Recent Trends in Gelatin Electrospun Nanofibers: Advances in Fabrication, Functionalization, and Applications
Abstract
1. Introduction
2. Methodology
3. Electrospinning of Gelatin Nanofibers
4. Physicochemical Properties of Gelatin Nanofibers
4.1. Crosslinking Strategies for Gelatin Nanofibers
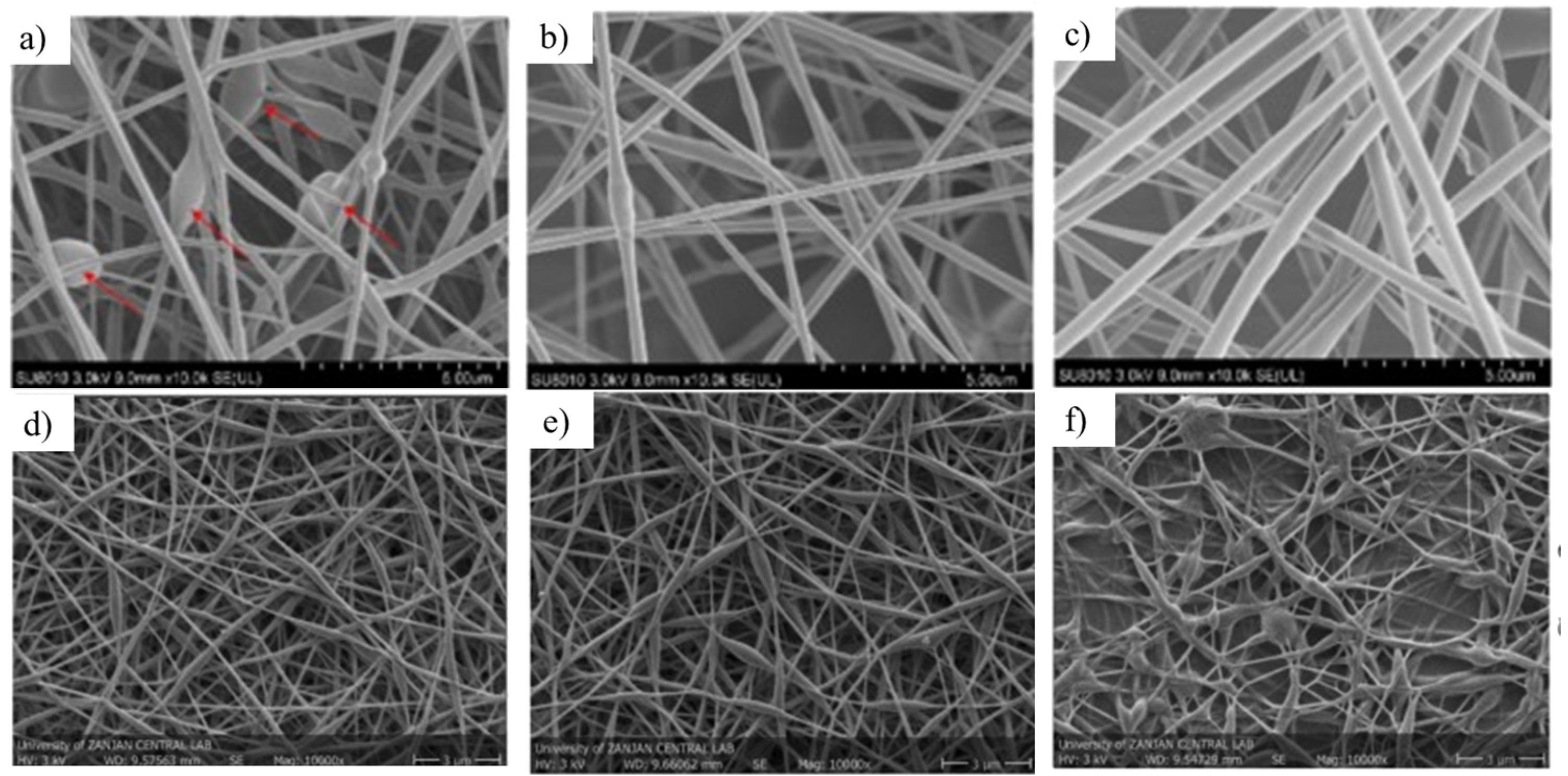
4.2. Morphology, Thermal and Mechanical Properties
4.3. Bioactive Compound Encapsulation and Functional Properties
4.4. Overall Trends in Physicochemical Improvements
| Nanofiber Material | Preparation Method | Post-Processing Treatment | Physicochemical Effects | Thermal Stability | Ref. |
|---|---|---|---|---|---|
| Bilayer film of balangu seed mucilage–gelatin (base layer) and polyvinyl acetate (PVA)–gelatin nanofiber layer with Fe3O4 nanoparticles | Casting for base layer; electrospinning of PVA–gelatin layer with Fe3O4 dispersed by sonication | - | The electrospun layer improved mechanical strength, hydrophobicity, and barrier properties. It decreased moisture content (37.4%), solubility (28.5%), swelling (35%), water vapor permeability (61.8%) and oxygen permeability (31.5%). | Tg and Tm increased with the increase in PVA content in the fibers (Tg: 104–126 °C Tm: 245–265 °C). | [47] |
| Polyamide/gelatin nanofibers with cerium-doped hydroxyapatite (Ce-HA) | Electrospinning of PA/gelatin solution with Ce-HA nanoparticles (prepared by precipitation) | - | Cerium doping increased hydrophilicity (contact angle from 38° to 0°), degradation rate, and mechanical properties. | - | [53] |
| Anthocyanin-enriched wheat gluten/gelatin electrospun nanofiber films | Electrospinning from wheat gluten/gelatin solutions (dissolution + stirring) | - | Compared with pure wheat gluten, an adjusted ratio of the wheat gluten to gelatin (16:9) improved physicochemical properties, reducing viscosity from 3.56 to 2.75 Pa·s and increasing conductivity from 1.53 to 2.06 mS/cm. SEM showed a transition from bead-like to fibrous morphology (691.6 nm diameter). The films exhibited improved thermal properties, and increased water solubility from 38% to 45%. | Gelatin confers better thermal stability than wheat gluten (Tg: 67–75 °C; Tm: 124–148 °C). | [48] |
| Silk fibroin/gelatin nanofibers | Electrospinning from silk fibroin/gelatin blends (various ratios) in formic acid | - | The addition of gelatin to silk fibroin increased fiber diameter, hydrophilicity, and mass loss, but decreased Young’s modulus, tensile strength, and porosity. | - | [54] |
| Gelatin/poly(sulphonic acid diphenyl aniline) (PSDA) nanofibers | Electrospinning of gelatin in 1% acetic acid with PSDA (10%–20%) | - | Improved thermal stability, storage modulus, and oxidation current with PSDA content. | Thermal stability gradually increases as the PSDA ratio increases in Gel/PSDA nanofibers (Tg and Tm data not available) | [39] |
| Gelatin/xanthan gum nanofibers with chitin and black barberry anthocyanins | Electrospinning from gelatin (12 g) and xanthan gum (1 g) in ethanol/acetic acid/water (45:45:10) | - | The nanofibers containing chitin and anthocyanins presented: improved thermal stability; decreased crystallinity, tensile strength, solubility, and water vapor permeability; enhanced antioxidant properties. compared to the straight nanofibers. Other properties of the nanofibers containing chitin and anthocyanins, including tensile strength, water vapor permeability, moisture content, and water solubility, were significantly lower than the straight nanofibers. | The increase in chitin and anthocyanins concentration increased the thermal stability of the nanofibers (Tg and Tm data not available) | [46] |
| Lycopene-loaded gelatin nanofibers (tri-layer structure) | Gelatin (30% w/v) dissolved in 30% acetic acid, with lycopene (2% w/v) and Tween 80® (0.5%) added; emulsion prepared by low-energy emulsification (40 °C, 1000 rpm); | - | Lycopene–gelatin nanofibers had average diameter of 139 ± 29 nm and showed improved structural stability and crystallinity. Tri-layer system enhanced molecular interactions among gelatin, chitosan, and lycopene, increasing lycopene bioaccessibility to 28.5%. | Tg was lower in gelatin nanofibers compared to lycopene-loaded electrospun nanofibers (Tg: 63–89 °C). | [18] |
| Polymethyl-methacrylate (PMMA)/gelatin nanofibers with propolis | Electrospinning of PMMA/Gelatin (70:30); propolis added at 10%–50% w/v | - | Homogeneous morphology; increased diameter with propolis; highest wettability (∼70°) and water vapor transmission rate (∼250 g/m2·24 h) for PMMA70/Gel30. | - | [50] |
| PLGA/gelatin nanofibers with quercetin and ciprofloxacin | Electrospinning of PLGA (15%) and gelatin (15%) in HFIP; DMSO used to solubilize quercetin and ciprofloxacin | - | High water absorption; drug-loaded scaffolds exhibited slower degradation due to hydrogen bonding with gelatin. | - | [55] |
| Eugenol-loaded gelatin nanofibers | Electrospinning of gelatin (2 g) and eugenol (0.16 mg) in acetic acid/ethanol/water (3:2:3) | - | Smooth, uniform fibers; increased fiber diameter (∼125 nm) due to eugenol encapsulation. | - | [52] |
| Gelatin/chitosan nanofibers with curcumin | Electrospinning from chitosan and gelatin in acetic acid; curcumin added at 0.1%–0.3% | - | Diameter ∼160–180 nm; enhanced tensile strength and thermal stability at 0.2% curcumin; improved antioxidant and antimicrobial properties. | The addition of curcumin decreased thermal stability (Tg and Tm data not available). | [51] |
| Ethyl cellulose/poly caprolactone/gelatin nanofibers | Electrospinning from ethyl cellulose/poly caprolactone/gelatin (70:20:10 or 70:10:20) in chloroform/ethanol; ZnO (3%) and zataria multiflora essential oil (ZEO) (10%–50%) added | The ethyl cellulose/poly caprolactone/gelatin/ZEO/ZnO nanofiber exhibited uniform morphology with a mean diameter of 362–467 nm. The material presented improved thermal and mechanical properties: young’s modulus (437.49 ± 18), tensile strength (7.88 ± 0.7), elongation at break (5.02 ± 0.6) and water contact angle (61.13 ± 0.5). | The addition of ZnO enhanced thermal stability (Tg and Tm data not available). | [56] |
| Nanofiber Material | Preparation Method | Post-Processing Treatment | Physicochemical Effects | Thermal Stability | Ref. |
|---|---|---|---|---|---|
| Gelatin nanofibers | Electrospinning of gelatin solution | Crosslinking via glutaraldehyde (25% v/v) vapor | Crosslinking improved water resistivity and thermal stability of the material. MEV analyses show morphological changes due to hydrophilicity. TGA analyses show that weight loss increased after cross linking. Controlled drug release modulated by pH and crosslinking time. | Crosslinking increased thermal stability (Tg and Tm data not available). | [41] |
