Research on the Effect of Argon–Nitrogen Ratio on the Mechanical Properties and Corrosion Behavior of CrN-Ag Self-Lubricating Coatings
Abstract
1. Introduction
2. Experimental Section
2.1. Preparation of CrN-Ag Coating
2.2. Measurement Apparatus and Characterization Methods
- (1)
- Chemical composition and morphology
- (2)
- Structure
- (3)
- Mechanical properties
- (4)
- High-temperature stability
- (5)
- Electrochemical Corrosion
3. Results and Discussion
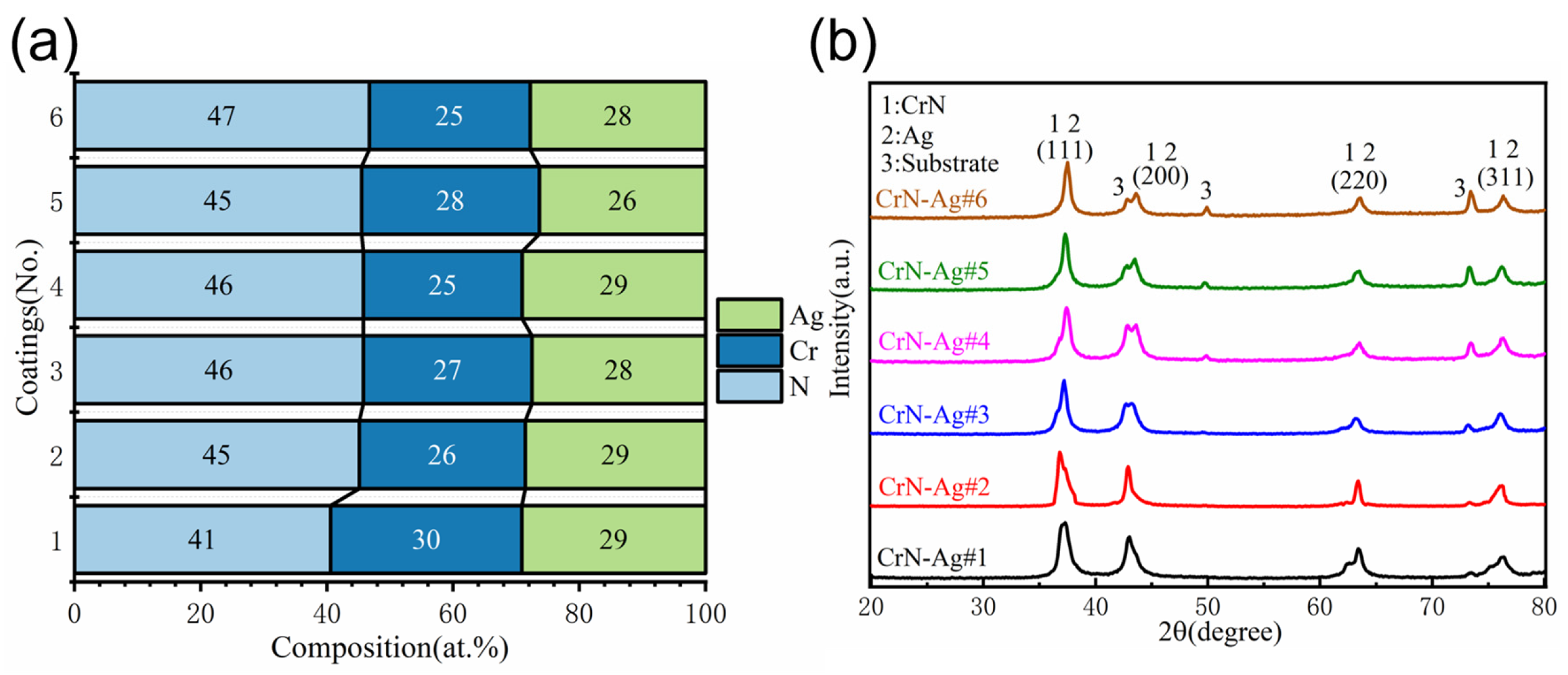
3.1. Morphology, Structure, and Chemical Composition of CrN-Ag Coating
3.2. High-Temperature Stability of CrN-Ag Coating
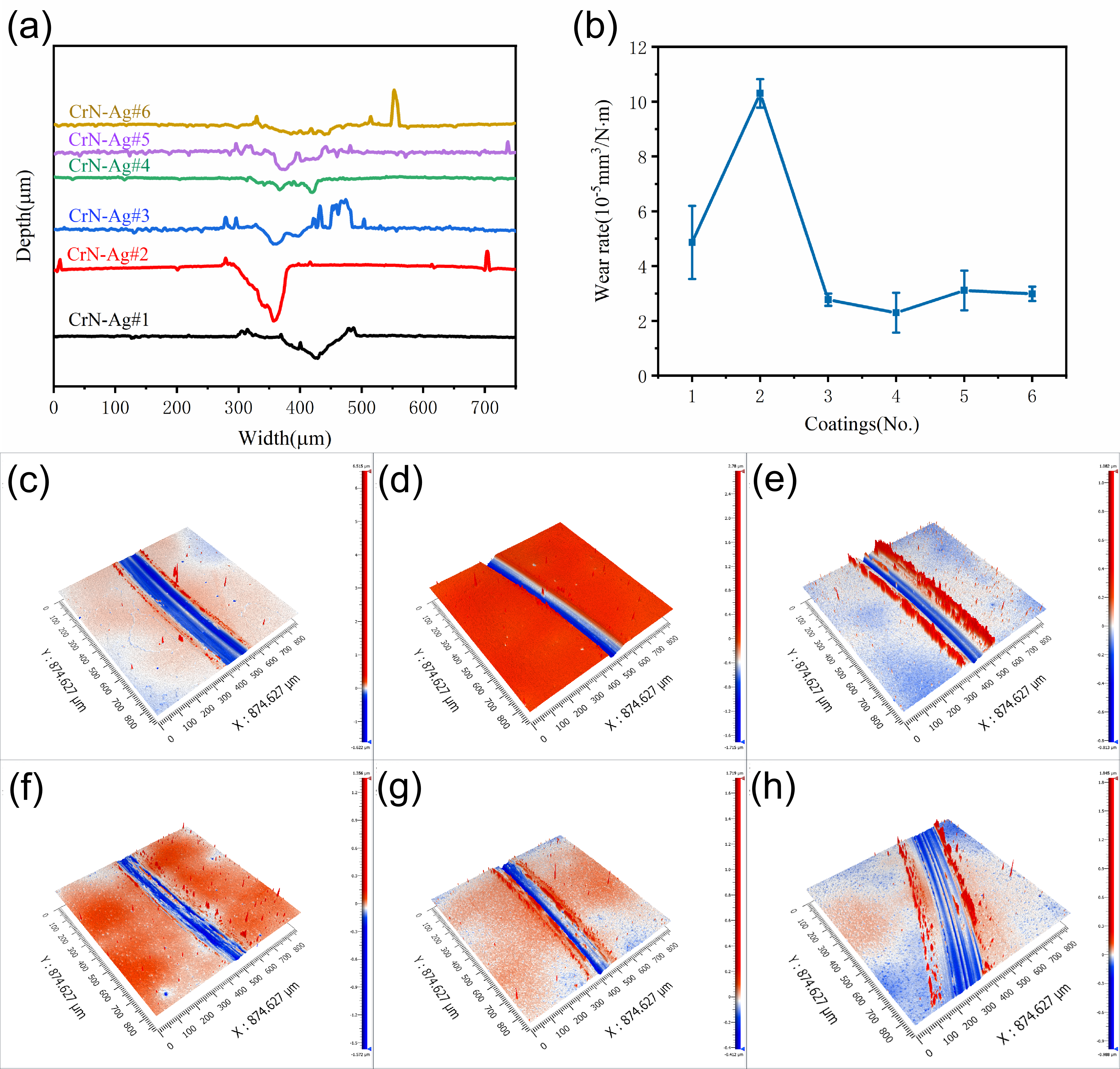
3.3. Mechanical Properties of CrN-Ag Coating
3.4. Corrosion Resistance of CrN-Ag Coating
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Zeng, Z.; Zeng, X.; Pelenovich, V.; Wan, Q.; Pogrebnjak, A.; Xue, L.; Chen, Y.; Yang, B. Hardening mechanism and plastic deformation behavior in super-hard AlCrNbSiTiN high-entropy nitride nanocomposite coatings. Mater. Res. Lett. 2024, 13, 103–112. [Google Scholar] [CrossRef]
- Holmberg, K.; Erdemir, A. Influence of tribology on global energy consumption, costs and emissions. Friction 2017, 5, 263–284. [Google Scholar] [CrossRef]
- Mulligan, C.P.; Gall, D. CrN–Ag self-lubricating hard coatings. Surf. Coat. Technol. 2005, 200, 1495–1500. [Google Scholar] [CrossRef]
- Barshilia, H.C.; Prakash, M.S.; Jain, A.; Rajam, K.S. Structure, hardness and thermal stability of TiAlN and nanolayered TiAlN/CrN multilayer films. Vacuum 2005, 77, 169–179. [Google Scholar] [CrossRef]
- Mulligan, C.P.; Blanchet, T.A.; Gall, D. CrN–Ag nanocomposite coatings: Tribology at room temperature and during a temperature ramp. Surf. Coat. Technol. 2010, 204, 1388–1394. [Google Scholar] [CrossRef]
- Incerti, L.; Rota, A.; Valeri, S.; Miguel, A.; García, J.A.; Rodríguez, R.J.; Osés, J. Nanostructured self-lubricating CrN-Ag films deposited by PVD arc discharge and magnetron sputtering. Vacuum 2011, 85, 1108–1113. [Google Scholar] [CrossRef]
- Zhang, S.; Gong, X.; Yu, R.; Xing, Q.; Xu, L.; Wang, Z. Self-lubrication and wear-resistance mechanism of graphene-modified coatings. Ceram. Int. 2020, 46, 15915–15924. [Google Scholar]
