Abstract
Photoinitiators (PIs) are pivotal in enabling energy-efficient, spatiotemporally controlled photopolymerization for coatings. To address application-specific demands of coatings, diverse systems of Norrish-Type I (e.g., oxime esters, acylphosphine oxides) and Type II (e.g., onium salts, ketones) PIs have been engineered through systematic molecular design strategies. A comprehensive review necessitates highlighting recent achievements in designing PIs by various molecular engineering approaches. The π-conjugation extension, push–pull structures, and auxochrome incorporation boost strong and long-wavelength absorption; unimolecular PI systems with hydrogen-donor modifications improve reactivity and reduce oxygen inhibition; photobleaching via cleavable bonds and blocking conjugation enables colorless coating and deep-penetration curing; polymerizable macromolecular designs enhance migration resistance; organosilicon-functionalized structures optimize monomer compatibility. These strategies bridge molecular innovations with advanced applications in biomedical and deep-cured coatings.
1. Introduction
Photopolymerization for coatings is a convenient and efficient technique for utilizing inexpensive, renewable light energy to initiate the crosslinking of monomers and pre-polymers. During a typical photopolymerization process, photoinitiators (PIs) absorb light photons with appropriate energy and generate active radicals or ionic species [1]. These active species can be the primary products of PIs or secondary products derived from their reactions with solvents, monomers, or even photolytic byproducts [1]. These light-induced reactive species trigger monomers through various mechanisms, such as conventional free radical polymerization (FRP) and cationic polymerization (CP). Additionally, photoinitiated living controlled polymerization methods have also been studied, including reversible addition–fragmentation chain transfer and atom transfer radical polymerizations [2,3,4,5,6,7,8,9].
The considerable interest in photopolymerization arises from its energy-efficient, environmentally friendly, safe, and highly controllable nature compared to conventional thermally initiated systems [10,11]. Photoinitiated polyreactions proceed with rapid kinetics, often completing within seconds to minutes under mild conditions due to an autocatalytic mechanism; their polymerization kinetics possess reduced activation energy, and high-rate constants [12,13,14,15,16]. Furthermore, low photoinitiation temperatures minimize side reactions and reduce energy consumption. Photoinitiated living polymerizations allow for low polydispersity, favorable tacticity, and enhanced molecular weights of the resulting polymers [17,18,19,20,21,22]. Additionally, the low release of volatile organic compounds from coatings aligns with the principles of green chemistry. Tuning the duration, intensity, and spatial position of light irradiation offers a straightforward approach for achieving precise spatial and temporal control over reaction kinetics and patterning. Spatiotemporal control of light is vital to optimize photopolymerization performance. The interplay between spatial coherence and temporal precision guarantees uniform curing depth and reduced line-edge roughness. As a result of these advantages, photopolymerization has found widespread applications in fields such as photocuring, light-sensitive coatings, 3D printing, optoelectronics, and dental restorations [9,23,24,25,26]. Photolithography stands as one of the most advanced applications of photopolymerization, enabling intricate semiconductor fabrication with features as fine as sub−5 nm patterning assisted by extreme ultraviolet lithography [27]. The advancement of photolithography critically relies on spatiotemporal control of light. Spatially, the techniques of multiphoton absorption confinement and spatial light modulators promote high-precision patterning for fabricating complicated 3D microstructures [28]. On the other hand, the ultrafast photopolymerization kinetics allow for synchronized polymerization with lithographic exposure timing to minimize feature distortion during high-throughput fabrication [29].
Among various components of photoinitiation systems, PIs are the central ingredients in coating formulations since they directly influence the efficiency of photoinitiation and the resultant properties of the coatings. Depending on the formation routes of reactive radicals, the two primary photoinitiating systems are Norrish-Type I (cleavage) and Type II (hydrogen abstraction) [30,31,32]. Type I systems are single-component systems consisting solely of photosensitizers (PSs) with photocleavable bonds. Upon light absorption, Type I PIs in singlet or triplet excited states undergo homolytic cleavage of chemical bonds via single-molecule reactions to generate reactive species. In contrast, multi-component Type II systems rely on non-photocleavable PS compounds, excited from the ground state to singlet states and subsequently converted to long-lived triplet states through intersystem crossing. These excited PS molecules then undergo intermolecular reactions with co-initiators to produce reactive radicals. Notably, mono-component Type II PIs capable of intramolecular hydrogen abstraction, such as thioxanthone derivatives, have also been developed. The molecular structures of representative PIs are listed in Figure 1. Table 1 summarizes the characteristic differences between Type I and Type II PIs. The comparative elements cover the photoinitiation mechanisms, curing kinetics, oxygen inhibition, applications, and exemplary molecular skeletons [30,31,32,33].
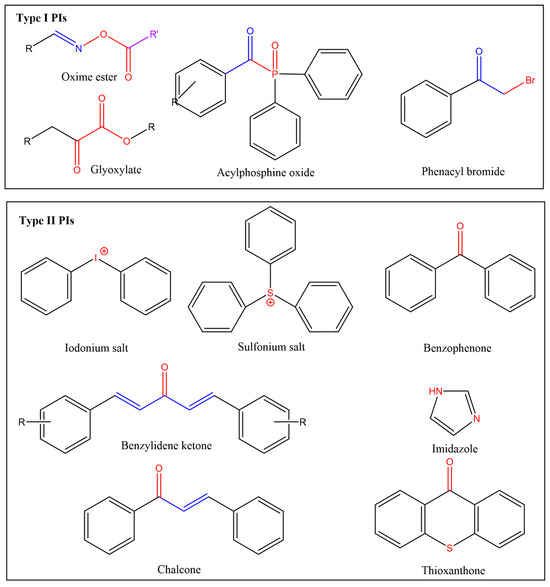
Figure 1.
Representative core structures of Type I and II PIs.

Table 1.
Summary of characteristic differences between Type I and Type II PIs.
2. Future Directions in Next-Generation Photoinitiators
The rational design of efficient photoinitiating systems is crucial for achieving colorless, eco-friendly, and deep-cured coatings through controllable reaction mechanisms. Current research on PIs for advanced coatings primarily focuses on several key directions, as outlined below (Figure 2) [33]:
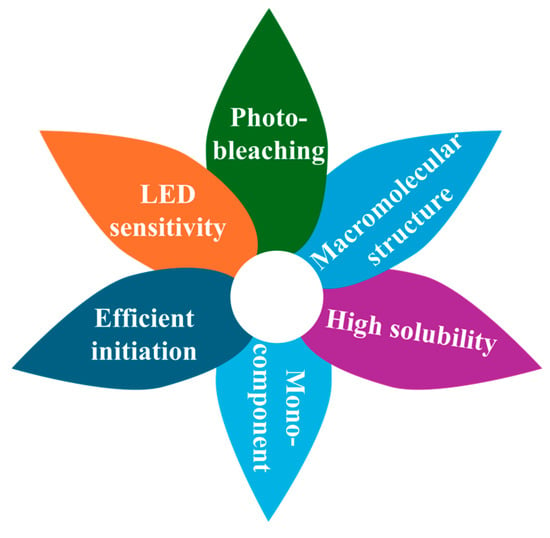
Figure 2.
Illustration of directions for next-generation photoinitiators (PIs).
High Efficiency. High photo-initiation efficiency is essential for high-speed applications and for reducing concentrations of PIs to mitigate side effects (discoloration and poor mechanical performance). Metrics for assessing PI efficiency include the final conversion of reactive groups, which are monitored via Fourier-transform infrared spectroscopy. Some factors are critical for evaluating efficiency, such as light absorption capabilities, quantum yield of radical production, the reactivity of generated species, and photocleavage routes from singlet or triplet excited states [32,34,35]. Additionally, sufficient excited-state lifetimes are required for PS molecules to transfer absorbed light energy to co-initiators [36]. However, it is noteworthy that the reactivity maximum does not always coincide with the absorption maximum [37].
Efficient Mono-Component PIs with High Oxygen Tolerance. Oxygen tolerance is crucial for photopolymerizations conducted under aerobic conditions. While mono-component Type I systems are simple and widely used, they often suffer from poor oxygen inhibition. Conversely, multi-component Type II PIs exhibit better oxygen inhibition but tend to have poor initiation activity in high-viscosity formulations [38,39]. Furthermore, the co-initiators in Type II systems can introduce drawbacks such as unpleasant odors, toxicity, yellowing, and compatibility issues [40]. Striking a balance between oxygen tolerance and photoinitiation efficiency remains challenging. A promising solution is the development of mono-component Type I or II PIs that incorporate covalently bonded hydrogen donors or iodonium salts [38,41,42,43].
LED-Sensitive PIs. Mercury lamps have been the primary ultraviolet (UV) light source for traditional photopolymerization. However, the use of UV light poses safety risks to humans, generates toxic ozone, and suffers from shallow penetration depths and high energy consumption [44]. In contrast, light-emitting diode (LED) devices offer advantages such as energy efficiency, safety, and long lifetimes; Besides, LED enables deep light penetration into coatings (up to several centimeters), benefited by the wide spectral range from violet to visible light and near-infrared regions [45]. Consequently, LED light sources are efficient for curing thick samples. Accordingly, developing LED-compatible PIs that absorb near-UV, visible, or near-infrared light is vital for expanding photopolymerization applications [3].
Photobleachable PIs. One challenge with visible-light PIs is the formation of colored photolytic residues in coatings, limiting their use in applications requiring optical transparency, including dental and optical devices. Additionally, visible-light PIs often suffer from reduced light intensity across thick polymer matrices, worsening their curing depth and uniformity. Residual PI molecules may also degrade the polymeric coatings by generating radicals, leading to premature aging. Developing photobleachable PIs offers a promising solution for high-performance coatings [46,47]. Such PIs undergo photochemical reactions that diminish or eliminate visible-light absorption during or after exposure, allowing the production of optically clear and thick polymeric coatings.