| Gelatin/camellia oil oleogels | Electrospinning of gelatin-based spinning solutions | Crosslinking | Adding gelatin nanofiber in electrospun fiber-based oleogels enhanced oil binding capacity (up to 79.3%) and thixotropic recovery (83.2%). Crosslinking reduced free fatty acid release (final: 50.3%) and stabilized structure. | Crosslinking increased thermal stability (Tg and Tm data not available). | [38] |
| PVA/gelatin (PG) nanofibers with Cu-based metal–organic frameworks (MOFs) | Electrospinning of PG solution with MOFs (stirring + sonication) | Crosslinking via glutaraldehyde vapor | Pure PG nanofibers had highest water uptake (∼349%). MOF-loaded PG fibers showed reduced water uptake but higher swelling capacity. | The incorporation of MOFs enhanced thermal stability nanofibers (Tg and Tm data not available). | [45] |
| Quercetin-loaded gelatin nanofibers with shellac coating | Electrospinning from gelatin solution (20% w/v) + quercetin (2.5%–7.5%) in acetic acid; shellac solution (30% w/v) stirred overnight | Shellac coating | Uniform nanofibers (∼206 nm); pH-responsive wettability due to shellac; stable in gastrointestinal tract; quercetin release: 4.75%–12.54%. | The addition of quercetin and shellac increased the thermal stability of nanofibers (Tg and Tm data not available). | [46] |
| Gelatin/oxidized xanthan gum nanofibers with propolis | Electrospinning of gelatin/oxidized xanthan gum mixture with propolis; precise method not detailed | Schiff base crosslinking | The fibers with more oxidized xanthan gum exhibited tensile strength up to 13.2 MPa (10× higher than neat gelatin); lower water vapor permeability and water solubility; higher porosity, antioxidant and antibacterial activity. | The increase on oxidized xanthan gum concentration increased thermal stability (Tg and Tm data not available). | [44] |
| Core–shell PVA/gelatin nanofibers crosslinked with microbial transglutaminase (mTG) | Core–shell electrospinning; gelatin phase crosslinked with mTG (0.5%–4%) | mTG crosslinking | Nanofibers cross-linked with mTG maintained fiber morphology at optimal mTG concentration and time; improved stability. | Crosslinking increased thermal stability (Tg: 70–90 °C). | [43] |
| Core–shell gelatin/gum arabic nanofibers from O/W emulsion | Emulsion electrospinning; genipin added (5% w/w) and allowed to crosslink for 0–24 h | Genipin crosslinking | The crosslinking increased viscosity and elasticity of emulsion. It also led to thicker, more stable fibers; decomposition only above 250 °C, associated with the chemical bonds formed between primary amines on the protein chains. | Crosslinking did not affect thermal stability (Tm: 392–395 °C). | [42] |
| Gelatin nanofibers crosslinked with 1,4-butanediol diglycidyl ether (BDDGE) | Electrospinning of gelatin followed by in situ crosslinking | BDDGE crosslinking (2%–6%, up to 72 h at 37 °C) | Fibers with 4% and 6% BDDGE (72 h) showed high crosslinking and stable diameters (339 ± 91 nm and 276 ± 88 nm). 4% BDDGE gave the best balance of crosslinking and mechanical strength. | - | [40] |
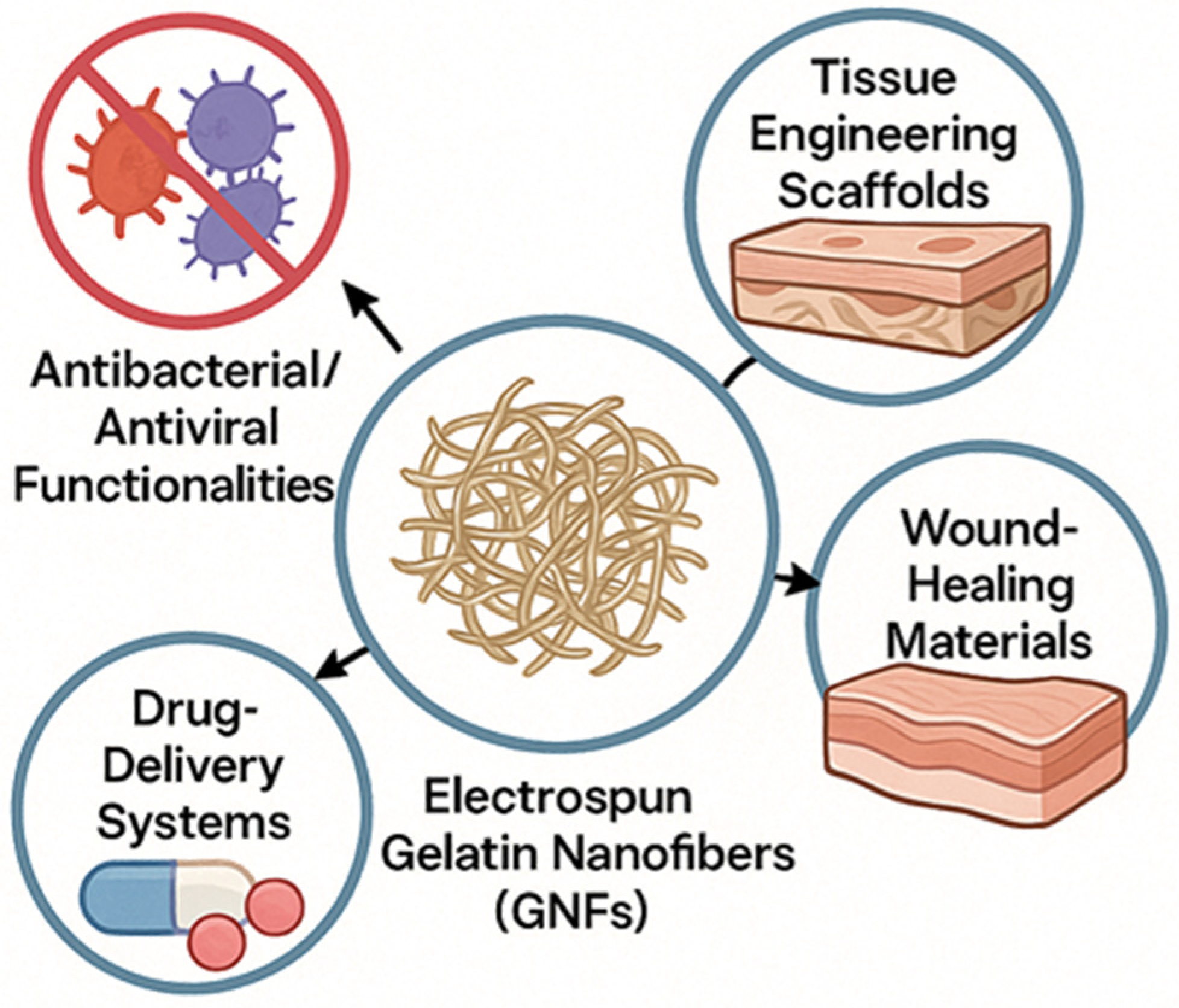
5. Biomedical Applications
5.1. Tissue Engineering Scaffolds
5.2. Wound Healing Materials
5.3. Drug Delivery Systems
5.4. Antibacterial/Antiviral Functionalities
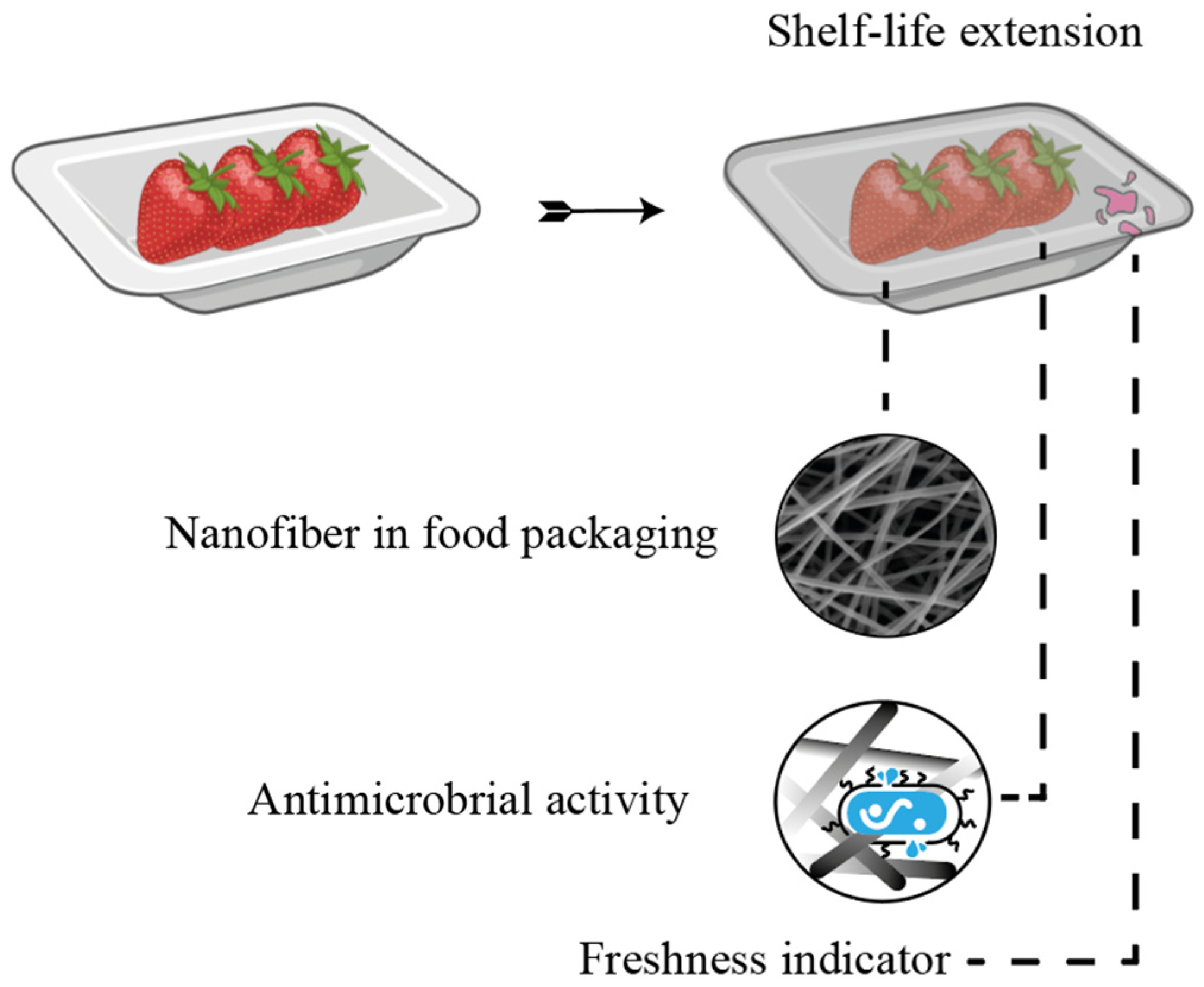
6. Food and Packaging Applications
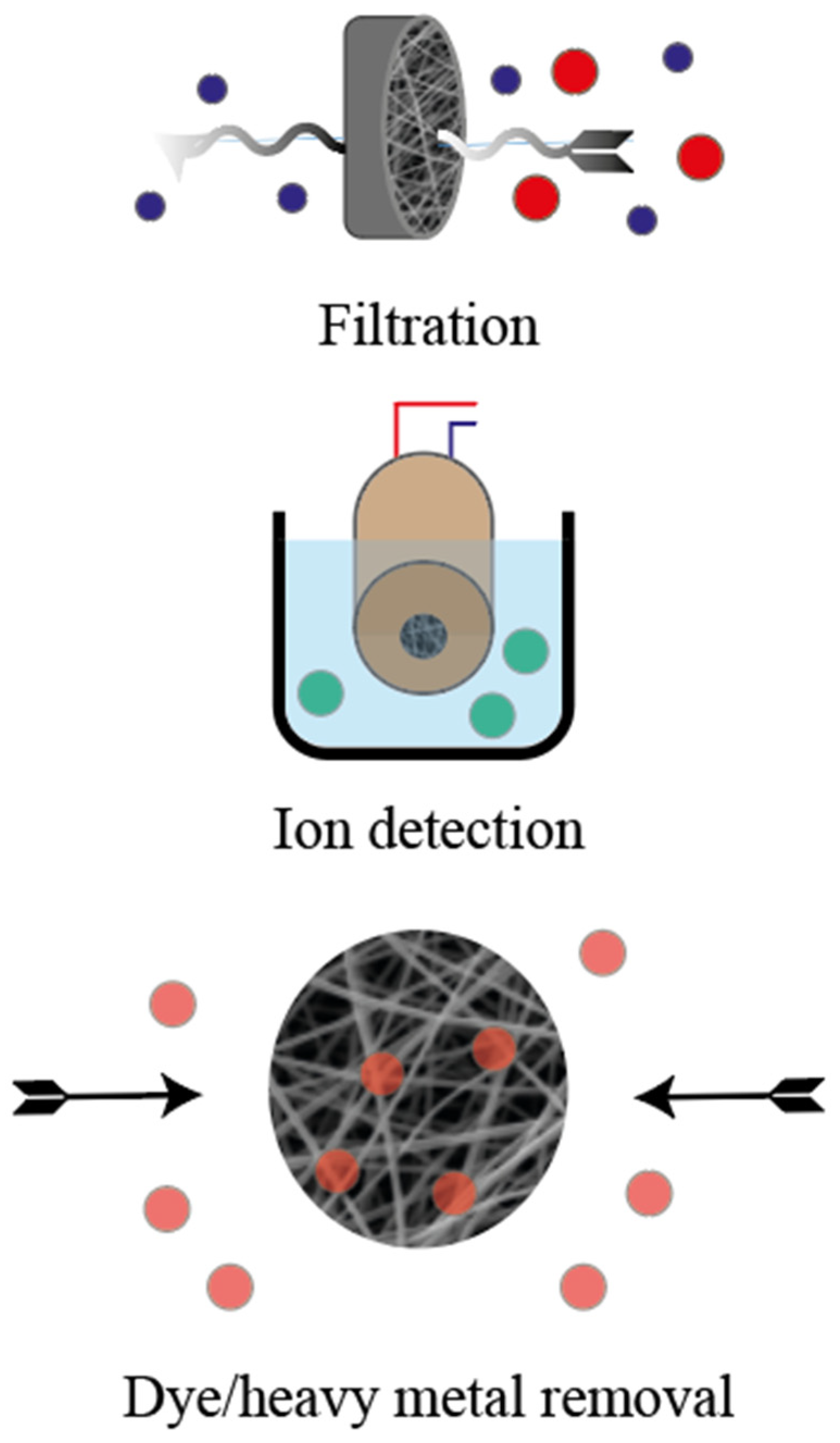
7. Environmental Applications
| Material | Application | Filtration Efficiency (%) | Adsorption Capacity | Reusability | Reference |
|---|---|---|---|---|---|
| Gelatin/PCL | Water Detection | - | - | - | [113] |
| Gelatin/PVA/Graphene Oxide | Oil/Water Separation | - | - | - | [114] |
| Gelatin/Calcium Alginate | Water Adsorption | - | 1937 mg g−1 (Methylene Blue) | 4 cycles | [116] |
| Fish Gelatin | Water Adsorption | - | 60 mg g−1 (Methylene Blue) | 6 cycles | [117] |
| Cellulose Nanofibers/Gelatin | Air Filtration | >90% (PM2.5) | - | - | [118] |
| Gelatin/β−CD | Air Filtration | >95% | High (VOCs) | - | [119] |
| EC/Gelatin/β-CD/Curcumin | Multifunctional Air Filtration | >99.25% (0.3 µm) | 442 µg g−1 (Formaldehyde) | - | [120] |
| Gelatin/ZIF-67 | Uranium Removal | - | 612.24 mg g−1 | - | [121] |
8. Biosensing Applications
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Seedi, H.R.; Said, N.S.; Yosri, N.; Hawash, H.B.; El-Sherif, D.M.; Abouzid, M.; Abdel-Daim, M.M.; Yaseen, M.; Omar, H.; Shou, Q.; et al. Gelatin Nanofibers: Recent Insights in Synthesis, Bio-Medical Applications and Limitations. Heliyon 2023, 9, e16228. [Google Scholar] [CrossRef]
- Gavande, V.; Nagappan, S.; Seo, B.; Lee, W.K. A Systematic Review on Green and Natural Polymeric Nanofibers for Biomedical Applications. Int. J. Biol. Macromol. 2024, 262, 130135. [Google Scholar] [CrossRef]
- Majidi, S.S.; Slemming-Adamsen, P.; Hanif, M.; Zhang, Z.; Wang, Z.; Chen, M. Wet Electrospun Alginate/Gelatin Hydrogel Nanofibers for 3D Cell Culture. Int. J. Biol. Macromol. 2018, 118, 1648–1654. [Google Scholar] [CrossRef]
- Ribeiro, E.S.; de Farias, B.S.; Sant’Anna Cadaval Junior, T.R.; de Almeida Pinto, L.A.; Diaz, P.S. Chitosan–Based Nanofibers for Enzyme Immobilization. Int. J. Biol. Macromol. 2021, 183, 1959–1970. [Google Scholar] [CrossRef]
- Lu, W.; Ma, M.; Xu, H.; Zhang, B.; Cao, X.; Guo, Y. Gelatin Nanofibers Prepared by Spiral-Electrospinning and Cross-Linked by Vapor and Liquid-Phase Glutaraldehyde. Mater. Lett. 2015, 140, 1–4. [Google Scholar] [CrossRef]
- de Farias, B.S.; Rizzi, F.Z.; Ribeiro, E.S.; Diaz, P.S.; Sant’Anna Cadaval Junior, T.R.; Dotto, G.L.; Khan, M.R.; Manoharadas, S.; de Almeida Pinto, L.A.; dos Reis, G.S. Influence of Gelatin Type on Physicochemical Properties of Electrospun Nanofibers. Sci. Rep. 2023, 13, 15195. [Google Scholar] [CrossRef]
- Sharma, G.K.; Jalaja, K.; Ramya, P.R.; James, N.R. Electrospun Gelatin Nanofibres—Fabrication, Cross-Linking and Biomedical Applications: A Review. Biomed. Mater. Devices 2023, 1, 553–568. [Google Scholar] [CrossRef]
- Yıkar, E.; Demir, D.; Bölgen, N. Electrospinning of Gelatin Nanofibers: Effect of Gelatin Concentration on Chemical, Morphological and Degradation Characteristics. Turk. J. Eng. 2021, 5, 171–176. [Google Scholar] [CrossRef]
- Su, X.N.; Khan, M.F.; Xin-Ai; Liu, D.L.; Liu, X.F.; Zhao, Q.L.; Cheong, K.L.; Zhong, S.Y.; Li, R. Fabrication, Modification, Interaction Mechanisms, and Applications of Fish Gelatin: A Comprehensive Review. Int. J. Biol. Macromol. 2025, 288, 138723. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.S.G.; Bandeira, S.F.; Pinto, L.A.A. Characteristics and Chemical Composition of Skins Gelatin from Cobia (Rachycentron Canadum). LWT 2014, 57, 580–585. [Google Scholar] [CrossRef]