- Lv, X.; Zou, G.; Ling, K.; Yang, W.; Mo, Q.; Li, W. Tribological properties of MAO/MoS2 self-lubricating composite coating by microarc oxidation and hydrothermal reaction. Surf. Coat. Technol. 2021, 406, 126630. [Google Scholar] [CrossRef]
- Li, J.; Chen, B.; Dong, Z.; Yang, B.; Qiu, S.; Zhang, H.; Wang, S.; Zhang, K. Improved tribological performance of epoxy self-lubricating composite coating by BNNSs/Ag. Prog. Org. Coat. 2022, 171, 107020. [Google Scholar] [CrossRef]
- Ren, P.; Zhang, S.; Qiu, J.; Yang, X.; Wang, W.; Li, Y.; Si, Y.; Wang, G.; Wen, M. Self-lubricating behavior of VN coating catalyzed by solute Ag atom under dry friction and oil lubrication. Surf. Coat. Technol. 2021, 409, 126845. [Google Scholar] [CrossRef]
- Shi, X.; Zhai, W.; Wang, M.; Xu, Z.; Yao, J.; Song, S.; Wang, Y. Tribological behaviors of NiAl based self-lubricating composites containing different solid lubricants at elevated temperatures. Wear 2014, 310, 1–11. [Google Scholar] [CrossRef]
- Lv, Y.; Sun, Y.; Deng, C.; Fan, X.; Li, S.; Wang, C.; Yang, Y.; Zhang, Y.; He, C. Preparation, corrosion and tribological properties evolution of self-lubricating Al2O3-TiO2 coatings with superior anti-corrosion. Surf. Coat. Technol. 2025, 498, 131849. [Google Scholar] [CrossRef]
- Liu, X.; Chen, H.; Zhao, Y.; Zhang, J.; Meng, X.; Xiao, G.; Chen, Z.; Yi, M.; Liu, W.; Xu, C. Tribology and mechanical performance of Al2O3/CaF2 self-lubricating ceramics prepared by gelcasting. Ceram. Int. 2025, 51, 20554–20564. [Google Scholar] [CrossRef]
- Wang, W.; Qiu, P.; Ye, S.; Zhang, X.; Niu, M.; Cheng, J.; Guo, Y.; Xie, Q. Investigation of microstructure, mechanical properties, and high-temperature self-lubrication capacities of CaF2/BaF2 reinforced CoCrFeNiV matrix composites. Vacuum 2025, 240, 114516. [Google Scholar] [CrossRef]
- Wang, D.; Tan, H.; Chen, W.; Zhu, S.; Cheng, J.; Yang, J. Tribological behavior of Ni3Al–Ag based self-lubricating alloy with Ag2MoO4 formed by high temperature tribo-chemical reaction. Tribol. Int. 2021, 153, 106659. [Google Scholar] [CrossRef]
