Biopolymer Paperboard Impregnation Based on Chitosan and Nanocellulose with Addition of Caffeine and Gallic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Paperboard Impregnation Formulations
2.3. Preparation of Impregnated Paperboard
2.4. Antibacterial Activities
2.5. Mechanical Properties of Impregnated Paperboard
2.5.1. Tensile Strengths and Elongations at Break
2.5.2. Puncture Resistance and Burst Strength
2.6. Water Absorption Value
2.7. Infrared Spectroscopy
2.8. Scanning Electron Microscopy
2.9. Statistical Analysis
3. Results and Discussion
3.1. Biological Performance of the Solution
Antibacterial Activities
3.2. Mechanical Parameters
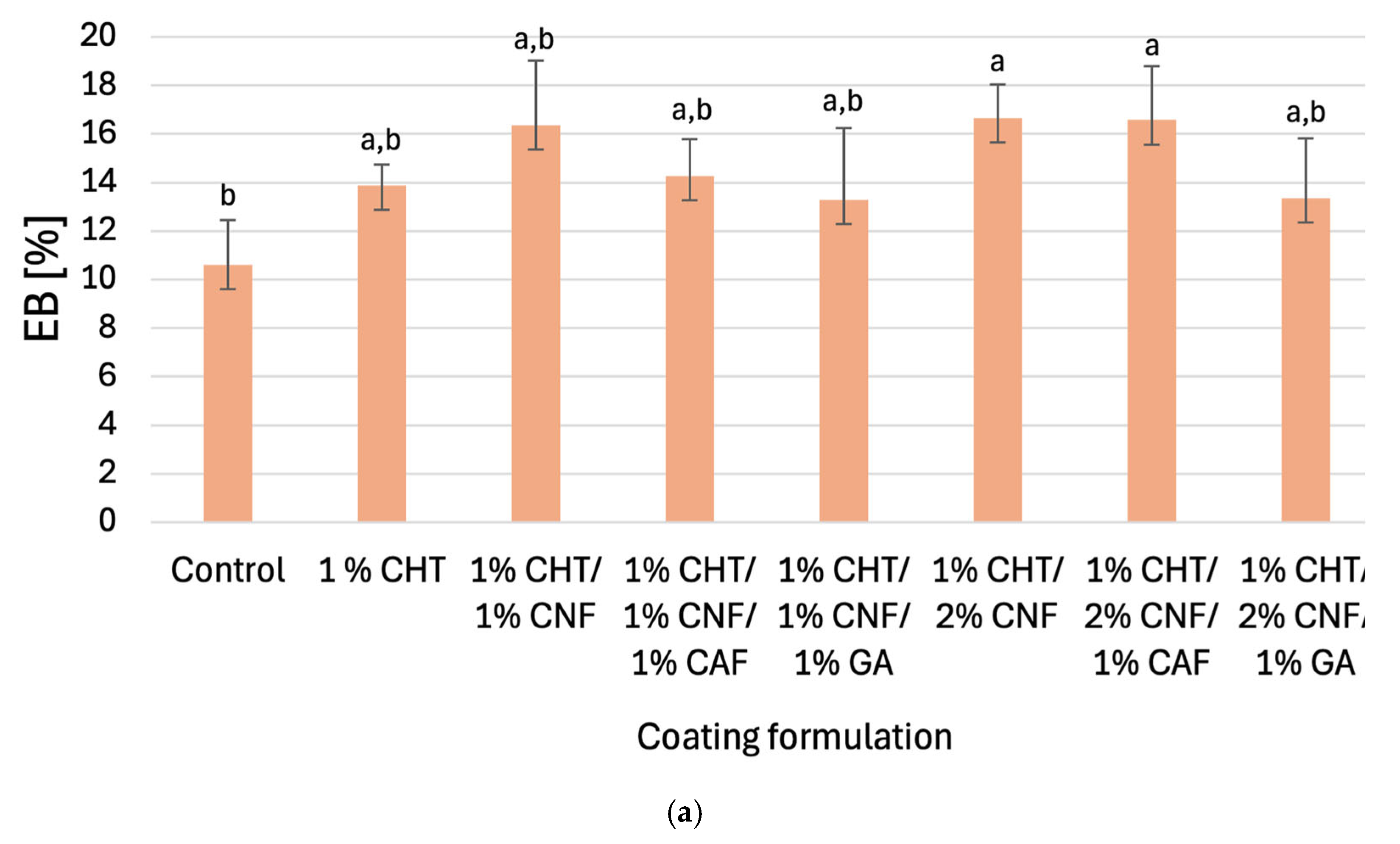
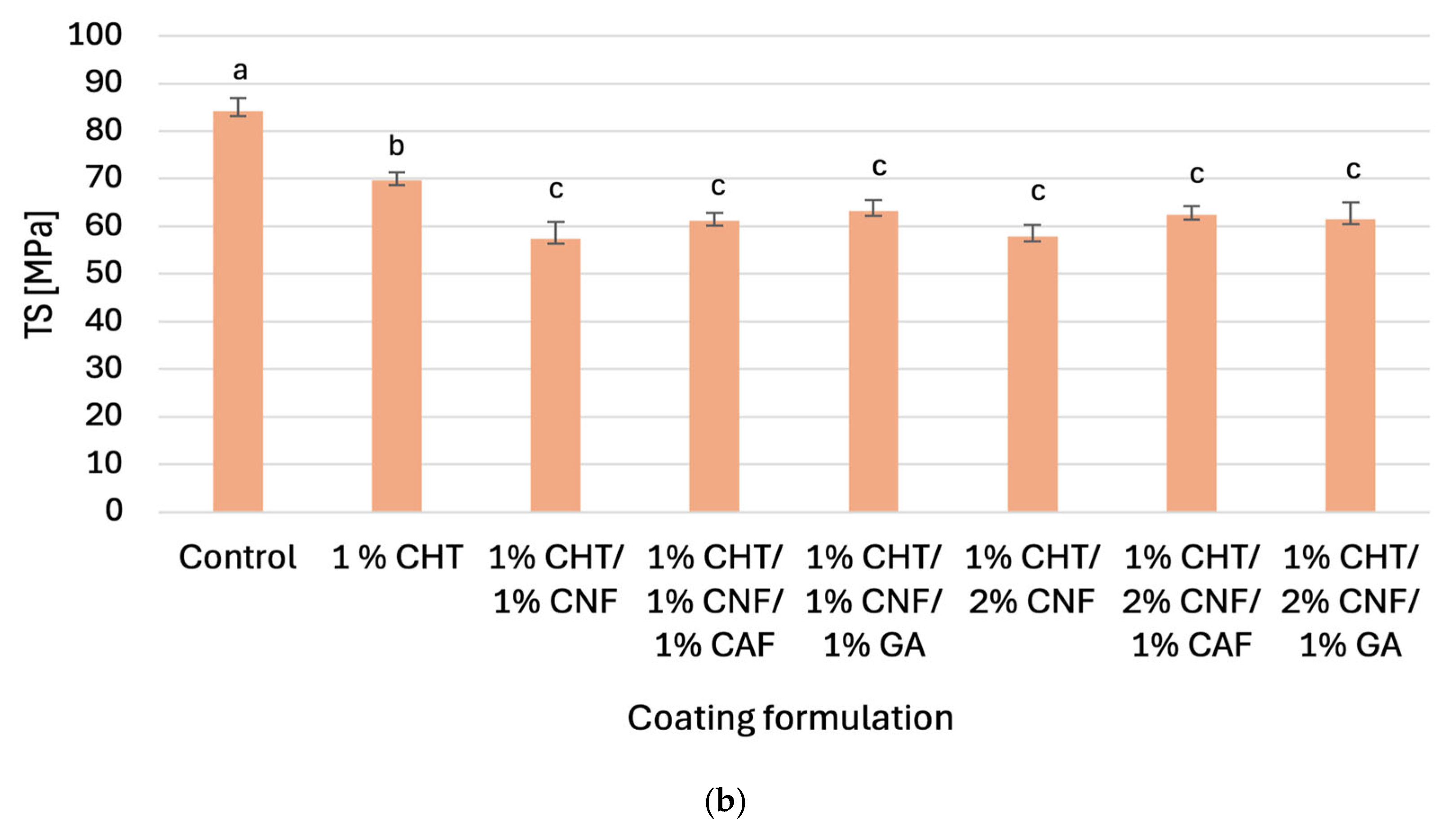
3.2.1. Tensile Strengths and Elongations at Break
3.2.2. Puncture Resistance and Burst Strength
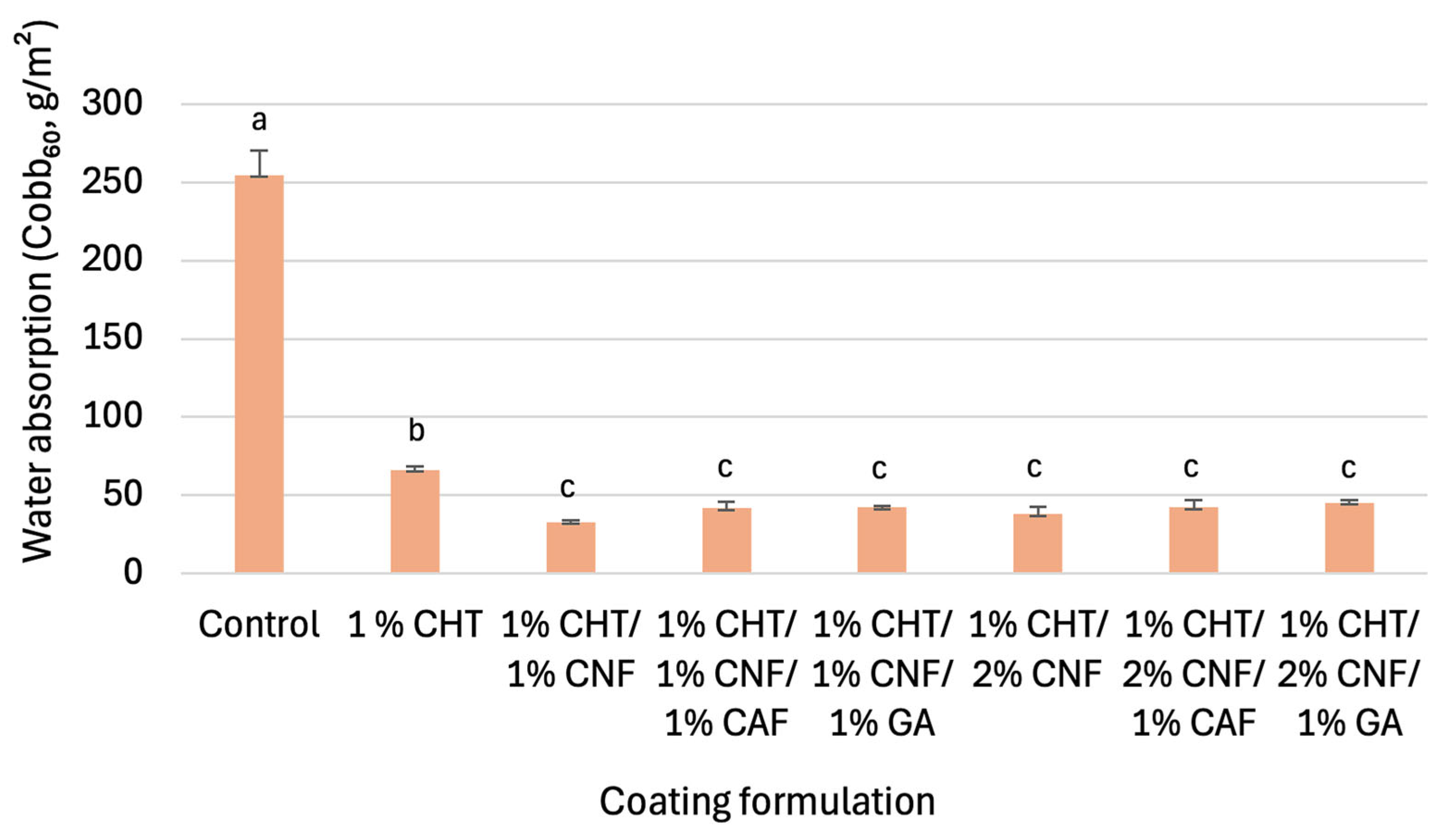
3.3. Water Absorption Performance
3.4. Structural Characteristic
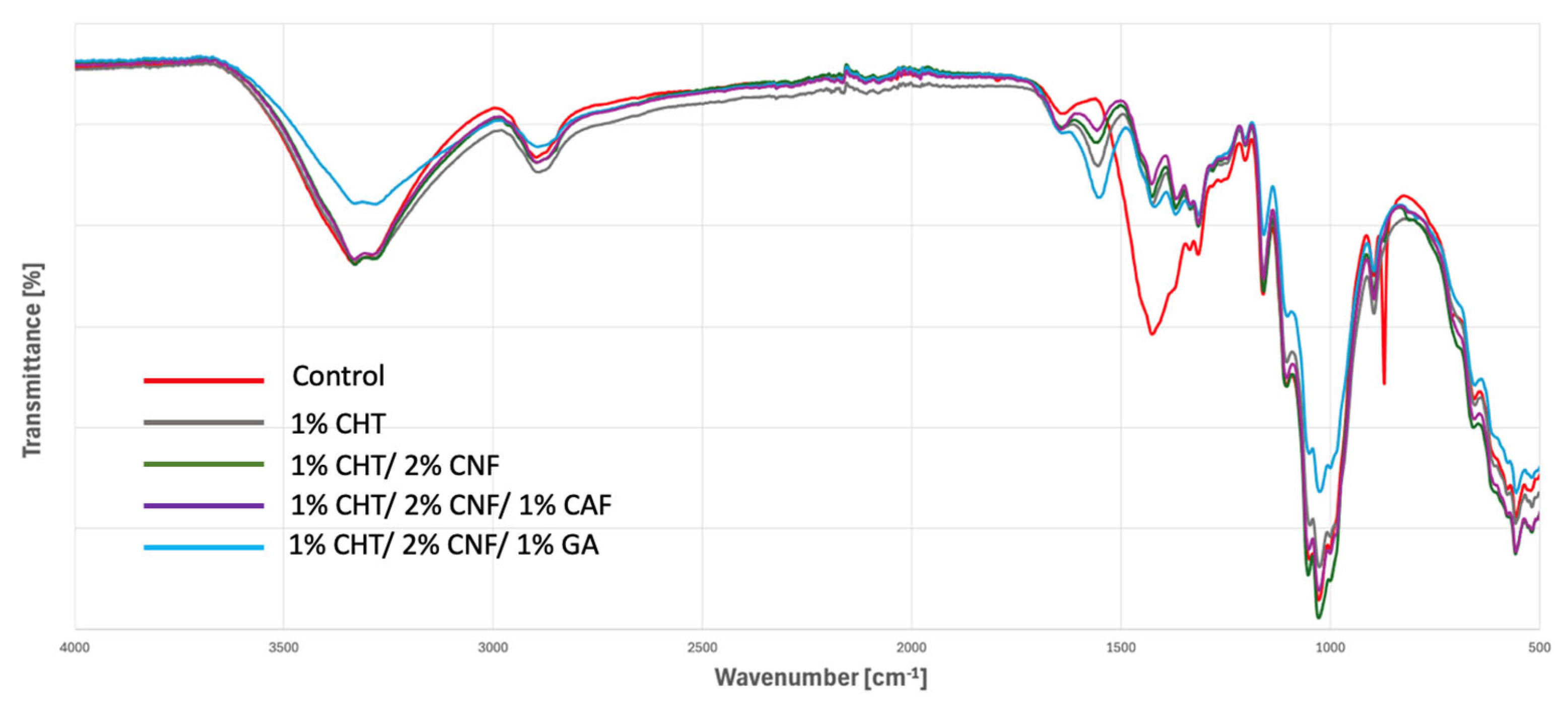
3.4.1. Infrared Spectroscopy
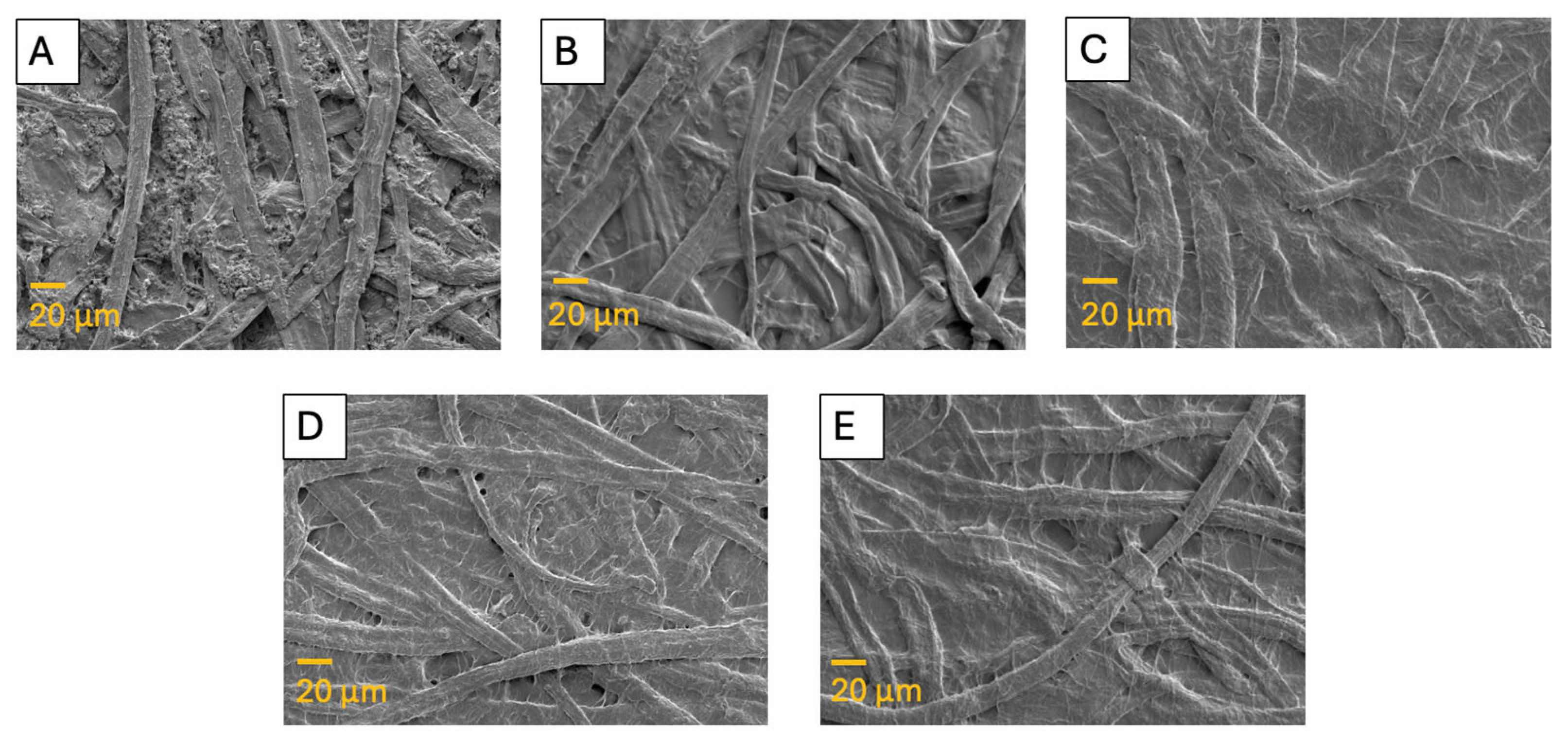
3.4.2. Scanning Electron Microscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khalid, M.Y.; Arif, Z.U. Novel Biopolymer-Based Sustainable Composites for Food Packaging Applications: A Narrative Review. Food Packag. Shelf Life 2022, 33, 100892. [Google Scholar] [CrossRef]
- Deshwal, G.K.; Panjagari, N.R.; Alam, T. An Overview of Paper and Paper Based Food Packaging Materials: Health Safety and Environmental Concerns. J. Food Sci. Technol. 2019, 56, 4391–4403. [Google Scholar] [CrossRef] [PubMed]
- Mendes, J.F.; Martins, J.T.; Manrich, A.; Sena Neto, A.R.; Pinheiro, A.C.M.; Mattoso, L.H.C.; Martins, M.A. Development and Physical-Chemical Properties of Pectin Film Reinforced with Spent Coffee Grounds by Continuous Casting. Carbohydr. Polym. 2019, 210, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Yang, D.; Qiu, X. Biomimetic Supertough and Strong Biodegradable Polymeric Materials with Improved Thermal Properties and Excellent UV-Blocking Performance. Adv. Funct. Mater. 2019, 29, 1806912. [Google Scholar] [CrossRef]
