Theoretical Insight into the Cooperative Strengthening of Interstitial Atoms(C/N/O) and Cr in Dilute Fe-Cr System
Abstract
1. Introduction
2. Computational Methods
3. Results and Discussion
3.1. The Effect of Cr Occupation on Structural Stability in Fe-Cr System
3.2. The Effect of IAs (C/N/O) on the Thermostability in Fe-Cr System
3.2.1. Site Preference of IAs at Interstitial Sites
3.2.2. The Effect of IAs on the Stability in Cr-Rich Regions of Fe-Cr System
3.2.3. The Diffusion of IA (C/N/O) in Fe-Cr System
3.3. The Interaction Mechanism Between IA (C/N/O) and Cr in Fe53Cr1X
3.3.1. The Occupancy Stability of C/N/O IAs in Fe53Cr1X
3.3.2. An In-Depth Analysis of the Interaction Mechanism Between IAs and Cr
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MSs | martensitic steels |
| IAs | interstitial atoms |
| O-sites | octahedral interstitial sites |
| T-sites | tetrahedral interstitial sites |
| DFT | density functional theory |
| PBE | Perdew–Burke–Ernzerhof |
| GGA | generalized gradient approximation |
| SCF | self-consistent calculation |
| EOS | equation of state |
| ELF | Electron Localization Function |
| COHP | crystal orbital Hamiltonian population |
| CDD | charge density difference |
| BCC Fe | body-centered cubic iron |
| CI-NEB | climbing image nudged elastic band |
| VASP | Vienna ab initio Simulation Package |
| PAW | projector-augmented wave |
| 1NN | the first nearest neighbor |
| 2NN | the second nearest neighbor |
| PDOS | projected density of states |
Appendix A

| K-Mesh | Energy per Atom (eV) | Energy Error (meV) | K-Spacing Value (2π/Å) |
|---|---|---|---|
| 2 × 2 × 2 | −8.428 | −191.315 | 0.050 |
| 3 × 3 × 3 | −8.236 | 0.815 | 0.040 |
| 4 × 4 × 4 | −8.238 | −0.889 | 0.030 |
| 5 × 5 × 5 | −8.236 | 0.630 | 0.025 |
| 6 × 6 × 6 | −8.237 | 0.000 | 0.020 |

| Reaction Coordinate | Em(eV) | ||
|---|---|---|---|
| Fe53Cr1C1 | Fe53Cr1N1 | Fe53Cr1O1 | |
| 0 | 0.000 | 0.000 | 0.000 |
| 1 | 0.834 | 0.719 | 0.493 |
| 2 | 0.321 | 0.148 | −0.044 |
| 3 | 1.420 | 0.652 | 0.136 |
| 4 | 0.158 | −0.116 | −0.345 |
| Configurations | Ef (eV) | Eb (eV) | d (Å) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fe53Cr1C1 | Fe53Cr1N1 | Fe53Cr1O1 | Fe53Cr1C1 | Fe53Cr1N1 | Fe53Cr1O1 | Cr-C | Cr-N | Cr-O | |
