Polymer-Metallic Systems Functionalizing Polylactide Nonwovens as a Greener Alternative to Modified Polypropylene-Based Textiles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Procedures
2.2.1. Polymerization of Methacrylate-N,N-Dimethylaminoethyl
2.2.2. Applying PDMAEMA Solution to the Surface of Nonwovens
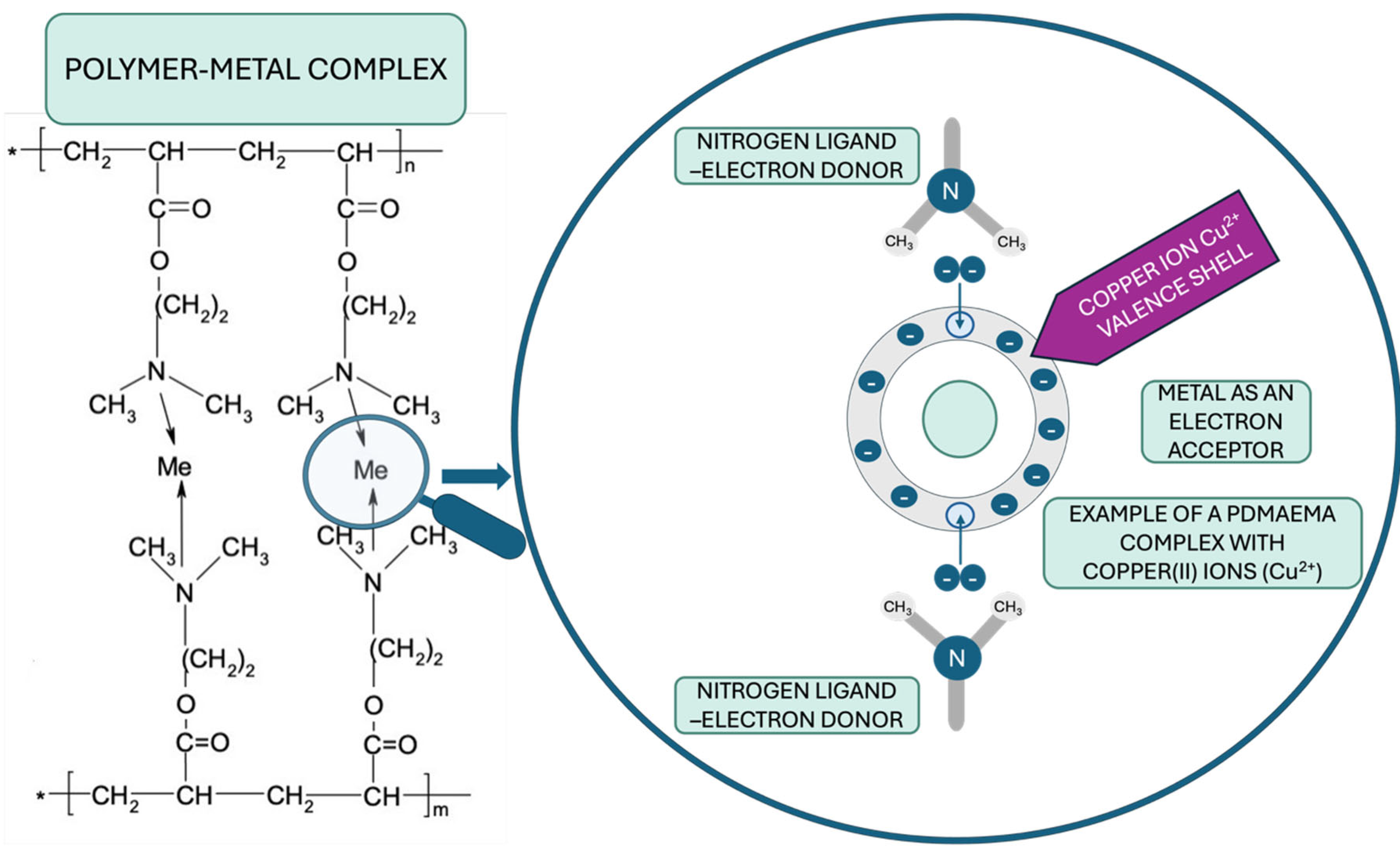
2.2.3. Complexation of PDMAEMA with Selected Divalent Metal Salts
2.3. Characterization
2.3.1. Scanning Electron Microscopy
2.3.2. Streaming Potential
2.3.3. Antimicrobial Activity of Functionalized Nonwovens
3. Results
3.1. Deposition of the Polymeric Layer
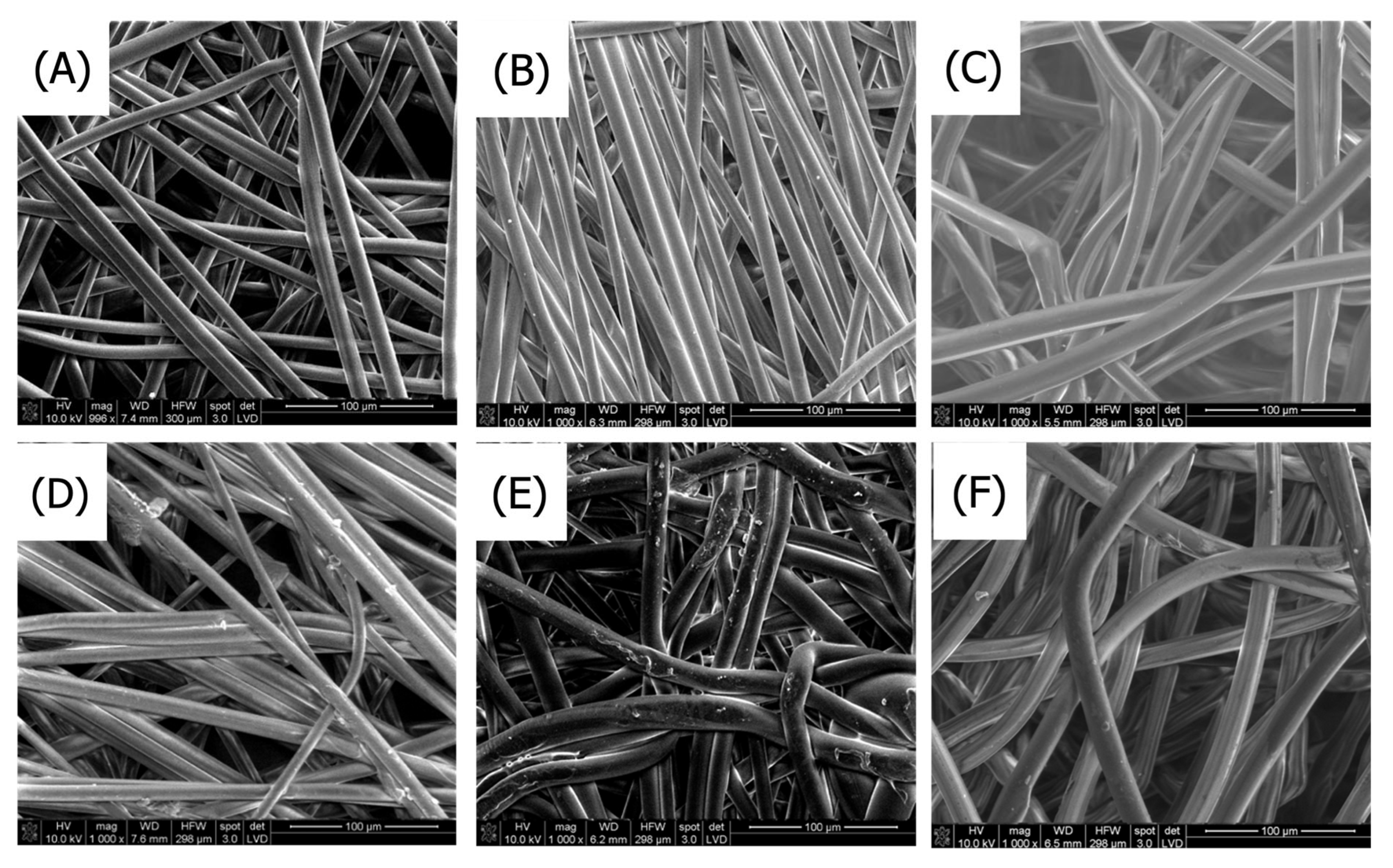
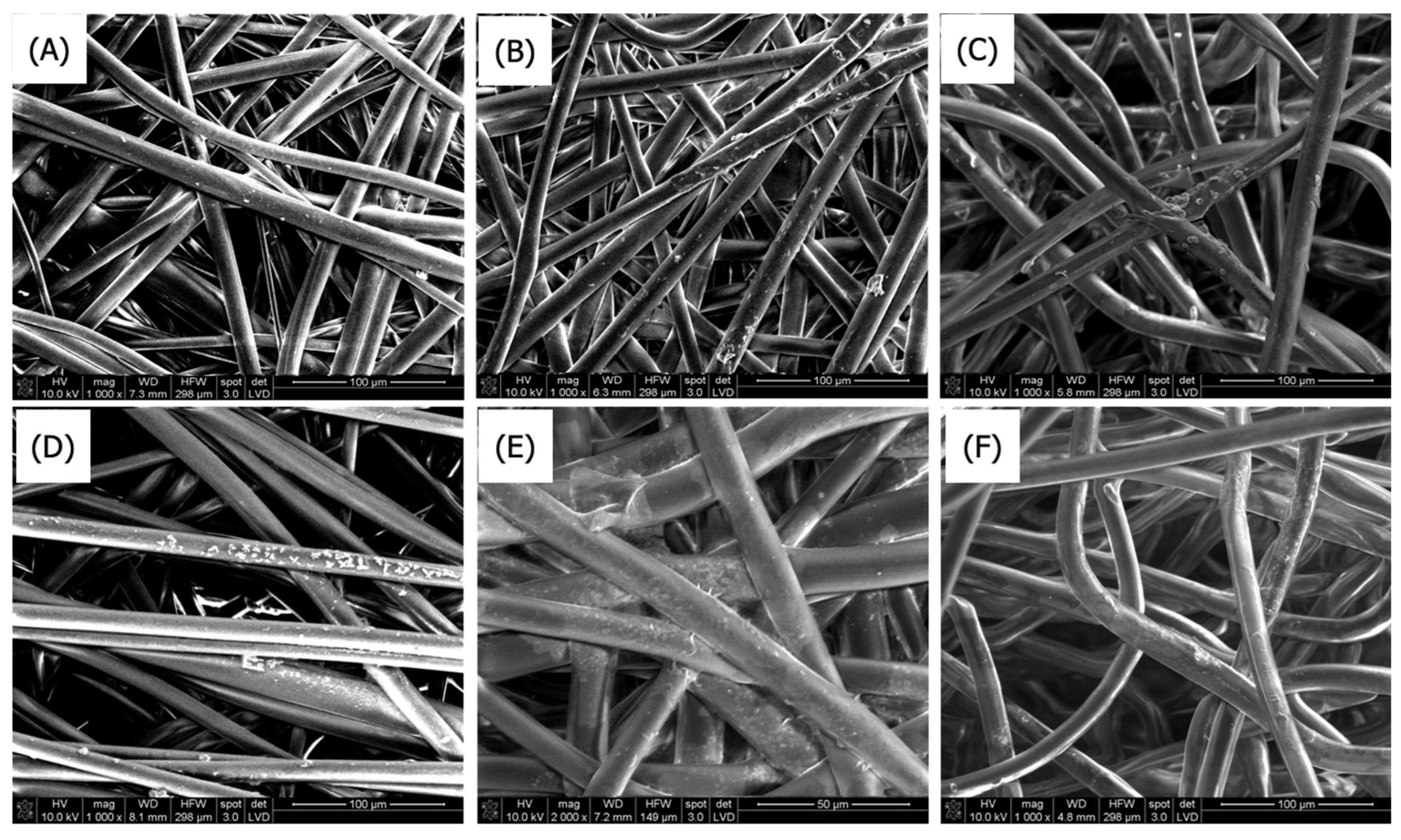
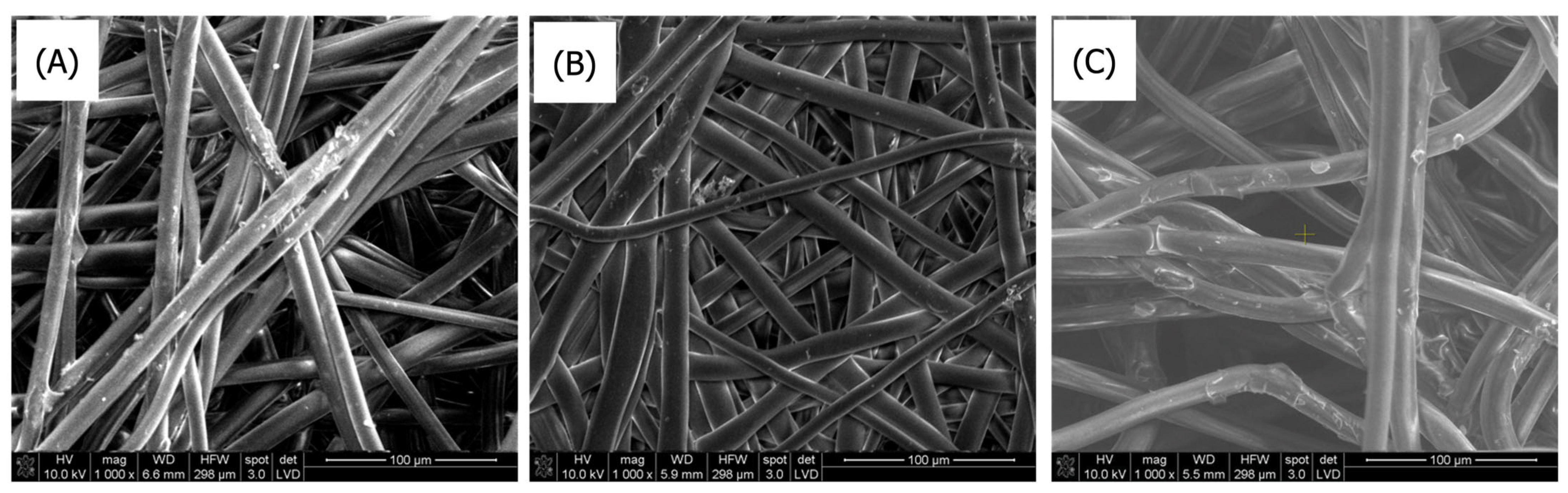
3.1.1. Scanning Electron Microscopy
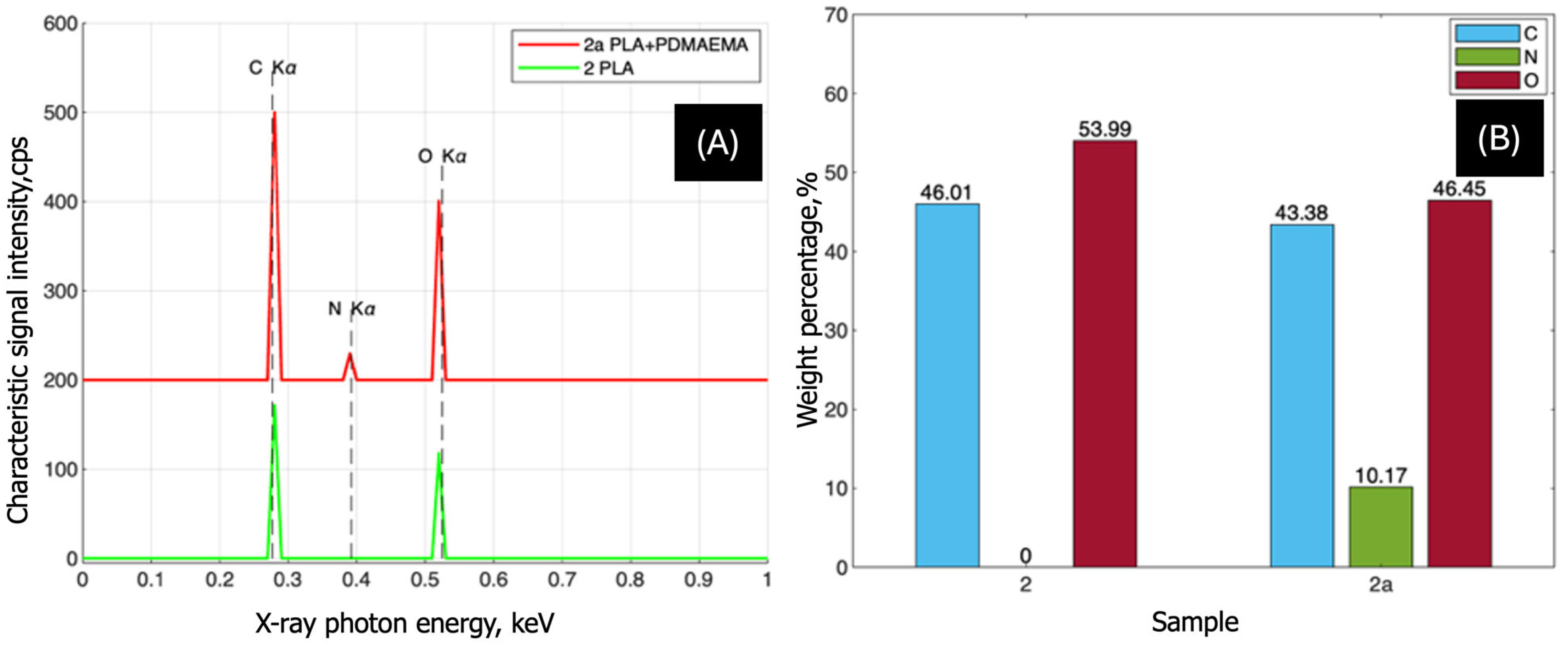
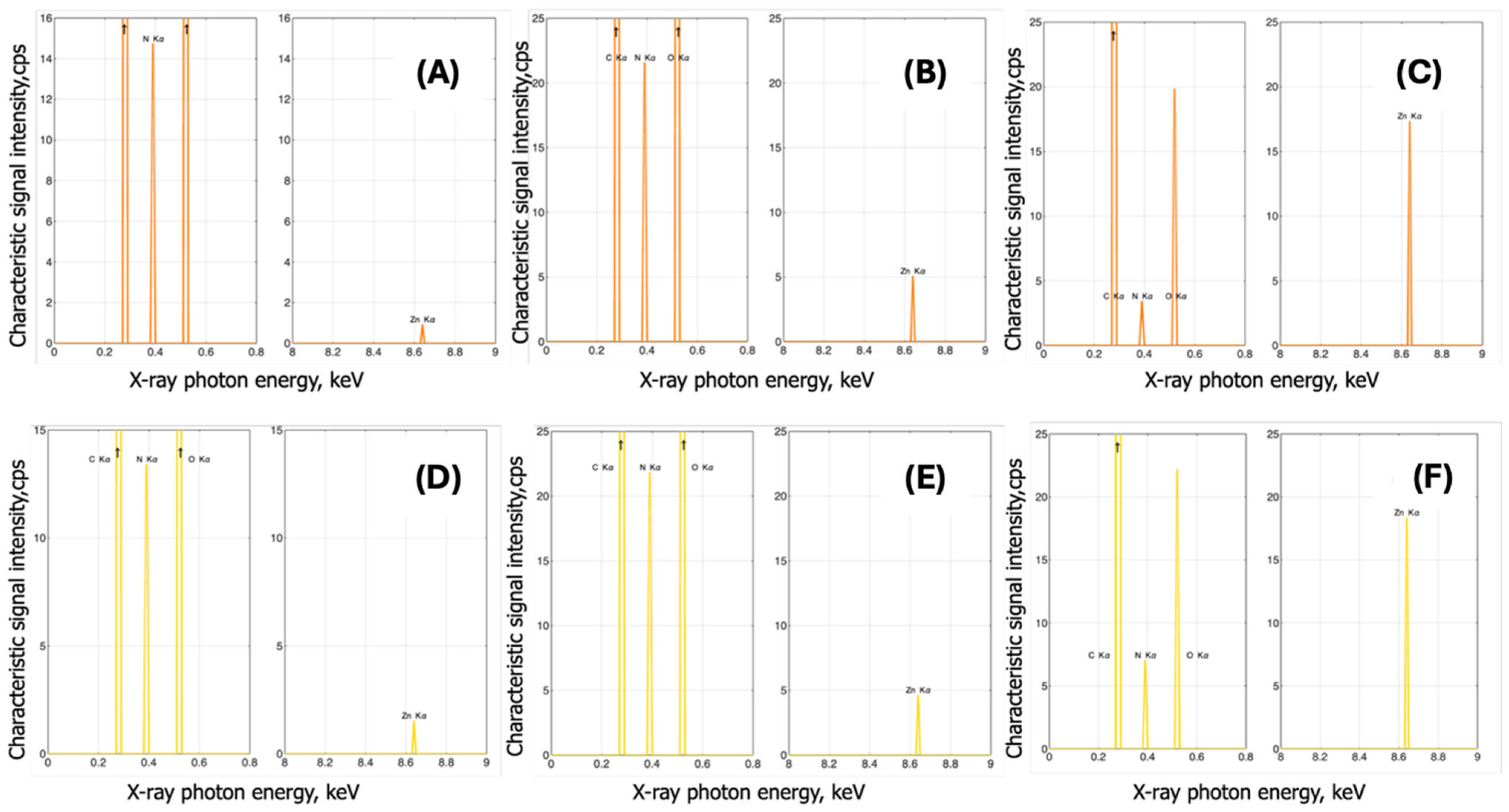
3.1.2. EDX Analysis
3.1.3. Electrokinetic Analysis
3.2. Metallic Complexation
3.2.1. Complexes with Zinc
SEM Analysis
EDX Analysis
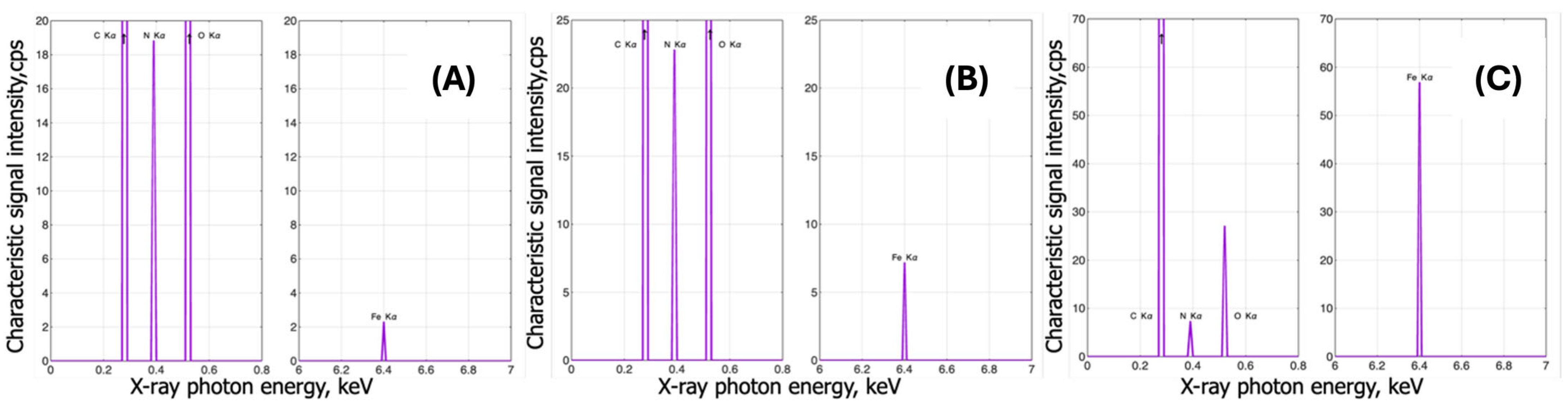
3.2.2. Complexes with Iron
SEM Analysis
EDX Analysis
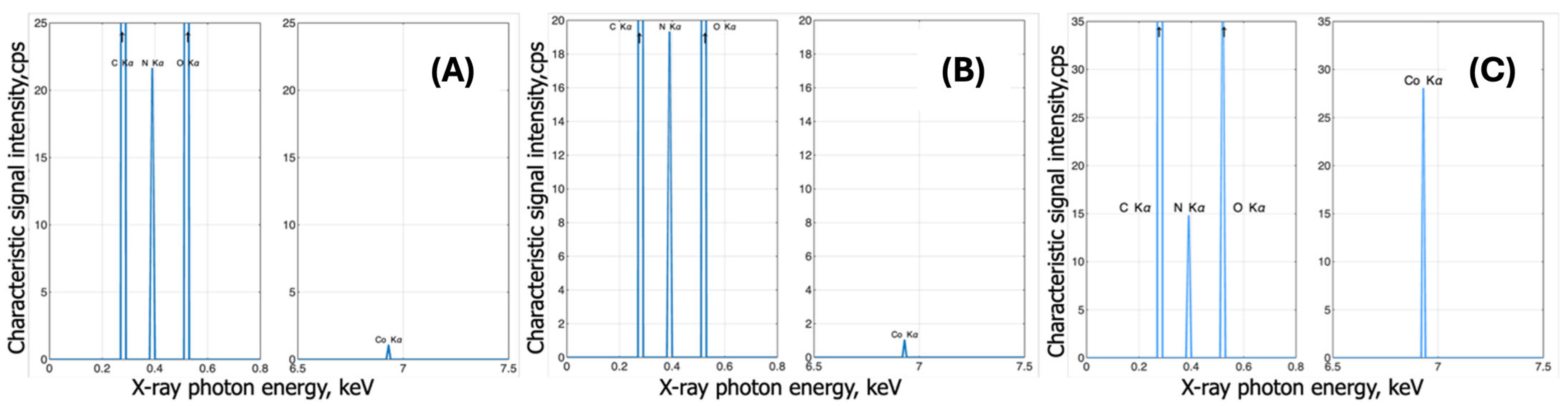
3.2.3. Complexes with Cobalt
SEM Analysis
EDX Analysis
3.2.4. Complexes with Copper
SEM Analysis
EDX Analysis
3.3. Studies on the Antibacterial Activity of Polylactide Nonwovens
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Action Plan on Antimicrobial Resistance. 2015. Available online: https://iris.who.int/bitstream/handle/10665/193736/9789241509763_eng.pdf?sequence=1 (accessed on 14 March 2025).
- Stawski, D.; Sarkar, A.K.; Połowiński, S.; Banerjee, A.; Ranganath, A.; Puchalski, M.; Stańczyk, K. Antibacterial properties of polypropylene textiles modified by poly(2-(N,N-dimethyloamino ethyl) methacrylate). J. Text. Inst. 2013, 104, 883–891. [Google Scholar] [CrossRef]