- Liu, X.; Shi, X.; Huang, Y.; Deng, X.; Yan, Z.; Xue, B. The Sliding Wear and Frictional Behavior of M50-10 wt.%(Sn-Ag-Cu) Self-Lubricating Materials at Elevated Temperatures. J. Mater. Eng. Perform. 2018, 27, 4291–4299. [Google Scholar] [CrossRef]
- Kumar, R.; Hussainova, I.; Rahmani, R.; Antonov, M. Solid Lubrication at High-Temperatures—A Review. Materials 2022, 15, 1695. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, T.; Mei, H.; Yun, J.M.; Jeong, S.H.; Wang, Q.; Kim, K.H. Effects of Negative Bias Voltage and Ratio of Nitrogen and Argon on the Structure and Properties of NbN Coatings Deposited by HiPIMS Deposition System. Coatings 2018, 8, 10. [Google Scholar] [CrossRef]
- Ren, T.; Fang, H.; Zhao, H.; He, J. Influence of Ag Target Power on Microstructure and Properties of TiN-Si3N4-Ag Composite Coatings. J. Mater. Eng. Perform. 2024, 33, 8425–8433. [Google Scholar] [CrossRef]
- Huang, K.; Cao, X.; Kong, L.; Lu, Z.; Zhang, G.; Ding, Q.; Hu, H. Effect of Ag content on friction and wear properties of Ag and V co-doped CrN coatings at 25–700 °C. Ceram. Int. 2021, 47, 35021–35028. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Huang, T.; Guo, F.; Dai, L.; Liu, Y.; Zhang, B. Tribological Properties of CrN/DLC and CrN Coatings under Different Testing Conditions. Coatings 2024, 14, 1002. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, F.-C.; Chen, W.-C.; Lee, J.-W.; Chang, C.-L. Effect of nitrogen-argon flow ratio on the microstructural and mechanical properties of AlSiN thin films prepared by high power impulse magnetron sputtering. Surf. Coat. Technol. 2017, 320, 138–145. [Google Scholar] [CrossRef]
- Meunier, C.; Vives, S.; Bertrand, G. X-ray diffractometry analysis of r.f.-magnetron-sputtered chromium/chromium nitride coatings. Surf. Coat. Technol. 1998, 107, 149–158. [Google Scholar] [CrossRef]
- Olaya, J.; Rodil, S.; Muhl, S.; Sánchez, E. Comparative study of chromium nitride coatings deposited by unbalanced and balanced magnetron sputtering. Thin Solid Films 2005, 474, 119–126. [Google Scholar] [CrossRef]
- Papi, P.; Mulligan, C.; Gall, D. CrN–Ag nanocomposite coatings: Control of lubricant transport by diffusion barriers. Thin Solid Films 2012, 524, 211–217. [Google Scholar] [CrossRef]