| 1 | 0.264 | −0.665 | −1.408 | −0.265 | 0.074 | 0.216 | 1.830 | 1.784 | 1.783 |
| 2 | 0.243 | −0.543 | −1.236 | −0.244 | −0.048 | 0.044 | 2.061 | 1.965 | 1.932 |
| 3 | 0.263 | −0.523 | −1.323 | −0.264 | −0.068 | 0.131 | 1.830 | 1.782 | 1.780 |
| 4 | 0.221 | −0.429 | −1.160 | −0.221 | −0.162 | −0.032 | 2.061 | 1.991 | 1.944 |
| 5 | 0.088 | −0.467 | −1.015 | −0.089 | −0.124 | −0.177 | 3.224 | 3.261 | 3.265 |
| 6 | 0.037 | −0.621 | −1.091 | −0.038 | 0.030 | −0.101 | 3.586 | 3.587 | 3.598 |
| 7 | 0.030 | −0.548 | −1.117 | −0.031 | −0.043 | −0.075 | 4.272 | 4.317 | 4.317 |
References
- Li, J.H.; Zhan, D.P.; Jiang, Z.H.; Zhang, H.S.; Yang, Y.K.; Zhang, Y.P. Progress on improving strength-toughness of ultra-high strength martensitic steels for aerospace applications: A review. J. Mater. Res. Technol. 2023, 23, 172–190. [Google Scholar] [CrossRef]
- Sun, H.; Liu, M.; Zeng, Y.; Liu, J. Influence of Chromium Content in Alloys on Corrosion in Saline Water Saturated with Supercritical CO2. Processes 2025, 13, 1334. [Google Scholar] [CrossRef]
- Singh, R.; Singh, J.; Chauhan, B.S.; Bansal, A.; Aggarwal, V. Microstructure and mechanical properties of the microwave welded dissimilar joints between SS-430 and plain carbon steel. Phys. Scr. 2025, 100, 055902. [Google Scholar] [CrossRef]
- Ei-Labban, H.F.; Mahmoud, E.R.I.; Al-Wadai, H. Formation of VC-composite surface layer on high C-Cr bearing tool steel by laser surface cladding. J. Manuf. Process. 2015, 20, 190–197. [Google Scholar] [CrossRef]
- Russell, Z.; Amegadzie, M.Y.; David, A.M.S.; Plucknett, K.P. Scratch Resistance and Damage Mechanisms Arising in Titanium Carbide-Nickel Aluminide-Based Laser DED Clads on D2 Tool Steel. Coatings 2025, 15, 330. [Google Scholar] [CrossRef]
- Saharudin, M.F.; Zulkifli, N.W.M.; Goh, Y.; Ibrahim, M.Z.; Morina, A.; Mehtab, R. Investigation of the tribological and mechanical properties of FeCrMoCB coating on AISI 52100 bearing steel. Tribol. Int. 2025, 203, 1100401. [Google Scholar] [CrossRef]
- Bolli, E.; Fava, A.; Ferro, P.; Kaciulis, S.; Mezzi, A.; Montanari, R.; Varone, A. Cr Segregation and Impact Fracture in a Martensitic Stainless Steel. Coatings 2020, 10, 843. [Google Scholar] [CrossRef]
