The Effect of LaPO4 Crystal Morphology on Gas-Phase Catalytic Synthesis of Anisole
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Synthesis
2.2. Evaluation of Catalytic Performance
2.3. Characterization
2.4. Calculation of Adsorption Energy and Bond Length
3. Results and Discussion
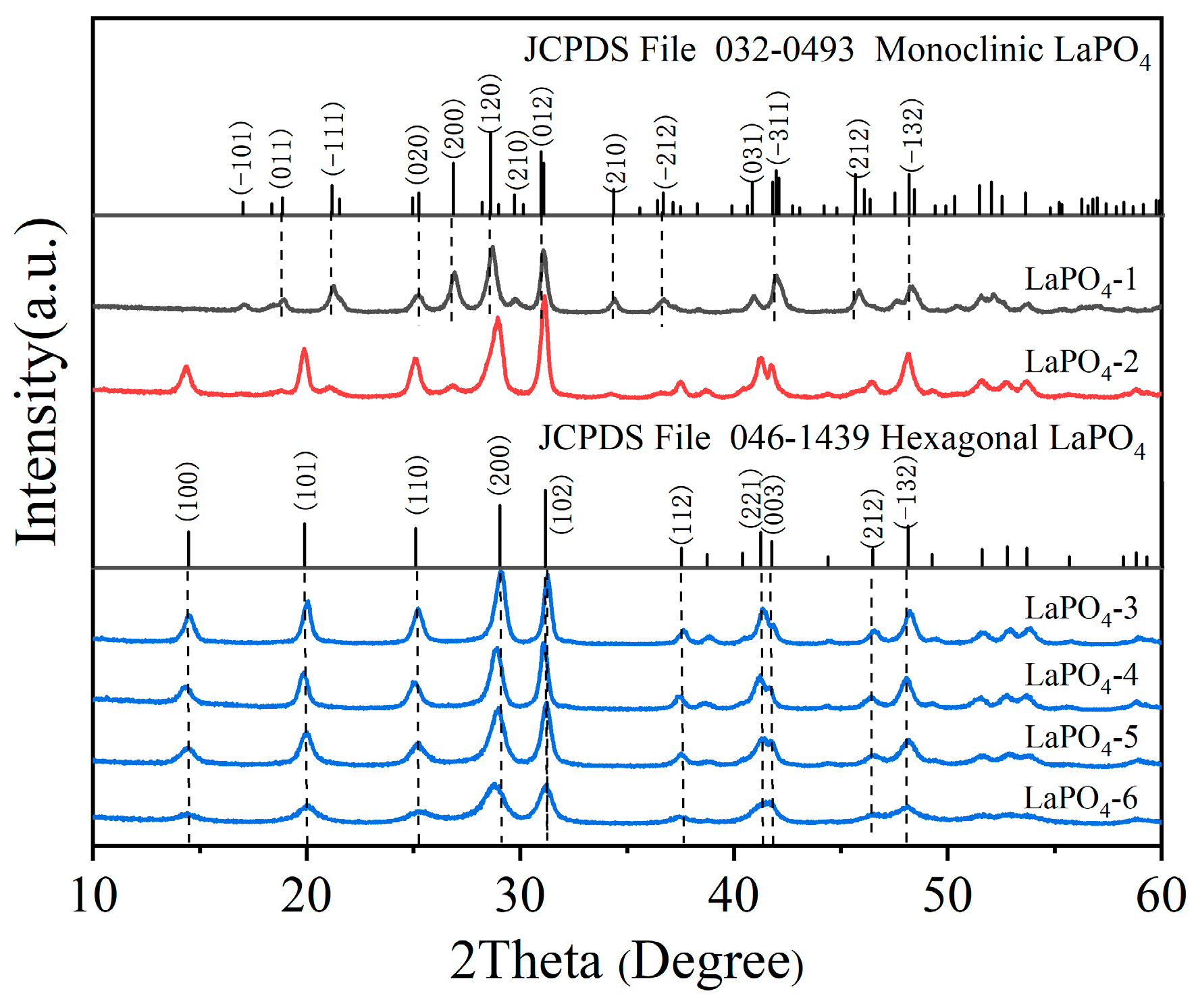
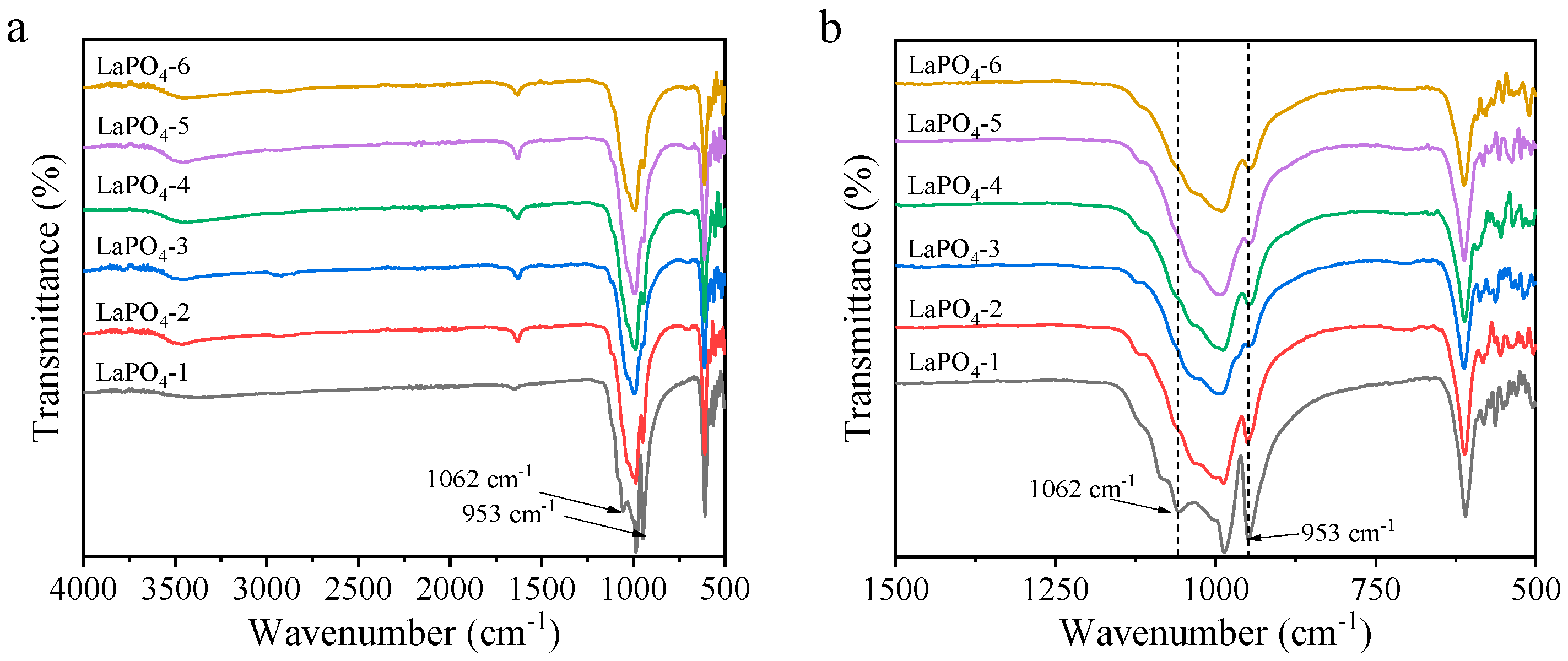
3.1. Structural Properties of LaPO4 Samples
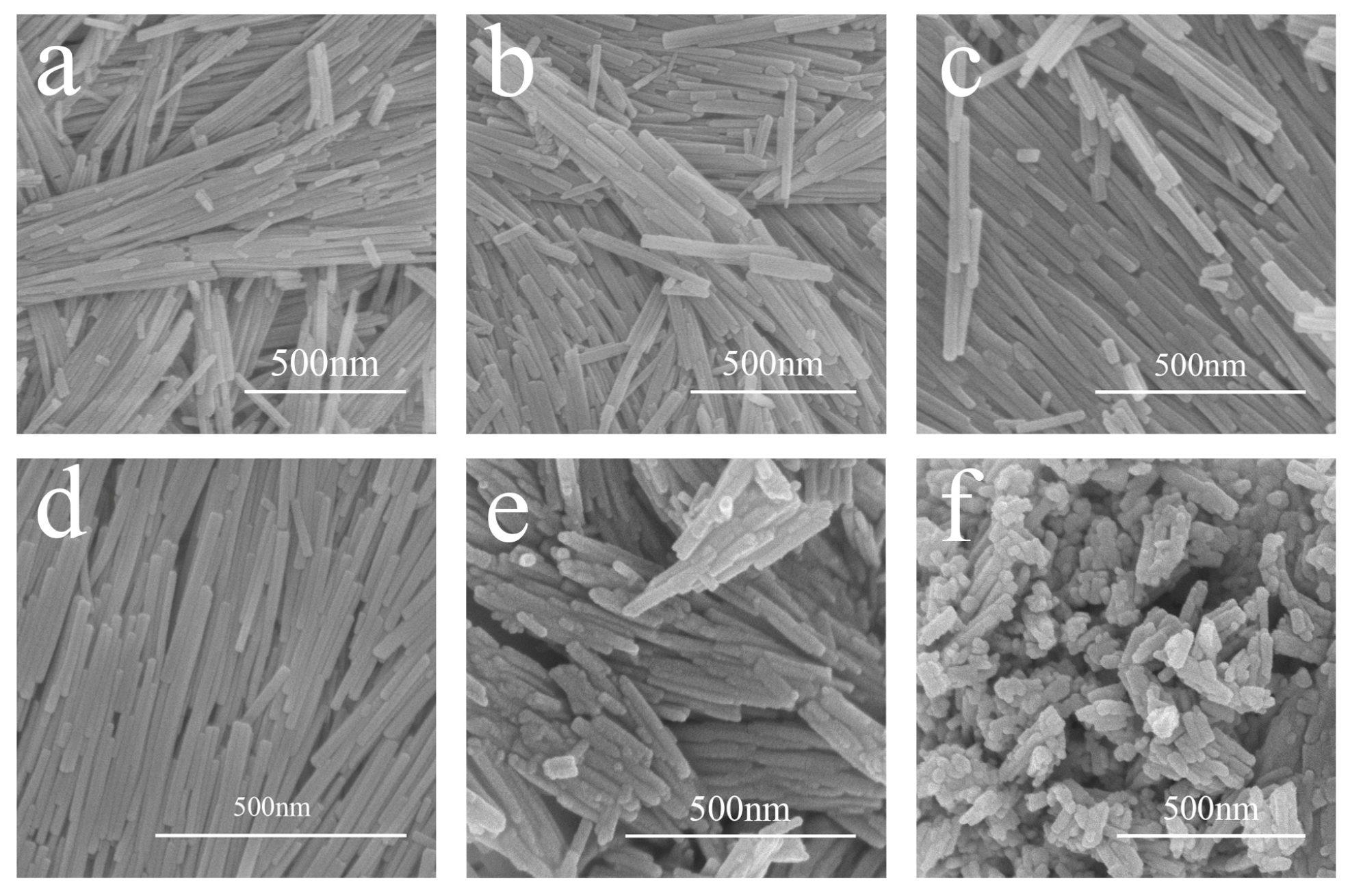
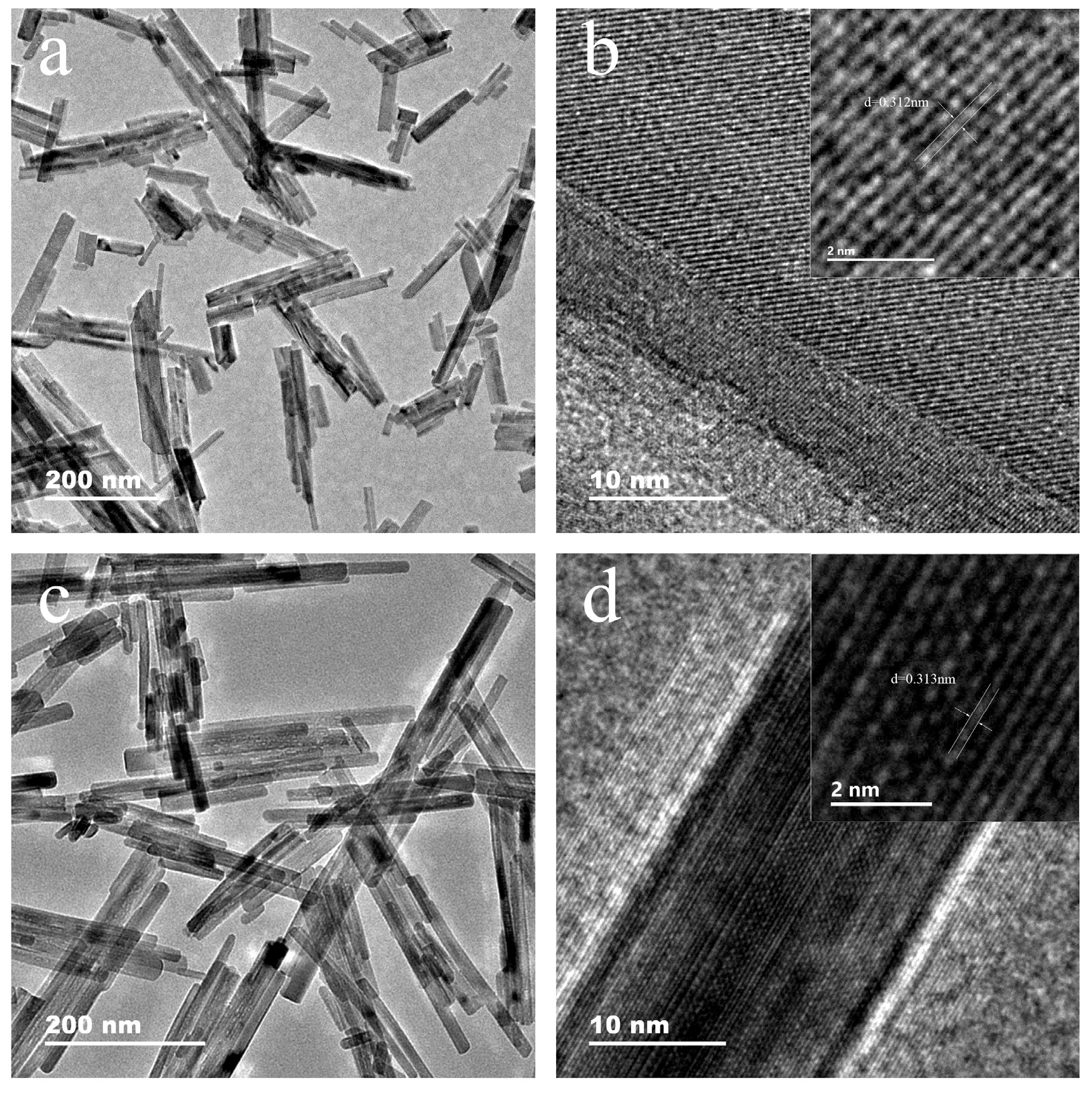
3.2. Crystal Morphology of LaPO4 Samples
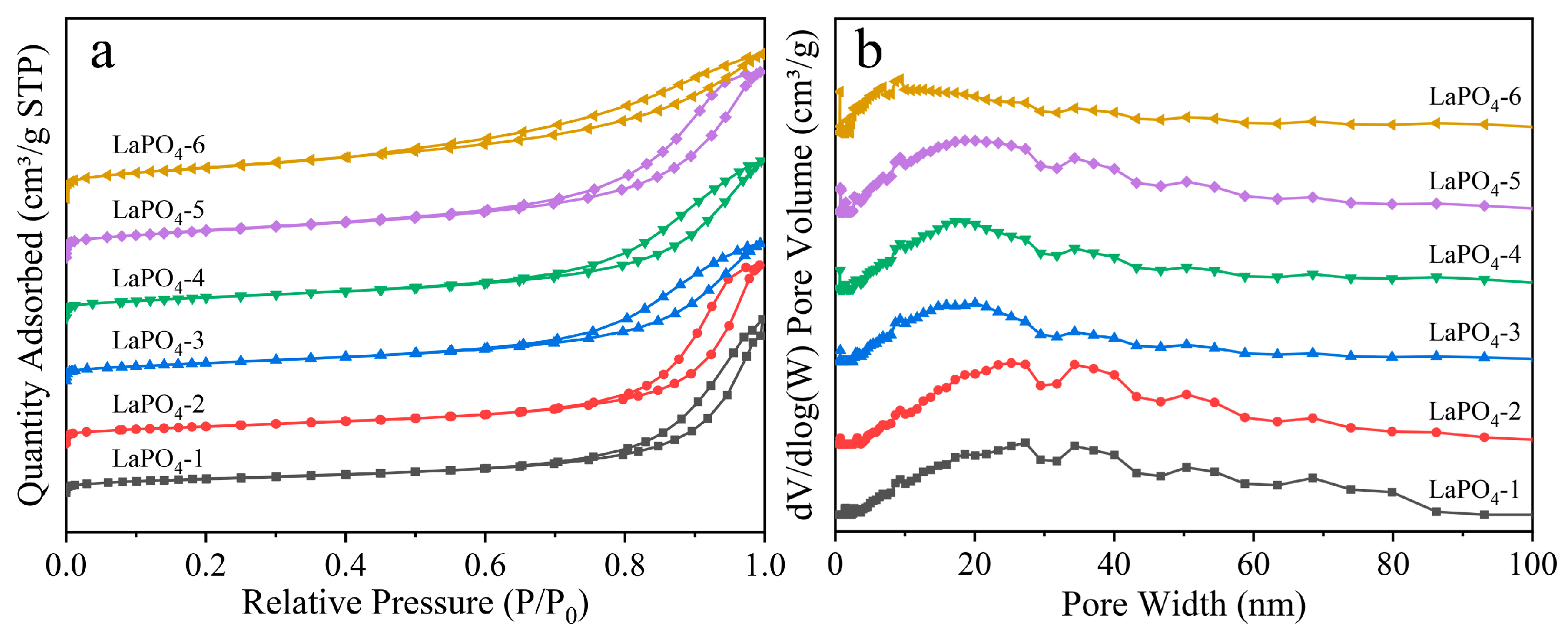
3.3. Textural Properties of LaPO4 Samples
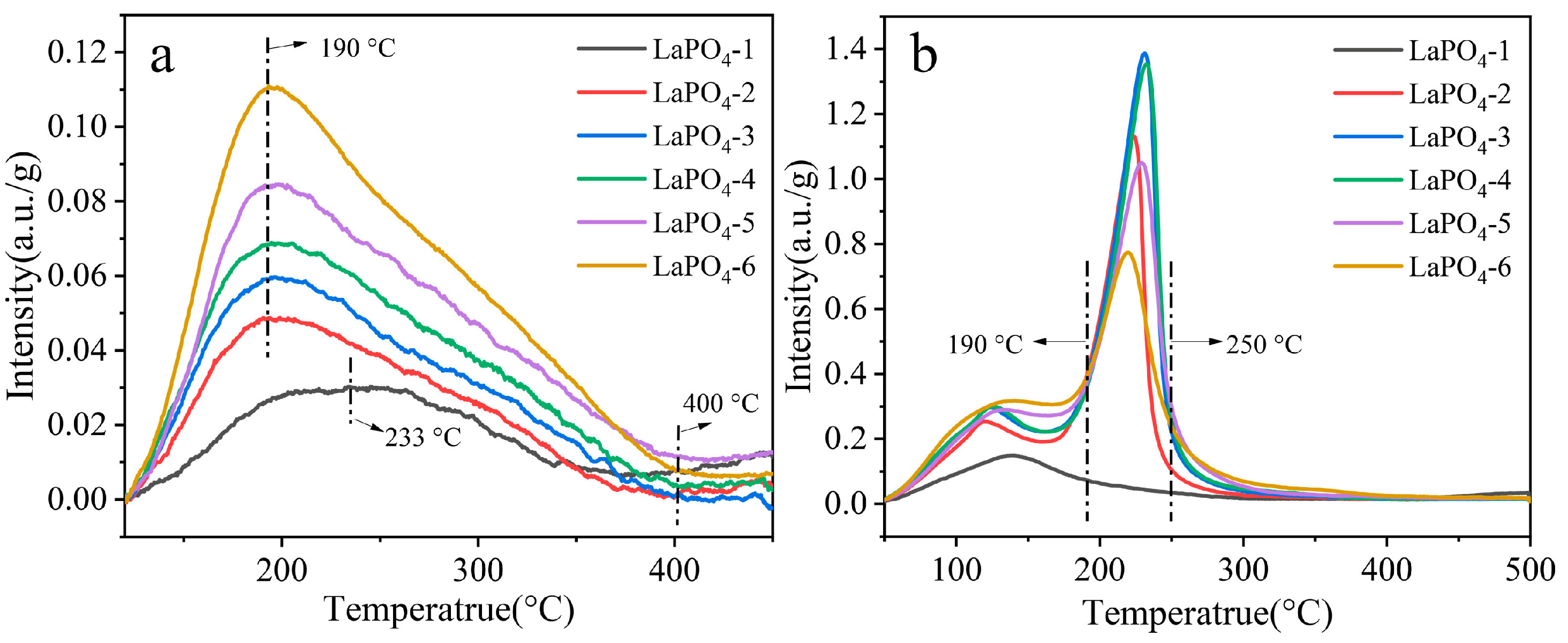
3.4. Acidity and Alkalinity Characterization of Samples
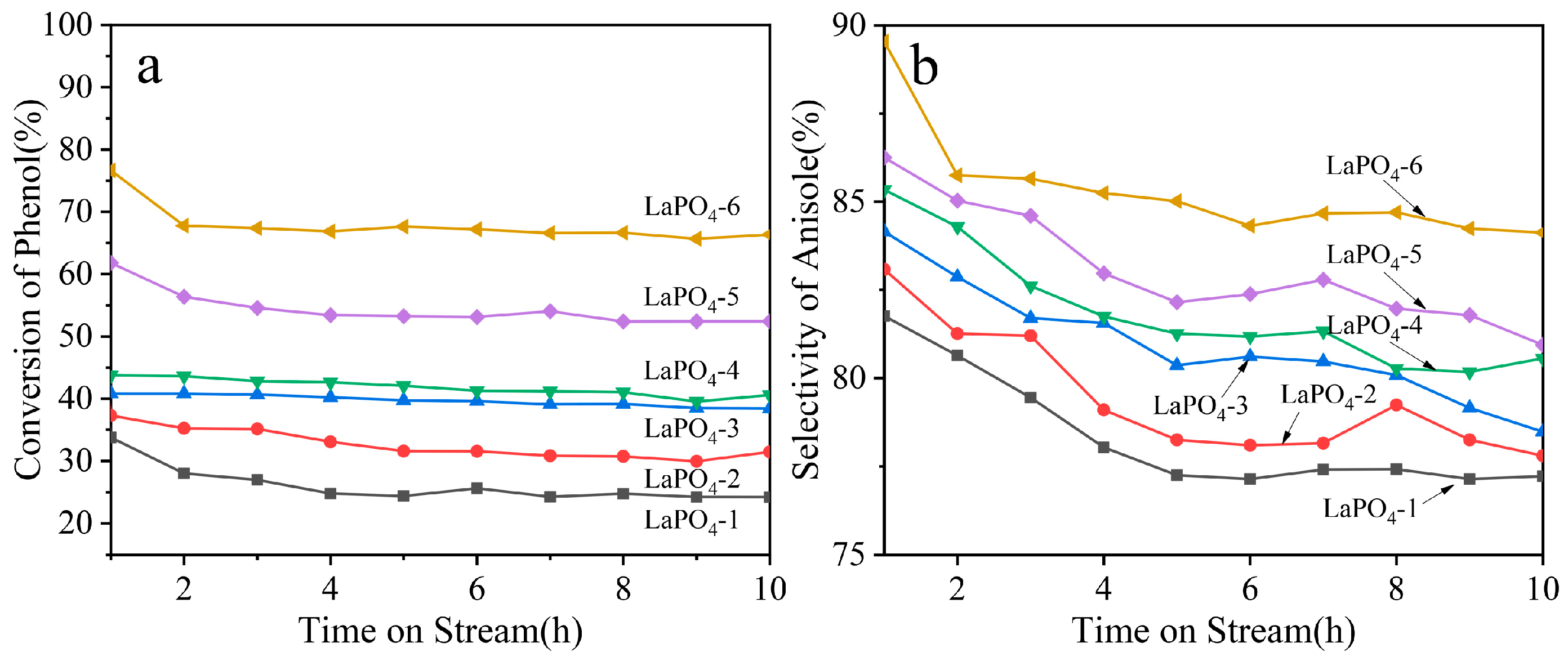
3.5. Catalytic Performance
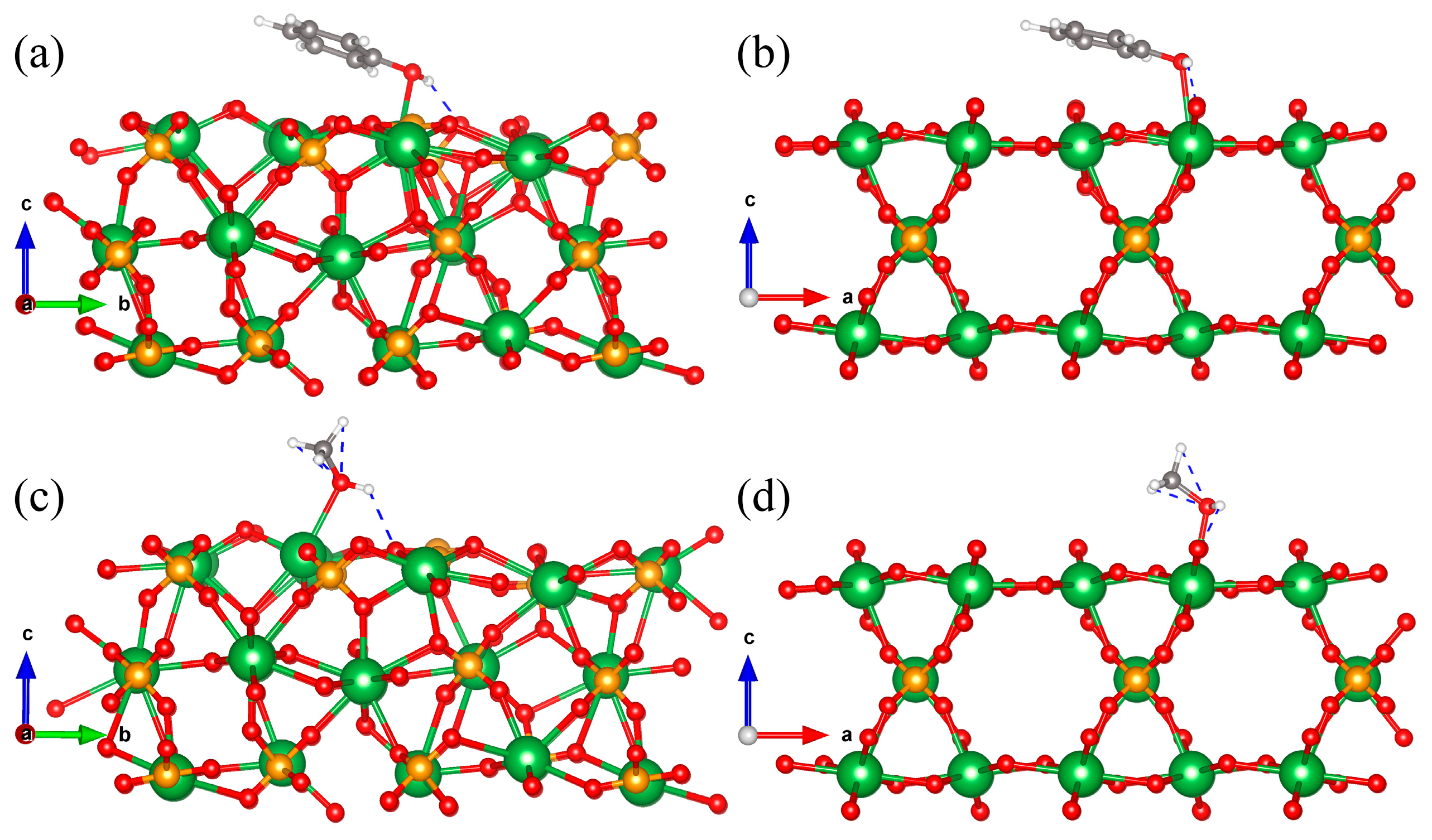
3.6. Calculation of Adsorption Energy and Hydrogen Bond Length
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cruz, J.J.; Escudero, F.; Verdugo, I.; Rivera, P.; Gutiérrez-Cáceres, N.; Yon, J.; Fuentes, A. Sooting propensity and maturity of gasoline/anisole blends in a laminar coflow diffusion flame. Fuel 2023, 345, 128091. [Google Scholar] [CrossRef]
- Shrabanti, R.; Omid, A. Detailed kinetics for anisole oxidation under various range of operating conditions. Fuel 2022, 325, 124907. [Google Scholar] [CrossRef]
- De Oliveira, M.P.; Delolo, F.G.; Villarreal, J.A.A.; dos Santos, E.N.; Gusevskaya, E.V. Hydroformylation and one-pot hydroformylation/epoxy ring cleavage of limonene oxide: A sustainable access to biomass-based multi-functional fragrances. Appl. Catal. A-Gen. 2021, 616, 118082. [Google Scholar] [CrossRef]
