Low-Temperature Deposition of Alumina Films by Ultrasonic Spray Pyrolysis with a Water-Based Precursor
Abstract
1. Introduction
2. Experimental Methods
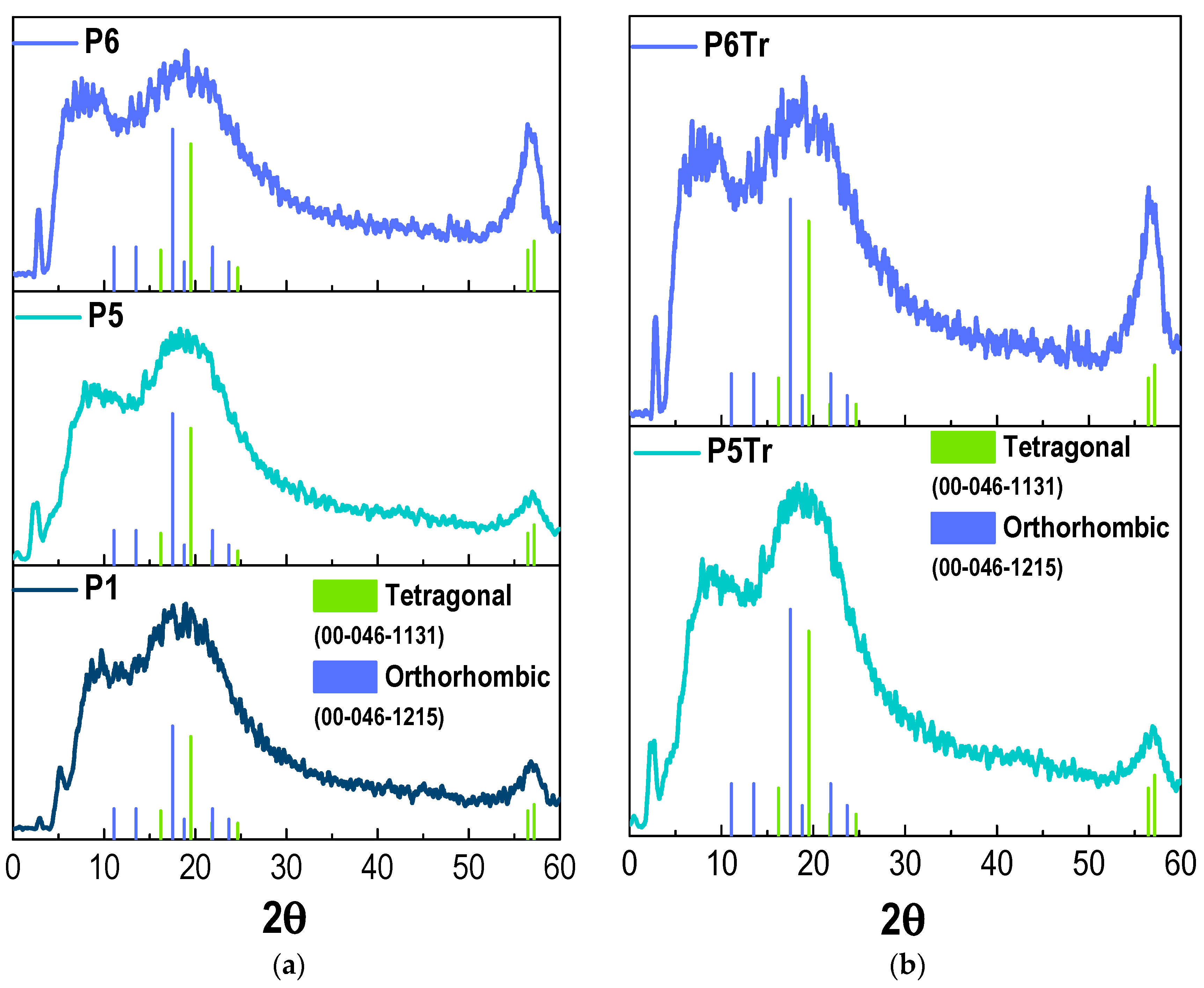
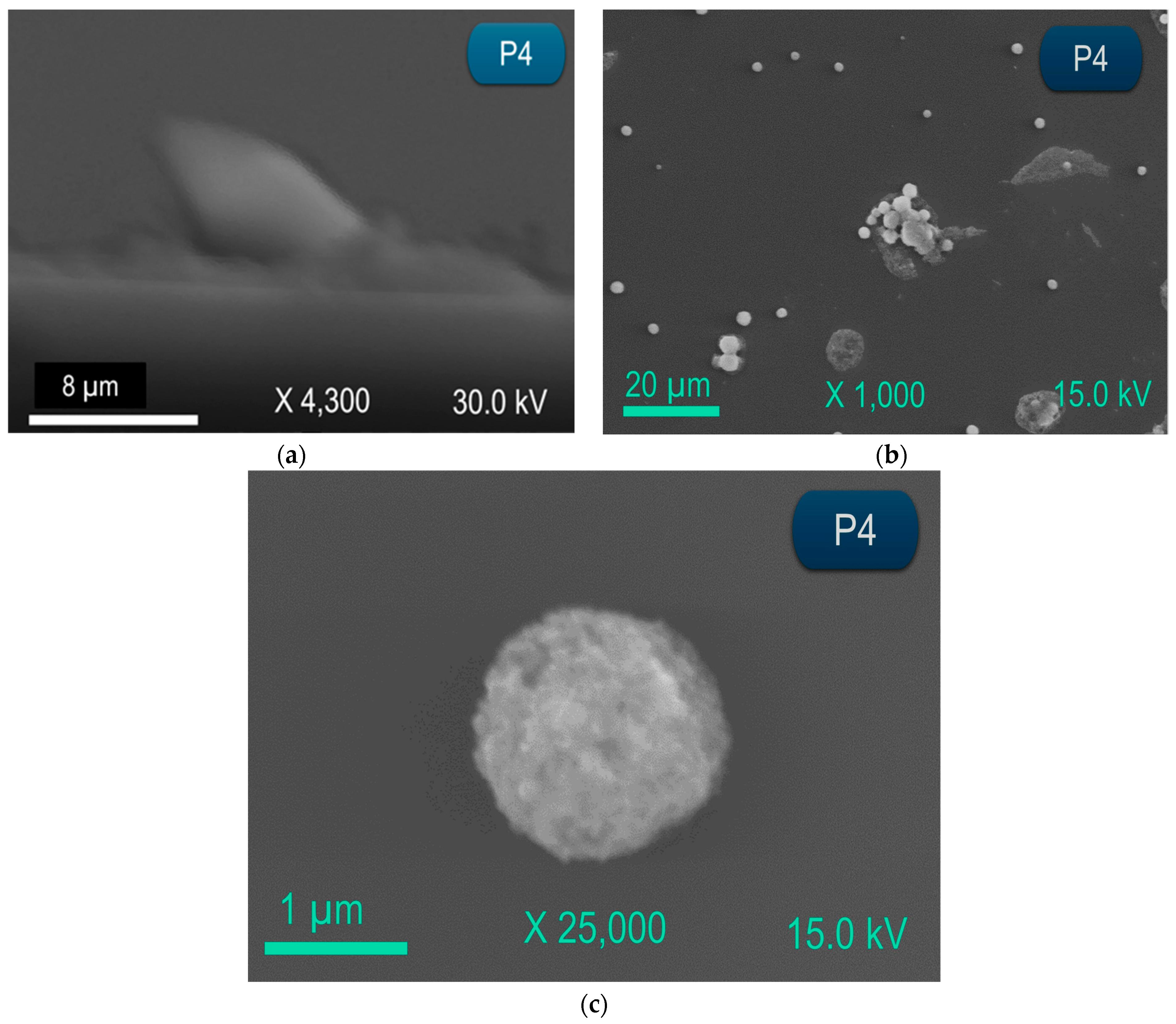
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nathan, A.; Jeon, S. Oxide electronics: Translating materials science from lab-to-fab. MRS Bull. 2021, 46, 1028–1036. [Google Scholar] [CrossRef]
- Regis, V.; Šadl, M.; Brennecka, G.; Bradeško, A.; Tomc, U.; Uršič, H. Investigation of Structural and Electrical Properties of Al2O3/Al Composites Prepared by Aerosol Co-Deposition. Crystals 2023, 13, 850. [Google Scholar] [CrossRef]
- Altuntas, H.; Kaplan, K. Electrical conduction mechanisms and dielectric relaxation in Al2O3 thin films deposited by thermal atomic layer deposition. Mater. Sci. Semicond. Process. 2018, 86, 111–114. [Google Scholar] [CrossRef]
- Shamala, K.S.; Murthy, L.C.S.; Radhakrishna, M.C.; Rao, K.N. Characterization of Al2O3 thin films prepared by spray pyrolysis method for humidity sensor. Sens. Actuators A Phys. 2007, 135, 552–557. [Google Scholar] [CrossRef]
- Schepis, D.J.; Seshan, K. Introduction to Chapter 1. In Handbook of Thin Film Deposition, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2012; Volume 9. [Google Scholar] [CrossRef]
- Jbara, A.S.; Othaman, Z.; Ati, A.A.; Saeed, M.A. Characterization of γ- Al2O3 nanopowders synthesized by Co-precipitation method. Mater. Chem. Phys. 2017, 188, 24–29. [Google Scholar] [CrossRef]
- Li, Q.; Wu, J.; Li, Q.; Wang, F.; Cheng, Y. Sediment Instability Caused by Gas Production from Hydrate-Bearing Sediment in Northern South China Sea by Horizontal Wellbore: Sensitivity Analysis. Nat. Resour. Res. 2025, 34, 1667–1699. [Google Scholar] [CrossRef]