Low Migration, Low Toxicity, and High Storage Stability. The migration of low molecular weight PIs and their photolytic residues raises concerns about health risks and environmental pollution. Migration levels are typically assessed through extraction experiments and monitored via UV–visible absorption spectroscopy. Developing PIs with low migration and low toxicity is critical for sensitive applications of coatings, such as food packaging [48]. Recent strategies include developing macromolecular or polymerizable PIs and using bio-sourced chromophores like coumarin, anthraquinone, chalcone, chlorophyll, capsanthin, and flavone [49,50,51]. Furthermore, next-generation PIs must exhibit excellent storage stability and compatibility with additives such as hydrogen donors (water, alcohols, or amines) and monomers [39,52].
This review aims to provide a comprehensive overview of newly developed PIs with improved performance for functional coatings, achieved through diverse molecular engineering strategies since 2021. Though molecular design strategies have been explored, few reviews have systematically highlighted these advances. This review covers recent progress in Type I and Type II PIs. Multiple directions are highlighted, including extended conjugation and push–pull groups, hydrogen-donor incorporation, photobleaching designs, macromolecular/polymerizable systems, silane-modified structures, and auxochrome-functionalized PIs. The review excludes discussions of two-photon polymerization without PIs, metal-containing complexes, and inorganic particle PIs; These topics have been extensively reported elsewhere [11,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67]. By focusing on molecular engineering strategies, this review provides insights into advancing photoinitiating systems to meet the demands of functional coatings for diverse applications.
3. Photocleavable Type I PIs
Classical Norrish-Type I PIs have been widely used in FRP and CP due to their straightforward reaction mechanisms. Notable examples of Type I PIs that have been commercialized for industrial applications include Irgacure 1173, Irgacure 819, Irgacure 369, and Irgacure 651. These PIs are based on key structural motifs such as α-hydroxy ketones, phosphine oxides, acetophenones, oxime esters, benzoins, α-oximino ketones, and α-dicarbonyl compounds. This section focuses on recent progress in Type I PIs, emphasizing oxime esters (OXEs), acylphosphine oxides (APOs), phenacyl bromides, and glyoxylates. Figure 3 presents the representative photoinitiation mechanism. Table 2 summarizes the polymerization performance of acrylate or epoxy monomers in a thin laminate sample. Strategies for improving these PIs have been applied to address the limitations in traditional coating applications. These approaches include introducing diverse chromophores, extending conjugated structures, or incorporating hydrogen donors.
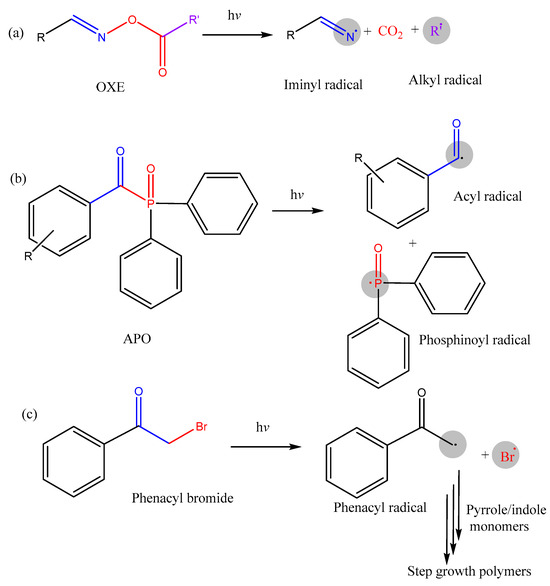
Figure 3.
Photoinitiation mechanism for Type I PIs, including (a) oxime ester (OXE), (b) acylphosphine oxide (APO), and (c) phenacyl bromide.

Table 2.
Summary of the initiation capabilities of some reported PIs in a laminate with a thickness of 25 mm under the irradiation of a 405 nm LED light source.
3.1. Oxime Esters (OXEs)
As monocomponent Norrish-Type I PIs, OXEs possess a generic chemical structure of (R1, R2)−C=N−O−C(=O)−R3 and are characterized by photocleavable N−O bonds. Upon exposure to light, photoexcited OXEs in singlet or triplet states undergo homolytic cleavage of the N−O bond, resulting in the formation of iminyl and acyloxy free radicals (Figure 3a) [35]. Subsequently, the decarboxylation of the acyloxy radical produces initiating alkyl radicals, with the simultaneous release of carbon dioxide. Examples of commercialized OXE PIs include OXE-01 and OXE-02, developed by BASF, which are well-suited for opaque formulations of coatings in industrial fields. Among the various Type I PIs, OXEs are notable for their advantages, including facile synthesis from inexpensive reagents, high thermal stability, and reduced oxygen inhibition in FRP due to the carbon dioxide release during photolysis. OXEs also exhibit low energy barriers for N−O bond cleavage, enabling rapid initiation kinetics. Furthermore, the photoreactivity of OXEs can be finely tuned by modifying the substituents, as these structural variations influence bond cleavage pathways and the enthalpies of N−O bond dissociation [75].
To develop LED-sensitive and efficient OXEs, various chromophore scaffolds have been integrated into OXE structures [35]. These designs included anthraquinones [75], N-naphthalimide [68], thioxanthone [76,77,78], naphthoquinone-imidazolyl [69], triphenylamine [79,80,81,82,83,84], carbazole [37,85,86,87,88,89], chalcone [90,91], indole [92], thiophene [93,94], phenothiazine [95,96], and coumarin [89,97]. The final conversion of TMPTA monomer could reach up to 85%, higher than the benchmark PI, when using the newly developed naphthoquinone-imidazolyl-OXE PIs (Table 2) [69]. Novel bis-chalcone-based OXEs bearing two diphenyl sulfide units have also been synthesized, enabling electron delocalization for enhanced conjugated systems [98]. These OXEs exhibited superior FRP capabilities for acrylic monomers/oligomers when exposed to 365 nm light. Fused systems, such as carbazole—coumarin and phenothiazine—carbazole hybrids, have also been explored for their promising photo-initiation properties [70,99]. Additionally, charge transfer complexes comprised of electron-deficient ketoxime esters and electron-rich aromatic amines, often incorporating pentafluorophenone rings, demonstrated high initiation efficiencies [100]. Another novel structural approach involved the introduction of allyloxy groups to the ortho-position of ketoxime esters, inducing intramolecular cyclization and forming highly reactive carbon-centered radicals [101].
3.2. Acylphosphine Oxides (APOs)
APOs as traditional Type I PIs are distinguished by their high electron density on phosphorus atoms, which contributes to their exceptional photoreactivity. Under light exposure, photoexcited APOs undergo homolytic cleavage of carbon–phosphorus bonds, producing phosphinoyl and acyl radicals via α-scission to initiate polymerization (Figure 3b). Unlike OXEs, APOs do not release carbon dioxide during curing. APOs are particularly advantageous due to their strong light absorption range from 350 to 400 nm (attributed to n-π* transitions), compatibility with LED sources, and photobleaching properties, which prevent yellowing during curing (visible at around 410–440 nm). Commercially available APOs include monoacylphosphine oxide, bisacylphosphine oxide, and 2,4,6-trimethylbenzoyldiphenylphosphine oxide (TPO, known as Irgacure 819). The TPO with its higher molecular weight and low migration properties is widely used in 3D printing applications [102]. TPO was commonly applied as a benchmark PI to assess the photoinitiation capacity of newly developed Type I and II PIs (Table 2). However, TPO and other APOs face challenges of poor biocompatibility due to genotoxicity and cytotoxicity, high migration, and low compatibility with macromonomers. These issues restrict their applications in fields such as food packaging and eco-friendly coatings.
Several strategies have been employed to overcome these limitations. For instance, physically mixing bisacylphosphine oxide with optical brighteners has enabled singlet-singlet energy transfer photosensitization, increased initiation efficiency, and addressed yellowing concerns [36]. Functionalizing APOs with organosilicon groups improves compatibility with polydimethylsiloxane, expanding their utility in silicone coatings [103]. LED-sensitive APO derivatives have also been created by linking phosphoryloxy groups with conjugated chromophores, such as phenothiazines, ferrocenes, and carbazoles; These kinds of conjugated structures enabled red-shifted absorption up to 667 nm for near-infrared photopolymerization [63,104,105]. The electron-donating and hydrogen-donor groups of diallyl amino groups endowed APOs with red-shift absorption and multiple functionalities for both Types I and II [106]. To address migration and toxicity issues, TPO derivatives have been modified with polymerizable unsaturated bonds or block copolymer nanoparticles to produce low-migration and bio-friendly macrophotoinitiators [4,102,107]. Examples include functional groups such as 2-hydroxyethyl acrylate or 2,3-dihydroxypropyl acrylate [102]. Additionally, less toxic TPO derivatives with substituents including 2,4,6-trimethoxyphenyl and 2,6-dimethyl-4-alkoxyphenyl groups have been synthesized [108,109]. Cyclic conjugated structures, such as 9,10-dihydro-9-oxa-10-phosphaphenanthrene 10-oxide motifs, have also shown potential for improved thermal stability and reduced toxicity [71]. The TPO analog exhibited better photoinitiation efficiency of FRP for TMPTA monomers than TPO (75% vs. 65% conversion) (Table 2) [71].
3.3. Phenacyl Bromides and Glyoxylates
Phenacyl bromides are a newly developed class of monocomponent Type I PIs, notable for their nonionic nature and high versatility in advanced photopolymerization techniques beyond traditional FRP and CP. Photoexcitation of phenacyl bromides initiates a series of processes—photolysis, electron transfer, proton release, and coupling—ultimately forming reactive polymer structures (Figure 3c) [6]. Phenacyl bromides have been employed in sophisticated polymerization applications, such as chain-end functionalization and step-growth polymerizations. For instance, bromine end-functional polymers have been synthesized using phenacyl bromides during FRP of methyl methacrylate and styrene under UV light [110]. The achieved poly (methyl methacrylate) polymers demonstrated a number average molecular weight up to 9.0 kg mol−1 with a polydispersity low to 2.1 [110]. These brominated polymers further served as macroinitiators for synthesizing block copolymers. Additionally, phenacyl bromides enabled efficient step-growth photoreactions of conjugated monomers, such as N-methylpyrrole and N-methylindole, under near-UV exposure [6]. To enhance light absorption, electron-donating coumarin moieties have been introduced into phenacyl bromide analogs, enabling visible and near-infrared photopolymerizations of N-ethylcarbazole conjugated monomers [111].