- Sreedhar, I.; Kantamneni, H.; Suresh Kumar Reddy, K.; Raghavan, K.V. Optimal process conditions for zeolite catalyzed acylation of anisole. Kinet. Catal. 2014, 55, 229–232. [Google Scholar] [CrossRef]
- Shahbaz, M.A.; Jahangir, S.; Kaiser, S.A. Imaging of flame propagation and temperature distribution in an all-metal gasoline engine with endoscopic access via anisole fluorescence. Exp. Fluids 2023, 64, 196. [Google Scholar] [CrossRef]
- Lewis, H.F.; Shaffer, S.; Trieschmann, W.; Cogan, H. Methylation of phenol by dimethyl sulfate. Ind. Eng. Chem. 1930, 22, 397–398. [Google Scholar] [CrossRef]
- Shiori, M.; Tatsuya, M.; Kotaro, M.; Yuko, N.; Yosinao, K. Three Cases of Chemical Burns Caused Due to Dimethyl Sulfate Poisoning. Cureus 2024, 16, e57060. [Google Scholar] [CrossRef]
- Memoli, S.; Selva, M.; Tundo, P. Dimethylcarbonate for eco-friendly methylation reactions. Chemosphere 2001, 43, 115–121. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, Z.; Li, S.; Chen, S.; Tang, Y. Selective O-methylation of phenol with dimethyl carbonate over catalysts supported on CaO. Kinet. Catal. 2021, 62, 496–506. [Google Scholar] [CrossRef]
- Huang, F.; Li, L.; Guan, M.; Hong, Z.; Miao, L.; Zhao, G.; Zhu, Z. A review of the process on vapor phase methylation of phenol with methanol. Catal. Lett. 2023, 153, 754–769. [Google Scholar] [CrossRef]