- Stawski, D.; Zielińska, D. Quaternary Ammonium Salts of Poly(N,N-dimethylaminoethyl methacrylate) as an Efficient Antibacterial Agent for Polylactide Textiles. J. Text. Polym. 2019, 7, 65–72. [Google Scholar]
- Gutarowska, B.; Stawski, D.; Skóra, J.; Herczyńska, L.; Pielech-Przybylska, K.; Połowiński, S.; Krucińska, I. PLA nonwovens modified with poly(dimethylaminoethyl methacrylate) as antimicrobial filter materials for workplaces. Text. Res. J. 2015, 85, 1083–1094. [Google Scholar] [CrossRef]
- Stawski, D.; Rolińska, K.; Zielińska, D.; Sahariah, P.; Hjálmarsdóttir, M.A.; Másson, M. Antibacterial properties of poly(N,N-dimethylaminoethyl methacrylate) obtained at different initiator concentrations in solution polymerization. R. Soc. Open Sci. 2022, 9, 211367. [Google Scholar] [CrossRef]
- ASTM E2149-10; Standard Test Method for Determining the Antimicrobial Activity of Immobilized Antimicrobial Agents Under Dynamic Contact Conditions. ASTM International: West Conshohocken, PA, USA, 2013. [CrossRef]
- Rawlinson, L.A.B.; Ryan, S.M.; Mantovani, G.; Syrett, J.A.; Haddleton, D.M.; Brayden, D.J. Antibacterial effects of poly(2-(dimethylamino ethyl)-methacrylate) against selected Gram-positive and Gram-negative bacteria. Biomacromolecules 2010, 11, 443–453. [Google Scholar] [CrossRef]
- Huang, J.; Murata, H.; Koepsel, R.R.; Russell, A.J.; Matyjaszewski, K. Antibacterial polypropylene via surface-initiated atom transfer radical polymerization. Biomacromolecules 2007, 8, 1396–1399. [Google Scholar] [CrossRef]