- Miyawaki, O.; Omote, C.; Matsuhira, K. Thermodynamic Analysis of Sol-Gel Transition of Gelatin in Terms of Water Activity in Various Solutions. Biopolymers 2015, 103, 685–691. [Google Scholar] [CrossRef]
- Amirrah, I.N.; Lokanathan, Y.; Zulkiflee, I.; Wee, M.F.M.R.; Motta, A.; Fauzi, M.B. A Comprehensive Review on Collagen Type I Development of Biomaterials for Tissue Engineering: From Biosynthesis to Bioscaffold. Biomedicines 2022, 10, 2307. [Google Scholar] [CrossRef]
- Jafari, H.; Lista, A.; Siekapen, M.M.; Ghaffari-Bohlouli, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Fish Collagen: Extraction, Characterization, and Applications for Biomaterials Engineering. Polymers 2020, 12, 2230. [Google Scholar] [CrossRef]
- Haug, I.J.; Draget, K.I.; Smidsrød, O. Physical and Rheological Properties of Fish Gelatin Compared to Mammalian Gelatin. Food Hydrocoll. 2004, 18, 203–213. [Google Scholar] [CrossRef]
- Kwak, H.W.; Shin, M.; Lee, J.Y.; Yun, H.; Song, D.W.; Yang, Y.; Shin, B.S.; Park, Y.H.; Lee, K.H. Fabrication of an Ultrafine Fish Gelatin Nanofibrous Web from an Aqueous Solution by Electrospinning. Int. J. Biol. Macromol. 2017, 102, 1092–1103. [Google Scholar] [CrossRef]
- Zou, Y.; Chen, X.; Lan, Y.; Yang, J.; Yang, B.; Ma, J.; Cheng, M.; Wang, D.; Xu, W. Find Alternative for Bovine and Porcine Gelatin: Study on Physicochemical, Rheological Properties and Water-Holding Capacity of Chicken Lungs Gelatin by Ultrasound Treatment. Ultrason. Sonochemistry 2024, 109, 107004. [Google Scholar] [CrossRef]
- Huang, C.; Tang, J.; Chen, X.; Zeng, X.; Zhong, W.; Pang, J.; Wu, C. Novel Electrospun Gelatin Nanofibers Loaded with Purple Potato Anthocyanin and Syringic Acid for Multifunctional Food Packaging. Foods 2024, 13, 2538. [Google Scholar] [CrossRef]
- de Farias, B.S.; Rizzi, F.Z.; Silva, P.P.; Ribeiro, E.S.; Diaz, P.S.; Cadaval Junior, T.R.S.A.; de Almeida Pinto, L.A. Tri–Layer Nanostructured Lycopene Delivery System Based on Chitosan Nanospheres and Gelatin Nanofibers. Food Res. Int. 2025, 220, 117099. [Google Scholar] [CrossRef]
- Topçu, A.A. The Adsorption Performance of Magnetic Gelatin Nanofiber for Orange G Removal. Polym. Bull. 2023, 80, 1017–1029. [Google Scholar] [CrossRef]
- İnal, M.; Mülazımoğlu, G. Production and Characterization of Bactericidal Wound Dressing Material Based on Gelatin Nanofiber. Int. J. Biol. Macromol. 2019, 137, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yu, H.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Fabrication of Eugenol Loaded Gelatin Nanofibers by Electrospinning Technique as Active Packaging Material. LWT 2021, 139, 110800. [Google Scholar] [CrossRef]
- Teepoo, S.; Dawan, P.; Barnthip, N. Electrospun Chitosan-Gelatin Biopolymer Composite Nanofibers for Horseradish Peroxidase Immobilization in a Hydrogen Peroxide Biosensor. Biosensors 2017, 7, 47. [Google Scholar] [CrossRef]
- de Farias, B.S.; Sant’Anna Cadaval Junior, T.R.; de Almeida Pinto, L.A. Chitosan-Functionalized Nanofibers: A Comprehensive Review on Challenges and Prospects for Food Applications. Int. J. Biol. Macromol. 2019, 123, 210–220. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.K. A Comprehensive Review Summarizing the Effect of Electrospinning Parameters and Potential Applications of Nanofibers in Biomedical and Biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Derkach, S.R.; Kuchina, Y.A.; Baryshnikov, A.V.; Kolotova, D.S.; Voron’ko, N.G. Tailoring Cod Gelatin Structure and Physical Properties with Acid and Alkaline Extraction. Polymers 2019, 11, 1724. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xu, J.; Zhang, Y.; Wang, X.; Lorenzo, J.M.; Zhong, J. Gelatins as Emulsifiers for Oil-in-Water Emulsions: Extraction, Chemical Composition, Molecular Structure, and Molecular Modification. Trends Food Sci. Technol. 2020, 106, 113–131. [Google Scholar] [CrossRef]
- Vaca Chávez, F.; Hellstrand, E.; Halle, B. Hydrogen Exchange and Hydration Dynamics in Gelatin Gels. J. Phys. Chem. B 2006, 110, 21551–21559. [Google Scholar] [CrossRef]
- Okutan, N.; Terzi, P.; Altay, F. Affecting Parameters on Electrospinning Process and Characterization of Electrospun Gelatin Nanofibers. Food Hydrocoll. 2014, 39, 19–26. [Google Scholar] [CrossRef]
- Cooper, C.J.; Mohanty, A.K.; Misra, M. Electrospinning Process and Structure Relationship of Biobased Poly(Butylene Succinate) for Nanoporous Fibers. ACS Omega 2018, 3, 5547–5557. [Google Scholar] [CrossRef]
- Al-Abduljabbar, A.; Farooq, I. Electrospun Polymer Nanofibers: Processing, Properties, and Applications. Polymers 2022, 15, 65. [Google Scholar] [CrossRef]
- Ahmadi Bonakdar, M.; Rodrigue, D. Electrospinning: Processes, Structures, and Materials. Macromol 2024, 4, 58–103. [Google Scholar] [CrossRef]
- Ninan, G.; Joseph, J.; Aliyamveettil, Z.A. A Comparative Study on the Physical, Chemical and Functional Properties of Carp Skin and Mammalian Gelatins. J. Food Sci. Technol. 2014, 51, 2085–2209. [Google Scholar] [CrossRef]
- Chandra, M.V.; Shamasundar, B.A. Texture Profile Analysis and Functional Properties of Gelatin from the Skin of Three Species of Fresh Water Fish. Int. J. Food Prop. 2015, 18, 572–584. [Google Scholar] [CrossRef]
- Rather, J.A.; Akhter, N.; Ashraf, Q.S.; Mir, S.A.; Makroo, H.A.; Majid, D.; Barba, F.J.; Khaneghah, A.M.; Dar, B.N. A Comprehensive Review on Gelatin: Understanding Impact of the Sources, Extraction Methods, and Modifications on Potential Packaging Applications. Food Packag. Shelf Life 2022, 34, 100945. [Google Scholar] [CrossRef]
- Derkach, S.R.; Voron’ko, N.G.; Kuchina, Y.A.; Kolotova, D.S. Modified Fish Gelatin as an Alternative to Mammalian Gelatin in Modern Food Technologies. Polymers 2020, 12, 3051. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guillén, M.C.; Turnay, J.; Fernández-Díaz, M.D.; Ulmo, N.; Lizarbe, M.A.; Montero, P. Structural and Physical Properties of Gelatin Extracted from Different Marine Species: A Comparative Study. Food Hydrocoll. 2002, 16, 25–34. [Google Scholar] [CrossRef]
- Ninan, G.; Jose, J.; Abubacker, Z. Preparation and Characterization of Gelatin Extracted from the Skins of Rohu (Labeo Rohita) and Common Carp (Cyprinus Carpio). J. Food Process. Preserv. 2011, 35, 143–161. [Google Scholar] [CrossRef]
- Li, J.; Qin, Z.; Jiang, Q.; Chen, M.; Li, Y.; Zou, Y.; Zhang, H. Development of Gelatin/Camellia Oil Oleogels as Fat Substitutes via a Novel Strategy: Covalent Crosslinking of Electrospun Nanofiber. Food Hydrocoll. 2025, 166, 111340. [Google Scholar] [CrossRef]