- Liu, Z.-b.; Yang, Z.; Liang, J.-x.; Yang, Z.-y.; Sheng, G.-m. Atomic-scale characterization of multiple precipitating species in a precipitation-hardened martensitic stainless steel. J. Iron Steel Res. Int. 2022, 29, 207–214. [Google Scholar] [CrossRef]
- Ye, Z.; Lei, Y.; Zhang, J.; Zhang, Y.; Li, X.; Xu, Y.; Wu, X.; Liu, C.S.; Hao, T.; Wang, Z. Effects of oxygen concentration and irradiation defects on the oxidation corrosion of body-centered-cubic iron surfaces: A first-principles study. Chin. Phys. B 2022, 31, 086802. [Google Scholar] [CrossRef]
- Wang, C.Y.; Luo, K.Y.; Wang, J.; Lu, J.Z. Carbide-facilitated nanocrystallization of martensitic laths and carbide deformation in AISI 420 stainless steel during laser shock peening. Int. J. Plast. 2022, 150, 103191. [Google Scholar] [CrossRef]
- Song, Y.; Lu, Y.; Li, Y.; Sun, H.; Li, H.; Xu, H.; Wang, Y. Effect of nitrogen on the thermal deformation behavior of maternsitic stainless bearing steel. Mater. Tehnol. 2024, 58, 809–818. [Google Scholar] [CrossRef]
- Zhu, H.M.; Hu, W.F.; Li, Y.Z.; Li, B.C.; Qiu, C.J. Effect of Tempering Temperature on Microstructure and Properties of Laser-cladded Martensitic Stainless Steel Layer. Chin. J. Lasers 2019, 46, 1202001. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, H.; Zhang, P.; Wang, Z.; Wang, M.; Sha, G.; Lin, H.; Ma, J.; Zhang, Z.; Song, Y.; et al. An exceptionally strong, ductile and impurity-tolerant austenitic stainless steel prepared by laser additive manufacturing. Acta Mater. 2023, 250, 118868. [Google Scholar] [CrossRef]
- Zhu, H.M.; Ben, X.; Li, B.C.; Shen, L.Z.; Pan, C.L.; Qiu, C.J. Influence of Al microalloying on microstructure and mechanical properties of laser directed energy deposited AISI 420 steel. Mater. Today Commun. 2025, 42, 111355. [Google Scholar] [CrossRef]
- Moriyama, J.; Takakuwa, O.; Yamaguchi, M.; Ogawa, Y.; Tsuzaki, K. The contribution of Cr and Ni to hydrogen absorption energy in Fe-Cr-Ni austenitic systems: A first-principles study. Comp. Mater. Sci. 2024, 232, 112650. [Google Scholar] [CrossRef]
- Shahboub, A.; Chen, P.; Deng, C.; Ding, W.; Zheng, M. Investigation of the anti-corrosion behavior of Fe-Cr oxide layer in LBE: A first-principles study. J. Nucl. Mater. 2025, 605, 155592. [Google Scholar] [CrossRef]