- Yuan, Q.; Shah, J.; Hein, S.; Misra, R.D.K. Controlled and Extended Drug Release Behavior of Chitosan-Based Nanoparticle Carrier. Acta Biomater. 2010, 6, 1140–1148. [Google Scholar] [CrossRef]
- Tsai, L.C.; Tsai, M.L.; Lu, K.Y.; Mi, F.L. Synthesis and Evaluation of Antibacterial and Anti-Oxidant Activity of Small Molecular Chitosan–Fucoidan Conjugate Nanoparticles. Res. Chem. Intermed. 2018, 44, 4855–4871. [Google Scholar] [CrossRef]
- Dutta, P.K.; Yadav, S.; Mehrotra, G.K. Modified Chitosan Films/Coatings for Active Food Packaging. In Chitosan for Biomaterials III: Structure-Property Relationships; Jayakumar, R., Prabaharan, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 203–232. ISBN 978-3-030-83807-2. [Google Scholar]
- Temple, J.L.; Bernard, C.; Lipshultz, S.E.; Czachor, J.D.; Westphal, J.A.; Mestre, M.A. The Safety of Ingested Caffeine: A Comprehensive Review. Front. Psychiatry 2017, 8, 80. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Safety of Caffeine. EFSA J. 2015, 13, 4102. [Google Scholar] [CrossRef]
- Carrillo, J.A.; Benitez, J. Clinically Significant Pharmacokinetic Interactions Between Dietary Caffeine and Medications. Clin. Pharmacokinet. 2000, 39, 127–153. [Google Scholar] [CrossRef]
- Salas-Ambrosio, P.; Vexler, S.; Rajalakshmi, P.S.; Chen, I.A.; Maynard, H.D. Caffeine and Cationic Copolymers with Antimicrobial Properties. ACS Bio. Med. Chem. Au 2023, 3, 189–200. [Google Scholar] [CrossRef]