- Huner, A. Fast-Dissolving Electroactive Drug Delivery Systems: Gelatin/Poly(Sulphonic Acid Diphenyl Aniline) Electrospun Nanofibers. Food Biosci. 2024, 61, 104924. [Google Scholar] [CrossRef]
- Dias, J.R.; Baptista-Silva, S.; de Oliveira, C.M.T.; Sousa, A.; Oliveira, A.L.; Bártolo, P.J.; Granja, P.L. In Situ Crosslinked Electrospun Gelatin Nanofibers for Skin Regeneration. Eur. Polym. J. 2017, 95, 161–173. [Google Scholar] [CrossRef]
- Laha, A.; Yadav, S.; Majumdar, S.; Sharma, C.S. In-Vitro Release Study of Hydrophobic Drug Using Electrospun Cross-Linked Gelatin Nanofibers. Biochem. Eng. J. 2016, 105, 481–488. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, P.; Li, J.; Zhang, H.; Weiss, J. Characterization of Core-Shell Nanofibers Electrospun from Bilayer Gelatin/Gum Arabic O/W Emulsions Crosslinked by Genipin. Food Hydrocoll. 2021, 119, 106854. [Google Scholar] [CrossRef]
- Sengor, M.; Ozgun, A.; Gunduz, O.; Altintas, S. Aqueous Electrospun Core/Shell Nanofibers of PVA/Microbial Transglutaminase Cross-Linked Gelatin Composite Scaffolds. Mater. Lett. 2020, 263, 127233. [Google Scholar] [CrossRef]
- Yavari Maroufi, L.; Norouzi, R.; Ramezani, S.; Ghorbani, M. Novel Electrospun Nanofibers Based on Gelatin/Oxidized Xanthan Gum Containing Propolis Reinforced by Schiff Base Cross-Linking for Food Packaging. Food Chem. 2023, 416, 135806. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Kamoun, E.A.; Ghaly, Z.S.; Shokr, A.-B.M.; El-Moslamy, S.H.; Abou-Elyazed, A.S. Copper and Fluorine-MOFs Loaded-Electrospun PVA/Gelatin Nanofibers for Enhancing the Antimicrobial Activity of Topical Wound Dressings: MOFs Synthesis and Spinning Conditions Optimization. Mater. Chem. Phys. 2025, 332, 130303. [Google Scholar] [CrossRef]
- Li, S.F.; Hu, T.G.; Wu, H. Development of Quercetin-Loaded Electrospun Nanofibers through Shellac Coating on Gelatin: Characterization, Colon-Targeted Delivery, and Anticancer Activity. Int. J. Biol. Macromol. 2024, 277, 134204. [Google Scholar] [CrossRef] [PubMed]
- Navaei, F.; Zandi, M.; Ganjloo, A.; Dardmeh, N. Fabrication of Magnetic Nanoparticle-Enhanced Bi-Layer Films: Fe3O4-Loaded Electrospun PVA/Gelatin Nanofibers on a Balangu Seed Mucilage-Gelatin Base. Ind. Crops Prod. 2025, 229, 121037. [Google Scholar] [CrossRef]
- Chen, M.; Yan, T.; Jiang, Q.; Li, J.; Ali, U.; Miao, Y.; Farooq, S.; Hu, Y.; Zhang, H. Real-Time Shrimp Freshness Detection by Anthocyanin-Enriched Wheat Gluten/Gelatin Electrospun Nanofiber Films. Food Chem. 2025, 469, 142542. [Google Scholar] [CrossRef]
- Mozaffari, A.; Parvinzadeh Gashti, M.; Alimohammadi, F.; Pousti, M. The Impact of Helium and Nitrogen Plasmas on Electrospun Gelatin Nanofiber Scaffolds for Skin Tissue Engineering Applications. J. Funct. Biomater. 2024, 15, 326. [Google Scholar] [CrossRef]
- Talib Al-Sudani, B.; Mahmoudi, E.; Adnan Shaker Al-Naymi, H.; Al-Musawi, M.H.; Al-Talabanee, I.B.N.; Ramezani, S.; Ghorbani, M.; Mortazavi Moghadam, F. Antibacterial and Wound Healing Performance of a Novel Electrospun Nanofibers Based on Polymethyl-Methacrylate/Gelatin Impregnated with Different Content of Propolis. J. Drug Deliv. Sci. Technol. 2024, 95, 105641. [Google Scholar] [CrossRef]
- Duan, M.; Sun, J.; Huang, Y.; Jiang, H.; Hu, Y.; Pang, J.; Wu, C. Electrospun Gelatin/Chitosan Nanofibers Containing Curcumin for Multifunctional Food Packaging. Food Sci. Hum. Wellness 2023, 12, 614–621. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; Hassanein, W.S.; Alkabaa, A.S.; Ceylan, Z. Electrospun Eugenol-Loaded Gelatin Nanofibers as Bioactive Packaging Materials to Preserve Quality Characteristics of Beef. Food Packag. Shelf Life 2022, 34, 100968. [Google Scholar] [CrossRef]
- Ekram, B.; Mousa, S.M.; El-Bassyouni, G.T.; Abdel-Hady, B.M.; Moaness, M. Novel Highly Proliferative Electrospun Cerium-Doped Hydroxyapatite/Polyamide/Gelatin Nanofibers for Guided Bone Regeneration Application. Mater. Chem. Phys. 2025, 342, 130975. [Google Scholar] [CrossRef]
- Dadras Chomachayi, M.; Solouk, A.; Akbari, S.; Sadeghi, D.; Mirahmadi, F.; Mirzadeh, H. Electrospun Nanofibers Comprising of Silk Fibroin/Gelatin for Drug Delivery Applications: Thyme Essential Oil and Doxycycline Monohydrate Release Study. J. Biomed. Mater. Res. A 2018, 106, 1092–1103. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, G.; Bonde, G.V.; Mittal, P.; Pandey, V.K.; Yadav, N.; Mishra, B. PLGA/Gelatin-Based Electrospun Nanofiber Scaffold Encapsulating Antibacterial and Antioxidant Molecules for Accelerated Tissue Regeneration. Mater. Today Commun. 2023, 35, 105633. [Google Scholar] [CrossRef]
- Beikzadeh, S.; Hosseini, S.M.; Mofid, V.; Ramezani, S.; Ghorbani, M.; Ehsani, A.; Mortazavian, A.M. Electrospun Ethyl Cellulose/Poly Caprolactone/Gelatin Nanofibers: The Investigation of Mechanical, Antioxidant, and Antifungal Properties for Food Packaging. Int. J. Biol. Macromol. 2021, 191, 457–464. [Google Scholar] [CrossRef]
- Sojobi, J.W.; Bankole, O.L.; Ulelu, D.C.; Fayomi, O.S.I. Examination into Nanomaterials, Nanofibers and Nanoparticles Advances for Wound Healing and Scaffolds in Tissue Engineering Application: A Review. Results Surf. Interfaces 2025, 18, 100380. [Google Scholar] [CrossRef]
- Shin, S.R.; Zihlmann, C.; Akbari, M.; Assawes, P.; Cheung, L.; Zhang, K.; Manoharan, V.; Zhang, Y.S.; Yüksekkaya, M.; Wan, K.; et al. Reduced Graphene Oxide-GelMA Hybrid Hydrogels as Scaffolds for Cardiac Tissue Engineering. Small 2016, 12, 3677–3689. [Google Scholar] [CrossRef]
- Kerscher, P.; Kaczmarek, J.A.; Head, S.E.; Ellis, M.E.; Seeto, W.J.; Kim, J.; Bhattacharya, S.; Suppiramaniam, V.; Lipke, E.A. Direct Production of Human Cardiac Tissues by Pluripotent Stem Cell Encapsulation in Gelatin Methacryloyl. ACS Biomater. Sci. Eng. 2017, 3, 1499–1509. [Google Scholar] [CrossRef]
- Jeong, S.I.; Lee, A.-Y.; Lee, Y.M.; Shin, H. Electrospun Gelatin/Poly(L-Lactide-Co-ε-Caprolactone) Nanofibers for Mechanically Functional Tissue-Engineering Scaffolds. J. Biomater. Sci. Polym. Ed. 2008, 19, 339–357. [Google Scholar] [CrossRef]
- Yang, J.; Deng, C.; Shafiq, M.; Li, Z.; Zhang, Q.; Du, H.; Li, S.; Zhou, X.; He, C. Localized Delivery of FTY-720 from 3D Printed Cell-Laden Gelatin/Silk Fibroin Composite Scaffolds for Enhanced Vascularized Bone Regeneration. Smart Mater. Med. 2022, 3, 217–229. [Google Scholar] [CrossRef]