- Singh, R.; Ulrich, R.K. High and low dielectric constant materials. Electrochem. Soc. Interface 1999, 8, 26–30. [Google Scholar] [CrossRef]
- Park, S.; Kim, C.H.; Lee, W.J.; Sung, S.; Yoon, M.H. Sol-gel metal oxide dielectrics for all-solution-processed electronics. Mater. Sci. Eng. R Rep. 2017, 114, 1–22. [Google Scholar] [CrossRef]
- Ortiz, A.; Alonso, J.C. High quality-low temperature aluminum oxide films deposited by ultrasonic spray pyrolysis. J. Mater. Sci. Mater. Electron. 2002, 13, 7–11. [Google Scholar] [CrossRef]
- Dominguez, M.; Luna-Lopez, J.A.; Flores, F.J. Semiconductor Materials by Ultrasonic Spray Pyrolysis and Their Application in Electronic Devices. In Pyrolysis; InTech: London, UK, 2017. [Google Scholar] [CrossRef]
- Bharthasaradhi, R.; Nehru, L.C. Structural and phase transition of α-Al2O3 powders obtained by co-precipitation method. Phase Transit. 2016, 89, 77–83. [Google Scholar] [CrossRef]
- Tsai, S.C.; Song, Y.L.; Tsai, C.S.; Yang, C.C.; Chiu, W.Y.; Lin, H.M. Ultrasonic spray pyrolysis for nanoparticles synthesis. J. Mater. Sci. 2004, 9, 3647–3657. [Google Scholar] [CrossRef]
- Filatova, E.O.; Konashuk, A.S. Interpretation of the Changing the Band Gap of Al2O3 Depending on Its Crystalline Form: Connection with Different Local Symmetries. J. Phys. Chem. C 2015, 119, 20755–20761. [Google Scholar] [CrossRef]
- Baronskiy, M.; Rastorguev, A.; Zhuzhgov, A.; Kostyukov, A.; Krivoruchko, O.; Snytnikov, V. Photoluminescence and Raman spectroscopy studies of low-temperature γ-Al2O3 phases synthesized from different precursors. Opt. Mater. 2016, 53, 87–93. [Google Scholar] [CrossRef]