Glyoxylates, represented by ketoester structures (R1−C(=O)−C(=O)−O−R2), have gained attention as photocleavable Type I PIs. This is due to the photobleaching properties, low toxicity, and ease of synthesis (via one-step Friedel–Crafts reactions). Upon light exposure, glyoxylates undergo homolytic cleavage of dicarbonyl C−C bonds, producing methoxy carbonyl radicals, which subsequently decarboxylate to form methyl radicals. Notable glyoxylate types are methylbenzoylformates [112]. Considering the weak visible light absorption of methylbenzoylformates, a series of glyoxylates was reported to develop visible light PIs by integrating photosensitive groups, such as carbazoles and coumarins [72,113]. The reported glyoxylates showed superior FRP of trimethylolpropane triacrylates and tri-(propylene glycol) diacrylates, better than benchmark PIs [72,112,113]. It has been discovered that the electronic effects of substituents were crucial for photoinitiation capabilities. The electron-withdrawing substituents endowed methylbenzoylformates and coumarin-based glyoxylates with improved photoinitiation ability, in contrast to electron-donating counterparts [72,112]. The sulfur-containing coumarin-glyoxylates showed a lower cleavage exothermicity than control samples (−122.65 vs. −99.94 kJ mol−1), contributing to the higher photoinitiation capacity (up to 68% final conversion) [72].
4. Hydrogen Abstraction Type II PIs
Unlike monocomponent Type I PIs, bi- or multicomponent Type II PIs rely on the combination of PSs and co-initiators to generate radicals through bimolecular reactions. As depicted in Figure 4, photo-activated PSs are first excited from their ground state to singlet states, which then transition into long-lived triplet states. These triplet-excited PS molecules subsequently react with co-initiators to produce both reducing and oxidizing radicals, driving FRP and CP reactions [114]. It is noted that the fluorescence quantum yield (related to the relaxation of excited singlet states to ground states) competes with the triplet quantum yield (Figure 4). Co-initiators in this system can include onium (ONI) salts and hydrogen donors such as tertiary amines or polyalcohols. A wide variety of PS scaffolds have been developed for Type II photoinitiating systems, including ketones, benzophenones, enones, chalcones, and thioxanthones. To adapt these PIs for LED illumination, many photosensitive groups have been modified with extended conjugation or push–pull structures to red-shift their light absorption.
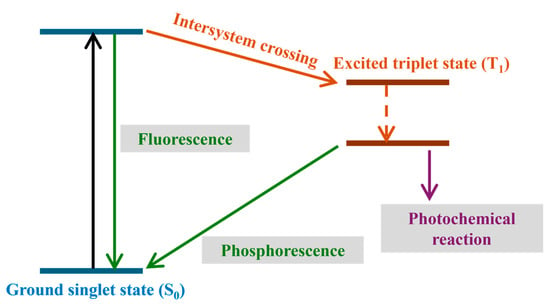
Figure 4.
Illustration of Jablonski diagrams showing triplet state formation through intersystem crossing.
To address some of the drawbacks of traditional Type II PIs—such as the toxicity, odor, yellowing, and migration caused by co-initiators—research has increasingly focused on creating one-component Type II systems. These systems involve hydrogen-donor-modified PS structures capable of intramolecular hydrogen abstraction. Another promising approach involves intermolecular hydrogen abstraction between the ground and triplet-excited states of the PI itself, utilizing skeletons such as naphthalimide, anthraquinone, and N-alkyl-substituted azacalixphyrins [38,115,116].
4.1. Onium (ONI) Salts
ONI salts are widely used as ionic Type II PIs or as co-initiators in multicomponent systems. These compounds typically consist of low-nucleophilic anions paired with cationic chromophores. In Type II systems, ONIs accept electrons from photoexcited PSs via photoinduced electron transfer, leading to homolytic and hydrogen abstraction reactions that generate reactive species. The photolytic decomposition of ONIs produces radicals, cationic species, and strong acids, enabling the initiation of CP, FRP, and hybrid polymerization processes with various monomers (epoxy and acrylic monomers) [117,118]. The anionic component of ONIs—often perfluorinated anions—must possess a large, non-nucleophilic structure. This ensures that the anions do not form π-bonds with macrocations in CP processes, thus enabling faster propagation during photopolymerization [42]. Common cationic variants include iodonium, sulfonium, and phenacyl salts.
Iodonium salts are well-known for their high photoreactivity and substantial photoacid yield, making them effective for initiating CP reactions. One widely used iodonium salt is diphenyl iodonium hexafluorophosphate. Iodonium salts absorb light up to 320 nm. During the interaction between iodonium salts and photoexcited PS molecules, phenyl radicals are generated for initiating polymerization processes (Figure 5a). Adding hydrogen donors of triethanolamine or 4-(dimethylamino)benzoate could further enhance phenyl radical production through the formation of hydrogen-modified PS molecules [119,120]. Thanks to their high redox potential, iodonium salts can be photosensitized by a wide variety of phenyl-free and aromatic PS molecules [121]. Table 3 presents photophysical parameters of some representative chromophores to sensitize iodonium salts. Recently developed aromatic PSs included carbazoles [34,47,119], capsanthin [50], squaraine dyes [39], chalcones [73,91,120,122,123,124], ketones [41,125], benzophenones [126,127], thioxanthones [128,129], naphthalimides [38,130], boron-dipyrromethenes (BODIPYs) [131,132], flavanols [48,51,133], phenothiazines [134,135], anthraquinones [74,115], coumarins [136,137], pyrazolines [138], pyrroles [139], phenanthroimidazoles [140], indanediones [141], and chlorophyll a derivatives [142]. The electron transfer quantum yield between chalcone-anthracene PSs in excited singlet states and iodonium salts was determined up to 84%, showing the effective electronic interactions (Table 2) [74]. A high electron transfer yield of 91% was reported for capsanthin dyes/iodonium systems, inducing 60% final conversion of acrylate monomers in a laminate [50].
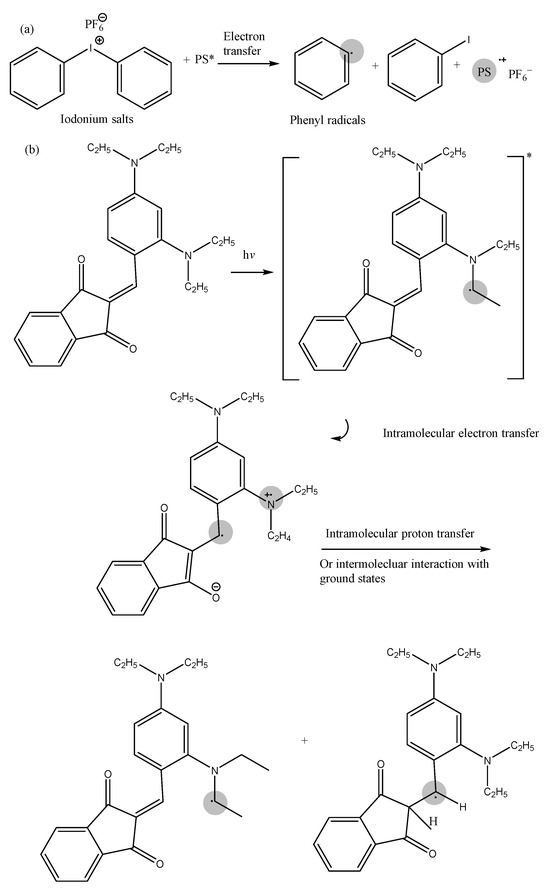
Figure 5.
Photoinitiation mechanism of (a) iodonium salts and (b) cinnamoyl-indanone PIs [47,143].
To address the efficiency limitations of multicomponent systems, researchers have developed one-component iodonium PIs by chemically attaching chromophoric groups to the iodonium salt. For instance, iodonium salts equipped with extended conjugated structures (such as stilbenes) exhibited improved quantum yields for superacid generation and enhanced photoinitiation properties [144]. Additionally, introducing electron-donating (methoxy) and electron-withdrawing groups (CN) on biphenyl-based iodonium salts led to red-shifted absorption peaks and accelerated photolysis for FRP reactions [145]. Modifications replacing one phenyl ring with π-conjugated structures, such as benzylidene [1,43], carbazole [47,146], or BODIPY [42], have also been reported. The unimolecular benzylidene-iodonium salts exhibited a rate of acid generation up to 1.63% and the conversion of epoxy CP reactions up to 45% under 365 nm LED irradiation [1]. Most recently, introducing stilbene chromophores further improved the photoacid generation yield up to 13%, greatly facilitating CP reactions of cyclic epoxy monomers (up to 69% conversion; 365 nm LED) [144]. Furthermore, the choice of perfluorinated anion in the ONI salt could significantly influence proton acid yields and initiation efficiencies. For example, BODIPY-based iodonium salts incorporating triflate anions demonstrated better photoactivity compared to tosylate, hexafluorophosphate, and hexafluoroantimonate anions [42].
Sulfonium salts offer several advantages over iodonium salts, including better light absorption, simpler synthesis, cost-effectiveness, and high thermal and storage stability. One widely used example is triarylsulfonium hexafluorophosphate, which absorbed light up to 360 nm and exhibited good stability at high temperatures (up to 370 °C) or during long-term storage (up to 30 days at 50 °C) [147]. However, the lower redox potential of sulfonium salts can limit their sensitization potential. To address these limitations, research into photosensitizers for sulfonium salts has expanded to coumarin-based glyoxylates [113], chalcone-anthracene [74], benzophenones [126], and pyrrole-carbazoles [124]. Furthermore, one-component sulfonium systems have been developed by chemically linking chromophoric groups, such as coumarins, to the sulfonium cation to enhance visible light absorption [117,147]. The coumarin sulfonium salts showed efficacy for the CP reaction of epoxy monomers with a conversion up to 65% under 365 nm LED irradiation [117].