- Chakraborty, P.; Dastidar, D.G.; Paul, P.; Dutta, S.; Basu, D.; Sharma, S.R.; Basu, S.; Sarker, R.K.; Sen, A.; Sarkar, A.; et al. Inhibition of Biofilm Formation of Pseudomonas Aeruginosa by Caffeine: A Potential Approach for Sustainable Management of Biofilm. Arch. Microbiol. 2020, 202, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Woziwodzka, A.; Krychowiak-Maśnicka, M.; Gołuński, G.; Łosiewska, A.; Borowik, A.; Wyrzykowski, D.; Piosik, J. New Life of an Old Drug: Caffeine as a Modulator of Antibacterial Activity of Commonly Used Antibiotics. Pharmaceuticals 2022, 15, 872. [Google Scholar] [CrossRef]
- Depaula, J.; Farah, A. Caffeine Consumption through Coffee: Content in the Beverage, Metabolism, Health Benefits and Risks. Beverages 2019, 5, 37. [Google Scholar] [CrossRef]
- Chanwitheesuk, A.; Teerawutgulrag, A.; Kilburn, J.D.; Rakariyatham, N. Antimicrobial Gallic Acid from Caesalpinia Mimosoides Lamk. Food Chem. 2007, 100, 1044–1048. [Google Scholar] [CrossRef]
- Xu, Y.; Tang, G.; Zhang, C.; Wang, N.; Feng, Y. Gallic Acid and Diabetes Mellitus: Its Association with Oxidative Stress. Molecules 2021, 26, 7115. [Google Scholar] [CrossRef]
- Yadav, S.; Mehrotra, G.K.; Dutta, P.K. Chitosan Based ZnO Nanoparticles Loaded Gallic-Acid Films for Active Food Packaging. Food Chem. 2021, 334, 127605. [Google Scholar] [CrossRef]
- Lamarra, J.; Giannuzzi, L.; Rivero, S.; Pinotti, A. Assembly of Chitosan Support Matrix with Gallic Acid-Functionalized Nanoparticles. Mater. Sci. Eng. C 2017, 79, 848–859. [Google Scholar] [CrossRef]
- Rahmawati, I.; Pratama, A.W.; Pratama, S.A.; Khozin, M.N.; Firmanda, A.; Irawan, F.H.; Asranudin; Ansori, A.N.M.; Sucipto, T.H. Gallic Acid: A Promising Bioactive Agent for Food Preservation and Sustainable Packaging Development. Case Stud. Chem. Environ. Eng. 2024, 10, 100776. [Google Scholar] [CrossRef]
- Kutraite, I.; Malys, N. Development and Application of Whole-Cell Biosensors for the Detection of Gallic Acid. ACS Synth. Biol. 2023, 12, 533–543. [Google Scholar] [CrossRef]
