Novel Poly(butylene succinate-dilinoleic succinate) Films in Packaging Systems for Fresh Cut Chicory
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Films Preparation
2.3. Characterization of Films Properties
2.3.1. Thickness and Mechanical Properties of the Films
2.3.2. UV-Vis Spectroscopic Analysis
2.3.3. Color and Opacity Measurements
2.3.4. Water Vapor Transmission Rate Measurements
2.3.5. Water Contact Angle Measurement
2.3.6. Microscopic Evaluation of Films
2.4. Sachets Preparation
2.5. Microbial Quality Investigation
2.6. Moisture Content and Relative Humidity Investigation
2.7. Microscopic Analysis of Chicory Leaves
2.8. Statistical Analysis
3. Results and Discussion
3.1. Thickness and Mechanical Properties of the Films
3.2. UV-Vis Spectroscopic Analysis
3.3. Color and Opacity Measurements
3.4. WVTR and Water Contact Angle Analysis
3.5. Microscopic Evaluation of Films
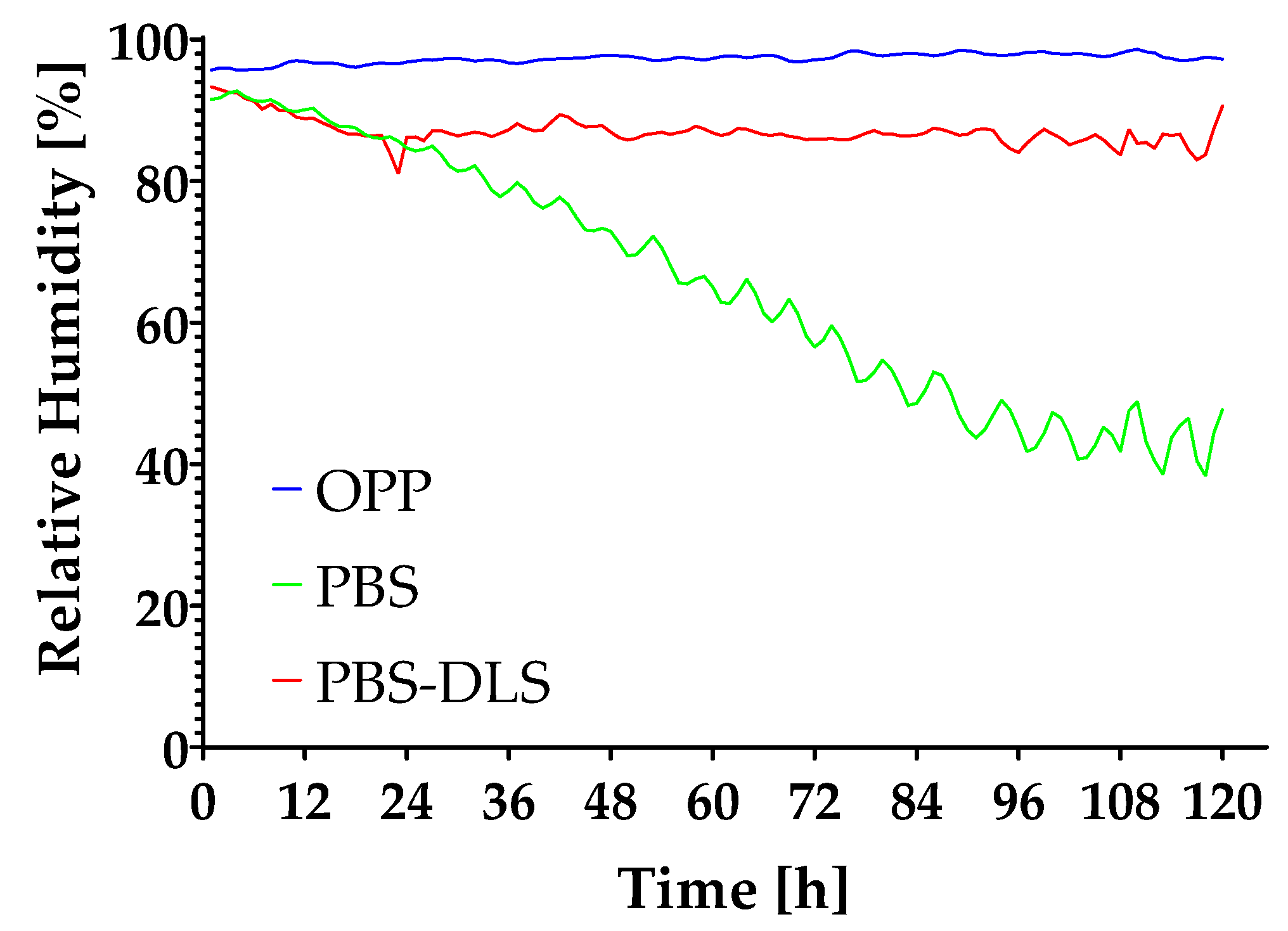
3.6. Moisture Content Investigation
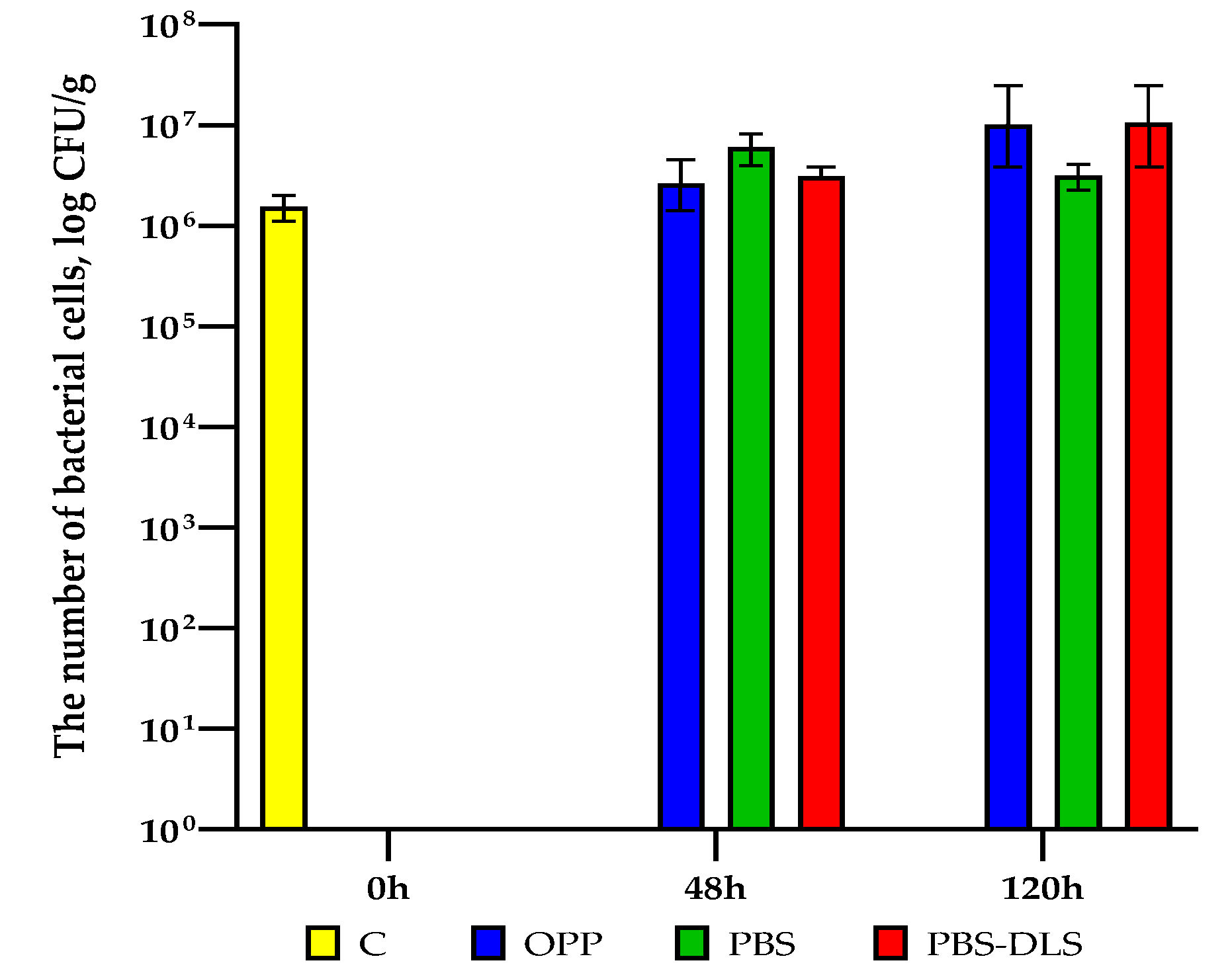
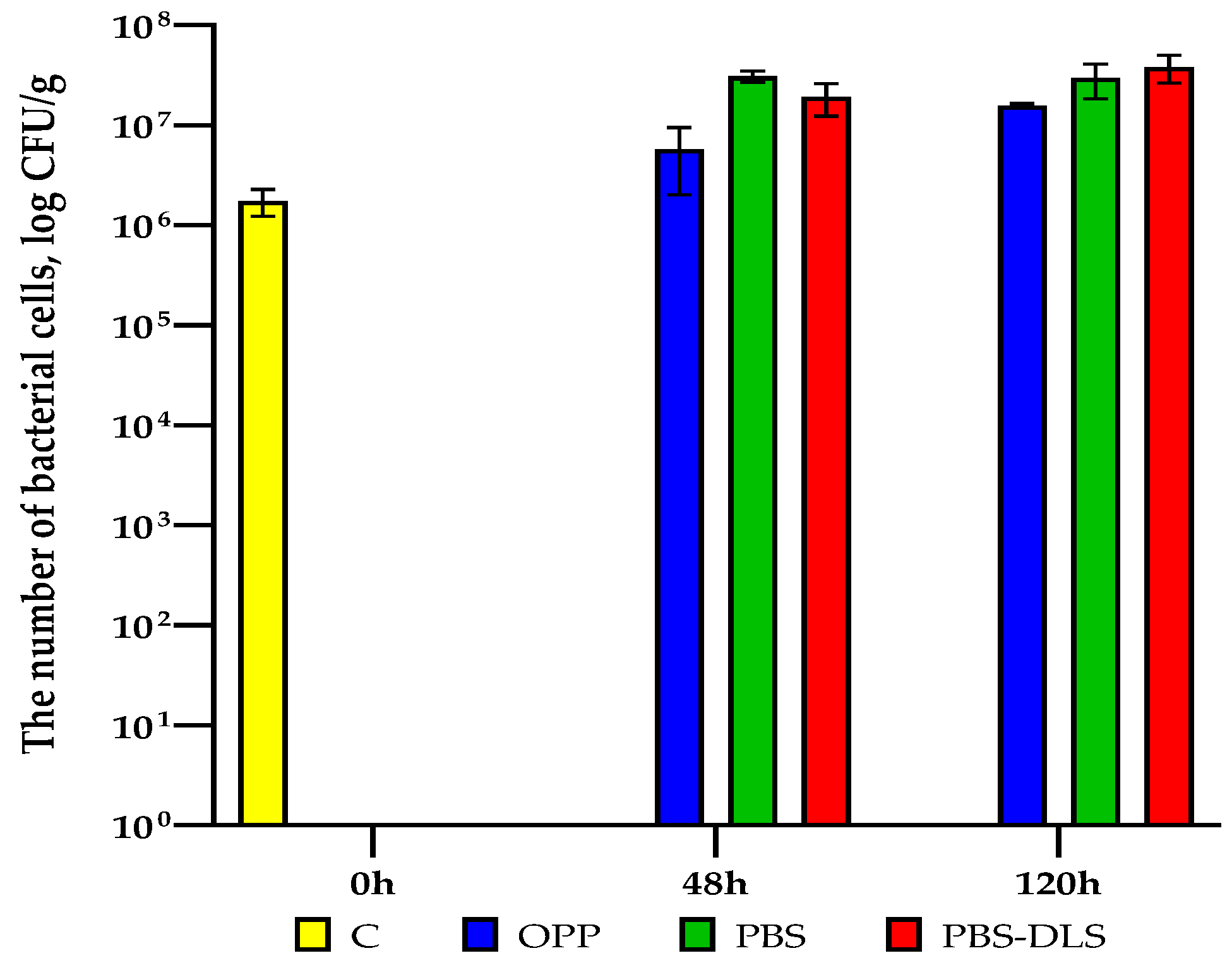
3.7. Microbial Quality Investigation
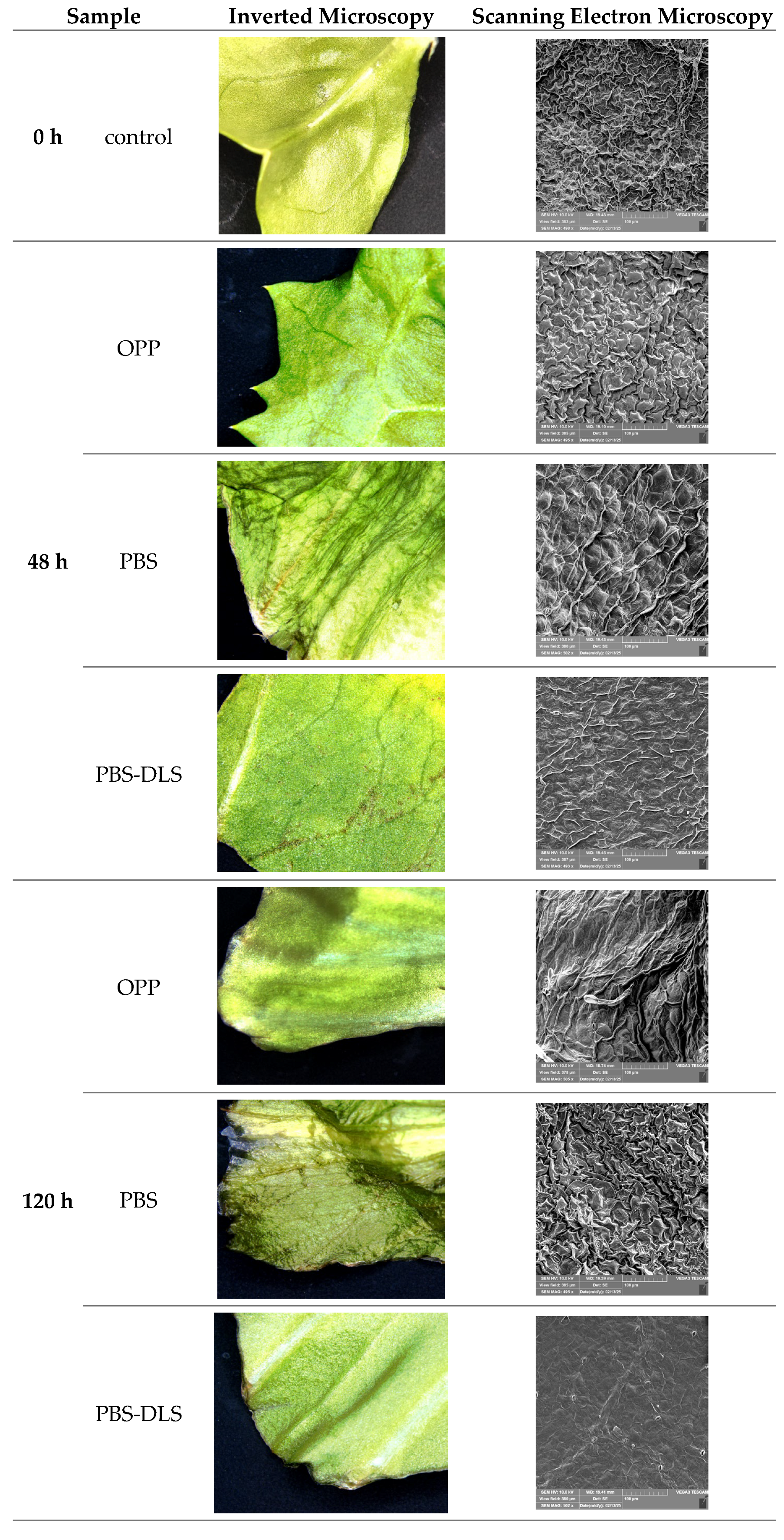
3.8. Microscopic and Scanning Electron Microscopic Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CFU | Colony forming units |
| EB | Elongation at break |
| OPP | Oriented polypropylene |
| PBS | Poly Butylene Succinate |
| PBS-DLS | Poly butylene succinate-co-dilinoleic succianate |
| PCA | Plate Count Agar |