Phenacyl salts, a newer class of onium salts, are attractive for their high photoinitiation efficiencies and flexible spectral tunability. For example, phenacyl phenothiazinium hexafluoroantimonates incorporating phenothiazine chromophores featured broad absorption spanning ultraviolet to near-infrared wavelengths [148].

Table 3.
Summary of photophysical parameters of reported Type II PIs.
Table 3.
Summary of photophysical parameters of reported Type II PIs.
| Chromophore | Absorption Peak (nm) | Molar Extinction Coefficient at Maximum Absorption (M−1 cm−1) | Fluorescence LifeTime (ns) | Fluorescence Quantum Yield | Ref. |
|---|---|---|---|---|---|
| Squaraine dyes | 569 | 192,000 | ___ | 0.0101 | [39] |
| Carbazole-coumarin-ketone dyes | 395 | 22,700 | ___ | 0.15 | [46] |
| Capsanthin | 455 | 5018 | 5.87 | ___ | [50] |
| Chalcone derivatives (C-TPA) | 405 | 33,600 | ___ | 0.027 | [91] |
| Indole-OXEs | 426 | 26,320 | 1.51 | 0.0019 | [92] |
| Thiobarbituric acid-aniline derivatives | 491 | 47,650 | 5.63 | 0.0071 | [149] |
Upon photoexcitation, phenacyl phenothiazinium hexafluoroantimonates generated phenacyl radicals to initiate FRP and phenothiazine radical cations for CP, as well as Brønsted superacids through hydrogen abstraction [148]. Similar analogs, such as phenacyl salts incorporating strong electron-donating coumarin moieties, have also demonstrated visible and near-infrared light sensitivity and dual capabilities for FRP and CP [5].
4.2. Ketones (Benzophenones, Enones, Benzylidene Ketones, and Chalcones)
Ketone-based PIs are widely used in Type II systems due to their relatively straightforward synthesis via one-step aldol condensation in alkaline aqueous solutions [150,151]. These compounds, functionalized as charge transfer photosensitizers, interact with ONI salts and hydrogen donors to produce radicals for FRP and/or CP. One of the most notable examples is the commercial α-hydroxy ketone-based PI Irgacure 2959, which offers excellent initiating efficiency, thermal stability, and color stability [152,153]. By extending the conjugated structures of α-hydroxy ketones with unsaturated double bonds, such as pyrrole rings or benzyl substituents, researchers have developed ketone derivatives with enhanced compatibility for LED light sources (405–465 nm) [152,154]. Other structural motifs have been explored through the incorporation of chromophoric peripheral groups, such as coumarins, quinolones, indanediones, and carbazoles [141,155,156,157,158]. Carbazole-coumarin-ketone derivatives with benzoyl substituents exemplify recent progress in unimolecular Type II PIs. These compounds exhibited intramolecular hydrogen abstraction, making them highly photobleachable and efficient [46]. The carbazole-coumarin-ketone manifested a low fluorescence quantum yield (0.14 for CCK-Ph; 0.01 for CCK-Tol) and high triplet quantum yield, accompanied by low singlet-triplet transition barriers (0.01 eV for CCK-Ph; 0.13 eV for CCK-Tol) [46]. Additionally, polymerizable or macromolecular derivatives of ketones have been synthesized for improved migration stability, such as Irgacure 907 derivatives with vinyl sulfide groups or polymer-bound Irgacure 2959 analogs [150,158,159,160]. The widely studied ketone dye scaffolds include benzophenones and enones.
Benzophenones are one of the low-cost PIs with excellent photoinitiation activity. Benzophenones can interact with tertiary amine co-additives through photoinduced electron transfer and hydrogen abstraction to generate aminoalkyl radicals for FRP of acrylate monomers [161,162]. Structural modifications by attaching aromatic amine-conjugated groups (triphenylamine, carbazole, salicylaldimine, and phenothiazine) have red-shifted their maximum absorption from 257 nm and enhanced initiation efficiency [119,126,127,163]. The triphenylamine-benzophenone PIs showed a high electron transfer quantum yield from their excited singlet states to iodonium (0.75), suggesting an enhanced capacity of photosensitization for FRP reaction (up to 77% final conversion) compared to benchmark 2-isopropylthioxanthone (61%) [127]. Schiff base-containing benzophenones have further extended light absorption [164]. Additionally, structural analogs like di-2-thienyl ketones with carbazole and phenylamine groups improved initiation kinetics via intramolecular charge transfer for visible light absorption [125]. Innovatively, photo-switchable, dual-color benzophenones linked to spiropyran cores have enabled advanced applications like two-photon photopolymerization and xolography techniques [165,166].
As α,β-unsaturated ketones, enones consist of ketone moieties connected by conjugated double bonds, which enable photobleaching properties. However, their photoinitiation efficiency is often limited by issues such as trans–cis isomerization, keto-enol tautomerization, and dimerization [167]. These challenges can be mitigated by introducing steric hindrance via alkyl bridges or push–pull conjugation systems, such as in pyrrole or thiobarbituric acid derivatives [149,155,168]. Substituted enones bearing aromatic amines support both multicomponent and monocomponent applications, producing reactive aminoalkyl and benzyl radicals through intramolecular charge transfer or electron–proton exchange (Figure 5b) [47,143]. Recent phenyl-free enone dyes with pyrrole or furan substituents offer better solubility and efficient PS sensitization of ONI salts [121,167].
As a part of enones, benzylidene ketones (BKs) feature a central ketone flanked by two double bonds, forming a planar structure for efficient electron delocalization and two-photon photoinitiation [41,151,157,169]. Synthesized via simple aldol condensation, BKs have been functionalized with chromophores including triphenylamine, carbazole, phenothiazine, and coumarin to optimize LED photoinitiation efficiency [41,135,170,171]. Double-bonded BKs tailored with specific substituents, such as methoxy groups, show superior initiation activity in the presence of hydrogen donors compared to other electron-donating groups [41,135,151].
Chalcones are natural flavonoids with enone structures, valued for their low toxicity, strong visible-light absorption, simple synthesis, and photobleaching capability [172]. Structurally modified chalcones have been developed for LED-sensitive PIs. These PIs showed enhanced intramolecular charge transfer and extended light absorption through autochrome substituents (halogens, amino groups, anthracene, ferrocene, triphenylamine, or phenothiazine) or prolonged conjugated spacers [74,91,122,172,173,174,175]. Coupled with iodonium salts, a triphenylamine-chalcone derivative showed a superior high quantum yield up to 0.097 of photoacid generation, benefiting the CP initiation of epoxides (up to 34.6% conversion; 405 nm LED) [74]. The phenothiazine-chalcone PSs showed a high electron transfer quantum yield (0.89%) with iodonium salts, generating a rapid FRP reaction of acylate monomers (90% final conversion; 440 nm LED irradiation) [122]. Push–pull chalcone architectures improved long-wavelength absorption, while sulfonic acid groups conferred water solubility [91]. Recently, unimolecular chalcones with dual functions were developed using nitrogen-containing structures (pyrrole-carbazole and triphenylamine-dimethylaniline), enabling hydrogen-donor-free applications or co-initiator systems [124,170]. These chalcones undergo hydrogen abstraction or electron transfer to produce radicals for FRP and CP. As a common issue for chalcones, photoisomerization has been mitigated with steric hindrance (intermolecular hydrogen bonding or alkyl chains) to improve storage and thermal stability under sunlight [176,177,178].
4.3. Thioxanthones and Imidazoles
Thioxanthones and their derivatives are widely used as hydrogen abstraction Type II PIs in industrial coatings [128,179,180]. In combination with hydrogen donor co-initiators, thioxanthones generate active radicals through electron and proton transfer while also photosensitizing ONI salts. Structural modifications have enhanced visible light absorption. Exemplary cases included di-substitution with amines and core extension via fusion with polynuclear aromatic chromophores (such as indoloquinoxalines) through thioxanthonation reactions [128,181,182,183]. The synthesized indene- and indoloquinoxaline derivatives showed high quantum yield of the triplet states (up to 0.22) and comparable initiation capacity (up to 104.3 mmol s−1 of initiation rates) to the benchmark camphorquinone PIs for dental filling materials [183]. Unimolecular Type II PIs were created by chemically attaching hydrogen donors to thioxanthone scaffolds. The isopropylthioxanthone is a well-known commercial example. Additional thioxanthone-based derivatives, such as naphthalene thioacetic acid, 1,4-naphthoquinone, dithioxanthone-disulfide, and 2-naphthalene-thioacetic acid, were synthesized by linking electron or hydrogen donors to thioxanthone structures, with or without spacers (e.g., methyl esters) [184,185,186,187,188]. These modifications extended light absorption to ~490 nm and enable photolysis to produce active alkyl radicals, driving FRP either directly or indirectly [184,185,186,187,188].
Imidazole is a planar, five-membered heterocycle with two nitrogen atoms at non-adjacent positions. These nitrogen atoms exhibit dual electronic properties, acting as pyridine-like electron-withdrawing and pyrrole-like electron-donating sites. The unique molecular and electronic properties endow imidazole PIs with dual functions of radical generation through both direct bond cleavage (Type I) or hydrogen abstraction (Type II) pathways. For instance, unimolecular imidazolium ionic liquids with high water solubility generate 4-alkylbenzyl radicals through C–N cleavage, initiating FRP of vinyl monomers under 310 nm UV light [189]. Functional modifications, such as introducing photoactive groups (phenanthrene or phenanthroline) into π-conjugated imidazole systems, enable blue LED-driven polymerizations without requiring photosensitizers [190]. Push–pull donor-π-acceptor (D-π-A) systems, based on phenanthroimidazoles, effectively photosensitize onium salts for FRP and CP reactions [140]. Hexaarylbiimidazole derivatives (such as Lencolo 5030 and 5033) are well-established for deep curing in coatings. A recently developed sensitizer, 2,4-bis [4-(diethylamino)-benzylidene]-cyclopentanone (C4), features a narrow bandgap, strong fluorescence, and low energy transfer barrier [114]. In the C4/hexaarylbiimidazole/2-mercaptobenzoxazole system, intermolecular energy transfer facilitated a double oxidative-reductive cycle for efficient radical production [114].