- Wianowska, D.; Olszowy-Tomczyk, M. A Concise Profile of Gallic Acid—From Its Natural Sources through Biological Properties and Chemical Methods of Determination. Molecules 2023, 28, 1186. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Nkambule, B.B.; Jack, B.; Mkandla, Z.; Mutize, T.; Silvestri, S.; Orlando, P.; Tiano, L.; Louw, J.; Mazibuko-Mbeje, S.E. Inflammation and Oxidative Stress in an Obese State and the Protective Effects of Gallic Acid. Nutrients 2019, 11, 23. [Google Scholar] [CrossRef]
- Bhuia, M.S.; Rahaman, M.M.; Islam, T.; Bappi, M.H.; Sikder, M.I.; Hossain, K.N.; Akter, F.; Al Shamsh Prottay, A.; Rokonuzzman, M.; Gürer, E.S.; et al. Neurobiological Effects of Gallic Acid: Current Perspectives. Chin. Med. 2023, 18, 27. [Google Scholar] [CrossRef]
- Singulani, J.D.L.; Scorzoni, L.; Gomes, P.C.; Nazaré, A.C.; Polaquini, C.R.; Regasini, L.O.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Activity of Gallic Acid and Its Ester Derivatives in Caenorhabditis Elegans and Zebrafish (Danio rerio) Models. Future Med. Chem. 2017, 9, 1863–1872. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Cao, J.; Jiang, W. Improving the Performance of Edible Food Packaging Films by Using Nanocellulose as an Additive. Int. J. Biol. Macromol. 2021, 166, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Emenike, E.C.; Iwuozor, K.O.; Saliu, O.D.; Ramontja, J.; Adeniyi, A.G. Advances in the Extraction, Classification, Modification, Emerging and Advanced Applications of Crystalline Cellulose: A Review. Carbohydr. Polym. Technol. Appl. 2023, 6, 100337. [Google Scholar] [CrossRef]
- Ludwicka, K.; Kaczmarek, M.; Białkowska, A. Bacterial Nanocellulose—A Biobased Polymer for Active and Intelligent Food Packaging Applications: Recent Advances and Developments. Polymers 2020, 12, 2209. [Google Scholar] [CrossRef]