| SEM | Scanning electron microscope |
| TS | Tensile strength |
References
- Dhanraj, N.D.; Hatha, A.A.M.; Jisha, M.S. Biodegradation of Petroleum Based and Bio-Based Plastics: Approaches to Increase the Rate of Biodegradation. Arch. Microbiol. 2022, 204, 258. [Google Scholar] [CrossRef]
- Narancic, T.; O’Connor, K.E. Plastic Waste as a Global Challenge: Are Biodegradable Plastics the Answer to the Plastic Waste Problem? Microbiology 2019, 165, 129–137. [Google Scholar] [CrossRef]
- Kliem, S.; Kreutzbruck, M.; Bonten, C. Review on the Biological Degradation of Polymers in Various Environments. Materials 2020, 13, 4586. [Google Scholar] [CrossRef]
- Conti, I.; Simioni, C.; Varano, G.; Brenna, C.; Costanzi, E.; Neri, L.M. Legislation to Limit the Environmental Plastic and Microplastic Pollution and Their Influence on Human Exposure. Environ. Pollut. 2021, 288, 117708. [Google Scholar] [CrossRef]
- Chen, G.-Q. Introduction of Bacterial Plastics PHA, PLA, PBS, PE, PTT, and PPP. Microbiol. Monogr. 2010, 14, 1–16. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, C.; Lu, A.; Dong, D.; Gong, W. Unveiling the Hidden Impact: How Biodegradable Microplastics Influence CO2 and CH4 Emissions and Volatile Organic Compounds (VOCs) Profiles in Soil Ecosystems. J. Hazard. Mater. 2024, 471, 134294. [Google Scholar] [CrossRef] [PubMed]