- Shao, Z.; Li, K.; Zhang, Y.; Liao, Z.; Li, C. Effect of Cr/Ni on the structural stability and mechanical properties of Fe-Cr-Ni-Mn alloys. Mater. Today Commun. 2025, 47, 113186. [Google Scholar] [CrossRef]
- Klaver, T.P.C.; Drautz, R.; Finnis, M.W. Magnetism and thermodynamics of defect-free Fe-Cr alloys. Phys. Rev. B 2006, 74, 094435. [Google Scholar] [CrossRef]
- Paduani, C.; Krause, J.C. A first-principles study of Cr impurities in iron. Braz. J. Phys. 2006, 36, 1262–1266. [Google Scholar] [CrossRef]
- Liu, M.; Aiello, A.; Xie, Y.; Sieradzki, K. The Effect of Short-Range Order on Passivation of Fe-Cr Alloys. J. Electrochem. Soc. 2018, 165, C830–C834. [Google Scholar] [CrossRef]
- Piochaud, J.B.; Klaver, T.P.C.; Adjanor, G.; Olsson, P.; Domain, C.; Becquart, C.S. First-principles study of point defects in an fcc Fe-10Ni-20Cr model alloy. Phys. Rev. B 2014, 89, 024101. [Google Scholar] [CrossRef]
- Wang, L.P.; Yang, W.; Ma, Z.B.; Zhu, J.H.; Li, Y.T. First-principles study of chromium diffusion in the ferritic Fe-Cr alloy. Comp. Mater. Sci. 2020, 181, 109733. [Google Scholar] [CrossRef]
- Yang, Z.H.; Zhang, C.; Wang, S.; Xue, C.P.; Tian, G.Y.; Su, H.; Yan, C.M.; Yan, Z.F.; Liu, X.G.; Wang, J.S. Towards quantum corrosion chemistry: Screening perfect Cr, Ni sites and stoichiometry on top of an Fe(110) surface using DFT. RSC Adv. 2023, 13, 9945–9953. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, N.; Henriksson, K.O.E.; Wallenius, J. Carbon impurity dissolution and migration in bcc Fe-Cr: First-principles calculations. Phys. Rev. B 2008, 78, 094110. [Google Scholar] [CrossRef]
- Wallenius, J.; Sandberg, N.; Henriksson, K. Atomistic modelling of the Fe-Cr-C system. J. Nucl. Mater. 2011, 415, 316–319. [Google Scholar] [CrossRef]
- Smirnova, E.A.; Ponomareva, A.V.; Shulyatev, D.A. Investigation of interstitial carbon in the disordered bcc FeCr alloys. J. Alloy Compd. 2024, 994, 174686. [Google Scholar] [CrossRef]
- Lv, Z.; Liu, T.; Wu, Z.; Guan, K.; Li, Y. Structural, electronic and magnetic properties of Fe20Cr6Mn6 austenitic alloys with interstitial carbon and nitrogen atoms from density functional theory. J. Magn. Magn. Mater. 2020, 504, 166663. [Google Scholar] [CrossRef]