- Wang, X.; Guo, J.; Ren, H.; Jin, J.; He, H.; Jin, P.; Wu, Z.; Zheng, Y. Research Progress of Nanocellulose-Based Food Packaging. Trends Food Sci. Technol. 2024, 143, 104289. [Google Scholar] [CrossRef]
- Du, P.; Ding, Q.; Zhao, C.; Jiang, Y.; Han, W.; Li, X. The Fluorine-Free Coating Has Excellent Hydrophobic and Oleophobic Properties for Porous Cellulose-Based Materials. Cellulose 2021, 28, 6133–6146. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.; Wang, H. Fabrication of Water/Oil-Resistant Paper by Nanocellulose Stabilized Pickering Emulsion and Chitosan. Int. J. Biol. Macromol. 2024, 275, 133609. [Google Scholar] [CrossRef]
- Xue, M.; Wen, Z.; Huang, R.; Chai, X.; Li, W.; Chen, C.; Chen, H. Preparation of Coated Paper Reinforced by a Blend of Anionic-Starch-Based Nanocellulose/Chitosan and Its Properties. RSC Adv. 2022, 12, 22402–22409. [Google Scholar] [CrossRef]
- Hassan, E.A.; Hassan, M.L.; Abou-zeid, R.E.; El-Wakil, N.A. Novel Nanofibrillated Cellulose/Chitosan Nanoparticles Nanocomposites Films and Their Use for Paper Coating. Ind. Crops Prod. 2016, 93, 219–226. [Google Scholar] [CrossRef]
- Roman, M.; Nechita, P.; Vasile, M.A.; Cantaragiu Ceoromila, A.M. Barrier and Antimicrobial Properties of Coatings Based on Xylan Derivatives and Chitosan for Food Packaging Papers. Coatings 2023, 13, 1761. [Google Scholar] [CrossRef]
- Stefanowska, K.; Woźniak, M.; Dobrucka, R.; Ratajczak, I. Chitosan with Natural Additives as a Potential Food Packaging. Materials 2023, 16, 1579. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, Z.; Kadouh, H.; Zhou, K. The Antimicrobial, Mechanical, Physical and Structural Properties of Chitosan-Gallic Acid Films. LWT 2014, 57, 83–89. [Google Scholar] [CrossRef]
- Costa, S.M.; Ferreira, D.P.; Teixeira, P.; Ballesteros, L.F.; Teixeira, J.A.; Fangueiro, R. Active Natural-Based Films for Food Packaging Applications: The Combined Effect of Chitosan and Nanocellulose. Int. J. Biol. Macromol. 2021, 177, 241–251. [Google Scholar] [CrossRef]