- Liu, N.; Mao, J.; Wu, Y.; Meng, X.; Shi, L. Alkylation of Phenol with Methanol over ZSM-5 Zeolite. Chem. Eng. Technol. 2024, 47, 984–993. [Google Scholar] [CrossRef]
- Lee, S.C.; Lee, S.W.; Kim, K.S.; Lee, T.J.; Kim, D.H.; Kim, J.C. O-alkylation of phenol derivatives over basic zeolites. Catal. Today 1998, 44, 253–258. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, F.; Chang, P.; Luo, X.; Miao, K.; Feng, G. One-step microwave synthesis of micron-sized ZSM-5/MCM-41 hierarchical porous materials for phenol hydroxyl alkylation. Inorg. Chem. Commun. 2022, 143, 109738. [Google Scholar] [CrossRef]
- Durgakumari, V.; Narayanan, S.; Guczi, L. Alkylation of phenol with methanol over AlPO and SAPO molecular sieves. Catal. Lett. 1990, 5, 377–384. [Google Scholar] [CrossRef]
- Samolada, M.C.; Grigoriadou, E.; Kiparissides, Z.; Vasalos, I.A. Selective O-alkylation of phenol with methanol over sulfates supported on γ-Al2O3. J. Catal. 1995, 152, 52–62. [Google Scholar] [CrossRef]
- Dang, D.; Wang, Z.; Lin, W.; Song, W. Synthesis of anisole by vapor phase methylation of phenol with methanol over catalysts supported on activated alumina. Chin. J. Catal. 2016, 37, 720–726. [Google Scholar] [CrossRef]
- Devi, G.S.; Giridhar, D.; Reddy, B.M. Vapour phase O-alkylation of phenol over alkali promoted rare earth metal phosphates. J. Mol. Catal. A Chem. 2002, 181, 173–178. [Google Scholar] [CrossRef]
- Chada, R.R.; Ketike, T.; Boilla, A.R.; Gangadharam, S.D.; Kamaraju, S.R.R.; Burri, D.R. Preparation and characterization of lanthanum phosphate catalysts for O-methylation of phenol to anisole in gas phase. Mol. Catal. 2017, 438, 224–229. [Google Scholar] [CrossRef]
