Abstract
Limited hardness and corrosion resistance restrict 7050 aluminum alloys in aggressive environments. Cr coatings, applied as single layers or over Ti, Al, or Ni buffer layers, were deposited onto 7050 aluminum alloy by direct-current magnetron sputtering; their microstructure, adhesion, mechanical properties, and corrosion behavior were examined. The results indicate that introducing a buffer layer significantly enhances the bonding strength between a Cr coating and an aluminum alloy substrate, with the Ni buffer layer exhibiting the highest bonding strength, nearly three times that of the Cr coating alone. Furthermore, the buffer layer influences the mechanical properties of the Cr coatings, with Ni/Cr and Al/Cr coatings demonstrating increased hardness and elastic modulus. The Ni/Cr coating achieved the highest values of 3.95 GPa and 62.09 GPa, respectively. Regarding corrosion performance, The Cr coatings containing buffer layers showed markedly better corrosion resistance than the bare 7050 Al alloy. A compact Cr2O3 passive film formed on their surfaces, cutting the corrosion current density by roughly two orders of magnitude. Among all samples, the Ti/Cr coating performed best, registering the lowest current density (1.687 × 10−6 A cm−2) and the highest charge-transfer resistance (6090 Ω cm2).
1. Introduction
Aluminum alloys are widely employed in transportation, aerospace, and other engineering sectors owing to their low density, high specific strength, excellent heat-treatability, and favorable formability [1,2]. In natural environments, they spontaneously develop a native oxide film; however, this layer is inherently thin, porous, and discontinuous. Aggressive ions readily infiltrate the film’s pores or mechanically damaged regions in acidic or chloride-rich media, initiating localized corrosion that can ultimately compromise structural integrity [3,4]. Consequently, enhancing aluminum alloys’ mechanical properties and corrosion resistance, thereby extending their service life, has become a central focus of current research in the aluminum alloy community [5,6,7,8].
Researchers have recently advanced several surface-coating techniques for aluminum alloys, including physical vapor deposition (PVD), chemical plating, cold spraying, and micro-arc oxidation, to enhance their performance in hostile environments [9,10,11,12]. Magnetron sputtering (MS), a PVD technique, can deposit metallic, alloy, or oxide films at relatively low substrate temperatures while permitting precise control over coating thickness, composition, and microstructure through the careful adjustment of target power, deposition temperature, and chamber pressure [13,14]. Chromium coatings attract particular interest among the candidate materials due to their excellent mechanical strength and chemical stability [15,16]. Li [17] and Wang [18] have confirmed that, in corrosive environments, Cr coatings rapidly develop a dense Cr2O3 passivation layer that markedly enhances their corrosion and oxidation resistance.
The low deposition temperature and high nucleation density inherent in MS films drive growth far from thermodynamic equilibrium, giving rise to columnar grains and defects such as pinholes, through-pores, and grain-boundary networks [19,20]. These flaws cannot be healed by atomic diffusion. Their continuous, vertically aligned paths weaken adhesion at the coating–substrate interface and serve as rapid channels for corrosive species, which compromises the corrosion resistance of Cr coatings [20,21]. To remedy this structural drawback, researchers have introduced multilayer architectures [21] and optimized coating compositions [16] to interrupt columnar growth, fill voids, and block penetration defects, ultimately improving the corrosion performance of Cr coatings [22].
Previous studies have shown that inserting metallic buffer layers such as Ti [23] or Al [24] and adopting multilayer architectures can relieve residual stresses at the coating–substrate interface, strengthen adhesion, and improve wear and corrosion resistance [17,25]. For instance, Qi et al. [24] deposited CrN films on Zr702 alloy with Cr, Ti, or Al buffer layers; all three buffer layers enhanced adhesion, whereas the Cr buffer layer afforded the best corrosion resistance because a continuous Cr2O3 passive film formed both on the coating surface and at the interfaces, effectively blocking electrolyte ingress. Likewise, Shugurov et al. [23] have reported that introducing Ti0.45Al0.55 or Ta buffer layers into Ti–Al–Ta–N coatings slightly reduced hardness but, through plastic cushioning and crack deflection at the multilayer interfaces, suppressed spallation and large-scale fragmentation, thereby increasing wear resistance. The amorphous, rough Ti0.45Al0.55 buffer layer interface was particularly effective at absorbing plastic deformation and arresting crack propagation, resulting in superior bonding. In general, metallic buffer layers dissipate strain energy during crack growth via plastic flow and interface deflection while simultaneously restricting columnar grain growth and reducing porosity; together, these effects comprehensively enhance the wear and corrosion performance of the coating [21,23,26].
Few studies have explored magnetron-sputtered Cr coatings on aluminum alloy substrates, and the influence of different metallic buffer layers on their microstructure, mechanical properties, and corrosion behavior remains unclear. There is a significant mismatch in aluminum alloy substrates’ physical and mechanical properties, such as the coefficient of thermal expansion, hardness, and elastic modulus between the substrate and the Cr coating. This mismatch results in different nucleation and growth behaviors of the coating. Three buffer materials—Ti, Al, and Ni—were selected to compare the effects of buffer layers with varying physical and chemical properties on the microstructure and overall performance of Cr coatings on aluminum alloy substrates. This work deposited monolithic Cr and Cr coatings incorporating Ti, Al, or Ni buffer layers on cast 7050 aluminum alloy by direct-current (DC) magnetron sputtering. The resulting coatings were systematically evaluated for microstructure, mechanical performance, and corrosion resistance, establishing a scientific basis for designing high-performance Cr coatings for aluminum alloy components.
2. Materials and Methods
2.1. Coating Preparation
Cr coatings prepared either as single layers or in combination with Ti, Al, or Ni buffer layers, were deposited on monocrystalline silicon (111) wafers and cast 7050 aluminum-alloy disks (Ø 28 mm × 3 mm) by DC magnetron sputtering. The alloy disks were cut by wire EDM, then sequentially ground with 400, 800, 1200, 1500, and 2000-grit SiC papers. All substrates underwent 10 min ultrasonic cleaning in acetone followed by ethanol and were air-dried to remove surface contaminants.