- SIST 14477:2004; Packaging-Flexible Packaging Material-Determination of Puncture Resistance-Test Methods. European Committee for Standardization: Brussels, Belgium, 2004.
- ISO 2759:2014; Board-Determination of Bursting Strength. International Organization for Standardization: Geneva, Switzerland, 2005.
- ISO 20535:2022; Footwear-Test Method for Insoles and Insocks-Dimensional Change After Cycle of Wetting and Drying. International Organization for Standardization: Geneva, Switzerland, 2022.
- Yu, C.; Chen, X.; Zhu, W.; Li, L.; Peng, M.; Zhong, Y.; Naeem, A.; Zang, Z.; Guan, Y. Synthesis of Gallic Acid-Loaded Chitosan-Grafted-2-Acrylamido-2-Methylpropane Sulfonic Acid Hydrogels for Oral Controlled Drug Delivery: In Vitro Biodegradation, Antioxidant, and Antibacterial Effects. Gels 2022, 8, 806. [Google Scholar] [CrossRef]
- Talebi, H.; Ghasemi, F.A.; Ashori, A. The Effect of Nanocellulose on Mechanical and Physical Properties of Chitosan-Based Biocomposites. J. Elastomers Plast. 2022, 54, 22–41. [Google Scholar] [CrossRef]
- Ling, N.Y.; Ilyas, R.A.; Jalil, R.; Ibrahim, R.; Hawanis, H.S.N.; Azriena, H.A.A.; Majid, R.A.; Hassan, N.H.M.; Atikah, M.S.N.; Nordin, A.H. Enhancing Papermaking with Nanocellulose and Chitosan: Synergistic Approaches for Eco-Friendly Production. Sustain. Mater. Technol. 2025, 43, e01252. [Google Scholar] [CrossRef]
- Almeida, T.; Karamysheva, A.; Valente, B.F.A.; Silva, J.M.; Braz, M.; Almeida, A.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Biobased Ternary Films of Thermoplastic Starch, Bacterial Nanocellulose and Gallic Acid for Active Food Packaging. Food Hydrocoll. 2023, 144, 108934. [Google Scholar] [CrossRef]