- Uesugi, T.; Ashino, S.; Takigawa, Y.; Higashi, K. Interactions between Interstitial and Substitutional Elements of Solute Diatomic and Triatomic Clusters in α-Fe from First-principles Calculations. ISIJ Int. 2024, 64, 1206–1217. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Y.; Wang, W.; Qian, L.; Xiao, S.; Lv, Z. Effect of interstitial nitrogen in Fe18Cr6Mn8 austenitic alloys from density functional theory. J. Magn. Magn. Mater. 2018, 463, 57–63. [Google Scholar] [CrossRef]
- Tong, K.; Ye, F.; Wang, Y.K. Short-range ordered structure and phase stability of supersaturated nitrided layer on austenitic stainless steel. Acta Mater. 2019, 175, 314–323. [Google Scholar] [CrossRef]
- Wang, X.; Posselt, M.; Fassbender, J. Influence of substitutional atoms on the diffusion of oxygen in dilute iron alloys. Phys. Rev. B 2018, 98, 064103. [Google Scholar] [CrossRef]
- Zhang, P.B.; Zou, T.T.; Sun, D.; Yin, Y.; Zhao, J.J. Oxygen interaction with alloying elements (Cr/Ni) and vacancies in dilute austenitic iron alloys: A first-principles study. Fusion. Eng. Des. 2021, 163, 112123. [Google Scholar] [CrossRef]
- Mathayan, V.; Gnanasekaran, J.; Chelladurai, L.; Balakrishnan, S.; Ramalingam, R.; Panigrahi, B.K.; Gangavarapu, A. Oxygen trapping in defect clusters in Fe and FeCr alloy by ion channeling and ab-initio study. J. Nucl. Mater. 2020, 532, 152032. [Google Scholar] [CrossRef]
- Kresse, G. Ab-intio molecular-dynamics for liquid-metals. J. Non-Cryst. Solids. 1995, 193, 222–229. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J.C.; Tang, G.; Geng, W.T. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]
- Henkelman, G.; Uberuaga, B.P.; Jonsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]
- Henkelman, G.; Jonsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 2000, 113, 9978–9985. [Google Scholar] [CrossRef]
- Birch, F. Finite elastic strain of cubic crystals. Phys. Rev. 1947, 71, 809. [Google Scholar] [CrossRef]
- Katsura, T.; Tange, Y. A Simple Derivation of the Birch-Murnaghan Equations of State (EOSs) and Comparison with EOSs Derived from Other Definitions of Finite Strain. Minerals 2019, 9, 745. [Google Scholar] [CrossRef]
- Dronskowski, R.; Bloechl, P.E. Crystal orbital Hamilton populations (COHP): Energy-resolved visualization of chemical bonding in solids based on density-functional calculations. J. Phys. Chem. 1993, 97, 8617–8624. [Google Scholar] [CrossRef]