- Sahnoun, M.; Charreyre, M.T.; Veron, L.; Delair, T.; D’Agosto, F. Synthetic and characterization aspects of dimethylaminoethyl methacrylate reversible addition fragmentation chain transfer (RAFT) polymerization. J. Polym. Sci. A Polym. Chem. 2005, 43, 3551–3565. [Google Scholar] [CrossRef]
- Shaban, S.E.; Ibrahiem, N.M.; El-Mongy, S.A.; Elshereafy, E.E. Validation of scanning electron microscope (SEM), energy dispersive X-ray (EDX) and gamma spectrometry to verify source nuclear material for safeguards purposes. J. Radioanal. Nucl. Chem. 2013, 296, 1219–1224. [Google Scholar] [CrossRef]
- Nica, I.C.; Stan, M.S.; Dinischiotu, A.; Popa, M.; Chifiriuc, M.C.; Lazar, V.; Pircalabioru, G.G.; Bezirtzoglou, E.; Iordache, O.G.; Varzaru, E.; et al. Innovative self-cleaning and biocompatible polyester textiles nano-decorated with Fe–N-doped titanium dioxide. Nanomaterials 2016, 6, 214. [Google Scholar] [CrossRef]
- Sonbati, A.Z.; Diab, M.A.; El-Bindary, A.A. Stoichiometry of polymer complexes. In Stoichiometry and Research: The Importance of Quantity in Biomedicine; Innocenti, A., Ed.; InTech: Rijeka, Croatia, 2012; pp. 147–148. [Google Scholar]
- Drewry, J.A.; Gunning, P.T. Recent advances in biosensory and medicinal therapeutic applications of zinc(II) and copper(II) coordination complexes. Coord. Chem. Rev. 2011, 255, 459–472. [Google Scholar] [CrossRef]
- Pellei, M.; Del Bello, F.; Porchia, M.; Santini, C. Zinc coordination complexes as anticancer agents. Coord. Chem. Rev. 2021, 445, 214088. [Google Scholar] [CrossRef]
- Gusev, A.; Braga, E.; Shul’gin, V.; Lyssenko, K.; Eremenko, I.; Samsonova, L.; Degtyarenko, K.; Kopylova, T.; Linert, W. Luminescent properties of Zn and Mg complexes on N-(2-carboxyphenyl)salicylidenimine basis. Materials 2017, 10, 897. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Roopa; Bhalla, V.; Kumar, M. Beyond zinc coordination: Bioimaging applications of Zn(II)-complexes. Coord. Chem. Rev. 2021, 427, 213550. [Google Scholar] [CrossRef]