5. Molecular Engineering Tactics
Several molecular engineering tactics of PIs have been developed to advance the application of photopolymerization techniques for broad applications. These strategies include the strong and long-wavelength absorption, photobleaching, polymerizable macromolecular, and silane-containing structures of PIs (Figure 6).
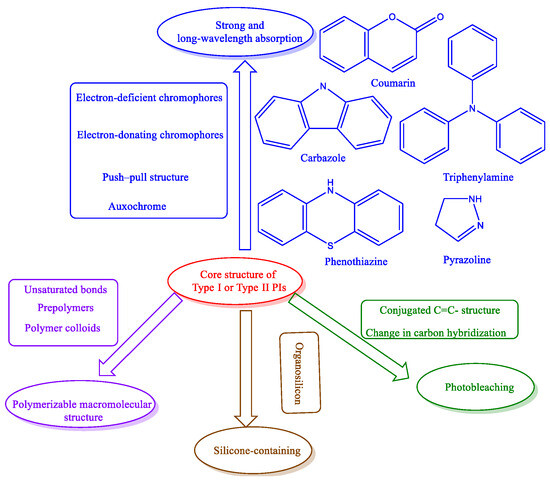
Figure 6.
Illustration of molecular engineering strategies for developing advanced PIs.
5.1. Extended Π-Conjugation Structures for Long-Wavelength Absorption
Classical PIs for coatings have limited visible light absorption. The development of PIs with long-wavelength absorption is crucial for adapting to LED light sources. Recently, natural dyes such as anthraquinone, capsanthin, and chlorophyll have been reported to exhibit light absorption up to 700 nm, along with promising long wavelength photoinitiation efficiencies (Figure 7) [49,50,51]. Structural modifications aimed at narrowing the bandgap and facilitating intramolecular electron transfer have been widely explored. This was achieved by introducing electron-donor or electron-acceptor moieties or extending the π-conjugated structures of chromophores.
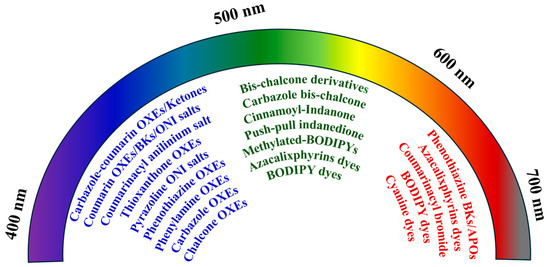
Figure 7.
Illustration of reported PIs or chromophore structures with different light absorption wavelengths.
Representative building blocks of conjugated chromophores include naphthalimide, pyrrole, carbazole, phenothiazine, thioxanthone, triphenylamine, coumarin, chalcone, anthracene, naphthalene, cyanine, BODIPY dyes, and azacalixphyrins. Electron-deficient chromophores (coumarin and pyrazoline) and electron-rich chromophores (carbazole, phenothiazine, and triphenylamine) have imparted photoinitiation capability to various PIs. These structurally modified PIs included OXEs, phenacyl salts, sulfonium salts, BKs, chalcones, and ketones under UV or blue LED illumination (311–468 nm) [5,46,47,79,84,85,88,89,90,91,95,96,97,117,136,137,138,156,171,191,192]. For example, carbazole-based OXEs containing aromatic substituents have shown blue light (460 nm) LED sensitivity [37]. Electron-rich groups (pyrrole, anthracene, and carbazole) have demonstrated photoactivity in chalcones under blue light (440–470 nm) and even green light (500–532 nm) illumination [74,174,175]. The introduction of a cyclopentane structure into bis-chalcone PIs resulted in an improvement in the planarity of the cross-conjugated system and an enhancement in the delocalization of π electrons, benefiting the redshift of the absorption wavelength (Figure 8a) [175]. Additionally, BODIPY derivatives with large planar π-structures and high rigidity have enabled FRP and CP photoinitiation within a three-component system under red light (660 nm) LED irradiation [132]. Notably, methylated-BODIPYs and azacalixphyrin derivatives are capable of functioning as Type I PIs under green (532 nm) and red (660 nm) LED illumination [116,193]. Furthermore, PIs (APOs, phenacyl, or BKs) containing electron-rich phenothiazine or coumarin architectures have exhibited efficiency under red light (620 nm) or near-infrared (850 nm) LED irradiation [104,111,135,148]. More recently, near-infrared-active cyanine chromophores were reported for use under 850 nm LED illumination, achieved through heterocyclic incorporation [194,195].
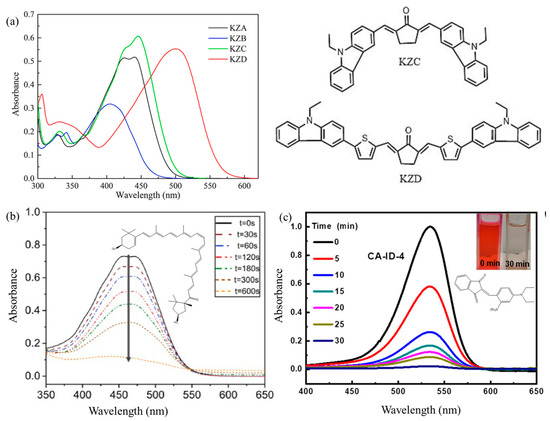
Figure 8.
(a) UV–vis spectra and molecular structures of carbazole-bis-chalcone PIs. Adapted from Ref. [175]; Copyright Elsevier. (b) Photolysis of capsanthin dyes in the presence of iodonium salts. Adapted from Ref. [50]; Copyright Elsevier. (c) Photolysis of CA-ID-4 dyes under LED exposure at 525 nm. Adapted from Ref. [143]; Copyright ACS.
Beyond modifying chromophore substituents, constructing push–pull structures represents an important strategy to enhance light absorption and improve molar absorptivity [47,145,196,197,198]. Push–pull architectures are typically composed of electron donors and electron acceptors bridged by a planar conjugated spacer (C=C bonds) or a saturated spacer [141]. These extended conjugation structures induce the “push-pull” effect via intramolecular electronic interactions and charge delocalization along the chromophore axis, leading to strong and broad absorption across the visible and near-infrared regions. This phenomenon can be validated by the emergence of a strong intramolecular charge transfer band [197]. Modulating the strength of electron-donating and withdrawing groups allows for tunability of absorption peaks [141,199]. Moreover, such electronic delocalization significantly enhances the molar extinction coefficients of ketone dyes, often reaching magnitudes of 105 M−1 cm−1 [168]. For instance, D-π-A cinnamoyl-indanone structures exhibited broad absorption up to 600 nm with high molar extinction coefficients [143]. Similarly, push–pull indanedione-based dyes exhibited light absorption spanning from blue to red, enabling photosensitization of onium salts [141]. Typical configurations of push–pull systems include D-π-A, symmetrical D-π-A-π-A or D-π-A-π-D, and asymmetrical D-π-A-π-A′ arrangements [73,90,92,120,123,140,143,149,155,168,174,176,200]. Ketone dyes—particularly chalcones—have been widely used as core structures due to their straightforward synthesis via Claisen–Schmidt reactions [123,143,155,174]. Common electron-acceptor moieties included malononitrile, indanedione, thiobarbituric acid, thioxanthone, OXEs, carbonyl, and dicyanovinyl groups [92,145,149,200,201,202,203]. Electron-donating groups included pyrrole, indole, carbazole, aniline, triphenylamine, phenothiazine, and hydroxyalkylphenone, in addition to substituents (methoxy, methylthio, or dimethylamino groups) [92,94,123,143,145,149,200,202,203,204,205]. It was noteworthy that the introduction of the thiophene π electron bridge could form a large conjugated structure, thereby red-shifting from 446 nm to 500 nm for bis-chalcone PIs (Figure 8a) [175].
5.2. Photobleachable Structures
Constructing extended π-conjugation is an effective strategy for red-shifting emission into the wavelength range of LED sources. However, such structures pose several challenges of intense coloration, complex synthesis processes, diminished photoactivity, and impaired light penetration depth in photocured coatings [139]. The yellowish color of LED-sensitive PIs limits their use in applications requiring colorless materials, such as clear coatings and dental products. Furthermore, the distribution gradient of light intensity along the radiation path can result in reduced curing depth and heterogeneity in material properties. Consequently, the development of photobleaching visible PIs is critical to achieving high-transparency polymers with deep curing capabilities. Photobleachable PIs diminish their coloration completely upon visible light exposure due to photochemical reactions that reduce their visible light absorbance. This allows light to penetrate up to several hundred microns. There are two main photobleaching mechanisms: molecular cleavage and conjugate blocking.
The molecular cleavage type of PIs undergoes photocleavage reactions to generate small molecules with no absorption of visible light, thus mitigating coloration. A representative example is conjugated C=C-containing structures. For instance, the conjugated C=C bond in Capsanthin chromophores could fragment under 405 nm LED exposure, imparting photobleaching properties in Type II photoinitiation systems (Figure 8b) [50]. The conjugate blocking type involves disrupting the conjugation system by changing the hybridization of carbons in ketone carbonyl bridges or C=C double bonds from sp2 to sp3. Several carbonyl- or double-bond-containing PIs (OXEs and enones) have demonstrated photobleaching capabilities [46,47,90,92,143,149,167]. For example, the push–pull D-π-A-π-A′ structures of diethylamino–ketone carbonyl–oxime acrylates displayed photobleaching when oxygen alkyl radicals were formed through electronic interactions between the nitrogen in diethylamino groups and the ketone carbonyl [90]. Similarly, D-π-A-π-A′ indole–ketone carbonyl–OXEs exhibited photobleaching because of the transformation of carbonyl bridges into hydroxyl alkyl radicals and disrupting the conjugation system [92]. Another example included D–π–A thiobarbituric acid–aniline and cinnamoyl-indanone, where aminoalkyl radicals interacted with ketone carbonyl moieties to destroy the conjugated π bridges (Figure 8c) [143,149]. Additionally, the C=C bonds in substituted cinnamoyl formate PIs were polymerized with the assistance of tertiary amine additives, thereby disrupting the conjugated systems for faded coloration [206].