- Mulligan, C.; Papi, P.; Gall, D. Ag transport in CrN–Ag nanocomposite coatings. Thin Solid Films 2012, 520, 6774–6779. [Google Scholar] [CrossRef]
- Mulligan, C.; Blanchet, T.; Gall, D. CrN–Ag nanocomposite coatings: High-temperature tribological response. Wear 2010, 269, 125–131. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Lu, F.-H. Oxidation behavior of chromium nitride films. Thin Solid Films 2006, 515, 2179–2184. [Google Scholar] [CrossRef]
- Moon, J.-J.; Kim, D.-J.; Lee, D.-B. High temperature oxidation of chromium nitrides. Met. Mater. Int. 2002, 8, 211–214. [Google Scholar] [CrossRef]
- Wu, Y.-P.; Chiang, H.-Y.; Hsiang, H.-I. AgCrO2 formation mechanism during silver inner electrode and Fe–Si–Cr alloy powder co-firing in metal multilayer chip power inductors. J. Mater. Sci. Mater. Electron. 2019, 30, 8080–8088. [Google Scholar] [CrossRef]
- Lu, H.; Wu, Y.; Liu, Z.; Wang, H.; Zheng, T.; Guo, J. Tribological performance evaluation of CrAlN-Ag and CrAlN/CrAlN-Ag coatings. Mater. Lett. 2025, 389, 138403. [Google Scholar]
- Mayrhofer, P.H.; Tischler, G.; Mitterer, C. Microstructure and mechanical/thermal properties of Cr–N coatings deposited by reactive unbalanced magnetron sputtering. Surf. Coat. Technol. 2001, 142–144, 78–84. [Google Scholar]
- Yu, X.; Wang, J.; Wang, L.; Huang, W. Fabrication and characterization of CrNbSiTiZr high-entropy alloy films by radio-frequency magnetron sputtering via tuning substrate bias. Surf. Coat. Technol. 2021, 412, 127074. [Google Scholar]
- Wang, J.; Han, B.; Chen, Z.; Hu, C.; Zhang, Q.; Wang, C. Study on tribological oxide mechanism of CoCrFeNiMo high entropy alloy. Wear 2023, 526–527, 204907. [Google Scholar] [CrossRef]
- Fan, Z.; Zhang, Z.; Yu, Z.; Chai, Z.; Chen, J.; Xu, C.; Yuan, M.; Wei, G. A pathway to modify tribological behavior of Ni-W coating in various tribo-pairs: Inhibition of delamination. Wear 2025, 578–579, 206176. [Google Scholar]
- Fillot, N.; Iordanoff, I.; Berthier, Y. Wear modeling and the third body concept. Wear 2007, 262, 949–957. [Google Scholar] [CrossRef]
- Han, X.; Liu, X.; Wang, D.; Wu, Z.; Duan, B. Copper-based tungsten coating by CVD: Microstructure, thermal shock resistance and interfacial bond force. Surf. Coat. Technol. 2021, 426, 127778. [Google Scholar] [CrossRef]