After the substrates were loaded, the chamber was evacuated to below 8 × 10−4 Pa and maintained at 30 °C with an Ar working pressure of 0.2 Pa. High-purity metal targets supplied by ZhongNuo Advanced Material (Beijing) Technology Co., Ltd. (Beijing, China), including Cr (99.95 wt %), Ti (99.995 wt %), Ni (99.995 wt %), and Al (99.999 wt %), were pre-sputtered in Ar for 10 min to remove native surface oxides. During deposition, the Cr target was powered at 250 W, and the Ti, Ni, and Al targets operated at 200 W. The deposition rates for each target under the selected sputtering conditions were quantified in preliminary experiments, allowing buffer layers of nearly identical thickness to be fabricated.
2.2. Characterization and Performance Testing
Cross-sectional morphology was examined by scanning electron microscopy (SEM, CIQTEK 5000X, Hefei, China) equipped with energy-dispersive X-ray spectroscopy (EDS), which also provided elemental compositions. Phase analysis of the Cr coatings with different buffer layers was carried out on an X-ray diffractometer (Bruker D8 Advance, Karlsruhe, Germany) using Cu Kα radiation (λ = 0.15405 nm); data were collected at 40 kV, a scan rate of 4°/min, and a 2θ range of 20° to 90°.
Nano-mechanical properties were measured with a nano-indenter (Bruker Hysitron TS 77, Minneapolis, MN, USA) having a displacement resolution of 0.2 nm and a load resolution of 75 nN. Each indentation was held for 3 s, and at least five indents per sample were averaged to obtain hardness and standard deviation. Adhesion was assessed with a progressive-load scratch tester up to a maximum normal load of 10 N. Electrochemical behavior was evaluated in naturally aerated 3.5 wt % NaCl. A saturated calomel electrode served as the reference, platinum as the counter, and the specimen as the working electrode with an exposed area of 1 cm2. The open-circuit potential was monitored for 60 min before testing. Electrochemical impedance spectroscopy was recorded from 100 kHz down to 0.01 Hz, and potentiodynamic polarization curves were obtained at 0.01 mV/s over a potential window of −2000 mV (vs. SCE) to +200 mV relative to the stabilized open-circuit potential.
3. Results
3.1. Microstructure
Figure 1a–d show SEM surface micrographs of single-layer Cr coatings and Cr coatings deposited on Ti, Al, or Ni buffer layers. All films are composed of uniformly sized particles with well-defined boundaries, with nanometre-scale pinhole defects sporadically distributed across the surfaces. The Cr and Ni/Cr coatings exhibit the finest grain sizes and the highest densification among the coatings. Figure 1e–h present cross-sectional SEM micrographs accompanied by the corresponding EDS line-scan profiles. The coatings display dense, continuous columnar structures, uniform overall thicknesses, and smooth surfaces, and the buffer layers have comparable thicknesses (approximately 0.14 µm). Pronounced through-pores are observed in the single-layer Cr coating. Along the EDS scan, the Cr signal rises progressively; once the scan reaches the buffer layer, the corresponding element (Ti, Al, or Ni) appears and then declines sharply at the coating/Si interface, forming a narrow transition zone with a compositional gradient. Except for these interfacial peaks, elemental distributions within the coatings remain uniform, confirming that Cr is the principal component and that the deposition process was stable and well-controlled.
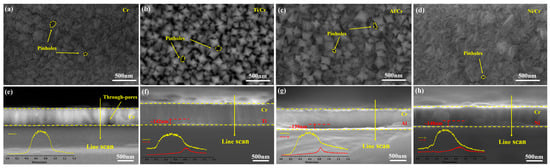
Figure 1.
Surface and cross-sectional morphologies, with EDS line scans, of different coatings: Cr (a,e); Ti/Cr (b,f); Al/Cr (c,g); and Ni/Cr (d,h).
Figure 2 shows the XRD pattern of the coating deposited on a silicon wafer, and the XRD spectrum of the Si substrate is given as a reference. The peaks at 44.39° and 81.72° in the prepared Cr coatings match the crystallographic facets of Cr(110) and Cr(211) (PDF#00-006-0694). Ti, Cr, and Ni peaks were detected in the XRD patterns of Ti/Cr, Al/Cr, and Ni/Cr coatings, respectively. All Cr coatings correspond to the strongest diffraction peaks at the (110) crystal plane, which is related to the energy of the growth mode of the Cr coatings prepared by magnetron sputtering [27,28]. Furthermore, Cr coatings deposited by alternative magnetron-sputtering methods exhibit comparable crystal structures and similar preferential orientations [29].
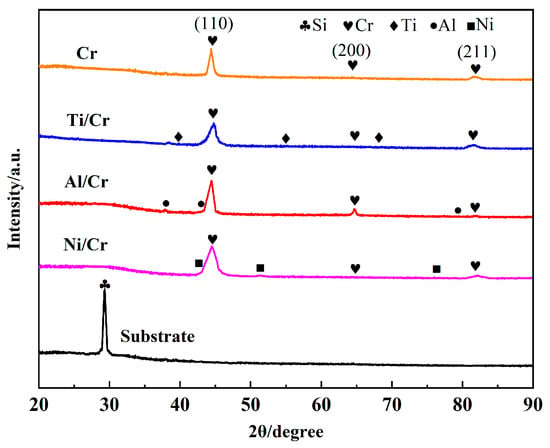
Figure 2.
XRD patterns of coatings.