- Ren, K.; Wang, Y.; Sun, T.; Yue, W.; Zhang, H. Electrospun PCL/Gelatin Composite Nanofiber Structures for Effective Guided Bone Regeneration Membranes. Mater. Sci. Eng. C 2017, 78, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.; Huang, L.; Shen, Y.; Wei, Z.; Li, Y.; Wang, J.; Chen, Z. Application of Gelatin-Based Composites in Bone Tissue Engineering. Heliyon 2024, 10, e36258. [Google Scholar] [CrossRef]
- Cabral, C.S.D.; de Melo-Diogo, D.; Ferreira, P.; Moreira, A.F.; Correia, I.J. Reduced Graphene Oxide–Reinforced Tricalcium Phosphate/Gelatin/Chitosan Light-Responsive Scaffolds for Application in Bone Regeneration. Int. J. Biol. Macromol. 2024, 259, 129210. [Google Scholar] [CrossRef] [PubMed]
- Shams, M.; Nezafati, N.; Hesaraki, S.; Azami, M. Gelatin-Containing Functionally Graded Calcium Sulfate/Bioactive Glass Bone Tissue Engineering Scaffold. Ceram. Int. 2024, 50, 31700–31717. [Google Scholar] [CrossRef]
- Omidian, H. Advancing Gelatin-Polycaprolactone Hybrids for Multifunctional Biomedical Applications. J. Bioact. Compat. Polym. 2025, 40, 303–335. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Pan, Z.; Sun, H.; Wang, J.; Yu, D.; Zhu, S.; Dai, J.; Chen, Y.; Tian, N.; et al. The Effects of Lactate and Acid on Articular Chondrocytes Function: Implications for Polymeric Cartilage Scaffold Design. Acta Biomater. 2016, 42, 329–340. [Google Scholar] [CrossRef]
- Orash Mahmoudsalehi, A.; Soleimani, M.; Stalin Catzim Rios, K.; Ortega-Lara, W.; Mamidi, N. Advanced 3D Scaffolds for Corneal Stroma Regeneration: A Preclinical Progress. J. Mater. Chem. B 2025, 13, 5980–6020. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, M.; Liu, L.; Mithieux, S.M.; Weiss, A.S. Polyglycerol Sebacate-based Elastomeric Materials for Arterial Regeneration. J. Biomed. Mater. Res. A 2024, 112, 574–585. [Google Scholar] [CrossRef]
- Palani, N.; Vijayakumar, P.; Monisha, P.; Ayyadurai, S.; Rajadesingu, S. Electrospun Nanofibers Synthesized from Polymers Incorporated with Bioactive Compounds for Wound Healing. J. Nanobiotechnol. 2024, 22, 211. [Google Scholar] [CrossRef]
- Samadian, H.; Salehi, M.; Farzamfar, S.; Vaez, A.; Ehterami, A.; Sahrapeyma, H.; Goodarzi, A.; Ghorbani, S. In Vitro and in Vivo Evaluation of Electrospun Cellulose Acetate/Gelatin/Hydroxyapatite Nanocomposite Mats for Wound Dressing Applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 964–974. [Google Scholar] [CrossRef]
- Hivechi, A.; Yousefmoumji, H.; Bahrami, S.H.; Brouki Milan, P. Fabrication and Characterization of in Situ Gelling Oxidized Carboxymethyl Cellulose/Gelatin Nanofibers for Wound Healing Applications. Int. J. Biol. Macromol. 2025, 298, 140033. [Google Scholar] [CrossRef]
- Yusuf Aliyu, A.; Adeleke, O.A. Nanofibrous Scaffolds for Diabetic Wound Healing. Pharmaceutics 2023, 15, 986. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.; Zhang, S.; Yang, R.; Sui, C. Enhancing the Mechanical Strength of 3D Printed GelMA for Soft Tissue Engineering Applications. Mater. Today Bio 2024, 24, 100939. [Google Scholar] [CrossRef] [PubMed]
- Naveedunissa, S.; Meenalotchani, R.; Manisha, M.; Ankul Singh, S.; Nirenjen, S.; Anitha, K.; Harikrishnan, N.; Prajapati, B.G. Advances in Chitosan Based Nanocarriers for Targetted Wound Healing Therapies: A Review. Carbohydr. Polym. Technol. Appl. 2025, 11, 100891. [Google Scholar] [CrossRef]
- Chhabra, H.; Deshpande, R.; Kanitkar, M.; Jaiswal, A.; Kale, V.P.; Bellare, J.R. A Nano Zinc Oxide Doped Electrospun Scaffold Improves Wound Healing in a Rodent Model. RSC Adv. 2016, 6, 1428–1439. [Google Scholar] [CrossRef]
- Kwak, H.W.; Woo, H.; Kim, I.-C.; Lee, K.H. Fish Gelatin Nanofibers Prevent Drug Crystallization and Enable Ultrafast Delivery. RSC Adv. 2017, 7, 40411–40417. [Google Scholar] [CrossRef]
- Razzaq, A.; Khan, Z.; Saeed, A.; Shah, K.; Khan, N.; Menaa, B.; Iqbal, H.; Menaa, F. Development of Cephradine-Loaded Gelatin/Polyvinyl Alcohol Electrospun Nanofibers for Effective Diabetic Wound Healing: In-Vitro and In-Vivo Assessments. Pharmaceutics 2021, 13, 349. [Google Scholar] [CrossRef]
- Chen, H.; Fan, L.; Peng, N.; Yin, Y.; Mu, D.; Wang, J.; Meng, R.; Xie, J. Galunisertib-Loaded Gelatin Methacryloyl Hydrogel Microneedle Patch for Cardiac Repair after Myocardial Infarction. ACS Appl. Mater. Interfaces 2022, 14, 40491–40500. [Google Scholar] [CrossRef]
- Carvalho, T.; Bártolo, R.; Pedro, S.N.; Valente, B.F.A.; Pinto, R.J.B.; Vilela, C.; Shahbazi, M.-A.; Santos, H.A.; Freire, C.S.R. Injectable Nanocomposite Hydrogels of Gelatin-Hyaluronic Acid Reinforced with Hybrid Lysozyme Nanofibrils-Gold Nanoparticles for the Regeneration of Damaged Myocardium. ACS Appl. Mater. Interfaces 2023, 15, 25860–25872. [Google Scholar] [CrossRef]
- Loan Khanh, L.; Thanh Truc, N.; Tan Dat, N.; Thi Phuong Nghi, N.; van Toi, V.; Thi Thu Hoai, N.; Ngoc Quyen, T.; Thi Thanh Loan, T.; Thi Hiep, N. Gelatin-Stabilized Composites of Silver Nanoparticles and Curcumin: Characterization, Antibacterial and Antioxidant Study. Sci. Technol. Adv. Mater. 2019, 20, 276–290. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Ismail, A.F.; Aziz, M.; Akbari, M.; Hadisi, Z.; Daroonparvar, M.; Chen, X.B. Antibacterial Activity and in Vivo Wound Healing Evaluation of Polycaprolactone-Gelatin Methacryloyl-Cephalexin Electrospun Nanofibrous. Mater. Lett. 2019, 256, 126618. [Google Scholar] [CrossRef]
- Dongargaonkar, A.A.; Bowlin, G.L.; Yang, H. Electrospun Blends of Gelatin and Gelatin–Dendrimer Conjugates as a Wound-Dressing and Drug-Delivery Platform. Biomacromolecules 2013, 14, 4038–4045. [Google Scholar] [CrossRef]
- Anitha, S.; Nadar, N.R.; Shivakumar, S.; Sharma, S.C.; Siddharth, P.; Surekha, V.V.; Lenka, R.; Rajadurai, S. Harnessing Natural Polymers and Nanoparticles: Synergistic Scaffold Design for Improved Wound Healing. Hybrid. Adv. 2025, 8, 100381. [Google Scholar] [CrossRef]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Ghayour, H.; Ismail, A.F.; Nur, H.; Berto, F. Electrospun Nano-Fibers for Biomedical and Tissue Engineering Applications: A Comprehensive Review. Materials 2020, 13, 2153. [Google Scholar] [CrossRef]
- Canafístula, F.V.C.; Oliveira, M.X.; Araújo, A.J.; Filho, J.D.B.M.; Sá, R.E.; Araujo-Nobre, A.R.; Araújo, S.S.M.; Ribeiro, F.O.S.; Jorge, R.J.B.; Oliveira, A.C.X.; et al. Gelatin-Guar Gum Hydrogel for Topical Application: Cytotoxicity, Antibacterial Activity Against Mrsa, and Non-Irritant Characteristics. Eur. Polym. J. 2025, 235, 114059. [Google Scholar] [CrossRef]
- Gulzar, S.; Tagrida, M.; Prodpran, T.; Benjakul, S. Antimicrobial Film Based on Polylactic Acid Coated with Gelatin/Chitosan Nanofibers Containing Nisin Extends the Shelf Life of Asian Seabass Slices. Food Packag. Shelf Life 2022, 34, 100941. [Google Scholar] [CrossRef]
