1. Introduction
Electroless cobalt (Co) and cobalt–boron (Co–B) coatings have been the subject of considerable research due to their unique properties, which include hardness, wear resistance, corrosion resistance, magnetic characteristics and catalytic activity [
1,
2,
3,
4]. These coatings are typically deposited without using an external electric current, instead relying on a chemical reduction process to deposit the metal onto a substrate. Electroless plating is a method used to deposit electrically conductive coatings onto both dielectric (non-conductive) materials such as glass, plastics and ceramics, as well as onto conductive substrates, using relatively simple equipment [
5]. Currently, these coatings are widely applied in various fields, including the chemical industry, microelectronics and electronics, and other machinery sectors. There is significant interest in the deposition of pure metal coatings because they offer superior mechanical, physical and catalytic properties [
6,
7]. Compared to other chemical and physical deposition methods, electroless plating is an attractive approach due to its relatively low cost, low-temperature requirements, minimal defects, and high conformality and uniformity. In particular, electroless cobalt coatings have been shown to provide excellent barrier and capping layers for copper. Autocatalytic cobalt reduction solutions are valuable tools in modern electronics and microelectronics, offering a means to deposit high-quality, conductive cobalt layers without the need for external electrical currents. These solutions provide uniform coatings and can be used on a wide variety of substrates. They are essential for precision applications such as semiconductor manufacturing and printed circuit board production. However, carefully controlling process parameters is essential to ensure consistent and high-quality deposits [
8]. The wide range of cobalt coating applications can be attributed to the well-known combination of cobalt’s properties and the unique characteristics of its alloys, including their effectiveness as barrier layers against copper diffusion, the uniformity of the coating, and their magnetic properties [
9]. In recent years, cobalt–phosphorus (CoP) alloys obtained using sodium phosphinate (hypophosphite) as a reducing agent have been studied [
5,
6,
7,
8,
9]. N. Petrov et al. presented electroless Co(P) and Co(W,P) systems for use as barrier/capping layers for copper interconnects [
10]. Their study considered cobalt, cobalt complexes, and aqueous solutions of tungstate and hypophosphite.
A novel electroless solution based on the cobalt sulfamate compound Co(NH
2SO
3)
2 was developed to produce soft magnetic CoWP alloy films [
11]. This study investigated how solution composition and deposition parameters affect the microstructure, electrical properties, and magnetic properties of deposits. Electroless deposition of CoWP alloys is also documented in [
12,
13].
Data from atomic force microscopy (AFM), X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS) confirmed that Co–W–P films deposited from glycine-containing solutions could serve as an effective diffusion barrier to prevent copper (Cu) diffusion [
14]. Autocatalytic Co–P/Au alloys containing about 4 wt% phosphorus have been shown to strongly limit interdiffusion and the formation of intermetallic compounds [
15].
Cobalt alloys containing phosphorus and boron have been produced using the electroless deposition method with two reducing agents, namely sodium hypophosphite [
16] and dimethylamine borane [
17], respectively. Experimental results suggest that the composition of the films in terms of phosphorus and boron content is determined by the surface adsorption rates of the reducing agents. Co–B–P–W alloys deposited using dimethylamine borane (DMAB) as the reducing agent have also been studied [
18]. This palladium-free activation process does not require palladium salts to activate the substrate, owing to the strong reducing power of DMAB. Co–B–P–W alloys have been extensively researched due to their interesting properties, particularly in the fields of catalysis, electronics, and materials science.
It is worth noting that pure cobalt coatings can be obtained using hydrazine [
19,
20,
21,
22] or Ti
3+ metal ions as reducing agents [
23]. These Ti
3+ ions are an environmentally friendly alternative to the other reducing agents used in electroless cobalt plating.
Some researchers have also explored the use of other organic reducing agents containing boron, such as morpholine borane (MB), C
4H
8ONH·BH
3, which has not yet been widely used for electroless metal deposition. It should be noted that MB is less volatile and less toxic than DMAB. Studies have shown that high-quality Co–B coatings can be obtained using MB even at relatively low temperatures, such as 30 °C [
24,
25]. CoBW alloys containing 5.5–16 at% boron and 5–14 at% tungsten have been successfully deposited [
26]. H. Einati et al. demonstrated that a Co–W–B coating containing 2.2 at% boron exhibited high resistance to air oxidation [
27].