- Sadasivuni, K.K.; Saha, P.; Adhikari, J.; Deshmukh, K.; Ahamed, M.B.; Cabibihan, J.J. Recent Advances in Mechanical Properties of Biopolymer Composites: A Review. Polym. Compos. 2020, 41, 32–59. [Google Scholar] [CrossRef]
- Ebhodaghe, S.O.; Ndibe, H. Mechanical Properties of Biopolymers. Handb. Biopolym. 2023, 1, 253–268. [Google Scholar] [CrossRef]
- Gowthaman, N.S.K.; Lim, H.N.; Sreeraj, T.R.; Amalraj, A.; Gopi, S. Advantages of Biopolymers over Synthetic Polymers: Social, Economic, and Environmental Aspects. Biopolym. Their Ind. Appl. 2021, 1, 351–372. [Google Scholar] [CrossRef]
- Oliveira, M.; Abadias, M.; Usall, J.; Torres, R.; Teixidó, N.; Viñas, I. Application of Modified Atmosphere Packaging as a Safety Approach to Fresh-Cut Fruits and Vegetables—A Review. Trends Food Sci. Technol. 2015, 46, 13–26. [Google Scholar] [CrossRef]
- Dey, A.; Dhumal, C.V.; Sengupta, P.; Kumar, A.; Pramanik, N.K.; Alam, T. Challenges and Possible Solutions to Mitigate the Problems of Single-Use Plastics Used for Packaging Food Items: A Review. J. Food Sci. Technol. 2021, 58, 3251–3269. [Google Scholar] [CrossRef]
- Sharma, P. Microplastic Contamination in Food Processing: Role of Packaging Materials. Food Sci. Eng. 2024, 2, 271–287. [Google Scholar] [CrossRef]
- Barletta, M.; Aversa, C.; Ayyoob, M.; Gisario, A.; Hamad, K.; Mehrpouya, M.; Vahabi, H. Poly(Butylene Succinate) (PBS): Materials, Processing, and Industrial Applications. Prog. Polym. Sci. 2022, 132, 101579. [Google Scholar] [CrossRef]