- Nieto, K. Thesis “Propiedades físicas de películas nanoestructuradas de semiconductores CdS, In2S3 y CdSe obtenidas por la técnica de baño químico”. Cent. Investig. Estud Av. 2018, 1, 100. [Google Scholar]
- Liu, A.; Zhu, H.; Sun, H.; Xu, Y.; Noh, Y.Y. Solution Processed Metal Oxide High-κ Dielectrics for Emerging Transistors and Circuits. Adv. Mater. 2018, 30, 1706364. [Google Scholar] [CrossRef]
- Tsybulya, S.V.; Kryukova, G.N. New X-ray powder diffraction data on δ-Al2O3. Powder Diffr. 2003, 18, 309–311. [Google Scholar] [CrossRef]
- Boumaza, A.; Favaro, L.; Lédion, J.; Sattonnay, G.; Brubach, J.B.; Berthet, P.; Huntz, A.M.; Roy, P.; Tétot, R. Transition alumina phases induced by heat treatment of boehmite: An X-ray diffraction and infrared spectroscopy study. J. Solid State Chem. 2009, 182, 1171–1176. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, H.S.; Park, N.K.; Lee, T.J.; Kang, M. Low temperature synthesis of α-alumina from aluminum hydroxide hydrothermally synthesized using [Al(C2O4)x(OH)y] complexes. Chem. Eng. J. 2013, 230, 351–360. [Google Scholar] [CrossRef]
- Quarto, F.D.; Zaffora, A. A generlized semiempirical approach to the modeling of the optical band gap of ternary Al-(Ga, Nb, Ta, W) oxides containing different alumina polymorphs. Inorg. Chem. 2021, 60, 1419–1435. [Google Scholar] [CrossRef]
- Ewelina, B.; Maksymilian, W. Influence of Anodization Temperature on Geometrical and Optical Properties of Porous Anodic Alumina (PAA)-Based Photonic Structures. Materials 2020, 13, 3185. [Google Scholar] [CrossRef]