Co and Co alloy coatings are widely applied as catalysts in glucose electrooxidation and total water splitting, as discussed in [
28,
29,
30,
31,
32,
33]. The aim of this study was therefore to investigate electroless cobalt deposition in detail using MB as a reducing agent with an additional ligand, diethylenetriamine (dien), in the plating solution. We propose a novel cobalt plating bath containing MB that enables boron-free coating deposition and allows for the acquisition of Co–B coatings with a controllable amount of boron. This study presents an experimental investigation of the morphology and composition of cobalt deposits, alongside an analysis of the deposition rate. It also includes AFM images of cobalt deposits obtained via electroless plating along with XRD investigation results.
2. Materials and Methods
The electroless deposition of boron-free cobalt and cobalt–boron coatings was performed on untreated silicon (Si) wafer substrates, which were initially coated with a TaN/Ta barrier layer and a Cu seed layer utilizing the physical vapor deposition (PVD) method (denoted as Cu/TaN/Ta/Si). Furthermore, Co–B coating deposition on the Cu/TaN/Ta/Si substrate was conducted without a prior activation step using Pd(II) ions.
Following the cleaning procedure, the Cu/TaN/Ta/Si substrate was immersed in the electroless cobalt deposition solution. The main solution’s composition was as follows (M): CoSO4-0.05; diethylenetriamine-0.015; and morpholine borane-0.05. The pH of the solution was 7.3and was adjusted using sulfuric acid. Co–B coating deposition was carried out from freshly prepared solutions at 30 °C.
Diethylenetriamine (99%), morpholine borane complex, and cobalt(II) sulfate heptahydrate were purchased from Sigma-Aldrich (Taufkirchen, Germany). Sulfuric acid (95%, Chempur) was also used. All the chemicals utilized in this study were of analytical grade and all solutions were prepared using deionized water.
The deposition rate of the coatings was determined gravimetrically.
The atomic force microscope (AFM) TopoMetrix Explorer SPM (Veeco, Santa Clara, CA, USA) was used for Co–B surface inspection.
XRD measurements were performed using a Smart Lab X-ray diffractometer (Rigaku, Tokyo, Japan) equipped with a 9 kW X-ray tube with a rotating Cu anode. The grazing incidence (GIXRD, Rigaku, Tokyo, Japan) method was used within the 2θ range of 35–80°. The angle between the parallel X-ray beam and the specimen surface (ω angle) was adjusted to 0.5°.
The morphology and structure of the Co–B coatings were characterized using an SEM-focused ion beam facility (Helios Nanolab 650, FEI, Eindhoven, The Netherlands).
3. Results and Discussion
The electroless cobalt deposition reaction consists of two simultaneous processes, namely the anodic oxidation of the reducing agent (morpholine borane) (Equation (1)) and the cathodic reduction of cobalt(II) ions (Equation (2)):
Both reactions occur simultaneously on the same surface to be plated. The overall autocatalytic process of electroless cobalt deposition in near-neutral solutions can be described by the following equations (Equations (3) and (4)):
In addition, some amount of morpholine borane can be consumed in the following catalytic reaction (Equation (5)):
Generally, during this process, the electrons involved in the anodic oxidation of morpholine borane are transferred through the surface to be plated to the adsorbed Co(II) ions, which leads to the autocatalytic reduction of cobalt.