- Chen, X.; Xu, C.; Xiang, Q.; Wang, S.; Li, J.; Hua, Y. The effect of the zinc salt on the electrochemical behaviors of Zn in ChCl-urea deep eutectic solvent. Ionics 2023, 29, 1255–1265. [Google Scholar] [CrossRef]
- Kaneti, Y.V.; Zhang, Z.; Yue, J.; Zakaria, Q.M.D.; Chen, C.; Jiang, X.; Yu, A. Crystal plane-dependent gas-sensing properties of zinc oxide nanostructures: Experimental and theoretical studies. Phys. Chem. Chem. Phys. 2014, 16, 11471–11480. [Google Scholar] [CrossRef]
- Bahojb Noruzi, E.; Kheirkhahi, M.; Shaabani, B.; Geremia, S.; Hickey, N.; Asaro, F.; Nitti, P.; Kafil, H.S. Design of a thiosemicarbazide-functionalized calix [4]arene ligand and related transition metal complexes: Synthesis, characterization, and biological studies. Front. Chem. 2019, 7, 663. [Google Scholar] [CrossRef] [PubMed]
- Borsukiewicz, J.; Zieliński, R. Zastosowanie modyfikowanych nanocząstek magnetycznych do obniżania twardości wody. Inż. Ap. Chem. 2011, 50, 64–65. [Google Scholar]
- Gan, Y.X.; Yu, C.; Panahi, N.; Gan, J.B.; Cheng, W. Processing iron oxide nanoparticle-loaded composite carbon fiber and the photosensitivity characterization. Fibers 2019, 7, 25. [Google Scholar] [CrossRef]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Chircov, C.; Bejenaru, I.T.; Nicoară, A.I.; Bîrcă, A.C.; Oprea, O.C.; Tihăuan, B. Chitosan-dextran-glycerol hydrogels loaded with iron oxide nanoparticles for wound dressing applications. Pharmaceutics 2022, 14, 2620. [Google Scholar] [CrossRef]