- Nelson, R.; Ertural, C.; George, J.; Deringer, V.L.; Hautier, G.; Dronskowski, R. LOBSTER: Local orbital projections, atomic charges, and chemical-bonding analysis from projector-augmented-wave-based density-functional theory. J. Comput. Chem. 2020, 41, 1931–1940. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Zhang, Q.; Li, R.; Hong, Y.; Zhang, X. Combined Effects of Carbon and Nitrogen in Fe20Cr6Mn6CN Alloys from First-Principles Calculation. J. Mater. Eng. Perform. 2021, 30, 8322–8335. [Google Scholar] [CrossRef]
- Wang, F.; Mi, T.; Chen, P.; Zhu, H.; Chen, Y.; Zhang, P.; Li, R.; Qiu, C. Synergistic Effect of Hetero Interstitial Atoms (C/N/O) on the Thermodynamic Stability in BCC Fe: A DFT Study. Coatings 2025, 15, 929. [Google Scholar] [CrossRef]
- Zhang, L.; Fu, C.-C.; Lu, G.-H. Energetic landscape and diffusion of He in α-Fe grain boundaries from first principles. Phys. Rev. B 2013, 87, 134107. [Google Scholar] [CrossRef]
- Luo, W.D.; Hou, T.P.; Liang, X.; Cheng, S.; Zheng, Y.H.; Li, Y.; Ye, X.F.; Wu, K.M. Structure and stability investigation of oxygen interaction with Fe in bcc-Fe. Vacuum 2023, 212, 112005. [Google Scholar] [CrossRef]
- Zou, C.; Li, J.; Wang, W.Y.; Zhang, Y.; Tang, B.; Wang, H.; Lin, D.; Wang, J.; Kou, H.; Xu, D. Revealing the local lattice strains and strengthening mechanisms of Ti alloys. Comp. Mater. Sci. 2018, 152, 169–177. [Google Scholar] [CrossRef]
- Raebiger, H.; Lany, S.; Zunger, A. Charge self-regulation upon changing the oxidation state of transition metals in insulators. Nature 2008, 453, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Martinez, E.; Fu, C.-C. Cr interactions with He and vacancies in dilute Fe-Cr alloys from first principles. Phys. Rev. B 2011, 84, 014203. [Google Scholar] [CrossRef]
- Razumovskiy, V.I.; Ruban, A.V.; Korzhavyi, P.A. First-principles study of elastic properties of Cr- and Fe-rich Fe-Cr alloys. Phys. Rev. B 2011, 84, 024106. [Google Scholar] [CrossRef]
- Olsson, P.; Abrikosov, I.A.; Wallenius, J. Electronic origin of the anomalous stability of Fe-rich bcc Fe-Cr alloys. Phys. Rev. B 2006, 73, 104416. [Google Scholar] [CrossRef]