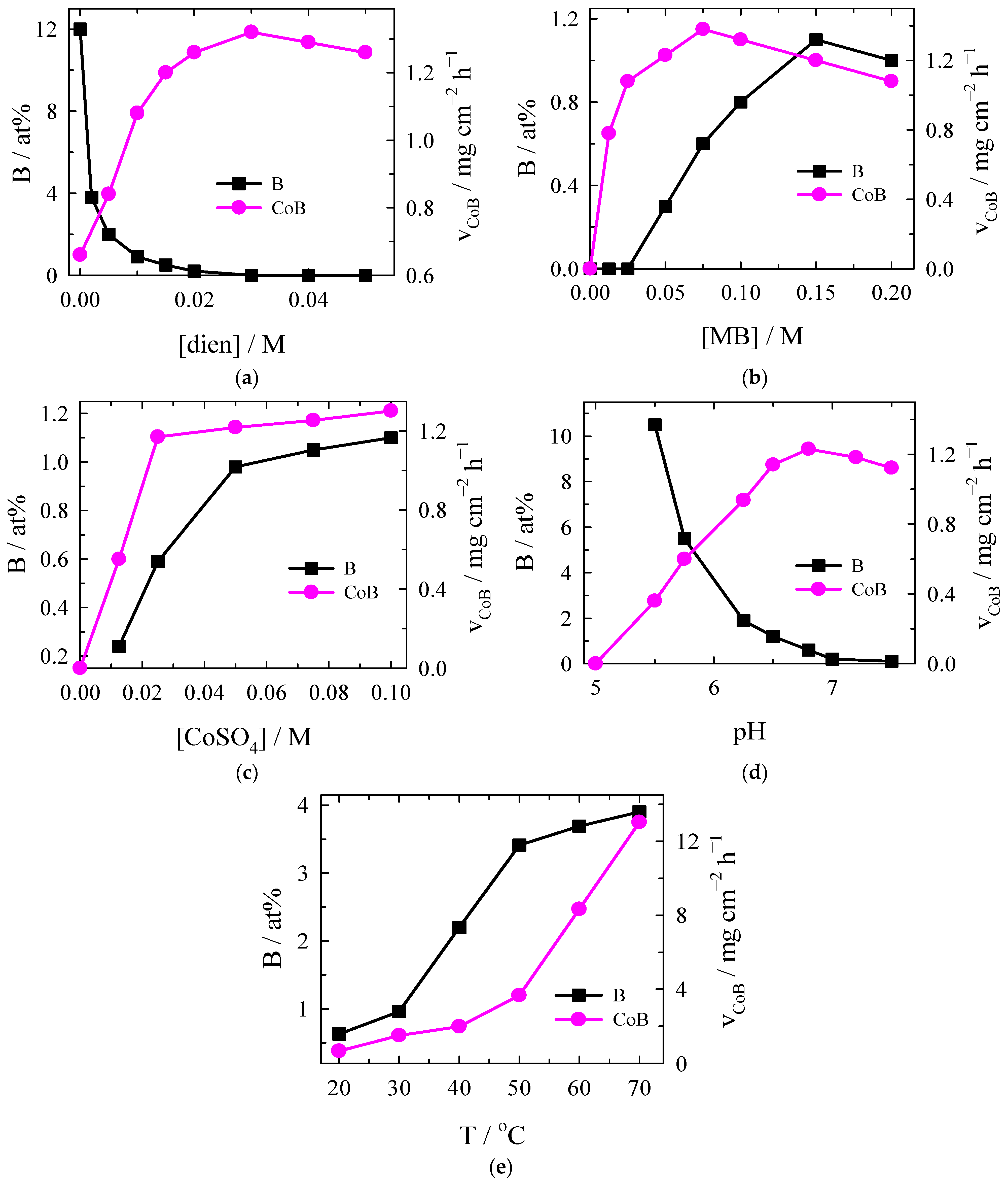
The data on kinetic investigations are presented in
Figure 1. When investigating the influence of diethylenetriamine, it can be seen that the coating deposition rate increases when the diethylenetriamine concentration is 0.03 M; later, it slightly decreases (
Figure 1a). The amount of boron decreases with the increase in diethylenetriamine concentration up to 0.03 M, and beginning from this concentration onwards, no boron is detected in the deposited coatings (
Figure 1a). The boron content in the coatings has been observed to decrease from 12 at% (without diethylenetriamine in the plating bath) to 0 at% as the diethylenetriamine concentration reaches 0.03 M or higher. This suggests that diethylenetriamine plays a crucial role in diminishing the boron content in the cobalt alloy films likely through its interaction with the cobalt(II)-dien complex. Boron does not co-deposit as part of the Co–B alloy because it is either oxidized or remains in solution due to the lack of sufficient reduction conditions for both cobalt and boron to exist simultaneously. The relationship between MB concentration and the cobalt alloy deposition rate is shown in
Figure 1b. Increasing the MB concentration up to 0.075 M resulted in an increase in the cobalt deposition rate, which reached 1.38 mg cm
−2 h
−1, with a slight decrease in the deposition rate at higher MB concentrations (
Figure 1b). It is worth noting that no boron is incorporated into the cobalt coating at MB concentrations up to and equal to 0.025 M, whereas further increases in MB concentration result in an increase in boron in the cobalt alloy coating, reaching a maximum value of 1.1 at% at an MB concentration of 0.15 M (
Figure 1b). An investigation of the influence of Co(II) concentration on the deposition rate and boron content in the cobalt alloy coatings (
Figure 1c) demonstrates a direct correlation between the increase in Co(II) concentration and the improvement of the cobalt alloy deposition rate, with the highest value (1.28 mg cm
−2 h
−1) being reached at the highest investigated Co(II) concentration (0.1 M). The boron content in the cobalt alloy coatings increases steadily with the increase in Co(II) salt concentration (