3.2. Mechanical Properties of Different Cr Coatings
Figure 3a presents the load–displacement curves for the 7050-Al substrate, the single-layer Cr coating, and the Cr coatings containing Ti, Al, or Ni buffer layers. All coatings display smooth loading and unloading segments, reflecting their dense, continuous microstructures. Figure 3b summarizes hardness and elastic modulus. Due to its partially columnar, porous microstructure, the single-layer Cr coating shows lower values than the aluminum substrate. Introducing Al or Ni buffer layers increases both parameters; the Ni/Cr coating attains the highest hardness and elastic modulus, at 3.95 GPa and 62.09 GPa, respectively. In contrast, adding a Ti buffer layer slightly decreases these properties compared with the pure Cr coating. Differences in elastic modulus among the buffer layers are a key factor influencing hardness [30].
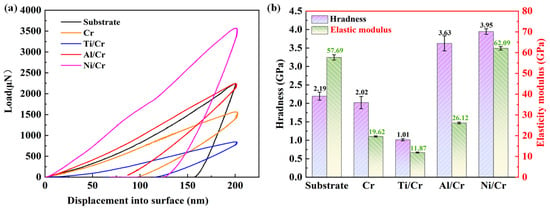
Figure 3.
Load–displacement curves of (a) nanoindentation and (b) hardness and modulus of elasticity of the aluminum alloy substrate, Cr monolayer, and Cr coatings with different buffer layers.
Figure 4 presents the scratch-test results for the Cr coatings with and without buffer layers. Figure 4a plots the acoustic emission (AE) signal and penetration depth curves for the monolithic Cr coating under a progressively increasing normal load; optical micrographs of the corresponding scratch tracks appear above the curves. As the load increases, coating spallation becomes more pronounced, and the AE intensity rises accordingly, indicating that failure at the coating–substrate interface intensifies with load [31]. In scratch testing, the first critical load at which cohesive cracks develop is designated Lc1 while the load at which extensive delamination exposes a continuous area of the substrate is termed Lc2; these two values are widely used to characterize intrinsic coating toughness and interfacial adhesion, respectively [32]. Figure 4b compares Lc1 and Lc2 for the single-layer Cr coating and Ti/Cr, Al/Cr, and Ni/Cr coatings. All coatings containing a buffer layer exhibit higher critical loads than the monolithic Cr coating, demonstrating improved adhesion to the aluminum alloy, and the Ni/Cr coating achieves the most significant enhancement. This superior performance is attributed to the higher hardness and elastic modulus of the Ni/Cr stack and to the formation of Ni-rich intermetallic compounds [7] at the Ni–Cr interface, which is highly compatible with Cr and effectively strengthens the bond [33].
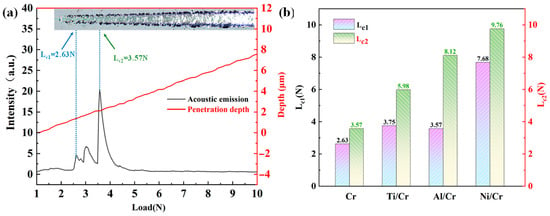
Figure 4.
(a) Acoustic emission profiles, penetration depth profiles, scratch images, and binding strength of Cr coatings and (b) different buffer layers, Lc1 and Lc2.
3.3. Electrochemical Properties of Different Cr Coatings
Figure 5 presents the potentiodynamic polarization curves for the bare aluminum alloy substrate and the four Cr coatings in 3.5 wt % NaCl. At the same time, the electrochemical parameters derived by Tafel extrapolation are compiled in Table 1. In general, a lower corrosion–current density (icorr) coupled with a higher corrosion potential (Ecorr) indicates superior corrosion resistance [34]. Relative to the uncoated alloy, all Cr-coated specimens exhibit a marked positive shift in Ecorr and a reduction in icorr of roughly two orders of magnitude, confirming that the coatings significantly mitigate the alloy’s corrosion tendency. A decrease in corrosion current density signifies a lower corrosion rate, indicating that the Cr coating effectively suppresses the electrochemical corrosion of the aluminum alloy substrate and substantially extends its service life in marine environments. This improvement stems from chromium’s strong self-passivating behavior—on contact with chloride media, the coating rapidly forms a dense, stable Cr2O3 film that isolates the substrate from the electrolyte, as described by the reaction given below [35].
2Cr + 3H2O → Cr2O3 + 6H+ + 6e−
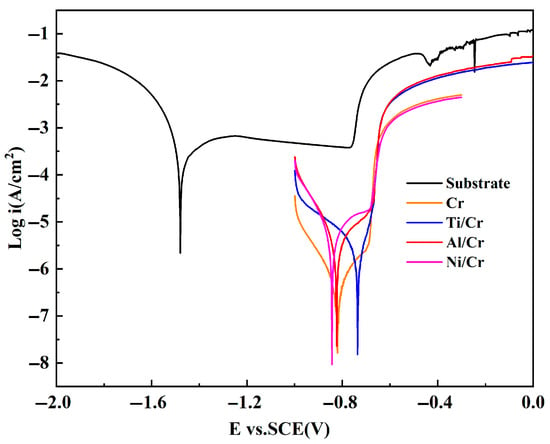
Figure 5.
Polarization curves of aluminum alloy substrate, Cr monolayer and Cr coatings with different buffer layers in 3.5 wt % NaCl solution.

Table 1.
Dynamic potentiodynamic polarization data for substrate and different Cr coatings.
Conversely, uncoated 7050 aluminum alloy is prone to micro-electrochemical-coupling corrosion and pitting in 3.5 wt % NaCl because second-phase particles and continuous grain-boundary precipitates act as local cathodes, creating a potential difference with the α-Al matrix and driving localized attack [36]. Applying a Cr coating physically separates these electrochemical heterogeneities from the electrolyte, lowering the alloy’s overall corrosion susceptibility [6,37]. During anodic polarization, the Cr-coated samples display a clear passivation region with a broad current plateau, confirming that the Cr2O3 film formed on the coating surface effectively suppresses anodic dissolution over a substantial potential range [38].