- Flieger, J.; Pasieczna-Patkowska, S.; Żuk, N.; Panek, R.; Korona-Głowniak, I.; Suśniak, K.; Pizoń, M.; Franus, W. Characteristics and antimicrobial activities of iron oxide nanoparticles obtained via mixed-mode chemical/biogenic synthesis using spent hop (Humulus lupulus L.) extracts. Antibiotics 2024, 13, 111. [Google Scholar] [CrossRef]
- Lee, S.; Ahn, S.; Lee, H.; Kim, J. Layer-by-layer coating of MIL-100(Fe) on a cotton fabric for purification of water-soluble dyes by the combined effect of adsorption and photocatalytic degradation. RSC Adv. 2022, 12, 14723–14731. [Google Scholar] [CrossRef] [PubMed]
- Sayed, F.N.; Polshettiwar, V. Facile and sustainable synthesis of shaped iron oxide nanoparticles: Effect of iron precursor salts on the shapes of iron oxides. Sci. Rep. 2015, 5, 9733. [Google Scholar] [CrossRef]
- Cheng, C.; Dai, J.; Li, Z.; Feng, W. Preparation and magnetic properties of CoFe2O4 oriented fiber arrays by electrospinning. Materials 2020, 13, 3860. [Google Scholar] [CrossRef]
- Zahradníková, E.; Pichon, C.; Duhayon, C.; Sutter, J.P.; Halaš, P.; Drahoš, B. Synthesis, structural and magnetic properties of cobalt(II) complexes with pyridine-based macrocyclic ligand containing two pyridine pendant arms. RSC Adv. 2024, 14, 28138–28147. [Google Scholar] [CrossRef]
- Gupta, K.C.; Kumar Sutar, A.; Lin, C.C. Polymer-supported Schiff base complexes in oxidation reactions. Coord. Chem. Rev. 2009, 253, 1926–1946. [Google Scholar] [CrossRef]
- Saeed, S.E.S.; Al-Harbi, T.M.; Alhakimi, A.N.; Abd El-Hady, M.M. Synthesis and characterization of metal complexes based on aniline derivative Schiff base for antimicrobial applications and UV protection of a modified cotton fabric. Coatings 2022, 12, 1181. [Google Scholar] [CrossRef]