Figure 1c). It is evident that the pH of a solution plays a significant role in the overall process of cobalt alloy deposition (
Figure 1d). Beginning from pH 5 up to pH 6.75, the cobalt alloy deposition rate increases gradually and reaches the maximum value at pH 6.75; further increases in the solution pH result in an insignificant decrease in the plating rate (
Figure 1d). In contrast to the plating rate, the amount of boron in coatings decreases in all cases with an increase in the pH value. Notably, the highest amount of boron was observed at pH 5.5 (10.6 at%), which decreases sharply up to pH 7.5 (1.2 at%), later, the decrease begins to slow and reaches 0.1 at% at pH 7.6 (
Figure 1d). The effect of temperature on the rate of electroless cobalt alloy plating is shown in
Figure 1e. It has been demonstrated that as the temperature increases from 20 °C to 70 °C, the rate of cobalt deposition also increases. Starting from 20 °C up to 50 °C, the plating rate increases from 0.9 mg cm
−2 h
−1 up to 3.9 mg cm
−2 h
−1. The further increase in temperature results in a sharp increase in the plating rate, which reaches 13 mg cm
−2 h
−1 at 70 °C (
Figure 1e).
It has been found that the content of boron in the coatings increases from 0.6 at% at 20 °C to 3.9 at% at 70 °C (
Figure 1e). This indicates that higher temperatures promote the incorporation of boron into the cobalt films.
When MB is used as a reducing agent, the release of boron occurs as a consequence of the reduction reaction. However, the boron tends to remain in solution or might form side products rather than being deposited onto the substrate. The underlying reason for this phenomenon is that cobalt reduction occurs preferentially, while boron exhibits a weak propensity to co-deposit with cobalt under typical electroless conditions. It is evident that boron has a lower reduction potential in comparison to cobalt. The standard reduction potential of Co2+/Co for the reduction of cobalt ions to cobalt metal is approximately −0.28 V. However, the reduction potential for boron B3+/B, is significantly lower, reaching a more negative value of approximately −1.09 V. It can thus be concluded that the presence of dien (>0.3 M) helps to achieve a B-free cobalt film, whilst the use of MB as a reducing agent does not result in boron incorporation within the final coating.