Further analysis of the polarization curves shows that the Ti/Cr coating gives the lowest corrosion–current density, 1.687 × 10−6 A cm−2, and shifts the corrosion potential positively, relative to the monolithic Cr coating. This improvement arises because the Ti buffer layer promotes a denser, more homogeneous microstructure and reduces through-thickness defects, effectively blocking the electrolyte’s access to the substrate. Adding Ni or Al buffer layers also lowers icorr compared with the single-layer Cr coating. However, the benefit is smaller: their looser microstructures provide additional diffusion paths for corrosive species [39], making the buffer layer more permeable and, in turn, weakening overall corrosion resistance.
To further understand the coatings’ corrosion behavior, electrochemical impedance spectroscopy (EIS) was performed. Figure 6 displays the Nyquist and Bode plots for the single-layer Cr coating and the Ti/Cr, Al/Cr, and Ni/Cr coatings, along with the equivalent circuit models used for fitting by the ZView 2 software. In the Nyquist spectra (Figure 6a), a larger capacitive arc in the low-frequency region corresponds to superior corrosion resistance in 3.5 wt % NaCl solution. The arc radius reflects the impedance linked to charge transfer at the coating surface and thus serves as a direct indicator of the coating’s capacity to impede electron flow during corrosion [24,40]. The figure shows that the Ti/Cr coating displays the largest capacitive arc radius, signifying the highest charge transfer resistance. In the Bode plots, a broader mid-frequency phase-angle peak, a larger low-frequency impedance modulus |Z|, and a maximum phase angle closer to −90° all correlate with superior corrosion protection [41,42]. As Figure 6b,c illustrate, the Ti/Cr coating has the widest mid-frequency phase-angle peak and the greatest low-frequency |Z|, confirming that it blocks the ingress of corrosive species most effectively.
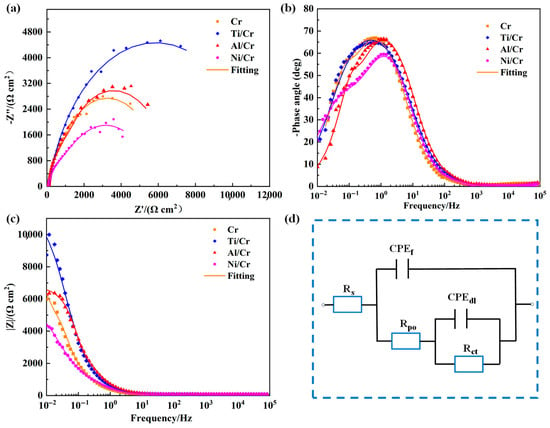
Figure 6.
EIS results for coatings: (a) Nyquist plot, (b) Bode phase angle plot, (c) Bode |Z| plot, and (d) equivalent circuit modeling of coatings.
Additionally, the impedance parameters of each coating were obtained by fitting its Nyquist and Bode plots with the equivalent circuit model, shown in Figure 6d. In this circuit, Rs represents the electrolyte resistance, Rpo is the pore resistance of the coating, and Rct is the charge transfer resistance. CPEf corresponds to the coating’s capacitance, whereas CPEdl is the double-layer capacitance connected in parallel with Rct [42,43]. Table 2 lists the parameters obtained from fitting the EIS data with the equivalent circuit. The Rct reflects how effectively a coating resists corrosive attack: a larger Rct means the coating blocks charge transfer more efficiently, lowering the corrosion rate [44]. Likewise, a higher Rpo indicates lower porosity. Among the coatings, the Ti/Cr sample shows by far the greatest Rct (6090 Ω cm2), confirming that the Ti buffer layer strongly impedes the intrusion of corrosive media. Its Rpo is also comparatively high, which is attributed to the good structural compatibility between the Ti buffer layer and the Cr layer [24]. Meanwhile, the Ti/Cr coating shows the lowest CPEdl and an n value close to 1, meaning that only a small interfacial area is active for corrosion, and the response is nearly that of an ideal capacitor. The Ni/Cr coating has a high Rct (4039 Ω cm2) but a relatively low Rpo (1604 Ω cm2), suggesting that charge transfer is effectively suppressed. However, the film is slightly less dense [39]. In contrast, Al/Cr combines a high Rpo with a lower Rct. This combination provides excellent physical shielding, yet it offers weaker inhibition of interfacial corrosion once the electrolyte penetrates the film.

Table 2.
Fitting parameters of equivalent circuits with different Cr coatings.
4. Discussion
To enhance the mechanical properties and corrosion resistance of 7050 Al alloy, a Cr coating was deposited on its surface by DC magnetron sputtering. Thin Ti, Al, and Ni buffer layers were inserted further between the substrate and the Cr top layer to improve the coating–substrate interface and overall performance.
Experimental results demonstrate that the buffer layer materials (Ti, Al, Ni) markedly affect the nucleation and growth behavior of sputtered Cr coatings on 7050 aluminum substrates, leading to distinct surface and cross-sectional morphologies. The Cr and Ni/Cr configurations exhibit relatively fine-grained and compact structures. Cr grows with a higher nucleation density in these cases, resulting in smaller, densely packed grains and reduced pronounced columnar features. This behavior is likely attributable to the superior crystallographic and chemical compatibility between Ni and Cr, which lowers the interfacial energy and enhances wetting. As a result, Cr adatoms are more prone to diffuse across the surface and form numerous nuclei rather than isolated islands [45,46]. Moreover, the Ni buffer layer offers a high density of nucleation sites and may facilitate interfacial mixing, leading to the formation of a continuous film from the initial stages of growth. Consequently, the surface exhibits a dense and uniform microstructure with minimal development of columnar grains [47]. In contrast, the Al/Cr and Ti/Cr coatings exhibit a more pronounced columnar structure, characterized by larger columnar grains extending throughout the coating thickness. This indicates that the initial nucleation density of Cr on Ti or Al buffer layers is relatively low, allowing the few nuclei that do form to grow competitively into columnar structures. This phenomenon is attributed to the differences in crystal structures and lattice parameters among Ti, Al, and Cr. The poor lattice matching results in limited incorporation of Cr adatoms into the growing film. Instead, these adatoms tend to aggregate into three-dimensional islands, which subsequently evolve into columnar crystals as the coating thickness increases [45].
