marengo, M. Outcomes from a drop impact on solid surfaces. At. Sprays 2001, 11, 155–165. [Google Scholar]
- Bernardin, J.D.; Mudawar, I. A Leidenfrost point model for impinging droplets and sprays. J. Heat Transf. 2004, 126, 272–278. [Google Scholar] [CrossRef]
- Mozumder, A.K.; Ullah, M.R.; Hossain, A.; Islam, M.A. Sessile drop evaporation and Leidenfrost phenomenon. Am. J. Appl. Sci. 2010, 7, 846–851. [Google Scholar] [CrossRef]
- Bernardin, J.D.; Stebbins, C.J.; Mudawar, I. Mapping of impact and heat transfer regimes of water drops impinging on a polished surface. Int. J. Heat Mass Transf. 1997, 40, 247–267. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Kim, J.H. Spray deposition of chalcogenide thin films. J. Korean Phys. Soc. 2010, 57, 1600–1604. [Google Scholar] [CrossRef]
- Daranfed, W.; Aida, M.S.; Attaf, N.; Bougdira, J.; Rinnert, H. Cu2ZnSnS4 thin films deposition by ultrasonic spray pyrolysis. J. Alloys Compd. 2012, 542, 22–27. [Google Scholar] [CrossRef]
- Soltman, D.; Subramanian, V. Inkjet-printed line morphologies and temperature control of the coffee effect. Langmuir 2008, 24, 2224–2231. [Google Scholar] [CrossRef]
- Kang, B.J.; Oh, J.H. Geometrical characterization of inkjet-printed conductive lines of nanosilver suspensions on a polymer substrate. Thin Solid Films 2010, 518, 2890–2896. [Google Scholar] [CrossRef]
- Teichler, A.; Eckardt, R.; Friebe, C.; Perelaer, J.; Schubert, U.S. Film formation properties of inkjet printed poly(phenylene-ethynylene)-poly(phenylene-vinylene)s. Thin Solid Film 2011, 519, 3695–3702. [Google Scholar] [CrossRef]
- Eslamian, M.; Ashgriz, N. Drop-On-Demand Drop Generators. In Handbook of Atomization and Sprays; Ashgriz, N., Ed.; Springer: New York, NY, USA, 2011; pp. 581–601. [Google Scholar]
- Scully, S.R. Exciton Transport, Charge Extraction, and Loss Mechanisms in Organic Photovoltaics. Ph.D. Dissertation, Stanford University, Stanford, CA, USA, 2008. [Google Scholar]
- Li, G.; Shrotriya, V.; Huang, J.; Yao, Y.; Moriarty, T.; Emery, K.; Yang, Y. High-efficiency solution processable polymer photovoltaic cells by self-organization of polymer blends. Nat. Mater. 2005, 4, 864–868. [Google Scholar] [CrossRef]
- Bielecka, U.; Lutsyk, P.; Janus, L.; Sworakowski, J.; Bartkowiak, W. Effect of solution aging on morphology and electrical characteristics of region regular P3HT FETs fabricated by spin coating and spray coating. Org. Electron. 2011, 12, 1768–1776. [Google Scholar] [CrossRef]
- Prehm, J.; Xin, L.; Möhwald, K.; Bach, F.-W. Coupled coating formation simulation in thermal spray processes using CFD and FEM. CFD Lett. 2011, 1, 89–99. [Google Scholar]
- SolarWindow™. New Energy Technologies Inc. Website. Available online: http://www.newenergytechnologiesinc.com/technology/solarwindow#spray (accessed on 13 January 2014).
- Jiang, X. Organic Semitransparent Photovoltaic Energy Converter (OSPEC)—A Green Solution to Today’s Energy Needs. Available online: http://www.azonano.com/article.aspx?ArticleId=2470 (accessed on 11 June 2013).
- Mitsubishi Spray-on Solar Power Technology. Solar Power Blog. Available online: http://solarpower.com/blog/mitsubishi-spray-on-solar-power-technology/ (accessed on 13 January 2014).
- Coxworth, B. Spray-on film turns windows into solar panels. Available online: http://www.gizmag.com/thin-film-turns-windows-into-solar-panels/16058/ (accessed on 17 August 2010).
- Vaughan, A. Colourful ‘solar glass’ means entire buildings can generate clean power. Available online: http://www.theguardian.com/environment/2013/feb/12/printed-solar-glass-panels-oxford-photovoltaics (accessed on 12 February 2013).
- Stohr, S. Scientists develop spray-on solar panels. Available online: http://www.greenlifestylemag.com.au/news/1193/scientists-develop-spray-solar-panels (accessed on 30 January 2009).
- Lower-Cost Solar Cells to Be Printed Like Newspaper, Painted on Rooftops. The University of Texas at Austin Website. Available online: http://www.utexas.edu/news/2009/08/24/printable_solar_cells/ (accessed on 24 August 2009).
- Spray-on Solar Panels. The National Science Foundation Website. Available online: http://www.nsf.gov/news/special_reports/science_nation/sprayonsolar.jsp (accessed on 14 February 2010).
- Cheap, spray-on solar cells developed by Canadian researchers. CBCnews. Available online: http://www.cbc.ca/m/touch/technology/story/1.1913086 (accessed on 7 October 2013).
- Tait, J.G.; Worfolk, B.J.; Maloney, S.A.; Hauger, T.C.; Elias, A.L.; Buriak, J.M.; Harris, K.D. Spray coated high-conductivity PEDOT:PSS transparent electrodes for stretchable and mechanically-robust organic solar cells. Sol. Energy Mater. Sol. Cells 2013, 110, 98–106. [Google Scholar] [CrossRef]
- Solar cell fabrication is simplified by spray painting. The University of Sheffield Website. Available online: http://www.shef.ac.uk/news/nr/solar-photovoltaic-pv-spray-painting-lidzey-1.251912 (accessed on 11 February 2013).
- McKeegan, N. New efficiency record advances spray-on solar cells. Available online: http://www.gizmag.com/quantum-dot-solar-cell-efficiency-record/19922/ (accessed on 23 September 2011).
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Eslamian, M. Spray-on Thin Film PV Solar Cells: Advances, Potentials and Challenges. Coatings 2014, 4, 60-84. https://doi.org/10.3390/coatings4010060
Eslamian M. Spray-on Thin Film PV Solar Cells: Advances, Potentials and Challenges. Coatings. 2014; 4(1):60-84. https://doi.org/10.3390/coatings4010060
Chicago/Turabian StyleEslamian, Morteza. 2014. "Spray-on Thin Film PV Solar Cells: Advances, Potentials and Challenges" Coatings 4, no. 1: 60-84. https://doi.org/10.3390/coatings4010060
APA StyleEslamian, M. (2014). Spray-on Thin Film PV Solar Cells: Advances, Potentials and Challenges. Coatings, 4(1), 60-84. https://doi.org/10.3390/coatings4010060