- Nozaki, F.; Kimura, I. A study of catalysis by metal phosphates. IV. the alkylation of phenol with methanol over metal phosphate catalysts. Bull. Chem. Soc. Jpn. 1977, 50, 614–619. [Google Scholar] [CrossRef]
- Velu, S.; Swamy, C.S. Effect of substitution of Fe3+/Cr3+ on the alkylation of phenol with methanol over magnesium-aluminium calcined hydrotalcite. Appl. Catal. A-Gen. 1997, 162, 81–91. [Google Scholar] [CrossRef]
- Rusu, O.A.; Hoelderich, W.F.; Wyart, H.; Ibert, M. Metal phosphate catalyzed dehydration of sorbitol under hydrothermal conditions. Appl. Catal. B Environ. 2015, 176–177, 139–149. [Google Scholar] [CrossRef]
- Touny, A.H.; Tammam, R.H.; Saleh, M.M. Electrocatalytic oxidation of formaldehyde on nanoporous nickel phosphate modified electrode. Appl. Catal. B Environ. 2018, 224, 1017–1026. [Google Scholar] [CrossRef]
- Sodesawa, T.; Kimura, I.; Nozaki, F. A study of catalysis by metal phosphates. V. The alkylation of toluene with methanol over metal phosphate catalysts. Bull. Chem. Soc. Jpn. 1979, 52, 2431–2432. [Google Scholar] [CrossRef]
- Subramaniam, T.; Balarabe Idris, M.; Harshini Sai, G.; Devaraj, S. The effect of the crystallographic form of MnO2 on the kinetics of oxygen reduction and evolution reaction. Mater. Chem. Phys. 2023, 303, 127845. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, J.; Sun, W.; Pei, Y.; An, J.; Li, Z.; Ren, J. A DFT study of dimethyl carbonate synthesis from methanol and CO2 on zirconia: Effect of crystalline phases. Comput. Mater. Sci. 2019, 159, 210–221. [Google Scholar] [CrossRef]