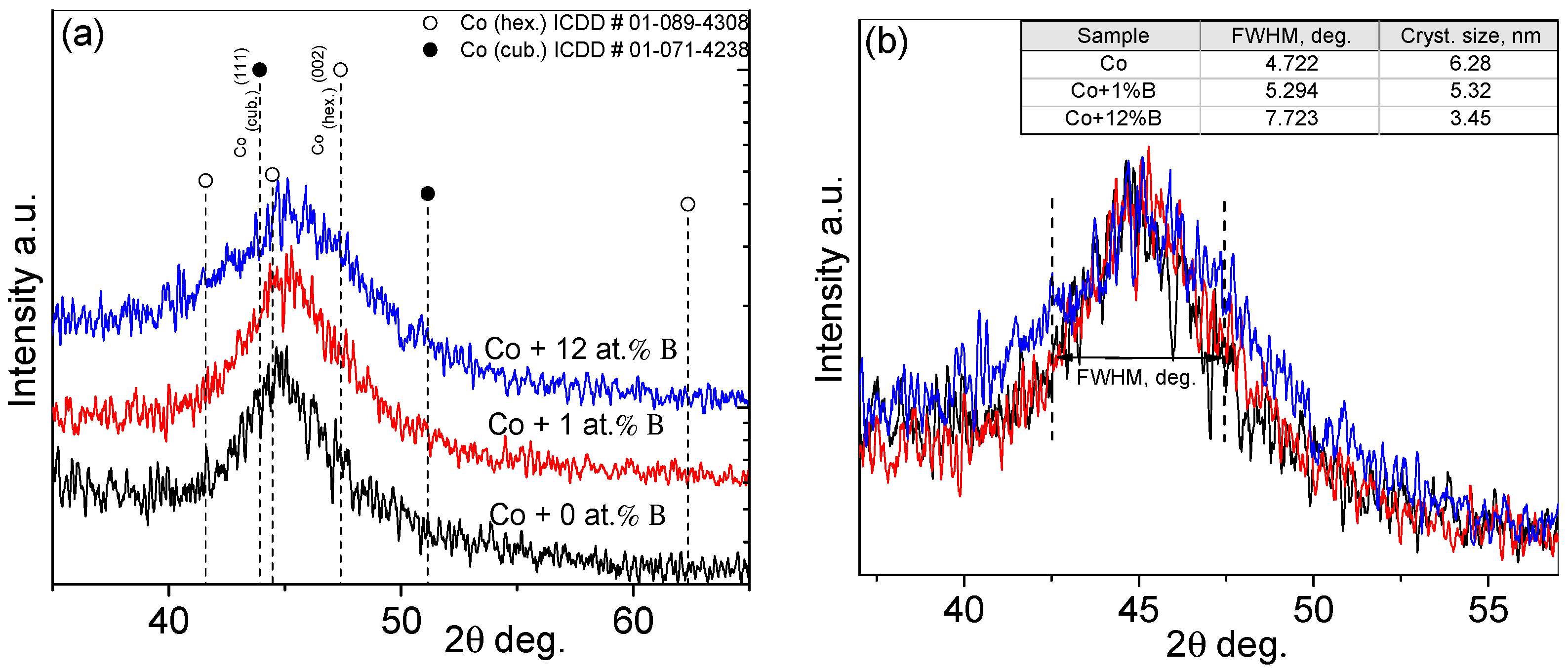
The crystalline structure of boron-free Co and Co–B coatings was investigated using X-ray diffraction (
Figure 2). These studies aimed to evaluate structural changes in Co coatings when boron is introduced.
Figure 2a shows the XRD patterns for the pure Co coating and coatings, containing 1 and 12 at% of B. XRD studies of the aforementioned Co coatings revealed that the electrolessly deposited coatings exhibited crystallinity. However, their significant expansion indicated that the coatings consisted of crystallites smaller than 10 nm. The XRD patterns (
Figure 2a) show only one broad Co peak, which may be a mixture of hexagonal (ICDD#01-089-4308) and cubic (ICDD#01-071-4238) Co structures. Adding B to the Co coating was found to reduce its crystallinity; that is, the Co diffraction peak broadens (
Figure 2b). The FWHM increases from 4.72 degrees without boron to 7.72 degrees with the addition of 12 at% boron. The width of the Co peak changes little when 1 at% of boron is added. However, the size of the Co crystallites forming the coatings, as calculated by the Halder-Wagner method, is only approximate since the calculations were complicated by the possible overlap of hexagonal and cubic Co structures within the coatings. Additionally, a more accurate estimate of the crystallite size requires a larger number of crystalline peaks of the compound. However, even these approximate calculations showed a decrease in the crystallinity of the Co coatings, from 6.3 nm to 3.4 nm, with the addition of boron.