- Dhanaraj, C.J.; Nair, M.S. Synthesis and characterization of cobalt(II) and zinc(II) complexes of poly(3-nitrobenzylidene-1-naphthylamine-co-succinic anhydride). J. Saudi Chem. Soc. 2014, 18, 479–485. [Google Scholar] [CrossRef]
- Piotrowska, A.; Drzeżdżon, J.; Jacewicz, D.; Chmurzyński, L. Antioxidant, antibacterial and antifungal properties of complex compounds of copper(III). Rocz. PZH 2017, 71, 3–4. [Google Scholar]
- Wu, W.; Yu, L.; Pu, Y.; Yao, H.; Chen, Y.; Shi, J. Copper-enriched Prussian blue nanomedicine for in situ disulfiram toxification and photothermal antitumor amplification. Adv. Mater. 2020, 32, 2000542. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, C.; Liu, J.; Yuan, Y.; Yao, C.; Liu, M.; Deng, L.; Sun, J.; Chen, Z.; Wang, L.; et al. Tumor specific in situ synthesis of therapeutic agent for precision cancer therapy. J. Nanobiotechnol. 2024, 22, 612. [Google Scholar] [CrossRef] [PubMed]
- Cieślak, M.; Kowalczyk, D.; Baranowska-Korczyc, A.; Kamińska, I.; Krzyżowska, M.; Janicka, M.; Kubacki, J. Viscose nonwoven fabric with copper and its multifunctional properties. Cellulose 2023, 30, 9843–9859. [Google Scholar] [CrossRef]
- Román, L.E.; Villalva, C.; Uribe, C.; Paraguay-Delgado, F.; Sousa, J.; Vigo, J.; Vera, C.M.; Gómez, M.M.; Solís, J.L. Textiles functionalized with copper oxides: A sustainable option for prevention of COVID-19. Polymers 2022, 14, 3066. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Baheti, V.; Militky, J.; Khan, Z.; Tunakova, V.; Naeem, S. Copper coated multifunctional cotton fabrics. J. Ind. Text. 2018, 48, 448–464. [Google Scholar] [CrossRef]



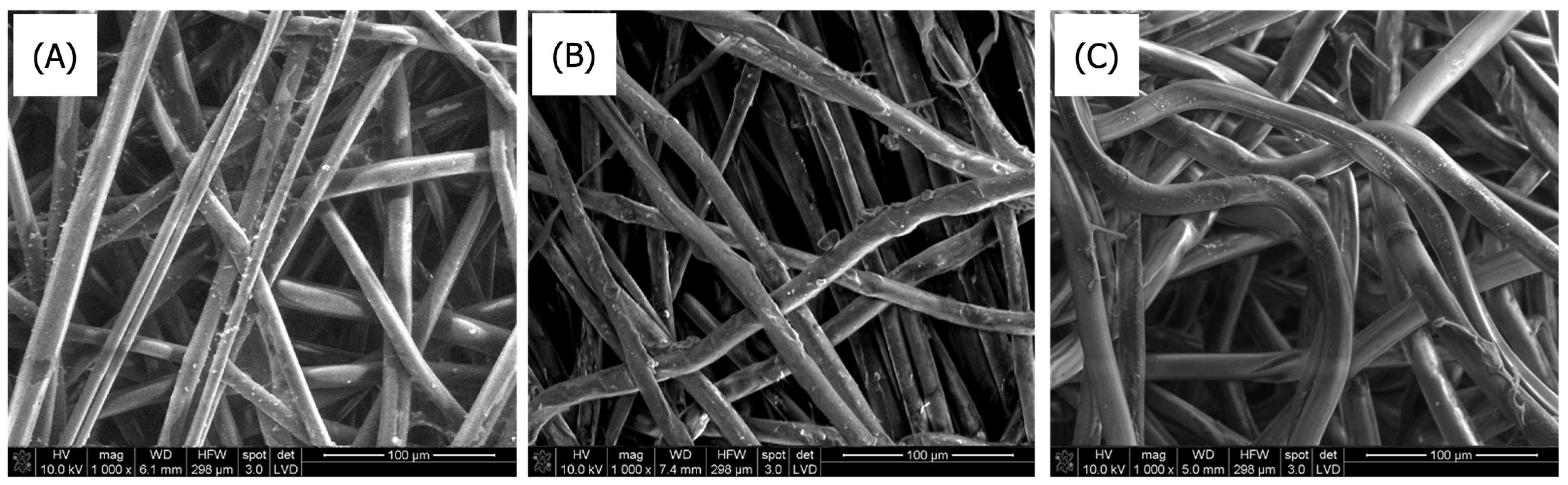