- Rahmat, N.; Yaakob, Z.; Pudukudy, M.; Rahman, N.A.; Jahaya, S.S. Single step solid-state fusion for MgAl2O4 spinel synthesis and its influence on the structural and textural properties. Powder Technol. 2018, 329, 409–419. [Google Scholar] [CrossRef]
- Su, Y.; Tang, Z.; Han, W.; Zhang, P.; Song, Y.; Lu, G. Influence of the pore structure of CeO2 supports on the surface texture and catalytic activity for CO oxidation. CrystEngComm 2014, 16, 5189–5197. [Google Scholar] [CrossRef]
- Shvets, O.V.; Konysheva, K.M.; Shamzhy, M.V.; Opanasenko, M.V.; Yaremov, P.S.; Xiao, C.; Zou, X.; Čejka, J. Mordenite nanorods and nanosheets prepared in presence of gemini type surfactants. Catal. Today 2019, 324, 115–122. [Google Scholar] [CrossRef]
- Zhou, P.; Liu, M.N.; Luo, Q.X.; Zhang, J.; Chen, H.; Ma, X.; Hao, Q.Q. Synthesis of hierarchical nanocrystalline β zeolite as efficient catalyst for alkylation of benzene with benzyl alcohol. RSC Adv. 2022, 12, 4865–4873. [Google Scholar] [CrossRef]
- Nguyen, T.T.N.; Ruaux, V.; Massina, L.; Lorentz, C.; Afanasiev, P.; Maugé, F.; Bellière-Baca, V.; Rey, P.; Millet, J.M.M. Synthesis, characterization and study of lanthanum phosphates as light alcohols dehydration catalysts. Appl. Catal. B Environ. 2015, 166–167, 432–444. [Google Scholar] [CrossRef]