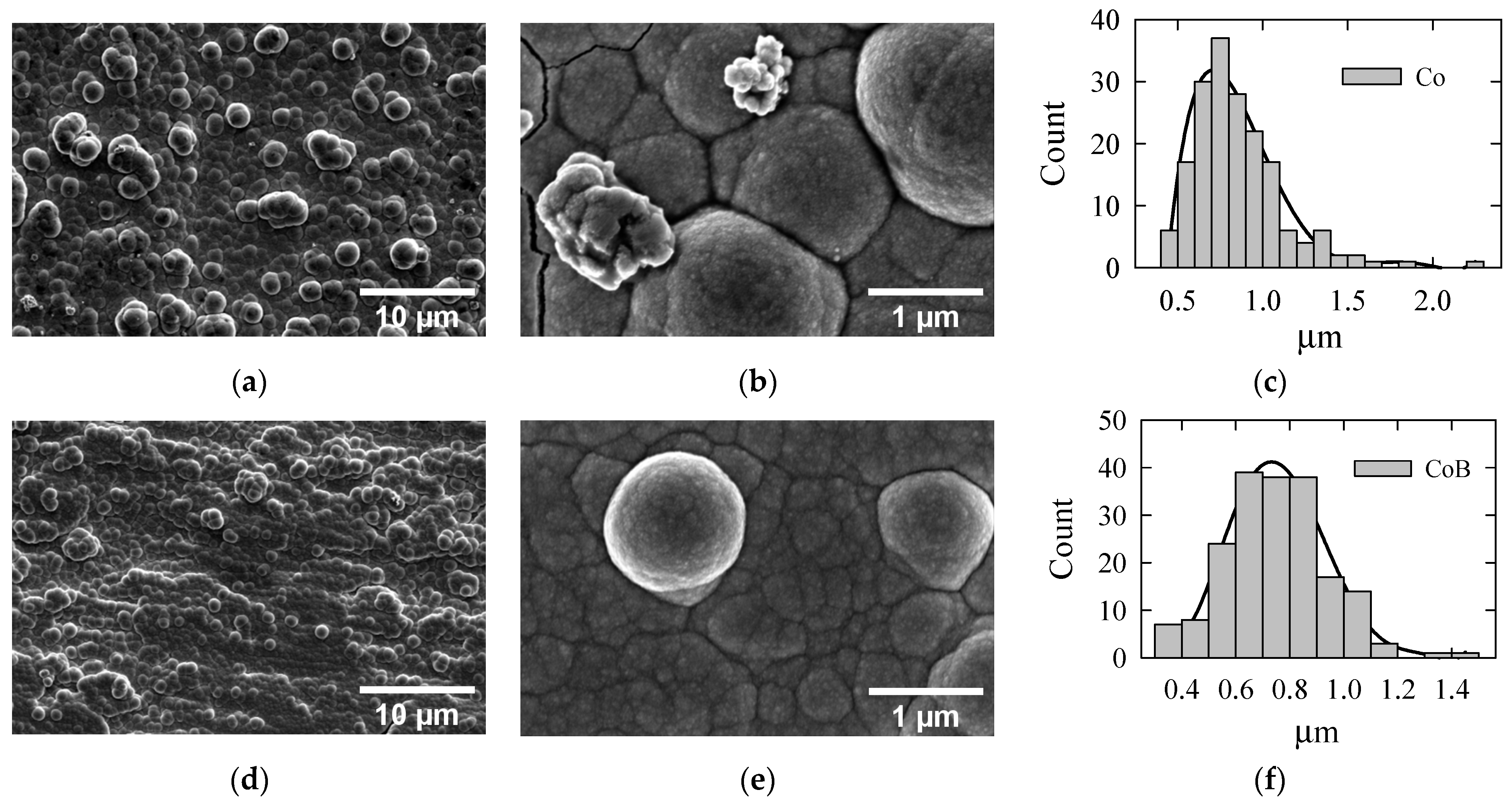
The morphology of the Co–B coatings was further investigated using scanning electron microscopy (SEM). The boron-free Co coating surface (
Figure 3a) is covered with densely packed spherical and hemispherical granules that form a uniform granular texture. These particles appear to range in diameter from 0.4 to 2 µm (
Figure 3c), indicating a nodular growth pattern. At higher magnification (
Figure 3b), the coating reveals larger, well-defined grains with a polygonal shape, closely packed together with clear grain boundaries. Some producing clusters or agglomerates are visible on the grain surface, likely secondary nucleation sites. This morphology is characteristic of crystalline Co coatings, where growth occurs through the formation of columnar grains and hemispherical nodules.