- Behera, S.K.; Kumar P, A.; Dogra, N.; Nosonovsky, M.; Rohatgi, P.K. Effect of Microstructure on Contact Angle and Corrosion of Ductile Iron: Iron–Graphite Composite. Langmuir 2019, 35, 16120–16129. [Google Scholar] [CrossRef]
- McCafferty, E. Validation of corrosion rates measured by the Tafel extrapolation method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, M.; Atrens, A. Measurement of the corrosion rate of magnesium alloys using Tafel extrapolation. Corros. Sci. 2010, 52, 579–588. [Google Scholar] [CrossRef]
- Cai, Q.; Li, S.; Pu, J.; Bai, X.; Wang, H.; Cai, Z.; Wang, X. Corrosion resistance and antifouling activities of silver-doped CrN coatings deposited by magnetron sputtering. Surf. Coat. Technol. 2018, 354, 194–202. [Google Scholar] [CrossRef]







| Coatings | No. | Argon–Nitrogen Ratio (Ar:N2) |
|---|---|---|
| CrN-Ag | 1 | 8:1 |
| 2 | 5:1 | |
| 3 | 2:1 | |
| 4 | 1:1 | |
| 5 | 1:2 | |
| 6 | 1:5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Hu, H.; Ma, X.; Chao, L.; Fu, Z.; Zeng, Z.; Yang, B. Research on the Effect of Argon–Nitrogen Ratio on the Mechanical Properties and Corrosion Behavior of CrN-Ag Self-Lubricating Coatings. Coatings 2025, 15, 1107. https://doi.org/10.3390/coatings15091107
Zhang Y, Hu H, Ma X, Chao L, Fu Z, Zeng Z, Yang B. Research on the Effect of Argon–Nitrogen Ratio on the Mechanical Properties and Corrosion Behavior of CrN-Ag Self-Lubricating Coatings. Coatings. 2025; 15(9):1107. https://doi.org/10.3390/coatings15091107
Chicago/Turabian StyleZhang, Yanbing, Huayong Hu, Xiangdong Ma, Liqing Chao, Zhiping Fu, Zhong Zeng, and Bing Yang. 2025. "Research on the Effect of Argon–Nitrogen Ratio on the Mechanical Properties and Corrosion Behavior of CrN-Ag Self-Lubricating Coatings" Coatings 15, no. 9: 1107. https://doi.org/10.3390/coatings15091107
APA StyleZhang, Y., Hu, H., Ma, X., Chao, L., Fu, Z., Zeng, Z., & Yang, B. (2025). Research on the Effect of Argon–Nitrogen Ratio on the Mechanical Properties and Corrosion Behavior of CrN-Ag Self-Lubricating Coatings. Coatings, 15(9), 1107. https://doi.org/10.3390/coatings15091107