- Cosquer, R.; Pruvost, S.; Gouanvé, F. Improvement of Barrier Properties of Biodegradable Polybutylene Succinate/Graphene Nanoplatelets Nanocomposites Prepared by Melt Process. Membranes 2021, 11, 151. [Google Scholar] [CrossRef]
- Debarshi, N.; Manjusri, M.; Fadi, A.-D.; Mohanty, A.K. Studies on Poly(Butylene Succinate) and Poly(Butylene Succinate- Co -Adipate)-Based Biodegradable Plastics for Sustainable Flexible Packaging and Agricultural Applications: A Comprehensive Review. RSC Sustain. 2025, 3, 1267–1302. [Google Scholar] [CrossRef]
- Rajendran, N.; Han, J. Techno-Economic Analysis and Life Cycle Assessment of Poly (Butylene Succinate) Production Using Food Waste. Waste Manag. 2023, 156, 168–176. [Google Scholar] [CrossRef]
- Rafiqah, S.A.; Khalina, A.; Harmaen, A.S.; Tawakkal, I.A.; Zaman, K.; Asim, M.; Nurrazi, M.N.; Lee, C.H. A Review on Properties and Application of Bio-Based Poly(Butylene Succinate). Polymers 2021, 13, 1436. [Google Scholar] [CrossRef] [PubMed]
- Peñas, M.I.; Pérez-Camargo, R.A.; Hernández, R.; Müller, A.J. A Review on Current Strategies for the Modulation of Thermomechanical, Barrier, and Biodegradation Properties of Poly (Butylene Succinate) (PBS) and Its Random Copolymers. Polymers 2022, 14, 1025. [Google Scholar] [CrossRef]
- Wei, Y.; Li, J.; Shi, D.; Liu, G.; Zhao, Y.; Shimaoka, T. Environmental Challenges Impeding the Composting of Biodegradable Municipal Solid Waste: A Critical Review. Resour. Conserv. Recycl. 2017, 122, 51–65. [Google Scholar] [CrossRef]