5.3. Polymerizable Macromolecular Structures
Small molecular PIs often suffer from migration issues, raising health concerns in applications of coatings in food packaging and dental materials. To overcome these issues without sacrificing mechanical properties, the construction of polymerizable macromolecular PIs—featuring structures similar to monomers—offers an effective solution. This approach lies in the principle of “like dissolves like” to improve solubility. Moreover, polymerizable/macromolecular PIs with bulky functional groups exhibit higher migration stability and lower toxicity compared to their small molecular counterparts. Polymerizable macromolecular PIs could be synthesized by embedding unreacted PIs into polymer matrices through the chemical linking of excess PIs with polymeric cross-linking networks. For instance, allyl-substituted carbazolyl APOs, methacrylic benzylidene ketones, and 10-undecenoyl lignin PIs showed enhanced solubility with monomers and better migration resistance compared to methyl or ethyl-substituted analogs [105,207,208]. Similarly, one-component acrylamide thioxanthones PIs demonstrated improved photoinitiation efficiency and reduced migration compared to commercial benchmark isopropylthioxanthones [209].
Another approach involved copolymerizing monomers or prepolymers with low-molecular-weight PIs containing polymerizable groups (vinyl, alkynyl, allyl, acryloyl, acryloyloxy, methacryloxy, styrene, or vinyl sulfide groups). For example, macromolecular PIs synthesized via the copolymerization of commercial Irgacure 907 and methyl methacrylate monomers exhibited enhanced migration stability and photoactivity (Figure 9a,b) [150]. This improvement arose from the steric hindrance effect, which stabilized reactive carbonyl radicals and prevented recombination quenching [150]. However, some polymerizable macromolecular PIs (such as those based on α-hydroxyalkyl acetophenone) might show reduced photoinitiation capability due to the electron-withdrawing effects of unsaturated ester groups [153]. This limitation could be addressed by introducing two-methylene spacers to block p-σ-π hyperconjugation, thus increasing the photocleavage rate to a level comparable to that of the parent PIs [153]. Polymerizable macromolecular PIs could also be prepared by functionalizing polymers or macromonomers with classical PIs. For instance, an aza-Michael addition reaction between amine-thioxanthones and poly(ethylene glycol) diacrylates produced a polymerizable macromolecular PI [210]. Compared to commercial Irgacure 2959, a macromolecular PI derived from Irgacure-functionalized poly(ethyleneimine) displayed significantly improved water solubility (35–45 g L−1) and a more than sevenfold increase in migration stability [159]. Similarly, a cyclic copolymer (Mn = 25,000 Da) formed between allyl-modified Irgacure 2959 and quaternary ammonium demonstrated improved photoactivity and dramatically enhanced migration stability (9% vs. 51%) compared to Irgacure 2959 [211]. Another example was a TPO-functionalized block copolymer colloid based on poly(ethylene glycol) methyl ether methacrylate (Mn = 500 g mol−1), synthesized via RAFT-mediated PISA processes; This colloidal PIs exhibited photoinitiation capabilities suitable for hydrogel fabrication (Figure 9c) [4]. Additionally, an acrylate-functionalized APO tethered to a polymer with structural similarities to macromonomers formed a biodegradable macrophotoinitiator with high solubility in monomers [102].
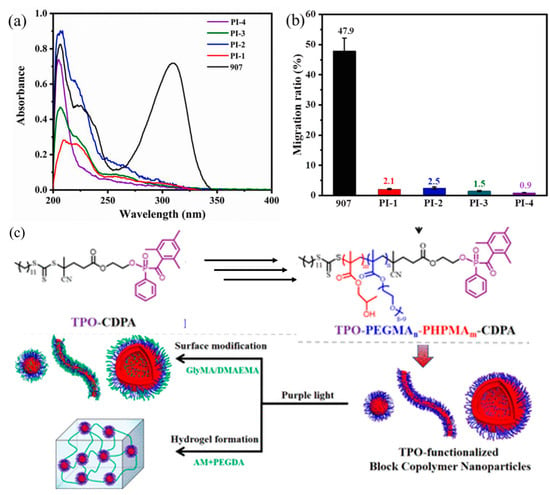
Figure 9.
The migration test for commercial 907 PI and polymerizable vinyl sulfide modified 907 derivatives: (a) UV–vis absorption spectra after being extracted from the polymer, (b) Migration ratio. Adapted from Ref. [150]; Copyright Elsevier. (c) Schematic illustration for the preparation of TPO-functionalized block copolymer nanoparticles by RAFT-mediated polymerization-induced self-assembly. Adapted from Ref. [4]; Copyright ACS.
5.4. Silicone- or Auxochrome-Containing Structures
Organosilicon polymers have garnered significant attention due to their chemical stability and biocompatibility, which make them widely applicable in coatings for medical devices and food packaging. However, classical PIs generally exhibit poor compatibility with silicone monomers or prepolymers, often leading to chain entanglement and phase separation. To address these issues, tethering PIs to silicon prepolymers has proven to be an effective strategy. As a proof of concept, chloromethyl flavonols were grafted onto thiol-containing oligosilsesquioxanes through a nucleophilic substitution reaction, resulting in polysesquioxane flavonols with high migration stability and excellent solubility (>50 wt.%) (Figure 10a) [133]. In a similar vein, chloride-containing TPO was reacted with small organosilicon compounds (such as γ-mercaptopropyltriethoxysilane) to achieve improved miscibility with methyl methacrylate-based polydimethylsiloxanes [103]. Additionally, benzophenone derivatives functionalized with bis(trimethylsilyl)amino groups exhibited enhanced oxygen inhibition and solubility [212]. This improved oxygen tolerance was attributed to the self-floating behavior of silicon-containing fragments generated from PIs in the presence of moisture, thereby effectively impeding oxygen penetration [212].
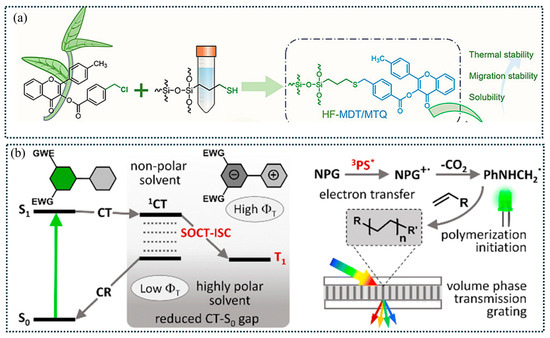
Figure 10.
(a) Illustration for preparing two flavonol PIs containing poly(sesquisiloxanes). Adapted from Ref. [133]; Copyright ACS. (b) Triplet state formation of Type II PIs through spin-orbit charge transfer intersystem crossing mechanism and photoinitiation routes. The symbol 3PS* indicates the triplet excited states of photosensitizers. Adapted from Ref. [213]; Copyright RSC.
The triplet states of excited PSs must possess sufficiently long lifetimes to enable efficient energy transfer to ONI salts or co-additives. Increasing polarization is a viable method to extend the lifetimes of excited states, achieved by introducing auxochrome groups (amino, imidazole, and other heteroatoms). Incorporating heavy atoms (halogen atoms or transition metals) further enhanced intersystem crossing of photoexcited PIs, enabling the formation of triplet excited states of chromophores [82]. For instance, a recent study on triphenylamine OXE PIs systematically investigated the effects of halogen substituents (iodine, chlorine, bromine, and fluorine) at the para-position of triphenylamine; The result revealed that iodine-containing PIs exhibited the best photocleavage efficiencies [82]. However, the use of heavy atoms raises concerns regarding environmental pollution and toxicity. As an alternative, heavy-atom-free BODIPY dyes have been synthesized, demonstrating comparable photoinitiation efficiencies for FRP to those of their heavy-atom-containing counterparts [193,213]. The electron donor-acceptor capabilities and triplet-state lifetimes of BODIPY dyes were fine-tuned through meso-position substitution with various moieties (anthracenyl, pyrenyl, and methoxyphenyl groups) [213]. Furthermore, BODIPY dyes functionalized with electron-withdrawing ethoxycarbonyl substituents facilitated electron transfer, enabling the formation of long-lived triplet excited states [213]. It is noted that the introduction of meso-aryl subunits endowed BODIPY dyes with a spin-orbit charge transfer intersystem crossing mechanism, which further enabled long-lived triplet lifetime (up to 70 ms) and high triplet quantum yield (up to 58%) (Figure 10b) [213].
6. Summary and Outlook
Photoinitiation plays a crucial role in determining coating performance. Various PIs have been developed for FRP and CP of benchmark monomers. Based on their photolysis mechanisms, PIs are generally categorized into photocleavable Type I systems (OXEs, APOs, glyoxylates, and phenacyl bromides) and hydrogen abstraction-based Type II systems (ONI salts, ketones, thioxanthones, and imidazoles). Extending the light absorption of PIs into the visible or near-infrared range has become essential with the increasing use of LED light sources and deep-curing technology. As depicted in Figure 11, the widely adopted strategies included attaching chromophores (pyrrole, carbazole, triphenylamine, coumarin, and phenothiazine), constructing push–pull structures for expanded conjugation, and introducing auxochromes to extend triplet excited-state lifetimes. However, visible-light PIs introduce challenges related to coloration, which can hinder transparency and deep curing. The development of photobleachable PIs through the incorporation of double bond-containing structures or carbonyl bridges has emerged as a promising solution for reducing coloration under light exposure. Additionally, small-molecule migration issues have been addressed through polymerizable macromolecular PIs, embedding PIs into polymer networks, copolymerizing them with monomers, or grafting them onto existing polymers. Functionalization with silanes has also improved PI solubility in silicon resins.