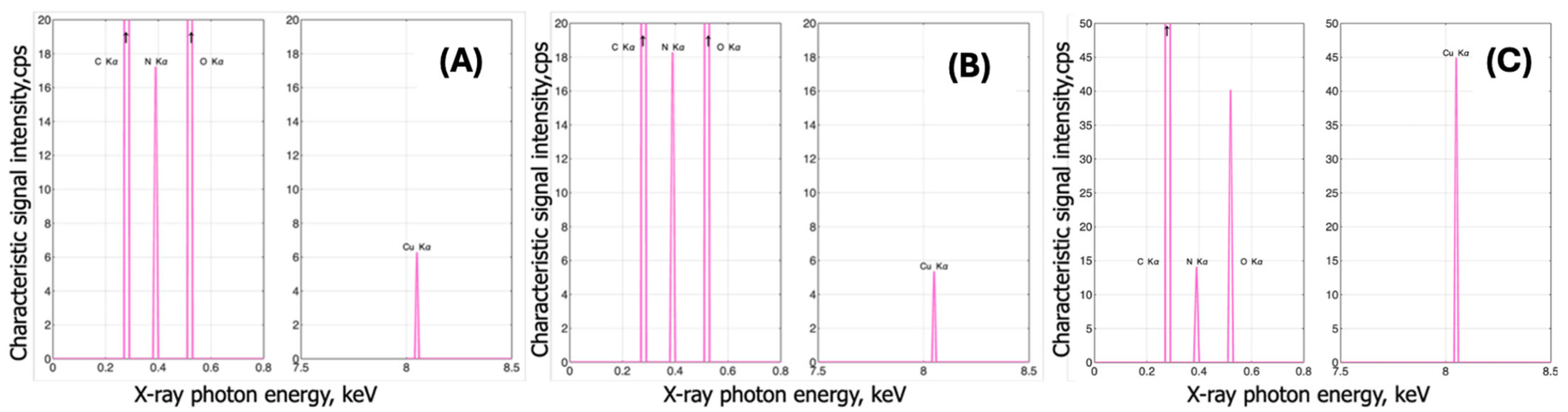
| Sample Number | Type of Polymer | Type of Nonwoven | Mass per Unit Area [g/m2] | Average Thickness of Nonwoven [mm] | Average Fiber Diameter in Nonwoven [µm] |
|---|---|---|---|---|---|
| 1 | PLA | Spunbond | 40 | 0.29 | 9–10 |
| 2 | PLA | Spunbond | 120 | 0.41 | 9–10 |
| 3 | PP | Needle punch | 65 | 1.98 | 14–16 |
| Sample | Material | Amount of PDMAEMA [%] |
|---|---|---|
| 1a | PLA | 1.365 |
| 2a | PLA | 1.415 |
| 3a | PP | 2.155 |
| Sample | Percentage of PDMAEMA [%] | Streaming Potential [mV] | Charge Density ×10−6 eq g−1 |
|---|---|---|---|
| 1 | - | −9.5 | 0.80 |
| 1a | 1.365 | +158 | 29.0 |
| 2 | - | −44 | 0.61 |
| 2a | 1.415 | +180 | 25.0 |
| 3 | - | −98 | 2.6 |
| 3a | 2.155 | +100 | 0.15 |
| Sample | Nonwoven | Type of salt |
|---|---|---|
| 1b1 | PLA1 | ZnCl2 |
| 1b2 | PLA1 | ZnSO4 · 7H2O |
| 2b1 | PLA2 | ZnCl2 |
| 2b2 | PLA2 | ZnSO4 · 7H2O |
| 3b1 | PP | ZnCl2 |
| 3b2 | PP | ZnSO4 · 7H2O |
| Sample | Element Content (wt%) | |||
|---|---|---|---|---|
| C | N | O | Zn | |
| 1b1 | 42.01 | 9.78 | 47.56 | 0.38 |
| 1b2 | 48.56 | 8.69 | 42.02 | 0.56 |
| 2b1 | 43.75 | 9.07 | 44.35 | 1.88 |
| 2b2 | 41.53 | 10.61 | 46.28 | 1.40 |
| 3b1 | 82.36 | 2.74 | 8.74 | 4.65 |
| 3b2 | 86.27 | 3.53 | 6.15 | 2.93 |
| Sample | Element Content (wt%) | |||
|---|---|---|---|---|
| C | N | O | Fe | |
| 1c | 42.37 | 8.96 | 48.10 | 0.43 |
| 2c | 42.09 | 8.44 | 47.07 | 1.99 |
| 3c | 81.26 | 4.09 | 8.45 | 5.10 |
| Sample | Element Content (wt%) | |||
| C | N | O | Co | |
| 1d | 41.56 | 9.64 | 48.58 | 0.22 |
| 2d | 42.20 | 9.88 | 47.73 | 0.19 |
| 3d | 81.66 | 6.16 | 9.92 | 2.26 |
| Sample | Element Content (wt%) | |||
|---|---|---|---|---|
| C | N | O | Cu | |