- Huang, C.L.; Jiao, L.; Zhang, J.J.; Zeng, J.B.; Yang, K.K.; Wang, Y.Z. Poly(Butylene Succinate)-Poly(Ethylene Glycol) Multiblock Copolymer: Synthesis, Structure, Properties and Shape Memory Performance. Polym. Chem. 2012, 3, 800–808. [Google Scholar] [CrossRef]
- Zarei, M.; El Fray, M. Synthesis of Hydrophilic Poly(Butylene Succinate-Butylene Dilinoleate) (PBS-DLS) Copolymers Containing Poly(Ethylene Glycol) (PEG) of Variable Molecular Weights. Polymers 2021, 13, 3177. [Google Scholar] [CrossRef]
- Stȩpień, K.; Miles, C.; McClain, A.; Wiśniewska, E.; Sobolewski, P.; Kohn, J.; Puskas, J.; Wagner, H.D.; El Fray, M. Biocopolyesters of Poly(Butylene Succinate) Containing Long-Chain Biobased Glycol Synthesized with Heterogeneous Titanium Dioxide Catalyst. ACS Sustain. Chem. Eng. 2019, 7, 10623–10632. [Google Scholar] [CrossRef]
- Macieja, S.; Piegat, A.; Mizielińska, M.; Stefaniak, N.; El Fray, M.; Bartkowiak, A.; Zdanowicz, M. The Effect of the Ratio of Butylene Succinate and Dilinoleic Diol in Their Copolyester (PBS-DLS) on the Physicochemical Properties and Biofilm Formation. Molecules 2025, 30, 1387. [Google Scholar] [CrossRef]
- ISO 2528:2017; Sheet Materials—Determination of Water Vapour Transmission Rate (WVTR)—Gravimetric (Dish). International Standard Organization (ISO): Geneva, Switzerland, 2017. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/07/23/72382.html (accessed on 25 February 2025).