- Zarandona, I.; Puertas, A.I.; Dueñas, M.T.; Guerrero, P.; de la Caba, K. Assessment of Active Chitosan Films Incorporated with Gallic Acid. Food Hydrocoll. 2020, 101, 105486. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Negi, Y.S. Effect of Varying Filler Concentration on Zinc Oxide Nanoparticle Embedded Chitosan Films as Potential Food Packaging Material. J. Polym. Environ. 2017, 25, 1087–1098. [Google Scholar] [CrossRef]
- Ruberto, Y.; Vivod, V.; Grkman, J.J.; Lavrič, G.; Graiff, C.; Kokol, V. Slot-Die Coating of Cellulose Nanocrystals and Chitosan for Improved Barrier Properties of Paper. Cellulose 2024, 31, 3589–3606. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, J.; Zhang, L. Structure and Properties of the Nanocomposite Films of Chitosan Reinforced with Cellulose Whiskers. J. Polym. Sci. B Polym. Phys. 2009, 47, 1069–1077. [Google Scholar] [CrossRef]
- De Carli, C.; Aylanc, V.; Mouffok, K.M.; Santamaria-Echart, A.; Barreiro, F.; Tomás, A.; Pereira, C.; Rodrigues, P.; Vilas-Boas, M.; Falcão, S.I. Production of Chitosan-Based Biodegradable Active Films Using Bio-Waste Enriched with Polyphenol Propolis Extract Envisaging Food Packaging Applications. Int. J. Biol. Macromol. 2022, 213, 486–497. [Google Scholar] [CrossRef] [PubMed]
| Samples | 1% Chitosan | 1% CNFs | 2% CNFs | 1% Caffeine | 1% Gallic Acid |
|---|---|---|---|---|---|
| Control | — | — | — | — | — |
| 1% CHT | ✓ | — | — | — | — |
| 1% CHT/1% CNF | ✓ | ✓ | — | — | — |
| 1% CHT/1% CNF/1% CAF | ✓ | ✓ | — | ✓ | — |
| 1% CHT/1% CNF/1% GA | ✓ | ✓ | — | — | ✓ |
| 1% CHT/2% CNF | ✓ | — | ✓ | — | — |
| 1% CHT/2% CNF/1% CAF | ✓ | — | ✓ | ✓ | — |
| 1% CHT/2% CNF/1% GA | ✓ | — | ✓ | — | ✓ |
| Bacterial Strains | 1% CHT | 1% CHT/1% CAF | 1% CHT/1% GA |
|---|---|---|---|
| Inhibition Zone (mm) | |||
| Gram-positive bacteria | |||
| L. monocytogenes | 12 | 15 | 13 |
| S. aureus | 12 | 14 | 13 |
| Gram-negative bacteria | |||
| E. coli | 12 | 17 | 19 |
| P. aeruginosa | 12 | 17 | 17 |