- Gulzar, S.; Tagrida, M.; Nilsuwan, K.; Prodpran, T.; Benjakul, S. Electrospinning of Gelatin/Chitosan Nanofibers Incorporated with Tannic Acid and Chitooligosaccharides on Polylactic Acid Film: Characteristics and Bioactivities. Food Hydrocoll. 2022, 133, 107916. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Y.; Sun, Z.; Wang, D.; Wu, H.; Du, L.; Wang, D. Preparation and Antibacterial Properties of ε-Polylysine-Containing Gelatin/Chitosan Nanofiber Films. Int. J. Biol. Macromol. 2020, 164, 3376–3387. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Miao, X.; Lan, X.; Luo, J.; Luo, T.; Zhong, Z.; Gao, X.; Mafang, Z.; Ji, J.; Wang, H.; et al. Angelica Essential Oil Loaded Electrospun Gelatin Nanofibers for Active Food Packaging Application. Polymers 2020, 12, 299. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Z.; Sun, Z.; Wang, D.; Liu, F. A Novel Gelatin/Chitosan-Based “Sandwich” Antibacterial Nanofiber Film Loaded with Perillaldehyde for the Preservation of Chilled Chicken. Food Chem. 2025, 465, 142025. [Google Scholar] [CrossRef]
- Wang, D.; Sun, J.; Li, J.; Sun, Z.; Liu, F.; Du, L.; Wang, D. Preparation and Characterization of Gelatin/Zein Nanofiber Films Loaded with Perillaldehyde, Thymol, or ɛ-Polylysine and Evaluation of Their Effects on the Preservation of Chilled Chicken Breast. Food Chem. 2022, 373, 131439. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Z.; He, S.; Liu, J.; Shao, W. Development of an Edible Food Packaging Gelatin/Zein Based Nanofiber Film for the Shelf-Life Extension of Strawberries. Food Chem. 2023, 426, 136652. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.; Sun, Z.; Liu, F.; Du, L.; Wang, D. Preparation and Characterization of Gelatin/Chitosan/3-Phenylacetic Acid Food-Packaging Nanofiber Antibacterial Films by Electrospinning. Int. J. Biol. Macromol. 2021, 169, 161–170. [Google Scholar] [CrossRef]
- Shi, Y.; Cao, X.; Zhu, Z.; Ren, J.; Wang, H.; Kong, B. Fabrication of Cellulose Acetate/Gelatin-Eugenol Core–Shell Structured Nanofiber Films for Active Packaging Materials. Colloids Surf. B Biointerfaces 2022, 218, 112743. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.O.; Leones, A.R.; de Farias, B.S.; da Silva, M.D.; Jaeschke, D.P.; Fernandes, S.S.; Ribeiro, A.C.; Cadaval, T.R.S.; Pinto, L.A.d.A. A Comprehensive Review of Agricultural Residue-Derived Bioadsorbents for Emerging Contaminant Removal. Water 2025, 17, 2141. [Google Scholar] [CrossRef]
- Sultana, M.; Rownok, M.H.; Sabrin, M.; Rahaman, M.H.; Alam, S.M.N. A Review on Experimental Chemically Modified Activated Carbon to Enhance Dye and Heavy Metals Adsorption. Clean. Eng. Technol. 2022, 6, 100382. [Google Scholar] [CrossRef]
- Wang, Z.; Bin Kang, S.; Yun, H.J.; Won, S.W. Efficient Removal of Arsenate from Water Using Electrospun Polyethylenimine/Polyvinyl Chloride Nanofiber Sheets. React. Funct. Polym. 2023, 184, 105514. [Google Scholar] [CrossRef]
- Varghese, A.G.; Paul, S.A.; Latha, M.S. Remediation of Heavy Metals and Dyes from Wastewater Using Cellulose-Based Adsorbents. Environ. Chem. Lett. 2019, 17, 867–877. [Google Scholar] [CrossRef]
- Kollarahithlu, S.C.; Balakrishnan, R.M. Adsorption of Pharmaceuticals Pollutants, Ibuprofen, Acetaminophen, and Streptomycin from the Aqueous Phase Using Amine Functionalized Superparamagnetic Silica Nanocomposite. J. Clean. Prod. 2021, 294, 126155. [Google Scholar] [CrossRef]
- Gonçalves, J.O.; Strieder, M.M.; Silva, L.F.O.; Dos Reis, G.S.; Dotto, G.L. Advanced Technologies in Water Treatment: Chitosan and Its Modifications as Effective Agents in the Adsorption of Contaminants. Int. J. Biol. Macromol. 2024, 270, 132307. [Google Scholar] [CrossRef] [PubMed]
- Di Marcantonio, C.; Chiavola, A.; Dossi, S.; Cecchini, G.; Leoni, S.; Frugis, A.; Spizzirri, M.; Boni, M.R. Occurrence, Seasonal Variations and Removal of Organic Micropollutants in 76 Wastewater Treatment Plants. Process Saf. Environ. Prot. 2020, 141, 61–72. [Google Scholar] [CrossRef]
- Choi, K.J.; Kim, S.G.; Kim, C.W.; Park, J.K. Removal Efficiencies of Endocrine Disrupting Chemicals by Coagulation/Flocculation, Ozonation, Powdered/Granular Activated Carbon Adsorption, and Chlorination. Korean J. Chem. Eng. 2006, 23, 399–408. [Google Scholar] [CrossRef]
- Dong, Y.; Jaleh, B.; Ashrafi, G.; Kashfi, M.; Rhee, K.Y. Mechanical Properties of the Hybrids of Natural (Alginate, Collagen, Chitin, Cellulose, Gelatin, Chitosan, Silk, and Keratin) and Synthetic Electrospun Nanofibers: A Review. Int. J. Biol. Macromol. 2025, 312, 143742. [Google Scholar] [CrossRef]
- Li, G.; Jankowski, W.; Kujawa, J.; Yalcinkaya, B.; Yalcinkaya, F.; Balogh-Weiser, D.; Tóth, G.; Ender, F.; Sepsik, N.; Kujawski, W. Recent Advances in the Preparation and Applications in Separation Processes of Electrospun Nanofiber-Based Materials. J. Environ. Chem. Eng. 2025, 13, 115174. [Google Scholar] [CrossRef]
- Salehi, M.; Sharafoddinzadeh, D.; Mokhtari, F.; Esfandarani, M.S.; Karami, S.; Salehi, M.; Sharafoddinzadeh, D.; Mokhtari, F.; Esfandarani, M.S.; Karami, S. Electrospun Nanofibers for Efficient Adsorption of Heavy Metals from Water and Wastewater. Clean Technol. Recycl. 2021, 1, 1–33. [Google Scholar] [CrossRef]
- Panthi, G.; Park, M.; Kim, H.Y.; Lee, S.Y.; Park, S.J. Electrospun ZnO Hybrid Nanofibers for Photodegradation of Wastewater Containing Organic Dyes: A Review. J. Ind. Eng. Chem. 2015, 21, 26–35. [Google Scholar] [CrossRef]
- Radoor, S.; Karayil, J.; Jayakumar, A.; Siengchin, S. Efficient Removal of Dyes, Heavy Metals and Oil-Water from Wastewater Using Electrospun Nanofiber Membranes: A Review. J. Water Process Eng. 2024, 59, 104983. [Google Scholar] [CrossRef]
- Talukder, M.E.; Pervez, M.N.; Jianming, W.; Stylios, G.K.; Hassan, M.M.; Song, H.; Naddeo, V.; Figoli, A. Ag Nanoparticles Immobilized Sulfonated Polyethersulfone/Polyethersulfone Electrospun Nanofiber Membrane for the Removal of Heavy Metals. Sci. Rep. 2022, 12, 5814. [Google Scholar] [CrossRef] [PubMed]
- Ahmadijokani, F.; Mohammadkhani, R.; Ahmadipouya, S.; Shokrgozar, A.; Rezakazemi, M.; Molavi, H.; Aminabhavi, T.M.; Arjmand, M. Superior Chemical Stability of UiO-66 Metal-Organic Frameworks (MOFs) for Selective Dye Adsorption. Chem. Eng. J. 2020, 399, 125346. [Google Scholar] [CrossRef]
- Gönen, S.Ö.; Erol Taygun, M.; Küçükbayrak, S. Evaluation of the Factors Influencing the Resultant Diameter of the Electrospun Gelatin/Sodium Alginate Nanofibers via Box–Behnken Design. Mater. Sci. Eng. C 2016, 58, 709–723. [Google Scholar] [CrossRef]
- Ahmetoglu, U.; Gungor, M.; Kilic, A. Alginate/Gelatin Blend Fibers for Functional High-Performance Air Filtration Applications. Int. J. Biol. Macromol. 2025, 294, 139389. [Google Scholar] [CrossRef]