- ISO 4833-2:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 2: Colony Count at 30 °C by the Surface Plating Technique. International Standard Organization (ISO): Geneva, Switzerland, 2013. Available online: https://www.iso.org/standard/59509.html (accessed on 25 February 2025).
- PN-ISO 4831:2007; Mikrobiologia żywności i pasz—Horyzontalna metoda wykrywania i oznaczania liczby bakterii z grupy coli—Metoda najbardziej prawdopodobnej liczby. Wersja Polska. ISO: Geneva, Switzerland, 2007. Available online: https://sklep.pkn.pl/pn-iso-4831-2007p.html (accessed on 25 February 2025).
- PN-ISO 21527-1:2009; Mikrobiologia żywności i pasz—Horyzontalna metoda oznaczania liczby drożdży i pleśni—Część 1: Metoda liczenia kolonii w produktach o aktywności wody wyższej niż 0.95. Wersja Polska. ISO: Geneva, Switzerland, 2009. Available online: https://sklep.pkn.pl/pn-iso-21527-1-2009p.html (accessed on 25 February 2025).
- Macieja, S.; Bartkowiak, A.; Mizielińska, M. Preparation and Characterization of Poly(Butylene Succinate) Films Modified with Sea Buckthorn (Hippophae Rhamnoides L.) Extract for Packaging Applications. Appl. Sci. 2025, 15, 2099. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Zdanowicz, M.; Macieja, S.; Kowalczyk, K.; Bartkowiak, A. Development and Characterization of Bioactive Poly(Butylene-Succinate) Films Modified with Quercetin for Food Packaging Applications. Polymers 2021, 13, 1798. [Google Scholar] [CrossRef]
- Wiles, D.M. Photostabilization of Macromolecules by Excited State Quenching. Pure Appl. Chem. 1978, 50, 291–297. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, S.J.; Lee, H.S.; Choi, J.H.; Jeong, C.M.; Sung, M.H.; Kim, D.H.; Park, H.J. Improvement of Oxygen Barrier of Oriented Polypropylene Films Coated by Gravure Ink-Containing Nanoclays. J. Appl. Polym. Sci. 2011, 121, 1788–1795. [Google Scholar] [CrossRef]
- Vukušić, T.; Vesel, A.; Holc, M.; Ščetar, M.; Jambrak, A.R.; Mozetič, M. Modification of Physico-Chemical Properties of Acryl-Coated Polypropylene Foils for Food Packaging by Reactive Particles from Oxygen Plasma. Materials 2018, 11, 372. [Google Scholar] [CrossRef]
- Kantor-Malujdy, N.; Skowron, S.; Michalkiewicz, B.; El Fray, M. Poly(Butylene-Succinate)-Based Blends with Enhanced Oxygen Permeability. Mater. Today Commun. 2022, 33, 104306. [Google Scholar] [CrossRef]