- Fang, Y.P.; Xu, A.W.; Song, R.Q.; Zhang, H.X.; You, L.P.; Yu, J.C.; Liu, H.Q. Systematic synthesis and characterization of single-crystal lanthanide orthophosphate nanowires. J. Am. Chem. Soc. 2003, 125, 16025–16034. [Google Scholar] [CrossRef]
- Lucas, S.; Champion, E.; Bregiroux, D.; Bernache-Assollant, D.; Audubert, F. Rare earth phosphate powders RePO4·nH2O(Re=La, Ce or Y)—Part I. synthesis and characterization. J. Solid State Chem. 2004, 177, 1302–1311. [Google Scholar] [CrossRef]
- Sankar, S.; Warrier, K.G. Aqueous sol–gel synthesis of lanthanum phosphate nano rods starting from lanthanum chloride precursor. J. Sol-Gel Sci. Technol. 2011, 58, 195–200. [Google Scholar] [CrossRef]
- Onoda, H.; Ishima, Y.; Takenaka, A.; Tanaka, I. Preparation of porous lanthanum phosphate with templates. Mater. Res. Bull. 2009, 44, 1712–1716. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B Condens. Matter 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B Condens. Matter 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B Condens. Matter 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B Condens. Matter 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Neupane, M.R.; Garrett, G.A.; Rudin, S.; Andzelm, J.W. Phase dependent structural and electronic properties of lanthanum orthophosphate (LaPO4). J. Phys.-Condens. Matter 2016, 28, 205501. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadeh, M.A.; Chini, S.F.; Sadeghi, A. Size and shape tailored sol-gel synthesis and characterization of lanthanum phosphate LaPO4 nanoparticles. Mater. Des. 2019, 181, 108058. [Google Scholar] [CrossRef]