- Hu, F.; Wu, X.; Wang, Y.; Lai, X. Ultrathin γ-Al2O3 nanofibers with large specific surface area and their enhanced thermal stability by Si-doping. RSC Adv. 2015, 5, 54053–54058. [Google Scholar] [CrossRef]
- Ienei, E.; Isac, L.; Duţǎ, A. Synthesis of alumina thin films by spray pyrolysis. Rev. Roum. Chim. 2010, 55, 161–165. [Google Scholar]
- Zeng, Y.; Pan, H. Improved Breakdown Strength and Restrained Leakage Current of Sandwich Structure Ferroelectric Polymers Utilizing Ultra-Thin Al2O3 Nanosheets. Nanomaterials 2023, 13, 2836. [Google Scholar] [CrossRef]
- Thompson, G.E. Porous anodic alumina: Fabrication, characterization and applications. Thin Solid Film. 1997, 297, 192–201. [Google Scholar] [CrossRef]
- Prashanth, P.A.; Raveendra, R.S.; Hari Krishna, R.; Ananda, S.; Bhagya, N.P.; Nagabhushana, B.M.; Lingaraju, K.; Naika, H.R. Synthesis, characterizations, antibacterial and photoluminescence studies of solution combustion-derived α-Al2O3 nanoparticles. J. Asian Ceram. Soc. 2015, 3, 345–351. [Google Scholar] [CrossRef]
- Kelekanjeri, V.S.K.G.; Carter, W.B.; Hampikian, J.M. Deposition of α-alumina via combustion chemical vapor deposition. Thin Solid Film. 2006, 515, 1905–1911. [Google Scholar] [CrossRef]
- Peitao, G.; Zhilin, X.; Yiyu, X.; Caihua, H.; Lixin, Z. Morphology and transmittance of porous alumina on glass substrate. Appl. Surf. Sci. 2011, 257, 3307–3312. [Google Scholar] [CrossRef]
- Patil, P.S. Versatility of chemical spray pyrolysis technique. Mater. Chem. Phys. 1999, 59, 185–198. [Google Scholar] [CrossRef]

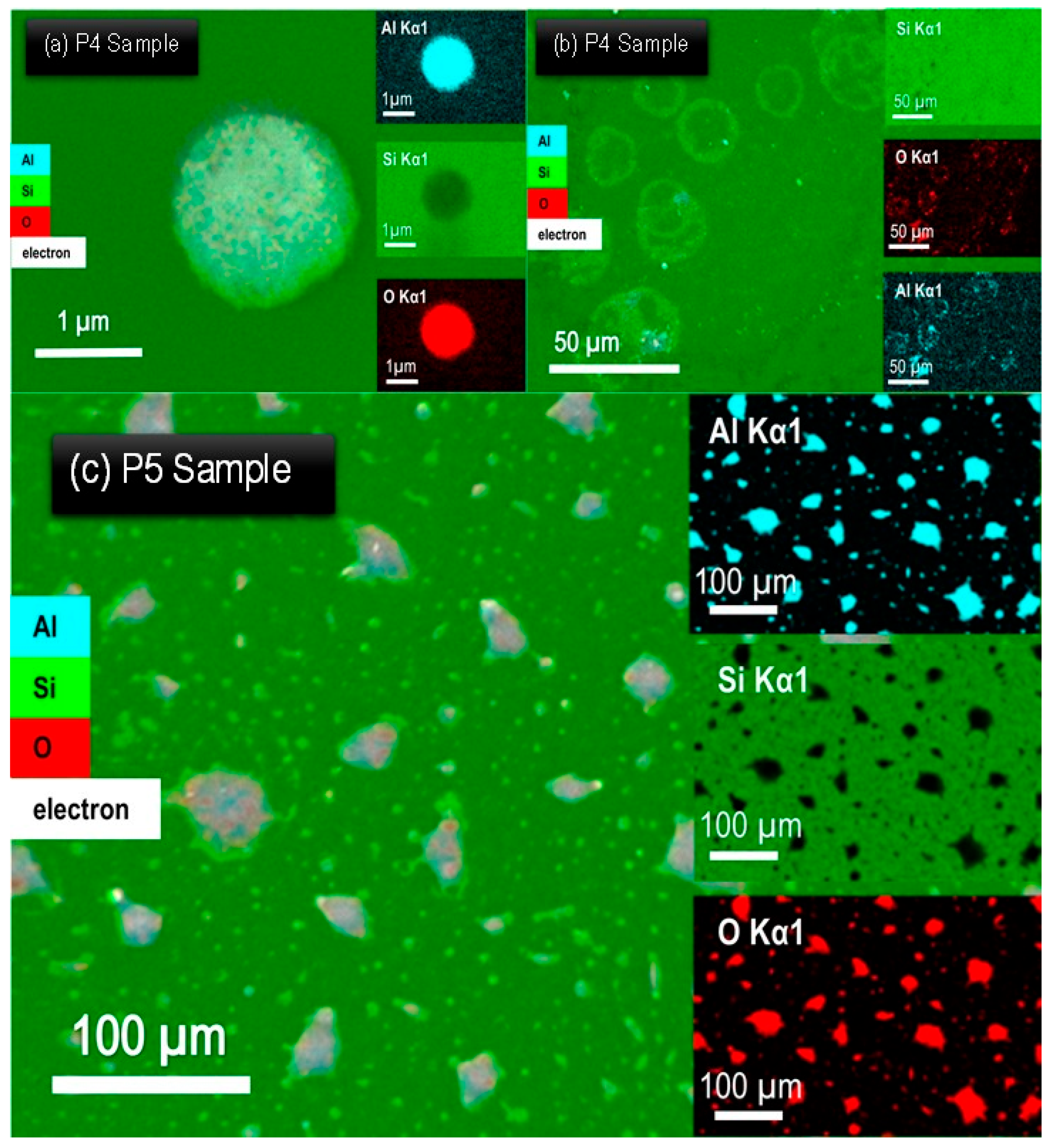

| Samples | Al (Wt%) | O (Wt%) |
|---|---|---|
| P4 | 44.7 | 55.3 |