- Mi Kim, Y.; Hyun Lee, D.; Young Jeong, J.; Ho Jang, S.; Suk Lee, Y.; Chang, M.-S.; Lee, J.-S.; Author, C. Changes in Freshness of Endive (Cichorium Endivia L.) by Different Packaging Types. Korean J. Packag. Sci. Technol. 2016, 22, 71–77. [Google Scholar] [CrossRef]
- Naknaen, P. Utilization Possibilities of Antimicrobial Biodegradable Packaging Produced by Poly(Butylene Succinate) Modified with Zinc Oxide Nanoparticles in Fresh-Cut Apple Slices. Int. Food Res. J. 2014, 21, 2413–2420. [Google Scholar]
- Sun, Y.; Zhao, X.; Ma, Y.; Ma, Z.; He, Z.; Zhao, W.; Wang, P.; Zhao, S.; Wang, D. Investigation on the Microbial Diversity of Fresh-Cut Lettuce during Processing and Storage Using High Throughput Sequencing and Their Relationship with Quality. Foods 2022, 11, 1683. [Google Scholar] [CrossRef]
- Chamorro, J.C.; Denoya, G.I.; Santamaria, B.; Fina, B.; Ferreyra, M.; Cejas, E.; Rodriguez, A.; Vaudagna, S.R.; Prevosto, L. Effects of the Plasma-Activated Water on the Quality and Preservation of Fresh-Cut Lettuce. IEEE Trans. Plasma Sci. 2023, 52, 1936–1946. [Google Scholar] [CrossRef]
- Lavelli, V.; Pagliarini, E.; Ambrosoli, R.; Zanoni, B. Quality of Minimally Processed Red Chicory (Cichorium Intybus L.) Evaluated by Anthocyanin Content, Radical Scavenging Activity, Sensory Descriptors and Microbial Indices. Int. J. Food Sci. Technol. 2009, 44, 994–1001. [Google Scholar] [CrossRef]
- Miceli, A.; Gaglio, R.; Francesca, N.; Ciminata, A.; Moschetti, G.; Settanni, L. Evolution of Shelf Life Parameters of Ready-to-Eat Escarole (Cichorium Endivia Var. Latifolium) Subjected to Different Cutting Operations. Sci. Hortic. 2019, 247, 175–183. [Google Scholar] [CrossRef]
- Raquel Wust Schmitz, F.; de Andrade Paulo, I.; Fernandes de Carvalho, L.; Leandro Bertoli, S.; Krebs de Souza, C. Advanced Methodology for Analysis of Changes in the Storage Lettuce Surface. MOJ Food Process. Technol. 2017, 5, 259–261. [Google Scholar] [CrossRef][Green Version]
- Ru, X.; You, W.; Zhang, J.; Xu, F.; Wu, Z.; Jin, P.; Zheng, Y.; Cao, S. γ-Aminobutyric Acid Treatment Inhibits Browning and Promotes Storage Quality by Regulating Reactive Oxygen Species and Membrane Lipid Metabolism in Fresh-Cut Stem Lettuce. Food Chem. 2024, 459, 140420. [Google Scholar] [CrossRef] [PubMed]