- Bezouhanova, C.; Al-Zihari, M.A. Alkylation of phenol with methanol over Mn3O4. Appl. Catal. A-Gen. 1992, 83, 45–49. [Google Scholar] [CrossRef]
- Berthou, H.; Jørgensen, C.K.; Bonnelle, C. Influence of the ligands on 3d photoelectron spectra of the first four lanthanides. Chem. Phys. Lett. 1976, 38, 199–206. [Google Scholar] [CrossRef]
- Wu, A.-P.; Bai, H.; Bao, J.-R.; Yang, K.-S.; Feng, L.-N.; Ma, Y.-Y.; Qiao, Y.; Li, W.-X.; Liu, Y.; Zhu, X.-W. Influence of citrate on phase transformation and photoluminescence properties in LaPO4 and LaPO4: Eu. RSC Adv. 2018, 8, 35813–35818. [Google Scholar] [CrossRef]
- Barman, S.; Pradhan, N.C.; Basu, J.K. Kinetics of alkylation of phenol with methanol over Ce-exchanged NaX zeolite. Catal. Lett. 2006, 111, 67–73. [Google Scholar] [CrossRef]

| Samples | Stirring Temperature (°C) | Stirring Time (h) | Crystallization Temperature (°C) | Crystallization Time (h) |
|---|---|---|---|---|
| LaPO4-1 | 50 | 0.5 | 160 | 12 |
| LaPO4-2 | 50 | 0.5 | 140 | 10 |
| LaPO4-3 | 50 | 0.5 | 120 | 10 |
| LaPO4-4 | 50 | 0.5 | 100 | 12 |
| LaPO4-5 | 90 | 1 | / | / |
| LaPO4-6 | 50 | 1 | / | / |
| Samples | LaPO4-1 | LaPO4-2 | LaPO4-3 | LaPO4-4 | LaPO4-5 | LaPO4-6 |
|---|---|---|---|---|---|---|
| SBET (m2·g−1) | 46.0 | 53.7 | 55.6 | 61.4 | 77.9 | 89.3 |
| Vt (cm3·g−1) | 0.088 | 0.100 | 0.113 | 0.121 | 0.139 | 0.134 |
| Da (nm) | 7.77 | 7.76 | 7.56 | 7.68 | 6.89 | 5.44 |
| Samples | LaPO4-1 | LaPO4-2 | LaPO4-3 | LaPO4-4 | LaPO4-5 | LaPO4-6 |
|---|---|---|---|---|---|---|