In addition, the introduction of a Ti buffer layer results in a reduction in the hardness of the Cr coating. In contrast, the incorporation of Al and Ni buffer layers leads to an increase in hardness. This variation is closely related to differences in the microstructure and density of the coatings and the disparity in elastic modulus among the various buffer layers [30]. In particular, the Ni/Cr coatings exhibit a dense, fine-grained microstructure, which results in high hardness due to the strengthening effect of Hall–Petch grain boundaries, along with a high elastic modulus [15]. In contrast, the Ti/Cr coatings show reduced hardness due to their columnar grain structure and the significant mismatch in elastic modulus between the Ti buffer layer, the substrate, and the Cr coating [48,49]. On the other hand, the Al buffer layer has properties similar to those of the substrate, which helps suppress the coarsening of columnar grains, hinders dislocation motion, and relieves the high stress gradient between the substrate and the Cr coating. As a result, the hardness of the coating is improved [50]. All buffer layers improved the bonding strength of the Cr coating to the 7050 Al alloy substrate, primarily because they can absorb residual stresses through plastic deformation. Among the layers, the Ni buffer layer provided the most significant enhancement. This is likely due to its ductility, which effectively relieves interfacial stresses [51]. Additionally, the formation of intermetallic compounds between Ni and Al exhibits a strong chemical affinity with the aluminum alloy matrix, further contributing to the coating’s improved bonding strength and mechanical performance [7].
The electrochemical experiment results show that Cr coatings, both with and without buffer layers, significantly enhance the corrosion resistance of the aluminum alloy substrate. The Ti/Cr coating exhibited the lowest current density and charge transfer resistance among all configurations. This improvement may be attributed to the multilayer structure, which refines grain size and impedes the penetration of corrosive media [52]. Furthermore, Ti is a highly passable metal capable of rapidly forming a stable TiO2 oxide film in neutral chloride environments. When pores exist in the Cr coating, the corrosive medium can reach the Ti buffer layer through these defects, triggering the oxidation of Ti to form TiO2. This in-situ generated oxide acts as a “self-healing” barrier that seals the defective pathways, thereby enhancing the overall corrosion resistance of the coating. Moreover, the composite oxide film formed by TiO2 [53] and Cr2O3 [54], which are generated from the Cr coating, exhibits smaller pores and greater resistance to dissolution and rupture in chloride-containing solutions. In comparison, although the Ni/Cr coatings possess superior mechanical properties, they demonstrate inferior corrosion resistance. This is primarily due to the significant differences in passivation behavior and electrochemical potential between Ni and Cr. Ni tends to form less stable and loosely adherent Ni(OH)2 and NiO films [22], which are susceptible to pitting corrosion in chloride environments. This degradation further compromises the passivation layer, as reflected by the lower Rpo values.
Our study revealed that introducing different buffer layers did not universally enhance the microstructure and properties of Cr coatings, which is consistent with the findings reported by Qi et al. [24]. First, the type of buffer layer alters the resulting surface morphology of the coating, a phenomenon closely related to changes in the nucleation and growth mechanisms. Second, variations in the elastic modulus of the buffer layers and differences in the crystallographic structures of the coatings play critical roles in determining their mechanical performance. While all buffer layers contribute to improved bonding strength between the coating and the substrate, their effects on corrosion resistance are not uniformly positive. The Al/Cr coating demonstrated a dense microstructure, favorable bonding strength, and mechanical properties among the studied buffer layer configurations. Additionally, the coating exhibited improved corrosion resistance compared with the bare aluminum alloy substrate. Therefore, the Al/Cr coating is a promising candidate for applications requiring the combined performance of high mechanical strength and resistance to corrosive environments.
There are also certain limitations in this study. For instance, while the porosity and columnar structures of Cr coatings with different buffer layers were qualitatively analyzed, their quantitative characterization remains incomplete, which may be associated with the observed variability in electrochemical behavior among the coatings. Additionally, parameters such as buffer layer thickness and deposition power are known to significantly influence the microstructure and properties of Cr coatings but were not systematically explored in this work.
Future research should focus on elucidating the film formation mechanisms of Cr coatings on aluminum alloys with various buffer layers and investigating the effects of deposition parameters on coating structure and performance. These insights would be of practical importance for guiding the selection and optimization of Cr coatings with different buffer layer types for diverse application scenarios involving aluminum alloys.
5. Conclusions
In summary, Cr coatings incorporating Ti, Al, or Ni buffer layers were deposited on 7050 Al alloy by direct-current magnetron sputtering, improving the alloy’s surface performance. Each coating (Cr, Ti/Cr, Al/Cr, and Ni/Cr) possessed a dense, continuous microstructure with uniform thickness, a flat surface, and homogeneous elemental distribution. Introducing a Ti buffer layer lowered hardness and elastic modulus, whereas Al and Ni buffers raised these properties. The Ni/Cr coating reached a maximum hardness of 3.95 GPa and a maximum modulus of 62.09 GPa. Scratch tests showed that every buffer layer enhanced adhesion compared with the single-layer Cr coating, and Ni/Cr produced the most significant increase. Electrochemical results in 3.5 wt % NaCl confirmed that all Cr-based coatings outperformed bare aluminum alloy by one to two orders of magnitude owing to the rapid formation of a protective Cr2O3 film. The Ti/Cr coating delivered the best corrosion protection, displaying the lowest icorr (1.687 × 10−6 A cm−2) and the highest Rct (6090 Ω cm2). Ni and Al’s buffers offered mechanical gains but slightly reduced corrosion resistance compared with Ti/Cr. Therefore, buffer layers should be selected according to the performance demands of each application. Doing so enables precise control and optimization of the coatings’ mechanical and electrochemical properties.