- Nguyen-Manh, D.; Lavrentiev, M.Y.; Dudarev, S.L. Ab initio and Monte Carlo modeling in Fe-Cr system: Magnetic origin of anomalous thermodynamic and kinetic properties. Comp. Mater. Sci. 2008, 44, 1–8. [Google Scholar] [CrossRef]
- Barouh, C.; Schuler, T.; Fu, C.-C.; Nastar, M. Interaction between vacancies and interstitial solutes (C, N, and O) in α-Fe: From electronic structure to thermodynamics. Phys. Rev. B 2014, 90, 054112. [Google Scholar] [CrossRef]
- Kadowaki, M.; Saengdeejing, A.; Muto, I.; Chen, Y.; Masuda, H.; Katayama, H.; Doi, T.; Kawano, K.; Miura, H.; Sugawara, Y.; et al. First-principles analysis of the inhibitive effect of interstitial carbon on an active dissolution of martensitic steel. Corros. Sci. 2020, 163, 108251. [Google Scholar] [CrossRef]
- Fedorov, M.; Wróbel, J.S.; London, A.J.; Kurzydlowski, K.J.; Fu, C.C.; Tadic, T.; Dudarev, S.L.; Nguyen-Manh, D. Precipitation of Cr-rich clusters in Fe-Cr alloys: Effects of irradiation from first principles modeling and experimental observations. J. Nucl. Mater. 2023, 587, 154715. [Google Scholar] [CrossRef]
- Yang, Z.-X.; Liu, W.-B.; Zhang, C.-Y.; He, X.-F.; Sun, Z.-Y.; Jia, L.-X.; Shi, T.-T.; Yun, D. Phase field simulation of grain boundary segregation and radiation-enhanced segregation in Fe-Cr alloys. Acta Phys. Sin.-Chim. Ed. 2021, 70, 20201840. [Google Scholar] [CrossRef]
- Müller, P.C.; Ertural, C.; Hempelmann, J.; Dronskowski, R. Crystal Orbital Bond Index: Covalent Bond Orders in Solids. J. Phys. Chem. C 2021, 125, 7959–7970. [Google Scholar] [CrossRef]
- Fernandez, N.; Ferro, Y.; Kato, D.J.A.M. Hydrogen diffusion and vacancies formation in tungsten: Density Functional Theory calculations and statistical models. Acta Mater. 2015, 94, 307–318. [Google Scholar] [CrossRef]
- Zhu, H.M.; Li, Y.Z.; Li, B.C.; Zhang, Z.Y.; Qiu, C.J. Effects of Low-Temperature Tempering on Microstructure and Properties of the Laser-Cladded AISI 420 Martensitic Stainless Steel Coating. Coatings 2018, 8, 415. [Google Scholar] [CrossRef]
- Zhao, D.; Yang, Q.; Wang, D.; Yan, M.; Wang, P.; Jiang, M.; Liu, C.; Diao, D.; Lao, C.; Chen, Z.; et al. Ordered nitrogen complexes overcoming strength-ductility trade-off in an additively manufactured high-entropy alloy. Virtual Phys. Prototy. 2020, 15, 532–542. [Google Scholar] [CrossRef]