- Yang, J.; Yuan, Y.; Zhang, R.; Liu, C.; Zhu, D.; Ji, D. Rapid Detection of Cu2+ and Cr3+ Ions in Industrial Wastewater Using Gelatin-Poly-ε-Caprolactone Nanofiber Fluorescent Sensors. J. Mol. Liq. 2025, 418, 126718. [Google Scholar] [CrossRef]
- Wang, J.; Shi, Q. Poly(Vinyl Alcohol) Nanofiber Incorporated Graphene Oxide/Gelatin Composite Aerogels Modified by Chemical Vapor Deposition with Superwetting Character for Efficient Separation of Oil and Water. Int. J. Biol. Macromol. 2025, 306, 141719. [Google Scholar] [CrossRef] [PubMed]
- Ghorani, B.; Emadzadeh, B.; Rezaeinia, H.; Russell, S.J. Improvements in Gelatin Cold Water Solubility after Electrospinning and Associated Physicochemical, Functional and Rheological Properties. Food Hydrocoll. 2020, 104, 105740. [Google Scholar] [CrossRef]
- Ma, Y.; Qi, P.; Ju, J.; Wang, Q.; Hao, L.; Wang, R.; Sui, K.; Tan, Y. Gelatin/Alginate Composite Nanofiber Membranes for Effective and Even Adsorption of Cationic Dyes. Compos. B Eng. 2019, 162, 671–677. [Google Scholar] [CrossRef]
- Kim, J.; Jung, S.; Kim, Y.; Yoon, J.; Kim, H.; Jin, H.J.; Kwak, H.W. Eco-Friendly Fish Gelatin Nanofibrous Membrane for Effective Cationic Dye Removal. Surf. Interfaces 2025, 72, 107096. [Google Scholar] [CrossRef]
- Laitinen, O.; Liimatainen, H. Gelatin-Reinforced Cellulose Nanofiber Composite Cryogels for Effective Separation of Small Particulate Matter in Air. Mater. Des. 2024, 238, 112654. [Google Scholar] [CrossRef]
- Kadam, V.; Truong, Y.B.; Schutz, J.; Kyratzis, I.L.; Padhye, R.; Wang, L. Gelatin/β–Cyclodextrin Bio–Nanofibers as Respiratory Filter Media for Filtration of Aerosols and Volatile Organic Compounds at Low Air Resistance. J. Hazard. Mater. 2021, 403, 123841. [Google Scholar] [CrossRef]
- Shao, Z.; Shen, R.; Gui, Z.; Xie, J.; Jiang, J.; Wang, X.; Li, W.; Guo, S.; Liu, Y.; Zheng, G. Ethyl Cellulose/Gelatin/β–Cyclodextrin/Curcumin Nanofibrous Membrane with Antibacterial and Formaldehyde Adsorbable Capabilities for Lightweight and High–Performance Air Filtration. Int. J. Biol. Macromol. 2024, 254, 127862. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Dai, Y.; Fang, C.; Xu, L.; Wang, Y.; Zhou, H.; Zhong, X.; Li, Z.; Tao, Q. Electrospun Gelatin Nanofibers in Situ Composite with ZIF-67 for Eco-Friendly and Efficient Uranium Removal. J. Radioanal. Nucl. Chem. 2024, 333, 3841–3855. [Google Scholar] [CrossRef]
- Furuno, K.; Wang, J.; Suzuki, K.; Nakahata, M.; Sakai, S. Gelatin-Based Electrospun Fibers Insolubilized by Horseradish Peroxidase-Catalyzed Cross-Linking for Biomedical Applications. ACS Omega 2020, 5, 21254–21259. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Meng, F.; Zhou, Y.; Mugo, S.M.; Zhang, Q. Graphene Oxide Films Prepared Using Gelatin Nanofibers as Wearable Sensors for Monitoring Cardiovascular Health. Adv. Mater. Technol. 2019, 4, 1900540. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Adhikary, P.; Jana, S.; Biswas, A.; Sencadas, V.; Gupta, S.D.; Tudu, B.; Mandal, D. Electrospun Gelatin Nanofiber Based Self-Powered Bio-e-Skin for Health Care Monitoring. Nano Energy 2017, 36, 166–175. [Google Scholar] [CrossRef]
- Kiefer, R.; Otero, T.F.; Harjo, M.; Le, Q.B. Chemically Polymerized Polypyrrole on Glucose-Porcine Skin Gelatin Nanofiber as Multifunctional Electrochemical Actuator-Sensor-Capacitor. Polymers 2025, 17, 631. [Google Scholar] [CrossRef]
- Deng, Z.; Bao, D.; Jiang, L.; Zhang, X.; Xi, W.; Zheng, W.; Xu, X. A Low Fouling and High Biocompatibility Electrochemical Sensor Based on the Electrospun Gelatin-PLGA-CNTs Nanofibers for Dopamine Detection in Blood. J. Appl. Polym. Sci. 2024, 141, e55969. [Google Scholar] [CrossRef]

| Gelatin Nanofiber Composition/Modification | Antimicrobial Agent/Mechanism | Antimicrobial Activity/Observed Effect | Reference |
|---|---|---|---|
| Gelatin/PMVE/MA/Zinc oxide nanoparticles | Zinc oxide nanoparticles (ZnO) | Antimicrobial action combined with pro-healing cues; nearly 99% wound healing within 10 days. | [85] |
| Gelatin incorporated with Silver Nanoparticles (AgNPs) | Silver Nanoparticles (AgNPs) | Dose-dependent antibacterial activity against Pseudomonas aeruginosa and Staphylococcus aureus. | [81] |
| PLGA/Gelatin methacryloyl loaded with cephalexin | Cephalexin (antibiotic) | Effective, controlled release and protection against Staphylococcus aureus and Escherichia coli. | [82,84] |
| Cellulose acetate/gelatin nanofibers with berberine | Berberine (alkaloid) | Antibacterial activity. | [71] |
| Gelatin/Dendrimer nanofibers | Cationic dendrimer architectures | Antibacterial activity against E. coli and S. aureus via membrane disruption; high biocompatibility. | [83] |
| Gelatin–rhodamine–chlorhexidine systems | Chlorhexidine | Potent activity against S. aureus and P. aeruginosa; broad-spectrum action; localized retention. | [83] |
| Gelatin with Silver Nanoparticles (AgNPs) | Silver Nanoparticles (AgNPs) | Additional antiviral properties to antibacterial effects. | [84] |
| Main Gelatin Nanofiber Type/Composition | Key Biomedical Application Categories | Key Results/Activities (Summary) | References |
|---|---|---|---|
| Pure and Coaxial Gelatin Nanofibers | Tissue Engineering (Vascular, Bone, Cardiac, Neural), Wound Healing. | Mimic ECM, support cell adhesion and proliferation, promote angiogenesis and dermal regeneration. Modulable mechanical properties and degradation rate. | [1,62] |
| Gelatin Methacrylate (GelMA) and its variants | Tissue Engineering (Cardiac, Bone), Targeted Drug Delivery. | High biocompatibility, induce cell differentiation (hiPSCs into cardiomyocytes), improve electrical conductivity and mechanical strength, allow sustained and targeted drug release. | [58,59,61,79] |
| Gelatin Composites with Natural Polymers | Wound Healing (Chronic, Diabetic), Tissue Engineering (Bone, Dermal), Antibacterial/Antiviral Functionalities. | High antibacterial efficacy (MRSA, P. aeruginosa), promote cell migration and accelerated healing, improve mechanical properties and biocompatibility. Structural versatility. | [67,72] |
| Gelatin Composites with Nanoparticles | Antibacterial/Antiviral Functionalities, Drug Delivery, Tissue Engineering (Bone, Vascular). | Broad-spectrum antibacterial/antiviral, controlled ion/drug release, improve mechanical properties (compressive strength, toughness), promote osteogenesis, vascularization, and cytocompatibility. | [64,76,81] |
| Gelatin Nanofibers for Drug Delivery | Controlled Drug Release, Wound Healing, Infection Treatment. | Adaptable release profiles (rapid, sustained), drug protection, pain reduction, angiogenesis, potential for diabetic wound and infection treatment. | [77,78,80] |