Figure 3d,e show the surface morphology of the Co–B coating consisting of 12 at% of B under different magnifications. As illustrated, the surface appears rough with a granular structure, containing numerous small spherical and semi-spherical particles, ranging in size from 0.3 to 1.5 µm (see
Figure 3f). The coating appears compact, with some agglomerated clusters of particles (
Figure 3d). At higher magnification, the spherical particles become more distinct, indicating the fine-grained, nanocrystalline, or amorphous-like structure typical of Co–B coatings with moderate boron content (
Figure 3e). It is evident that the incorporation of B into the Co coating results in denser, finer, and nodular surface morphology, as well as a decrease in grain size.
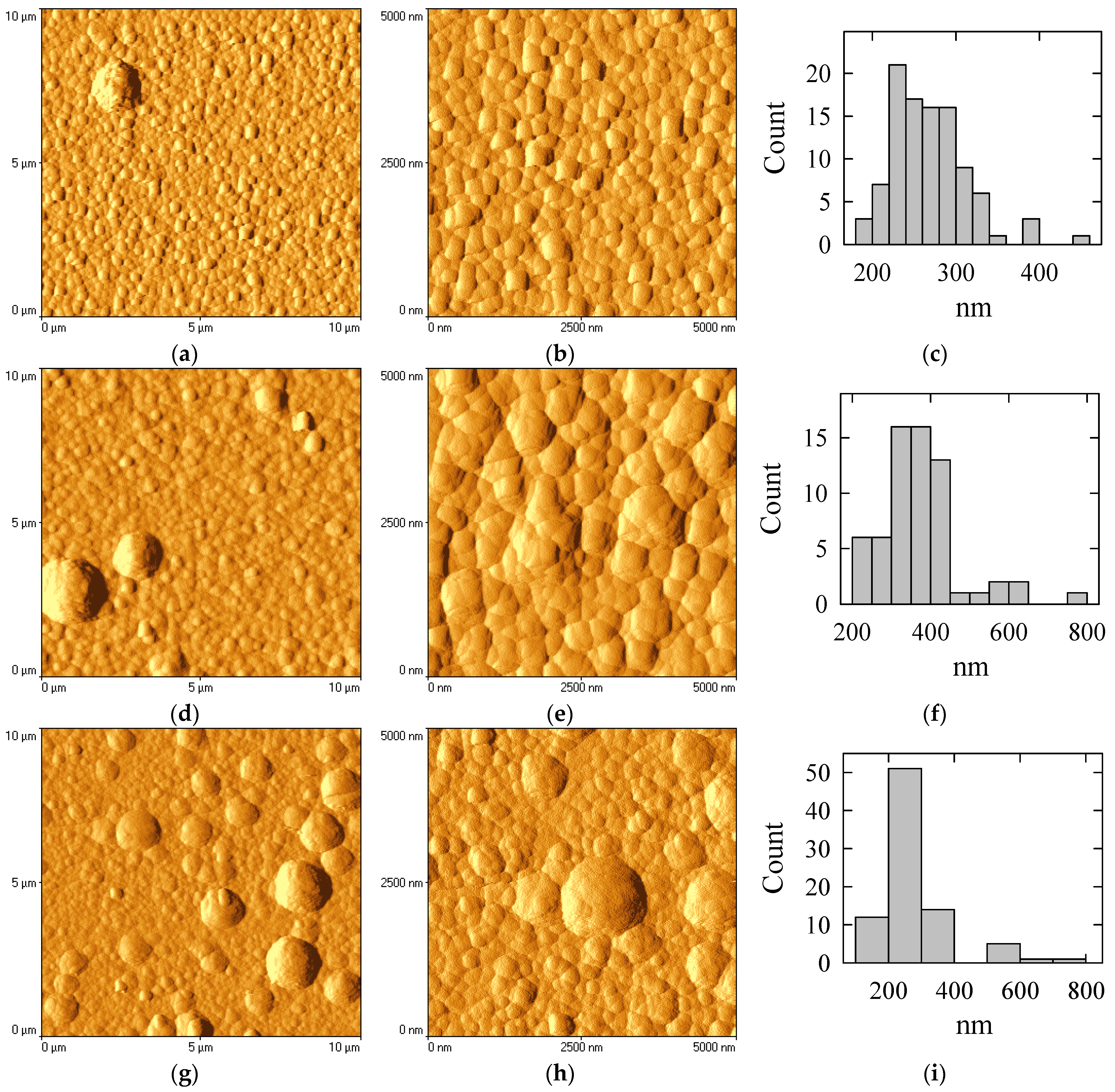
The surface structure of the deposited films was also analyzed using atomic force microscopy (AFM) (see
Figure 4). The AFM image of the boron-free Co coating shows that it is composed of densely packed grains with a clear polycrystalline texture (see
Figure 4a,b). Each grain appears as a rounded or faceted feature, closely adjoining its neighbors without large voids. Furthermore, the grains exhibit a uniform size distribution, with an average diameter of D = 250 ± 50 nm (
Figure 4c). The grains have well-defined edges, highlighting the polycrystalline nature of the Co structure, as also observed in the SEM images. As the boron content in the Co–B coatings increases, the grain size distribution becomes more heterogeneous. In the case of coatings containing 1 at% of B, the surface consists of densely packed grains, closely resembling the pure Co coating but with slightly refined features.
The structure remains predominantly polycrystalline, with only minor amorphous/nanocrystalline influence from the low addition of boron. The grains are also relatively uniform in the range of 300 ± 50 nm. A coating containing a higher amount of B (12 at%) exhibits a nodular surface structure with spherical and semi-spherical protrusions (see
Figure 4g,h). These nodules are larger and more prominent than those of the pure Co coating. Furthermore, the grains consist of both small and large crystallites, ranging in size from 0.25 to 1.5 µm. This suggests that the crystallization process in Co–B coatings is uneven, possibly due to the differing effects of boron on the growth of cobalt crystallites. Notably, the discrepancy in grain size becomes more pronounced as the boron content increases up to 12 at%. In this case, the small grains diminish in size, while the density of the larger grains increases. The results obtained correlate well with the XRD and SEM results.