| Total acid sites (mmol g−1) | 0.154 | 0.231 | 0.299 | 0.342 | 0.385 | 0.494 |
| Total base sites (mmol g−1) | 0.242 | 0.900 | 1.144 | 1.142 | 1.104 | 1.019 |
| Samples | Methanol | Phenol | ||
|---|---|---|---|---|
| Adsorption Energy (eV) | H a Bond Length (Å) | Adsorption Energy (eV) | H b Bond Length (Å) | |
| Monoclinic | −0.686 | 2.181 | −1.704 | 1.865 |
| Hexagonal | −0.942 | 2.086 | −1.261 | 1.877 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Zhang, Q.; Zhang, F.; Li, H.; Liu, Y.; Wei, K.; Zhao, Y.; Yu, S.; Li, Y.; Zhang, F.; et al. The Effect of LaPO4 Crystal Morphology on Gas-Phase Catalytic Synthesis of Anisole. Coatings 2025, 15, 1093. https://doi.org/10.3390/coatings15091093
Wang W, Zhang Q, Zhang F, Li H, Liu Y, Wei K, Zhao Y, Yu S, Li Y, Zhang F, et al. The Effect of LaPO4 Crystal Morphology on Gas-Phase Catalytic Synthesis of Anisole. Coatings. 2025; 15(9):1093. https://doi.org/10.3390/coatings15091093
Chicago/Turabian StyleWang, Wei, Qiwen Zhang, Fan Zhang, Hongyue Li, Ying Liu, Kemeng Wei, Yan Zhao, Songlin Yu, Yajun Li, Feng Zhang, and et al. 2025. "The Effect of LaPO4 Crystal Morphology on Gas-Phase Catalytic Synthesis of Anisole" Coatings 15, no. 9: 1093. https://doi.org/10.3390/coatings15091093
APA StyleWang, W., Zhang, Q., Zhang, F., Li, H., Liu, Y., Wei, K., Zhao, Y., Yu, S., Li, Y., Zhang, F., Yang, M., Hao, Q.-Q., & Luo, X. (2025). The Effect of LaPO4 Crystal Morphology on Gas-Phase Catalytic Synthesis of Anisole. Coatings, 15(9), 1093. https://doi.org/10.3390/coatings15091093