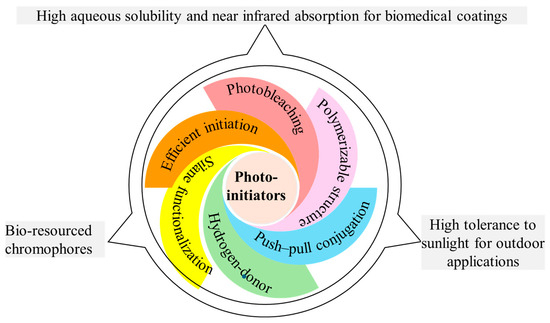
Figure 11.
Illustration of molecular engineering tactics for advanced PIs and proposed future research opportunities.
While significant progress has been made in advancing photoinitiation systems for functional coating, a few challenges and opportunities remain for broader implementation (Figure 11). For outdoor applications, such as architectural coating or oil pipe repairs, PIs must demonstrate high tolerance to sunlight for better storage and transport. Small-molecule fragments generated by photocleavable Type I and multi-component Type II systems raise concerns regarding migration, toxicity, and yellowing. It necessitates alternative approaches like macrocyclic PIs that produce linear oligomer radicals instead of small molecular products. Moreover, bio-based chromophores derived from renewable resources provide a sustainable and non-toxic alternative for long-term circular photochemistry. Furthermore, traditional organic solvents pose biotoxicity risks as their photodegradation byproducts denature proteins and compromise bioactivity, hindering biomaterial coating development. Strengthening biomedical relevance requires greater attention to solvent biocompatibility in molecularly engineered photoinitiating systems. Future research should prioritize aqueous-compatible or solvent-free PIs (such as ionic liquids and inorganic nanoparticles) to eliminate volatile organic compounds in biomedical coatings. Biomedical photopolymerization requires addressing cytotoxicity from short-wavelength light sources. Ultraviolet or blue light radiation might cause photochemical damage, such as DNA lesions, protein denaturation, and disrupted cellular processes. This constraint critically limits suitable PIs, demanding long-wavelength alternatives (>550 nm) with high cytocompatibility. By maintaining biocompatibility, more Type II chromophores (such as camphorquinone and curcumin derivatives) can be engineered to extend light absorption to red light or even near infrared regions. Achieving industrial scalability of PIs will require balancing high initiation efficiency with energy budgets. By uniting molecular design with system-level innovation, the future of coating technologies could be applied to diverse areas.
Author Contributions
Conceptualization, L.C. and X.B.; writing—original draft preparation, L.C. and X.D.; writing—review and editing, X.D., Y.W. and X.B.; visualization, L.C.; supervision, X.D. and X.B.; funding acquisition, X.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Zhejiang Provincial Natural Science Foundation of China (LQ23B030006).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
During the preparation of this manuscript/study, the authors used ChatGPT 2.0 for polishing. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest. Lijun Cao hereby discloses his conflict of interest as outlined by the MDPI guidelines. Lijun Cao is currently employed by Suzhou OMAY Optical Materials Co., Ltd, and was employed by Suzhou OMAY Optical Materials Co., Ltd. while contributing to this manuscript. Lijun Cao’s contributions to this work and manuscript were made independently without any requirement, guidance, or input from the employer. Lijun Cao received no financial compensation from any source for the contribution he made to this scientific work and manuscript.
Abbreviations
The following abbreviations are used in this manuscript:
| PI | Photoinitiator |
| PS | Photosensitizer |
| FRP | Free radical polymerization |
| CP | Cationic polymerization |
| UV | Ultraviolet |
| LED | Light-emitting diode |
| OXE | Oxime ester |
| APO | Acylphosphine oxide |
| TPO | 2,4,6-trimethylbenzoyldiphenylphosphine oxide |
| ONI | Onium |
| BK | Benzylidene ketone |
| BODIPY | Boron-dipyrromethene |
| C4 | 2,4-bis [4-(diethylamino)-benzylidene]-cyclopentanone |
References
- Petko, F.; Galek, M.; Hola, E.; Popielarz, R.; Ortyl, J. One-component cationic photoinitiators from tunable benzylidene scaffolds for 3D printing applications. Macromolecules 2021, 54, 7070–7087. [Google Scholar] [CrossRef]
- Hartlieb, M. Photo-iniferter RAFT polymerization. Macromol. Rapid Commun. 2022, 43, 2100514. [Google Scholar] [CrossRef]
- Lee, K.; Corrigan, N.; Boyer, C. Rapid high-resolution 3D printing and surface functionalization via type I photoinitiated RAFT polymerization. Angew. Chem. Int. Ed. 2021, 60, 8839–8850. [Google Scholar] [CrossRef]
- Du, Y.; Jia, S.; Chen, Y.; Zhang, L.; Tan, J. Type I photoinitiator-functionalized block copolymer nanoparticles prepared by RAFT-mediated polymerization-induced self-assembly. ACS Macro Lett. 2021, 10, 297–306. [Google Scholar] [CrossRef]
- Li, L.; Wan, M.; Li, Z.; Luo, Y.; Wu, S.; Liu, X.; Yagci, Y. Coumarinacyl anilinium salt: A versatile visible and NIR photoinitiator for cationic and step-growth polymerizations. ACS Macro Lett. 2023, 12, 263–268. [Google Scholar] [CrossRef]
- Kocaarslan, A.; Kaya, K.; Jockusch, S.; Yagci, Y. Phenacyl bromide as a single-component photoinitiator: Photoinduced step-growth polymerization of N-methylpyrrole and N-methylindole. Angew. Chem. Int. Ed. 2022, 61, e202208845. [Google Scholar] [CrossRef]
- Hughes, R.W.; Lott, M.E.; Olson, S.R.A.; Sumerlin, B.S. Photoiniferter polymerization: Illuminating the history; ascendency, and renaissance. Prog. Polym. Sci. 2024, 156, 101871. [Google Scholar] [CrossRef]
- Wu, Z.; Boyer, C. Near-infrared light-induced reversible deactivation radical polymerization: Expanding frontiers in photopolymerization. Adv. Sci. 2023, 10, 2304942. [Google Scholar] [CrossRef]
- Luo, X.; Zhao, S.; Chen, Y.; Zhang, L.; Tan, J. Switching between thermal initiation and photoinitiation redirects RAFT-mediated polymerization-induced self-assembly. Macromolecules 2021, 54, 2948–2959. [Google Scholar] [CrossRef]
- Zakrzewski; Ryu, C.Y.; Bae, C.; Picu, C.R. Photopolymerization-induced phase separation kinetics explored by intermittent irradiation. Polymer 2024, 290, 126526. [Google Scholar] [CrossRef]
- Hu, P.; Xu, H.; Pan, Y.; Sang, X.; Liu, R. Upconversion particle-assisted NIR polymerization enables microdomain gradient photopolymerization at inter-particulate length scale. Nat. Commun. 2023, 14, 3653. [Google Scholar] [CrossRef]
- Chandra, R.; Soni, R.K. Studies on kinetics of bulk polymerization of divinyl ester by radical-initiated thermal and photo-polymerization. Polym. Int. 1993, 31, 239–245. [Google Scholar] [CrossRef]
- Andrzejewska, E.; Lindén, L.-Å.; Rabek, J.F. Modelling the kinetics of photoinitiated polymerization of di (meth) acrylates. Polym. Int. 1997, 42, 179–187. [Google Scholar] [CrossRef]
- Lecamp, L.; Youssef, B.; Bunel, C.; Lebaudy, P. Photoinitiated polymerization of a dimethacrylate oligomer: 2. Kinetic studies. Polymer 1999, 40, 1403–1409. [Google Scholar] [CrossRef]
- Morancho, J.M.; Cadenato, A.; Fernández-Francos, X.; Salla, J.M.; Ramis, X. Isothermal kinetics of photopolymerization and thermal polymerization of bis-GMA/TEGDMA resins. J. Therm. Anal. Calorim. 2008, 92, 513–522. [Google Scholar] [CrossRef]
- Kamachi, M.; Kajiwara, A. Difference in propagation rate constants between photo-and thermal-initiated polymerization of styrene–effects of light irradiation. Macromol. Chem. Phys. 1997, 198, 787–795. [Google Scholar] [CrossRef]
- Perkowski, A.J.; You, W.; Nicewicz, D.A. Visible light photoinitiated metal-free living cationic polymerization of 4-methoxystyrene. J. Am. Chem. Soc. 2015, 137, 7580–7583. [Google Scholar] [CrossRef]
- Gardiner, J.; Hornung, C.H.; Tsanaktsidis, J.; Guthrie, D. Continuous flow photo-initiated RAFT polymerisation using a tubular photochemical reactor. Eur. Polym. J. 2016, 80, 200–207. [Google Scholar] [CrossRef]
- Shim, S.-H.; Ham, M.-K.; Huh, J.; Kwon, Y.-K.; Kwark, Y.-J. Simultaneous control over the molecular weight and tacticity of poly(vinyl acetate) using a low-temperature photoinitiated RAFT process in fluoroalcohols. Polym. Chem. 2013, 4, 5449–5455. [Google Scholar] [CrossRef]
- Allushi, A.; Jockusch, S.; Yilmaz, G.; Yagci, Y. Photoinitiated metal-free controlled/living radical polymerization using polynuclear aromatic hydrocarbons. Macromolecules 2016, 49, 7785–7792. [Google Scholar] [CrossRef]