| 1e | 44.00 | 9.44 | 44.09 | 2.47 |
| 2e | 41.99 | 9.62 | 46.95 | 1.44 |
| 3e | 79.03 | 5.70 | 9.35 | 5.29 |
| Sample | Modifier | CFU mL−1 at t0 | CFU mL−1 at t1 | Reduction (%) | Log10 Reduction |
|---|---|---|---|---|---|
| Culture only (DK) | — | 1.83 × 105 | 1.73 × 105 | — | — |
| 2 (PLA control) | — | — | 7.06 × 105 | 0 | — |
| 2a | PDMAEMA | — | 0 | 100 | 4 |
| 2b1 | PDMAEMA + Zn (ZnCl2) | — | 0 | 100 | 4 |
| 2b2 | PDMAEMA + Zn (ZnSO4) | — | 0 | 100 | 4 |
| 2c | PDMAEMA + Co | — | 0 | 100 | 4 |
| 2d | PDMAEMA + Fe | — | 0 | 100 | 4 |
| 2e | PDMAEMA + Cu | — | 0 | 100 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czajka, M.; Stawski, D.; Herczyńska, L.; Puchalski, M. Polymer-Metallic Systems Functionalizing Polylactide Nonwovens as a Greener Alternative to Modified Polypropylene-Based Textiles. Coatings 2025, 15, 996. https://doi.org/10.3390/coatings15090996
Czajka M, Stawski D, Herczyńska L, Puchalski M. Polymer-Metallic Systems Functionalizing Polylactide Nonwovens as a Greener Alternative to Modified Polypropylene-Based Textiles. Coatings. 2025; 15(9):996. https://doi.org/10.3390/coatings15090996
Chicago/Turabian StyleCzajka, Maria, Dawid Stawski, Lucyna Herczyńska, and Michał Puchalski. 2025. "Polymer-Metallic Systems Functionalizing Polylactide Nonwovens as a Greener Alternative to Modified Polypropylene-Based Textiles" Coatings 15, no. 9: 996. https://doi.org/10.3390/coatings15090996
APA StyleCzajka, M., Stawski, D., Herczyńska, L., & Puchalski, M. (2025). Polymer-Metallic Systems Functionalizing Polylactide Nonwovens as a Greener Alternative to Modified Polypropylene-Based Textiles. Coatings, 15(9), 996. https://doi.org/10.3390/coatings15090996