Author Contributions
Conceptualization, data curation, writing—original draft Y.D. and T.H.; methodology, X.D. and K.C.; methodology, resources, writing—review and editing, A.V., C.S. and J.L.; investigation, Y.W. and P.H.; supervision, T.H.; project administration, T.H.; funding acquisition, T.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (Grant No. 52275350), International Cooperation Research Platform Construction Project of Shanghai University of Engineering Science (Grant No. 0301006).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ji, S.; Li, J.; Yang, H.; Wang, S.; Qiao, J.; Wen, Q.; Mu, W. Deposition behavior and microstructure of WC-12Co coating deposited by HVOF on 7075 aluminum alloy substrates. Surf. Coat. Technol. 2024, 489, 131137. [Google Scholar] [CrossRef]
- Ding, Y.; He, T.; Zhang, J.-J.; Du, X.-Y.; Vereschaka, A. Microstructural Evolution and Kinetics of 7075 Al Alloy During Homogenization Treatment. JOM 2024, 77, 1196–1207. [Google Scholar] [CrossRef]
- Fernández-López, P.; Alves, S.A.; San-Jose, J.T.; Gutierrez-Berasategui, E.; Bayón, R. Plasma Electrolytic Oxidation (PEO) as a Promising Technology for the Development of High-Performance Coatings on Cast Al-Si Alloys: A Review. Coatings 2024, 14, 217. [Google Scholar] [CrossRef]
- Zhou, Y.T.; Jia, X.L.; Li, J.B.; Guan, G.G.; Ma, X.L.; Zhang, J.S. Unraveling the precipitate-induced discontinuity of the surface oxide film on Al alloy. Appl. Surf. Sci. 2022, 590, 153108. [Google Scholar] [CrossRef]
- Zhang, J.; He, T.; Du, X.; Alexey, V.; Song, M.; Chen, X. Enhanced mechanical properties in 7075 Al alloy fasteners processed by post ECAP-CU aging. Mater. Today Commun. 2025, 45, 112274. [Google Scholar] [CrossRef]
- Li, X.; Wang, K.; Jiang, B.; Chen, Y.; Zhang, Z.; Lu, K.; Liang, X. Enhancing corrosion and wear resistance of a 7075 aluminum alloy via depositing TC4 coating. J. Mater. Res. Technol. 2024, 32, 1736–1748. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.; Ning, H.; Ma, H.; Xie, R.; Kong, Y.; Fu, Y. Ni-based coating on 5083 aluminum alloy with Cu-Ni interlayer fabricated by ultra-high-speed laser directed energy deposition. Surf. Coat. Technol. 2023, 474, 130068. [Google Scholar] [CrossRef]
- Li, J.; He, T.; Du, X.-Y.; Vereschaka, A.; Zhang, J.-J. Regulating hardness homogeneity and corrosion resistance of Al-Zn-Mg-Cu alloy via ECAP combined with inter-pass aging. Mater. Charact. 2024, 218, 114489. [Google Scholar] [CrossRef]
- Xue, N.; Li, W.; Shao, L.; Chen, Y.; Wu, Y.; Luo, M.; Sajjad, K.; Dai, S.; Zhu, L. Characterization of monolayer and single-pass pure Cu coatings applied to 6061 T6 Al alloy and AZ31B Mg alloy substrates using high-pressure cold spray technology. J. Mater. Res. Technol. 2025, 36, 252–271. [Google Scholar] [CrossRef]
- Mahton, Y.; Kamde, M.A.; Saha, P. Influence of Cu addition on the microstructure, and corrosion behavior of electroless Ni-Cu-P coating on squeeze-cast Al-Cu-Mg alloy. Surf. Coat. Technol. 2024, 494, 131544. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Y. Microhardness, wear resistance, and corrosion resistance of AlxCrFeCoNiCu high-entropy alloy coatings on aluminum by laser cladding. Opt. Laser Technol. 2021, 134, 106632. [Google Scholar] [CrossRef]
- Ye, T.; Ma, J.; Jia, Z.; Li, T.; Liu, W.; Yu, W. Microstructure, mechanical properties and low-temperature tribological behavior of Cr/Cr-W/W-DLC/DLC multilayer coatings on 5A06 Al alloy. J. Mater. Res. Technol. 2022, 18, 810–819. [Google Scholar] [CrossRef]
- Li, C.; Li, G.; Lü, W.; Deng, J.; Song, Z.; Wang, Q. Effect of carbon content on the structure and mechanical properties of (TiAlTaCrZr)CN high entropy alloy carbonitride coatings prepared by magnetron sputtering. Ceram. Int. 2025, 51, 11499–11508. [Google Scholar] [CrossRef]
- Yang, X.; Du, Y.; Du, H.; Tang, Z. Tribocorrosion behavior of magnetron-sputtered MoS2-TiCr composite coatings. Ceram. Int. 2025. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Q.; Zhang, X.; Yu, Z.; Zhang, X.; Mao, Q.; Nie, J.; Zhao, Y. Electrodeposition of nanocrystalline Ni and NiCr alloy coatings: Effects of Cr content on microhardness and wear resistance improvement. J. Mater. Res. Technol. 2024, 30, 3584–3593. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, H.; Zhong, Y.; Zhang, Y.; Li, Q.; Wu, L.; Ning, Z.; Wu, X.; Yang, J. Exploring the effect of Si content in Cr coating on its microstructure and properties. Surf. Coat. Technol. 2024, 493, 131253. [Google Scholar] [CrossRef]
- Yu, J.-G.; Li, Z.-Y.; Ren, Q.-Y.; Jiao, Y.-J.; Cai, Z.-B. Corrosion and fretting wear behavior of Cr-based composite coatings on zirconium alloy. Vacuum 2025, 234, 114104. [Google Scholar] [CrossRef]