| Type of Coating | Puncture Force [N] | Puncture Elongation [mm] | Burst Strength [kPa] |
|---|---|---|---|
| Control | 27.98 a, b ± 2.15 | 2.78 a, b ± 0.10 | 369.77 b ± 28.17 |
| 1% CHT | 26.05 b ± 2.19 | 2.75 a, b ± 0.13 | 466.87 a ± 22.42 |
| 1% CHT/ 1% CNF | 30.78 a ± 1.42 | 2.88 a, b ± 0.05 | 431.14 a, b ± 33.31 |
| 1% CHT/ 1% CNF/ 1% CAF | 30.35 a, b ± 1.79 | 2.88 a, b ± 0.10 | 417.93 a, b ± 41.36 |
| 1% CHT/ 1% CNF/ 1% GA | 25.88 b ± 1.64 | 2.70 b ± 0.08 | 397.34 a, b ± 16.94 |
| 1% CHT/ 2% CNF | 29.88 a, b ± 1.03 | 2.98 a ± 0.10 | 440.07 a, b ± 28.17 |
| 1% CHT/ 2% CNF/ 1% CAF | 29.08 a, b ± 0.99 | 2.85 a, b ± 0.13 | 438.37 a, b ± 49.60 |
| 1% CHT/ 2% CNF/ 1% GA | 26.78 a, b ± 3.23 | 2.83 a, b ± 0.19 | 425.83 a, b ± 16.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Młodziejewska, J.; Woźniak, M.; Sip, A.; Dobrucka, R.; Ratajczak, I. Biopolymer Paperboard Impregnation Based on Chitosan and Nanocellulose with Addition of Caffeine and Gallic Acid. Coatings 2025, 15, 1034. https://doi.org/10.3390/coatings15091034
Młodziejewska J, Woźniak M, Sip A, Dobrucka R, Ratajczak I. Biopolymer Paperboard Impregnation Based on Chitosan and Nanocellulose with Addition of Caffeine and Gallic Acid. Coatings. 2025; 15(9):1034. https://doi.org/10.3390/coatings15091034
Chicago/Turabian StyleMłodziejewska, Joanna, Magdalena Woźniak, Anna Sip, Renata Dobrucka, and Izabela Ratajczak. 2025. "Biopolymer Paperboard Impregnation Based on Chitosan and Nanocellulose with Addition of Caffeine and Gallic Acid" Coatings 15, no. 9: 1034. https://doi.org/10.3390/coatings15091034
APA StyleMłodziejewska, J., Woźniak, M., Sip, A., Dobrucka, R., & Ratajczak, I. (2025). Biopolymer Paperboard Impregnation Based on Chitosan and Nanocellulose with Addition of Caffeine and Gallic Acid. Coatings, 15(9), 1034. https://doi.org/10.3390/coatings15091034