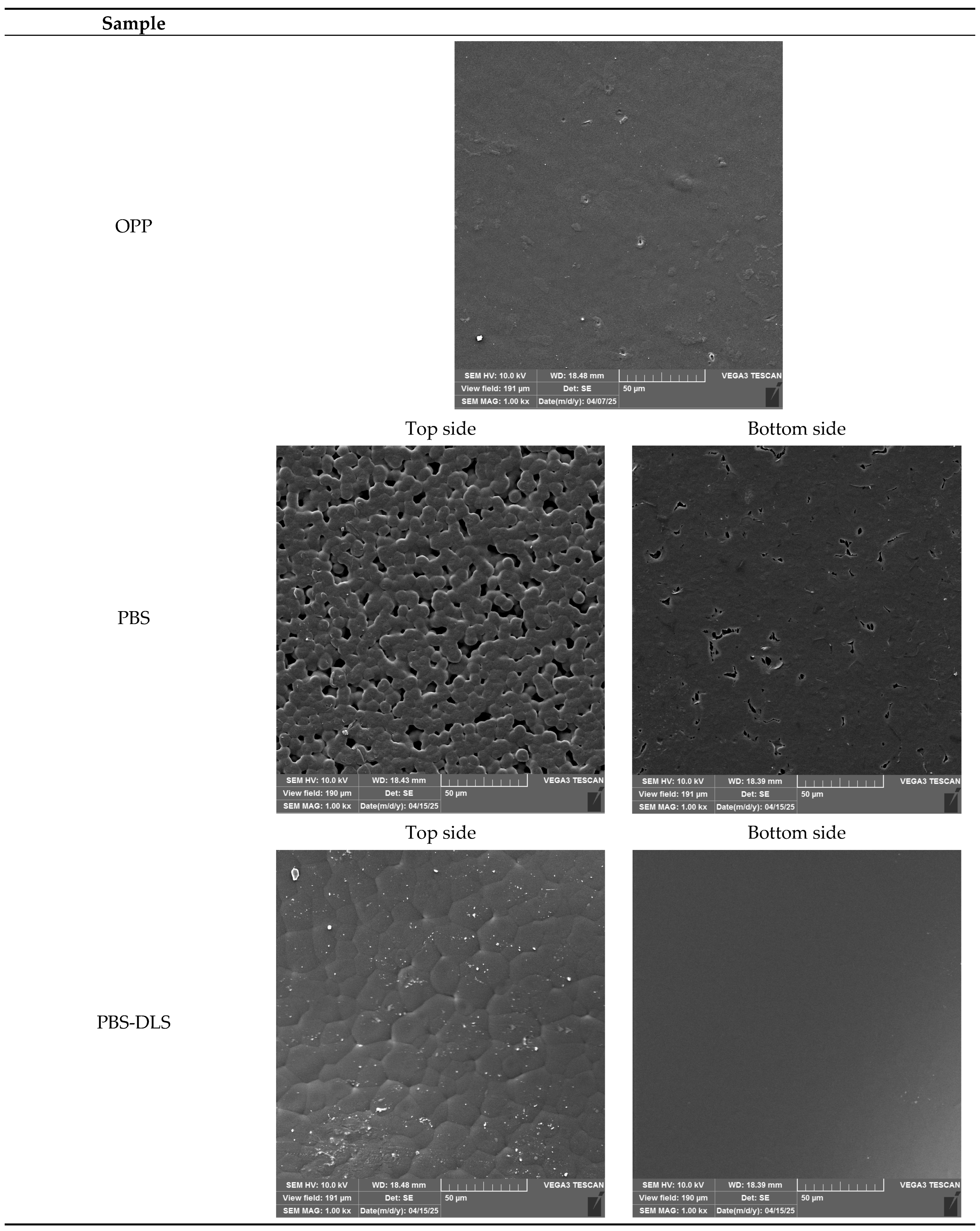


| Sample | Thickness (mm) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|
| OPP | 0.025 ± 0.003 a | 104.36 ± 10.03 b | 231.84 ± 20.30 c |
| PBS | 0.024 ± 0.002 a | 10.96 ± 0.68 a | 38.16 ± 12.36 a |
| PBS-DLS | 0.035 ± 0.004 b | 6.36 ± 0.62 a | 132.30 ± 25.08 b |
| Sample | L* | a* | b* | Opacity | T660 (%) | T280 (%) |
|---|---|---|---|---|---|---|
| OPP | 53.29 ± 0.54 a | 20.41 ± 0.16 a | 42.11 ± 1.72 a | 7.20 ± 0.12 a | 89.33 | 81.56 |
| PBS | 53.93 ± 0.71 b | 20.28 ± 0.30 a | 41.83 ± 0.38 a | 16.30 ± 0.91 b | 17.60 | 6.95 |
| PBS-DLS | 53.69 ± 0.31 a,b | 20.08 ± 0.14 b | 40.43 ± 1.43 b | 7.74 ± 0.09 a | 27.58 | 10.14 |
| Sample | WVTR [g/m2·day] | Average Water Contact Angle [°] |
|---|---|---|
| OPP | 6 ± 4 c | 68 ± 2 a |
| PBS | 1350 ± 294 a | 51 ± 4 b |
| PBS-DLS | 288 ± 26 b | 71 ± 5 a |
| Sample | Moisture Content [%] | |
|---|---|---|
| 0 h | Control | 94.66 ± 0.95 a |
| 48 h | OPP | 92.19 ± 1.93 a |
| PBS | 90.12 ± 0.62 a | |
| PBS-DLS | 92.71 ± 2.26 a | |
| 120 h | OPP | 91.63 ± 2.71 a |
| PBS | 59.73 ± 13.62 b | |
| PBS-DLS | 91.88 ± 0.35 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macieja, S.; Mizielińska, M.; El Fray, M.; Bartkowiak, A. Novel Poly(butylene succinate-dilinoleic succinate) Films in Packaging Systems for Fresh Cut Chicory. Coatings 2025, 15, 1095. https://doi.org/10.3390/coatings15091095
Macieja S, Mizielińska M, El Fray M, Bartkowiak A. Novel Poly(butylene succinate-dilinoleic succinate) Films in Packaging Systems for Fresh Cut Chicory. Coatings. 2025; 15(9):1095. https://doi.org/10.3390/coatings15091095
Chicago/Turabian StyleMacieja, Szymon, Małgorzata Mizielińska, Mirosława El Fray, and Artur Bartkowiak. 2025. "Novel Poly(butylene succinate-dilinoleic succinate) Films in Packaging Systems for Fresh Cut Chicory" Coatings 15, no. 9: 1095. https://doi.org/10.3390/coatings15091095
APA StyleMacieja, S., Mizielińska, M., El Fray, M., & Bartkowiak, A. (2025). Novel Poly(butylene succinate-dilinoleic succinate) Films in Packaging Systems for Fresh Cut Chicory. Coatings, 15(9), 1095. https://doi.org/10.3390/coatings15091095