- Han, Z.; Wang, Z.; Wang, Z.; Tan, S.; Wang, A.; Piao, Z.; Ke, P. Tailored high-temperature corrosion behavior of Cr coatings using high power impulse magnetron sputtering on ZIRLO alloys for accident-tolerant fuel application. Surf. Coat. Technol. 2024, 488, 130941. [Google Scholar] [CrossRef]
- Daure, J.L.; Voisey, K.T.; Shipway, P.H.; Stewart, D.A. The effect of coating architecture and defects on the corrosion behaviour of a PVD multilayer Inconel 625/Cr coating. Surf. Coat. Technol. 2017, 324, 403–412. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Li, H.; Ma, G.; Sun, L.; Guo, P.; Ke, P.; Lee, K.-R.; Wang, A. Controllable defect engineering to enhance the corrosion resistance of Cr/GLC multilayered coating for deep-sea applications. Corros. Sci. 2022, 199, 110175. [Google Scholar] [CrossRef]
- Cai, F.; Zhou, Q.; Chen, J.; Zhang, S. Effect of inserting the Zr layers on the tribo-corrosion behavior of Zr/ZrN multilayer coatings on titanium alloys. Corros. Sci. 2023, 213, 111002. [Google Scholar] [CrossRef]
- Du, H.; Wen, J.; Song, G.; Wu, H.; Yin, Y. Corrosion Behavior of Ni/NiCr/NiCrAlSi Composite Coating on Copper for Application as a Heat Exchanger in Sea Water. Nanomaterials 2023, 13, 3129. [Google Scholar] [CrossRef] [PubMed]
- Shugurov, A.R.; Kuzminov, E.D. Mechanical and tribological properties of Ti-Al-Ta-N/TiAl and Ti-Al-Ta-N/Ta multilayer coatings deposited by DC magnetron sputtering. Surf. Coat. Technol. 2022, 441, 128582. [Google Scholar] [CrossRef]
- Li, Y.; Cao, H.; Li, H.; Yang, J.; Qi, F.; Lu, L.; Zhao, N.; Zhou, Y.; Ouyang, X. Effect of buffer layer on oxidation and corrosion resistance of CrN coatings on Zr alloy prepared by FCVAD technology. Surf. Coat. Technol. 2022, 448, 128942. [Google Scholar] [CrossRef]
- He, T.; Valery, Z.; Vereschaka, A.; Keshin, A.; Huo, Y.; Milovich, F.; Sotova, C.; Seleznev, A. Influence of niobium and hafnium doping on the wear and corrosion resistance of coatings based on ZrN. J. Mater. Res. Technol. 2023, 27, 6386–6399. [Google Scholar] [CrossRef]
- Tao, H.; Zhylinski, V.; Vereschaka, A.; Chayeuski, V.; Yuanming, H.; Milovich, F.; Sotova, C.; Seleznev, A.; Salychits, O. Comparison of the Mechanical Properties and Corrosion Resistance of the Cr-CrN, Ti-TiN, Zr-ZrN, and Mo-MoN Coatings. Coatings 2023, 13, 750. [Google Scholar] [CrossRef]
- Huang, M.; Li, Y.; Ran, G.; Yang, Z.; Wang, P. Cr-coated Zr-4 alloy prepared by electroplating and its in situ He+ irradiation behavior. J. Nucl. Mater. 2020, 538, 152240. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, G.; Wang, C.; Wang, T.; Zhang, Y.; Xin, T. Insights into 1200 °C steam oxidation behavior of Cr coatings with different microstructure on Zircaloy-4 alloys for enhanced accident tolerant fuel cladding. Ann. Nucl. Energy 2025, 210, 110885. [Google Scholar] [CrossRef]
- Shi, W.; Peng, J.; Xu, Z.; Shen, Q.; Wang, C. Effect of Power on Structural and Mechanical Properties of DC Magnetron Sputtered Cr Coatings. Metals 2023, 13, 691. [Google Scholar] [CrossRef]
- Xiao, B.; Zhang, T.F.; Guo, Z.; Li, Z.; Fan, B.; Chen, G.; Xiong, Z.; Wang, Q. Mechanical, oxidation, and cutting properties of AlCrN/AlTiSiN nano-multilayer coatings. Surf. Coat. Technol. 2022, 433, 128094. [Google Scholar] [CrossRef]
- Cao, H.; Yang, J.; Li, Y.; Ren, L.; Qi, F.; Zhao, N.; Zhou, Y.; Li, B.; Ouyang, X. Effect of nitrogen pressure on the microstructure, mechanical and electrochemical properties of CrAlN coatings deposited by filter cathode vacuum arc. Ceram. Int. 2022, 48, 36570–36584. [Google Scholar] [CrossRef]
- Akhter, R.; Bendavid, A.; Munroe, P. Tailoring the scratch adhesion strength and wear performance of TiNiN nanocomposite coatings by optimising substrate bias voltage during cathodic arc evaporation. Surf. Coat. Technol. 2022, 445, 128707. [Google Scholar] [CrossRef]
- Kurbanbekov, S.; Rakhadilov, B.; Kakimzhanov, D.; Seitov, B.; Katpaeva, K.; Kurmantayev, A.; Dautbekov, M.; Kengesbekov, A. Research on the Structural–Phase and Physical–Mechanical Characteristics of the Cr3C2-NiCr Composite Coating Deposited by the HVOF Method on E110 Zirconium Alloy. Coatings 2024, 14, 1030. [Google Scholar] [CrossRef]
- Xu, X.; Li, W.; Wan, B.; Jin, S.; Chen, K.; Su, F. Extremely improved the corrosion resistance and anti-wear behavior of aluminum alloy in 3.5% NaCl solution via amorphous CrAlN coating protection. Corros. Sci. 2024, 230, 111952. [Google Scholar] [CrossRef]
- Ho, W.-Y.; Shen, C.-H.; Chang, C.-L.; Wang, D.-Y. Corrosion behaviors of Cr(N,O)/CrN multi-layered coatings by cathodic arc deposition. Surf. Coat. Technol. 2007, 202, 745–749. [Google Scholar] [CrossRef]