| P5 | 47.9 | 52.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luna Zempoalteca, A.; Hernández de la Luz, J.A.D.; Luna Flores, A.; Benítez Lara, A.; Hernández Simón, Z.J.; Mendoza Conde, G.O.; Monfil Leyva, K.; Flores Méndez, J.; Minquiz Xolo, G.M.; Luna López, J.A. Low-Temperature Deposition of Alumina Films by Ultrasonic Spray Pyrolysis with a Water-Based Precursor. Coatings 2025, 15, 1076. https://doi.org/10.3390/coatings15091076
Luna Zempoalteca A, Hernández de la Luz JAD, Luna Flores A, Benítez Lara A, Hernández Simón ZJ, Mendoza Conde GO, Monfil Leyva K, Flores Méndez J, Minquiz Xolo GM, Luna López JA. Low-Temperature Deposition of Alumina Films by Ultrasonic Spray Pyrolysis with a Water-Based Precursor. Coatings. 2025; 15(9):1076. https://doi.org/10.3390/coatings15091076
Chicago/Turabian StyleLuna Zempoalteca, Anayantzi, J. A. David Hernández de la Luz, Adan Luna Flores, Alfredo Benítez Lara, Zaira Jocelyn Hernández Simón, Gabriel Omar Mendoza Conde, Karim Monfil Leyva, Javier Flores Méndez, Gustavo M. Minquiz Xolo, and José Alberto Luna López. 2025. "Low-Temperature Deposition of Alumina Films by Ultrasonic Spray Pyrolysis with a Water-Based Precursor" Coatings 15, no. 9: 1076. https://doi.org/10.3390/coatings15091076
APA StyleLuna Zempoalteca, A., Hernández de la Luz, J. A. D., Luna Flores, A., Benítez Lara, A., Hernández Simón, Z. J., Mendoza Conde, G. O., Monfil Leyva, K., Flores Méndez, J., Minquiz Xolo, G. M., & Luna López, J. A. (2025). Low-Temperature Deposition of Alumina Films by Ultrasonic Spray Pyrolysis with a Water-Based Precursor. Coatings, 15(9), 1076. https://doi.org/10.3390/coatings15091076