| Other Specialized Compositions | Diverse Tissue Engineering, Wound Healing, Specific Functionalities. | Promote cell proliferation and high wound closure, exhibit antibacterial activity and biocompatibility, improve stem cell retention and survival. | [83] |
| Packaging | Nanofiber Film Composition | Main Function | Main Results | Others Result | Reference |
|---|---|---|---|---|---|
| Active | Gelatin (7.2%), chitosan (1.2%), and ε-polylysine (0.15%) | Antibacterial activity | Reduction in E. coli S7, K. pneumoniae B6, S. enteritidis H4, Pseudomonas aeruginosa M5, S. aureus G, and L. monocytogenes L1 | Increased thermal stability and decreased permeability to water vapor and oxygen of the film | [89] |
| Gelatin (8%), chitosan (1.6%), and PLA (2%) | Antibacterial activity | Reduction of more than 4 log CFU/mL of S. enterica Enteritidis and S. aureus in 30 min | Improved nanofiber network structure and morphology, heat capacity, moisture content, water solubility, and water vapor permeability | [94] | |
| Gelatin (30%), cellulose acetate (18%), and eugenol (10%) | Antibacterial activity | Inhibition of greater than 60% of the growth of E. coli and S. aureus within 24 h | Encapsulation efficiency is greater than 70%, with greater tensile strength and improved thermal stability | [95] | |
| Gelatin (18%), zein (2%), glucose (5%), cinnamaldehyde (1.378%), Tween 80 (1.5%), and thymol (0.32%) | Antibacterial activity | DPPH inhibitory activity of 99.9%; and inhibitory effects against E. coli with a bacteriostatic ratio of 67.5%, S. aureus, and L. monocytogenes with an antibacterial ratio of 100% | Keeps strawberries fresh for up to 7 days | [93] | |
| Gelatin (12.5%), zein (2.5%), and perillaldehyde (0.05%) | Antibacterial activity | Larger zones of inhibition for S. aureus (14.3 mm) and S. enteritidis (15.2 mm) | The film absorbed the water from the blood that overflowed from the chilled chicken breast and slowed down the deterioration of the meat | [92] | |
| Smart | Gelatin (25%), wheat gluten protein (25%), and blueberry-derived anthocyanin (22.7%) | Fresh quality monitoring | The film color ranged from white to red, with superior color stability under different temperatures and storage conditions, and a sensitive color response to acetic acid and ammonia gas | Improved thermal stability and mechanical properties were observed with high gelatin content | [48] |
| Active and smart | Gelatin (25%), chitosan (3%), and curcumin (0.3%) | Protect and monitor the freshness of foods of animal origin | DPPH inhibitory activity of 51.2%; and inhibitory effects for S. aureus (17.3 mm), and E. coli (16.1 mm) | Color changes from yellow to reddish orange in the presence of ammonia | [51] |
| Bilayer Films | Main Function | Main Results | Others Result | Reference | |
|---|---|---|---|---|---|
| Film | Nanofiber Film Composition | ||||
| PLA (4%) and nanomaterial (MgO and ZnO, 0.08%) | Gelatin (30%), and eugenol (0.0125%) | Antibacterial and antioxidant activities | Highest antioxidant activity (32.99 mg DPPH/g dry weight), radical scavenging activity (43.80%), and significant microbial growth inhibition with CFU of about 3 log units (E. coli) and 2.5 log units (S. aureus) lower than the control | High encapsulation efficiencies and loading capacity for eugenol | [21] |
| PLA (5%) | Gelatin (20%), chitosan (3%), tannic acid (0.1%), and chitooligosaccharides (0.1%) | Antibacterial and antioxidant activities | Larger zones of inhibition for S. aureus (12.3 mm), L. monocytogenes (14.6 mm), E. coli (19.3 mm), and P. aeruginosa (18.0 mm); and higher DPPH (71.9 mmol TE/g sample) and ABTS (95.4 mmol TE/g sample) radical scavenging activities | Increased mechanical resistance and decreased permeability to water vapor and light transmission | [88] |
| PLA (5%) | Gelatin (20%), chitosan (3%), and nisin (0.4%) | Antibacterial activity | Larger zones of inhibition for S. aureus (20.3 mm), L. monocytogenes (21.7 mm), E. coli (14.3 mm), and P. aeruginosa (12.7 mm) | The TBARS limit was only exceeded after the 12th day, and TVB after the 9th day of storage | [87] |
| Balangu seed mucilage (4%) and gelatin (2%) | Gelatin (2%), PVA (8%), and Fe3O4 nanoparticles (4%) | Antibacterial and antioxidant activities | DPPH inhibitory activity greater than 90%; and significant increase in antimicrobial activity against E. coli (18.2 mm), S. aureus (32.1 mm), C. albicans (14.4 mm), and A. niger (13.2 mm) | Increased the strength, barrier properties, and hydrophobicity of the film surface, and reduced moisture content, water solubility, and swelling rate | [47] |
| Gelatin (1.6%) and chitosan (0.3%) | Gelatin (4%), Tween 80 (1.6%), and perillaldehyde (2%) | Antibacterial activities | Increase in antimicrobial activity against P. lundensis (15.9 mm), A. hydrophila (17.5 mm), and B. thermosphacta (17.6 mm) | Increased shelf life of chilled chicken from 4 to 10 days | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Farias, B.S.; Christ Ribeiro, A.; Jaeschke, D.P.; Ribeiro, E.S.; Gonçalves, J.O.; Vergara, R.F.; Fernandes, S.S.; Dias, D.; Cadaval Jr., T.R.S.; de Almeida Pinto, L.A. Recent Trends in Gelatin Electrospun Nanofibers: Advances in Fabrication, Functionalization, and Applications. Coatings 2025, 15, 1110. https://doi.org/10.3390/coatings15091110
de Farias BS, Christ Ribeiro A, Jaeschke DP, Ribeiro ES, Gonçalves JO, Vergara RF, Fernandes SS, Dias D, Cadaval Jr. TRS, de Almeida Pinto LA. Recent Trends in Gelatin Electrospun Nanofibers: Advances in Fabrication, Functionalization, and Applications. Coatings. 2025; 15(9):1110. https://doi.org/10.3390/coatings15091110
Chicago/Turabian Stylede Farias, Bruna Silva, Anelise Christ Ribeiro, Débora Pez Jaeschke, Eduardo Silveira Ribeiro, Janaína Oliveira Gonçalves, Ricardo Freitas Vergara, Sibele Santos Fernandes, Daiane Dias, Tito Roberto Sant’Anna Cadaval Jr., and Luiz Antonio de Almeida Pinto. 2025. "Recent Trends in Gelatin Electrospun Nanofibers: Advances in Fabrication, Functionalization, and Applications" Coatings 15, no. 9: 1110. https://doi.org/10.3390/coatings15091110
APA Stylede Farias, B. S., Christ Ribeiro, A., Jaeschke, D. P., Ribeiro, E. S., Gonçalves, J. O., Vergara, R. F., Fernandes, S. S., Dias, D., Cadaval Jr., T. R. S., & de Almeida Pinto, L. A. (2025). Recent Trends in Gelatin Electrospun Nanofibers: Advances in Fabrication, Functionalization, and Applications. Coatings, 15(9), 1110. https://doi.org/10.3390/coatings15091110