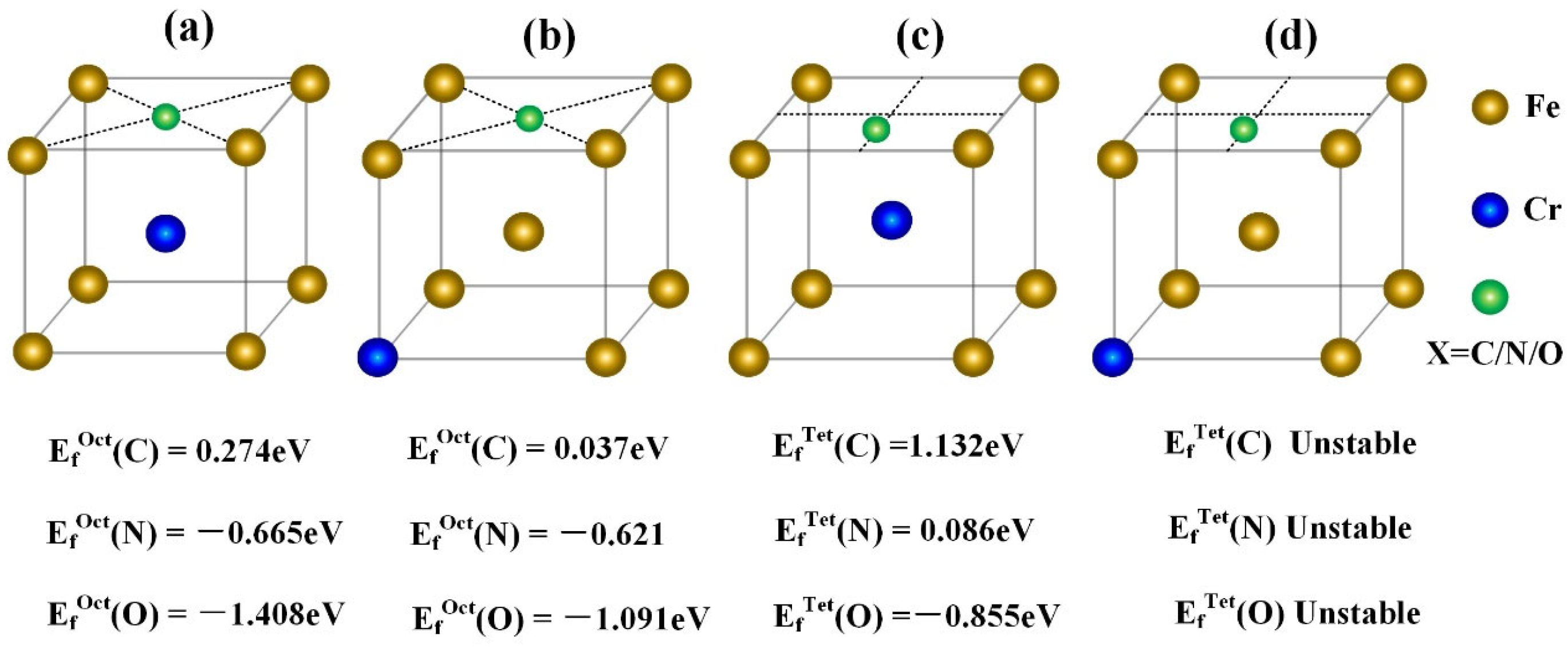
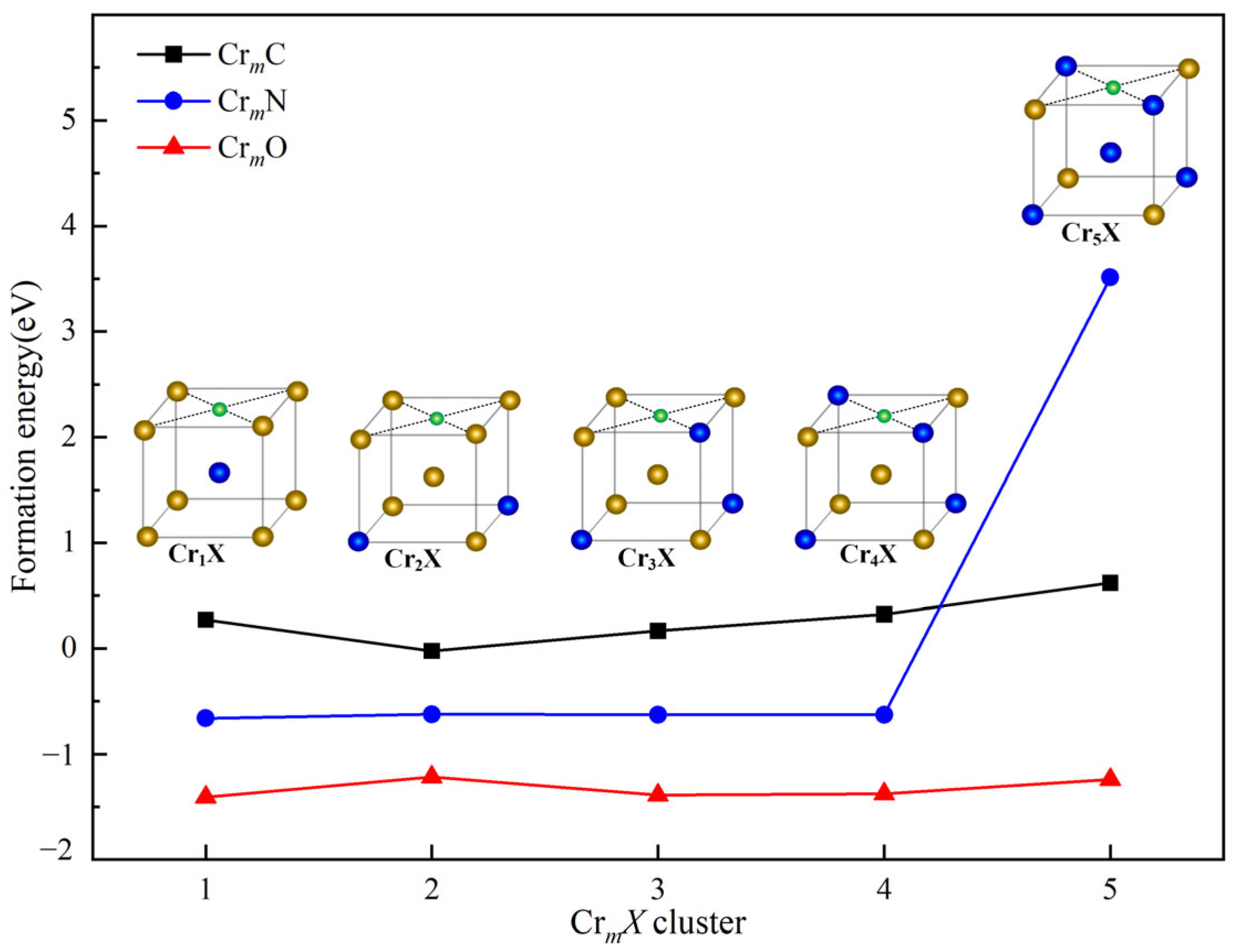
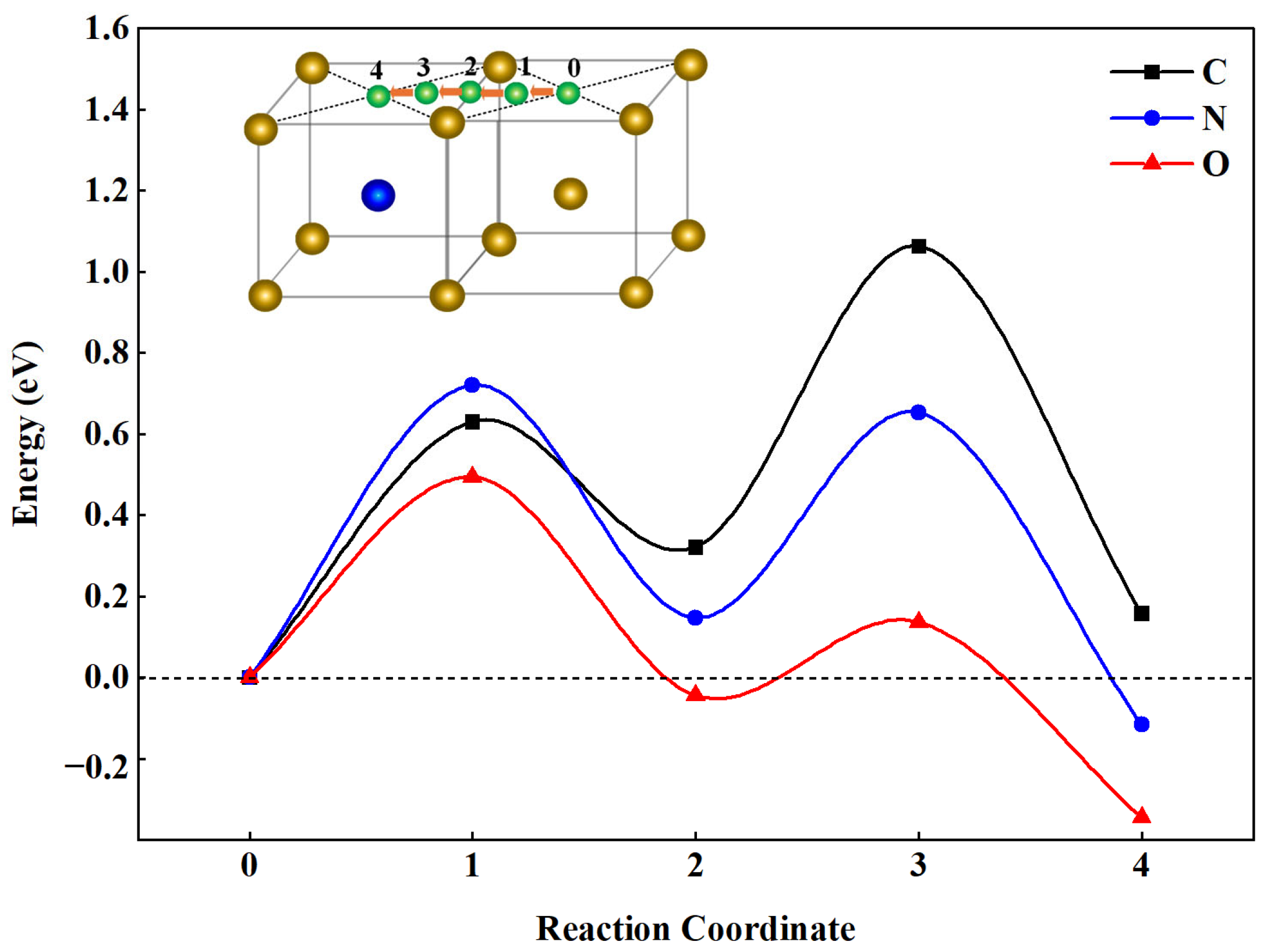

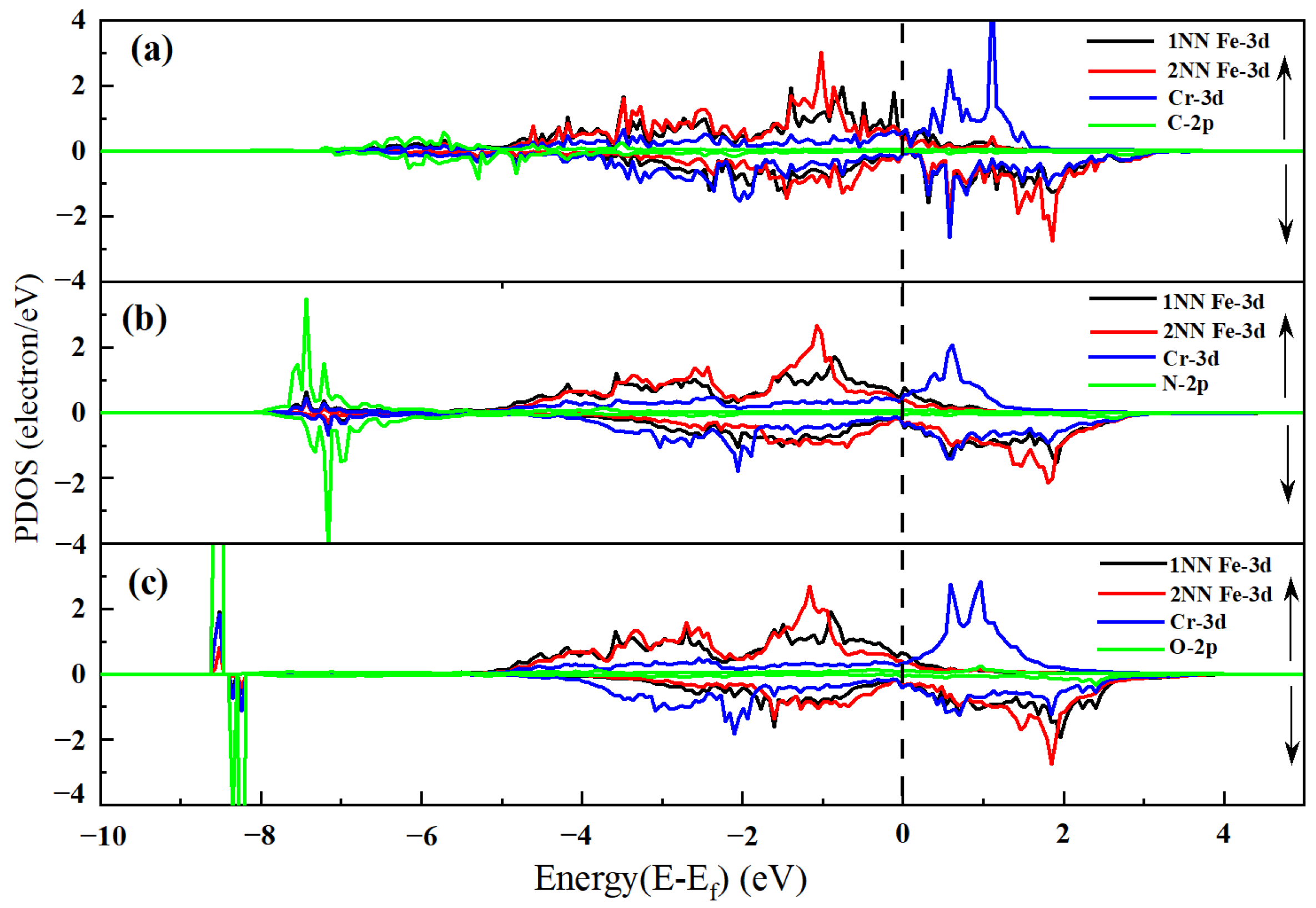
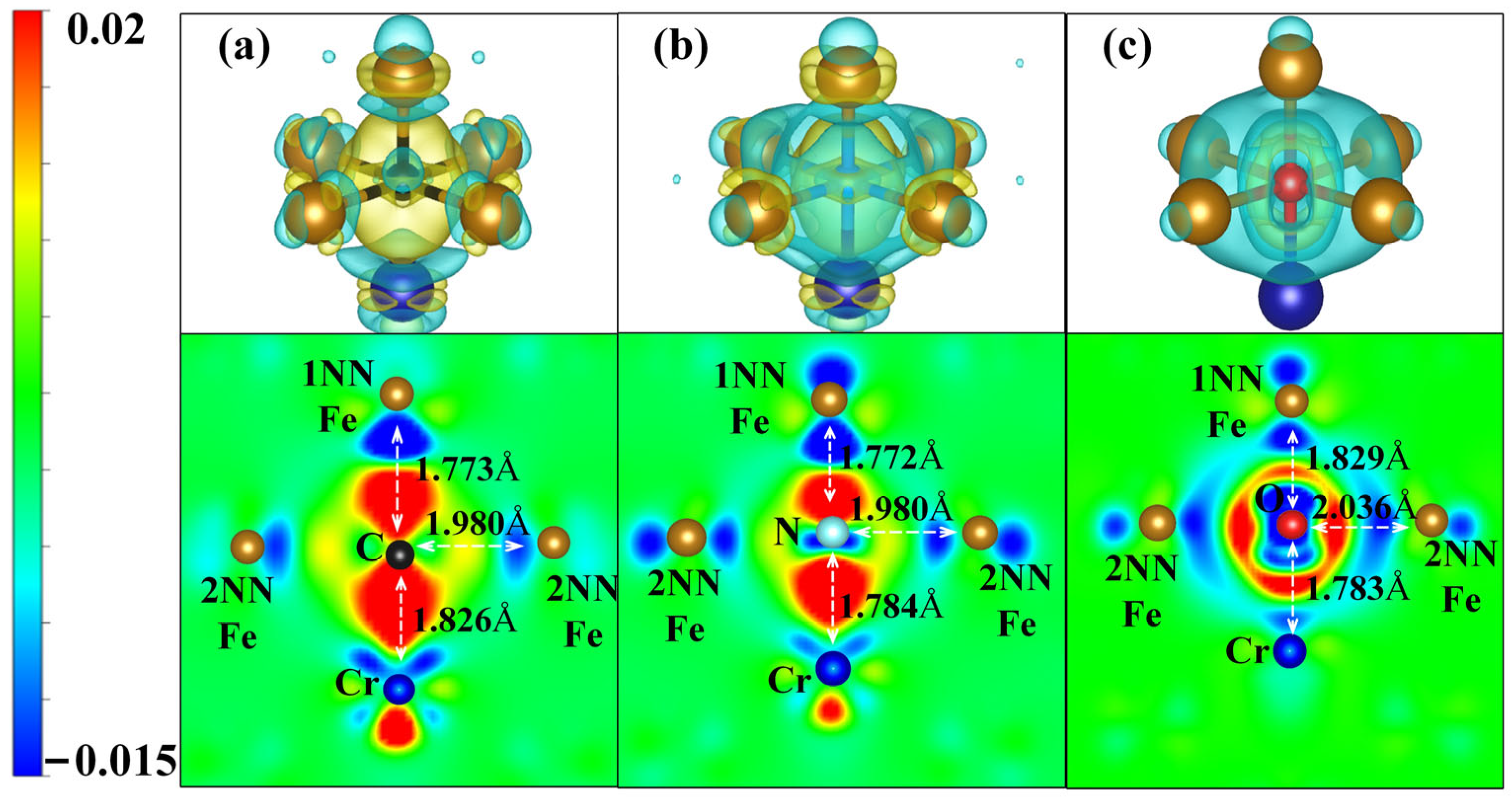
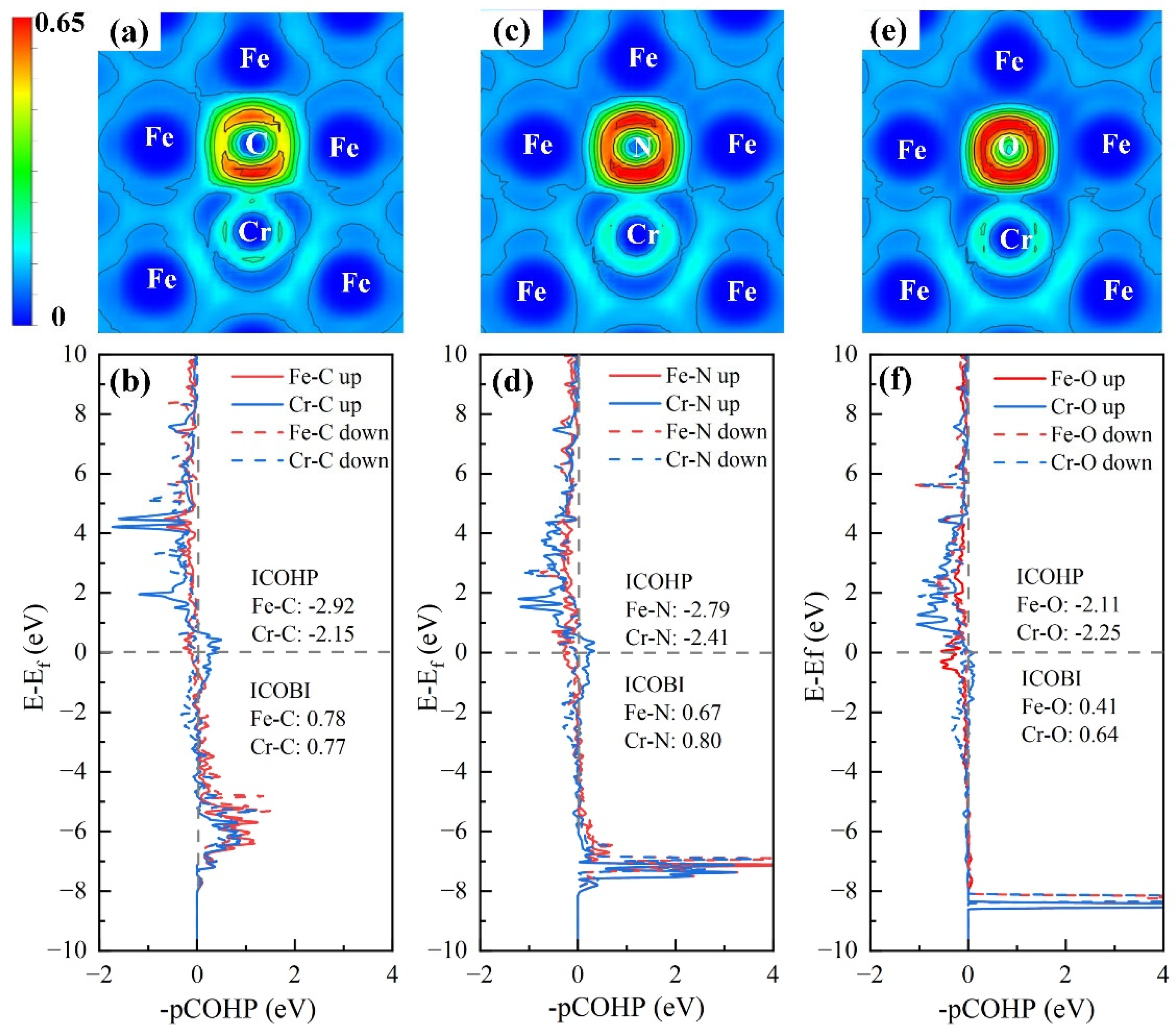
| Alloys | ∆V (Å3) | B (GPa) | M (μB) | Ef (eV) | Eld (eV) |
|---|---|---|---|---|---|
| Fe54 | 0.000 | 189.240 | 2.200 | - | - |
| Cr1-1 | 2.518 | 183.886 | 2.158 | −0.648 | −1.465 |
| Cr1-2 | 2.503 | 184.079 | 2.158 | −0.646 | −1.463 |
| Cr2-1 | 5.266 | 179.318 | 2.114 | −0.661 | −2.776 |
| Cr2-2 | 4.473 | 181.64 | 2.109 | −0.557 | −2.762 |
| Cr2-3 | 5.577 | 177.942 | 2.112 | −0.782 | −2.897 |
| Cr2-4 | 5.268 | 178.323 | 2.108 | −0.777 | −2.892 |
| Cr2-5 | 5.267 | 178.67 | 2.115 | −0.662 | −2.777 |
| Eb | Em | Edis | Tdis (K) | |
|---|---|---|---|---|
| Single C | - | 0.926 * | 0.926 | 325 |
| Single N | - | 0.784 * | 0.784 | 281 |
| Single O | - | 0.557 * | 0.557 | 202 |
| Cr1C→Cr + C | −0.265 | 1.262 | 0.997 | 347 |
| Cr1C→Cr + N | 0.074 | 0.768 | 0.842 | 302 |
| Cr1O→Cr + O | 0.223 | 0.481 | 0.704 | 254 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Mi, T.; Chen, P.; Zhu, H.; Chen, Y.; Zhang, P.; Li, R.; Qiu, C. Theoretical Insight into the Cooperative Strengthening of Interstitial Atoms(C/N/O) and Cr in Dilute Fe-Cr System. Coatings 2025, 15, 1096. https://doi.org/10.3390/coatings15091096
Wang F, Mi T, Chen P, Zhu H, Chen Y, Zhang P, Li R, Qiu C. Theoretical Insight into the Cooperative Strengthening of Interstitial Atoms(C/N/O) and Cr in Dilute Fe-Cr System. Coatings. 2025; 15(9):1096. https://doi.org/10.3390/coatings15091096
Chicago/Turabian StyleWang, Fang, Tengge Mi, Pinghu Chen, Hongmei Zhu, Yong Chen, Pengbo Zhang, Ruiqing Li, and Changjun Qiu. 2025. "Theoretical Insight into the Cooperative Strengthening of Interstitial Atoms(C/N/O) and Cr in Dilute Fe-Cr System" Coatings 15, no. 9: 1096. https://doi.org/10.3390/coatings15091096
APA StyleWang, F., Mi, T., Chen, P., Zhu, H., Chen, Y., Zhang, P., Li, R., & Qiu, C. (2025). Theoretical Insight into the Cooperative Strengthening of Interstitial Atoms(C/N/O) and Cr in Dilute Fe-Cr System. Coatings, 15(9), 1096. https://doi.org/10.3390/coatings15091096