It can by noted that in the case of cobalt electroless deposition of, when using boron-containing reducing agents, the resulting coatings are generally Co–B boron alloys because boron is incorporated into deposited films. In our study, we demonstrated that the use of diethylenetriamine hinders boron incorporation in coatings, enabling the production of pure cobalt films. Additionally, we showed that changing the concentration of diethylenetriamine enables the deposition of Co–B alloys with a controllable amount of boron. These results are novel and could be of interest to academia and industry when pure cobalt coatings need to be obtained by means of electroless deposition.
4. Conclusions
The present study has demonstrated that morpholine borane can effectively serve as a reducing agent in the electroless deposition of cobalt coatings in the presence of diethylenetriamine.
It has been demonstrated that even small additions of diethylenetriamine to the morpholine borane-based plating bath result in a substantial reduction in the boron content in the cobalt coatings. A rigorous examination was conducted to ascertain the impact of diethylenetriamine concentration on the boron content. The findings yielded significant insights, elucidating the correlation between solution composition and coating properties.
Depending on the concentration of diethylenetriamine in the plating solution, the deposited coatings contained between 0 and 12 at% of boron. It was clearly shown that diethylenetriamine suppresses the incorporation of boron into the Co–B alloy. The same tendency was also observed in the case of an increase in the solution pH, whereas higher temperatures promote the incorporation of boron into the cobalt films.
A neutral electroless cobalt deposition bath was developed using morpholine borane as the reducing agent. This enabled cobalt deposition at rates equal to 13 mg cm−2 h−1 at 70 °C. This process is promising for the fabrication of uniform boron-free cobalt coatings or Co–B alloys with controllable composition and thickness.
This study also investigated the effects of solution composition and deposition parameters on the morphology and microstructure of the deposited films. It has been demonstrated that these factors have a significant impact on the overall performance of the coatings, with notable variations in grain structure and crystallinity depending on the plating conditions.