- Li, J.; He, T.; Du, X.-Y.; Vereschaka, A. Enhancing the corrosion resistance of high-strength Al-Zn-Mg-Cu alloys after equal channel angular pressing by developing retrogression and re-aging strategies. Corros. Sci. 2025, 246, 112736. [Google Scholar] [CrossRef]
- Zhang, J.-J.; He, T.; Du, X.-Y.; Alexer, V.; Song, M.; Chen, X.-L.; Li, J. Effect of pre-heat treatment and subsequent ECAP-CU on microstructure and corrosion behavior of 7075 Al alloy fasteners. J. Cent. South Univ. 2025, 1–21. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, L.; Bai, J.; Liu, X. Corrosion behavior and microstructure of electrodeposited nano-layered Ni–Cr coatings. Thin Solid Film. 2015, 595, 36–40. [Google Scholar] [CrossRef]
- Chen, Q.; Su, M.; Pang, G.; Zhou, Q.; Liang, D.; Zhang, E. Improving the corrosion resistance and conductivity of 316L bipolar plates used for PEMFCs by applying Cr/CrN multilayer coating. Appl. Surf. Sci. 2025, 689, 162573. [Google Scholar] [CrossRef]
- Mi, B.; Chen, Z.; Wang, Q.; Li, Y.; Qin, Z.; Wang, H. Properties of C-doped CrTiN films on the 316L stainless steel bipolar plate for PEMFC. Int. J. Hydrogen Energy 2021, 46, 32645–32654. [Google Scholar] [CrossRef]
- Jin, J.; He, Z.; Zhao, X. Effect of Al content on the corrosion resistance and conductivity of metal nitride coating in the cathode environment of PEMFCs. Mater. Chem. Phys. 2020, 245, 122739. [Google Scholar] [CrossRef]
- Yang, J.; Shou, D.; Zhao, N.; Tang, Y.; Cao, H.; Qi, F.; Ouyang, X. Interfacial structure, mechanical properties, and corrosion resistance of Cr/TiSiN nano-multilayer coating by filtered cathode vacuum arc technique. J. Manuf. Process. 2025, 136, 267–281. [Google Scholar] [CrossRef]
- Song, G.-H.; Yang, X.-P.; Xiong, G.-L.; Lou, Z.; Chen, L.-J. The corrosive behavior of Cr/CrN multilayer coatings with different modulation periods. Vacuum 2013, 89, 136–141. [Google Scholar] [CrossRef]
- Haixiang, C.; Dejun, K. Comparison on electrochemical corrosion performances of arc and laser thermal sprayed Al–Ti–Ni coatings in marine environment. Mater. Chem. Phys. 2020, 251, 123200. [Google Scholar] [CrossRef]
- Lozovoy, K.A.; Korotaev, A.G.; Kokhanenko, A.P.; Dirko, V.V.; Voitsekhovskii, A.V. Kinetics of epitaxial formation of nanostructures by Frank–van der Merwe, Volmer–Weber and Stranski–Krastanow growth modes. Surf. Coat. Technol. 2020, 384, 125289. [Google Scholar] [CrossRef]
- Belosludtsev, A.; Sytchkova, A.; Baltrusaitis, K.; Vaicikauskas, V.; Jasulaitiene, V.; Gric, T. Growth of Magnetron-Sputtered Ultrathin Chromium Films: In Situ Monitoring and Ex Situ Film Properties. Coatings 2023, 13, 347. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Lee, J.-W.; Ho, L.-W.; Chen, H.-W.; Chan, Y.-C.; Duh, J.-G. Microstructure and mechanical property evaluation of pulsed DC magnetron sputtered Cr–B and Cr–B–N films. Surf. Coat. Technol. 2011, 206, 1711–1719. [Google Scholar] [CrossRef]
- Cao, H.; Qi, F.; Ouyang, X.; Zhao, N.; Zhou, Y.; Li, B.; Luo, W.; Liao, B.; Luo, J. Effect of Ti Transition Layer Thickness on the Structure, Mechanical and Adhesion Properties of Ti-DLC Coatings on Aluminum Alloys. Materials 2018, 11, 1742. [Google Scholar] [CrossRef]
- Kim, G.S.; Lee, S.Y.; Hahn, J.H.; Lee, B.Y.; Han, J.G.; Lee, J.H.; Lee, S.Y. Effects of the thickness of Ti buffer layer on the mechanical properties of TiN coatings. Surf. Coat. Technol. 2003, 171, 83–90. [Google Scholar] [CrossRef]
- Jin, Y.; Lu, B.; Tang, X. Effect of Ag buffer layer thickness on the microstructure and hardness distribution of Cu alloy coating on Al substrate. Surf. Coat. Technol. 2023, 470, 129840. [Google Scholar] [CrossRef]
- Wang, M.; Liu, G.; Huang, M.; Fu, Y.; Lin, C.; Wu, J.; Levchenko, V.A. Investigation of the Adhesion Strength, Fracture Toughness, and Stability of M/Cr2N and M/V2N (M = Ti, Ru, Ni, Pd, Al, Ag, and Cu) Interfaces Based on First-Principles Calculations. Coatings 2022, 12, 66. [Google Scholar] [CrossRef]
- Zhu, S.; Wu, Y.; Liu, T.; Tang, K.; Wei, Q. Interface structure and corrosion resistance of Ti/Cr nanomultilayer film prepared by magnetron sputtering on depleted uranium. ACS Appl. Mater. Interfaces 2013, 5, 6598–6602. [Google Scholar] [CrossRef] [PubMed]
- Shuai, J.; Zuo, X.; Wang, Z.; Guo, P.; Xu, B.; Zhou, J.; Wang, A.; Ke, P. Comparative study on crack resistance of TiAlN monolithic and Ti/TiAlN multilayer coatings. Ceram. Int. 2020, 46, 6672–6681. [Google Scholar] [CrossRef]
- Sun, H.; Xu, Z.; Zhang, D.; Peng, L.; Lai, X. Corrosion behavior of passivation layer Cr2O3 of uncoated stainless steel under the anodic and cathodic conditions: A first-principles study. Chem. Eng. J. 2024, 493, 152658. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).