- Su, X.; Zhao, Z.; Li, H.; Li, X.; Wu, P.; Han, Z. Stereocontrol during photo-initiated controlled/living radical polymerization of acrylamide in the presence of Lewis acids. Eur. Polym. J. 2008, 44, 1849–1856. [Google Scholar] [CrossRef]
- Zheng, J.; Lin, S.; Liu, J.; Yu, H.; Pan, Z.; Zheng, R.; Yi, H.; Wei, Y. Photochemical fabrication of responsive block copolymer nanoparticles: A self-assembly approach for temperature-sensitive applications. Chem. Pap. 2025, 79, 3895–3907. [Google Scholar] [CrossRef]
- Stevens, L.M.; Almada, N.T.; Kim, H.S.; Page, Z.A. Visible-light-fueled polymerizations for 3D printing. Accounts of Chemical Research. Acc. Chem. Res. 2025, 58, 250–260. [Google Scholar] [CrossRef]
- Liu, D.; Chen, Y.; Zhang, L.; Tan, J. Photoinitiated RAFT dispersion polymerization: A straightforward approach toward highly monodisperse functional microspheres. Macromolecules 2020, 53, 9725–9735. [Google Scholar] [CrossRef]
- Cao, L.; Jiang, Y.; Dai, X.; Zhang, X.; Peng, Y.; Liu, X. Using azo-compounds to endow biobased thermosetting coatings with potential application for reversible information storage. ACS Appl. Polym. Mater. 2020, 2, 4551–4558. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, Y.; Cao, L.; Jing, Y.; Xu, Z.; Tang, G.; Shu, Y.; Liu, X.; Zhang, X. Design and study of azobenzene-modified AESO photopolymerization coating for information reversible storage performance. J. Appl. Polym. Sci. 2025, 142, e56550. [Google Scholar] [CrossRef]
- Giannopoulos, I.; Mochi, I.; Vockenhuber, M.; Ekinci, Y.; Kazazis, D. Extreme ultraviolet lithography reaches 5 nm resolution. Nanoscale 2024, 16, 15533–15543. [Google Scholar] [CrossRef]
- Ding, C.; Liu, X.; Liu, Q.; Zhu, D.; Luo, M.; Gao, X.; Yang, Z.; Sun, Q.; Qian, Q.; Shen, X.; et al. Subdiffraction 3D nanolithography by two-Photon two-step absorption and photoinhibition. Photonics Rev. 2024, 18, 2300645. [Google Scholar] [CrossRef]
- Won, S.; Kim, T.; Choi, S.; Kim, B.; Kim, S.; Lee, J.; Kim, Y. Ultraviolet beam pattern control in second and third harmonic generation by spatial wavefront modulation of near-infrared driving wavelength. Nonlinear Freq. Gener. Convers. Mater. Devices XXIII 2024, 12869, 133–136. [Google Scholar]
- Garra, P.; Fouassier, J.P.; Lakhdar, S.; Yagci, Y.; Lalevée, J. Visible light photoinitiating systems by charge transfer complexes: Photochemistry without dyes. Prog. Polym. Sci. 2020, 107, 101277. [Google Scholar] [CrossRef]
- Crivello, J.V.; Sangermano, M. Visible and long-wavelength photoinitiated cationic polymerization. J. Polym. Sci. Part A 2001, 39, 343–356. [Google Scholar] [CrossRef]
- Noirbent, G.; Dumur, F. Photoinitiators of polymerization with reduced environmental impact: Nature as an unlimited and renewable source of dyes. Eur. Polym. J. 2021, 142, 110109. [Google Scholar] [CrossRef]
- Hola, E.; Morlet-Savary, F.; Lalevée, J.; Ortyl, J. Photoinitiator or photosensitizer? Dual behaviour of m-terphenyls in photopolymerization processes. Eur. Polym. J. 2023, 189, 111971. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Lara, D.M.; Noirbent, G.; Dumur, F.; Toufaily, J.; Hamieh, T.; Bui, T.-T.; Goubard, F.; Graff, B.; Gigmes, D.; et al. Carbazole derivatives with thermally activated delayed fluorescence property as photoinitiators/photoredox catalysts for LED 3D printing technology. Macromolecules 2017, 50, 4913–4926. [Google Scholar] [CrossRef]
- Hammoud, F.; Hijazi, A.; Schmitt, M.; Dumur, F.; Lalevée, J. A review on recently proposed oxime ester photoinitiators. Eur. Polym. J. 2023, 188, 111901. [Google Scholar] [CrossRef]
- Niederst, L.; Allonas, X.; Morone, M.; van den Branden, S. Employing singlet-singlet energy transfer for boosting the reactivity of Type I photoinitiators in radical photopolymerization. Angew. Chem. Int. Ed. 2024, 63, e202412625. [Google Scholar] [CrossRef]
- Kanchana, D.; Carroll, J.A.; Giacoletto, N.; Gigmes, D.; Kim, J.; Unterreiner, A.N.; Mundsinger, K.; Tuten, B.T.; Barner-Kowollik, C. Wavelength-resolved oxime ester photoinitiator decay in radical polymerization. Macromolecules 2024, 57, 9779–9787. [Google Scholar] [CrossRef]
- Ju, X.; Hu, X.; Gao, Y.; Nie, J.; Sun, F. Two hydrogen donor-containing naphthalimide benzyl thioether photoinitiators for LED photopolymerization. Prog. Org. Coat. 2022, 162, 106562. [Google Scholar] [CrossRef]
- Balcerak, A.; Kwiatkowska, D.; Kabatc, J. Novel photoinitiators based on difluoroborate complexes of squaraine dyes for radical polymerization of acrylates upon visible light. Polym. Chem. 2022, 13, 220–234. [Google Scholar] [CrossRef]
- Moreno, V.F.; Barboza, B.H.; Martins, L.M.; Gaglieri, C.; Bannach, G.; Batagin-Neto, A.; da Silva-Filho, L.C. Novel quinoline photoinitiators for dimethacrylate monomer photopolymerization under UV and blue light. Eur. Polym. J. 2024, 218, 113331. [Google Scholar] [CrossRef]
- Bao, B.; You, J.; Li, D.; Zhan, H.; Zhang, L.; Li, M.; Wang, T. Double benzylidene ketones as photoinitiators for visible light photopolymerization. J. Photochem. Photobiol. A Chem. 2022, 429, 113938. [Google Scholar] [CrossRef]
- Topa-Skwarczyńska, M.; Galek, M.; Jankowska, M.; Morlet-Savary, F.; Graff, B.; Lalevée, J.; Popielarz, R.; Ortyl, J. Development of the first panchromatic BODIPY-based one-component iodonium salts for initiating the photopolymerization processes. Polym. Chem. 2021, 12, 6873–6893. [Google Scholar] [CrossRef]
- Petko, F.; Galek, M.; Hola, E.; Topa-Skwarczyn, M.; Tomal, W.; Jankowska, M.; Pilch, M.; Popielarz, R.; Graff, B.; Morlet-Savary, F. Symmetric iodonium salts based on benzylidene as one-component photoinitiators for applications in 3D printing. Chem. Mater. 2022, 34, 10077–10092. [Google Scholar] [CrossRef]
- Dumur, F. The future of visible light photoinitiators of polymerization for photocrosslinking applications. Eur. Polym. J. 2023, 187, 111883. [Google Scholar] [CrossRef]
- Dumur, F. Recent advances on Anthraquinone-based photoinitiators of polymerization. Eur. Polym. J. 2023, 191, 112039. [Google Scholar] [CrossRef]
- Guo, X.; Mao, H.; Bao, C.; Wan, D.; Jin, M. Fused carbazole–coumarin–ketone dyes: High performance and photobleachable photoinitiators in free radical photopolymerization for deep photocuring under visible LED light irradiation. Polym. Chem. 2022, 13, 3367–3376. [Google Scholar] [CrossRef]
- Liao, W.; Liao, Q.; Xiong, Y.; Li, Z.; Tang, H. Design, synthesis and properties of carbazole-indenedione based photobleachable photoinitiators for photopolymerization. J. Photochem. Photobiol. A Chem. 2023, 435, 114297. [Google Scholar] [CrossRef]
- You, J.; Cao, D.; Hu, T.; Ye, Y.; Jia, X.; Li, H.; Hu, X.; Dong, Y.; Ma, Y.; Wang, T. Novel Norrish type I flavonoid photoinitiator for safe LED light with high activity and low toxicity by inhibiting the ESIPT process. Dye. Pigment. 2021, 184, 108865. [Google Scholar] [CrossRef]
- Chen, H.; Zhu, D.; Kavalli, T.; Xiao, P.; Schmitt, M.; Lalevée, J. Photopolymerization using bio-sourced photoinitiators. Polym. Chem. 2023, 14, 3543–3568. [Google Scholar] [CrossRef]
- Li, J.; Wu, X.; Chen, Y.; Deng, J.; Yuan, H.; Lian, H.; Xia, C. Capsanthin-iodonium salt system for free radical photopolymerization under LED irradiation. Dye. Pigment. 2024, 230, 112347. [Google Scholar] [CrossRef]
- Li, M.; Du, Y.; Zhan, H.; Zhang, L.; Wu, H.; Zhao, D.; Wang, Q.; Wang, T. Synthesis and photochemistry of flavonol camphorsulfonates photoinitiator with different substituents. Prog. Org. Coat. 2023, 183, 107810. [Google Scholar] [CrossRef]
- Li, J.; Ding, Q.; Cao, K.; Chen, Y.; Fu, Z.; Nie, J. Pyrrolidone based one-component photoinitiator for improving the storage stability of photocurable materials to sunlight via two beams of light excitation. Eur. Polym. J. 2023, 196, 112291. [Google Scholar] [CrossRef]
- Nakayama, A.; Kumamoto, Y.; Minoshima, M.; Kikuchi, K.; Taguchi, A.; Fujita, K. Photoinitiator-free two-photon polymerization of biocompatible materials for 3D micro/nanofabrication. Adv. Opt. Mater. 2022, 10, 2200474. [Google Scholar] [CrossRef]
- Li, R.; Guo, H.; Luo, X.; Wang, Q.; Pang, Y.; Li, S.; Liu, S.; Li, J.; Strehmel, B.; Chen, Z. Type I photoinitiator based on sustainable carbon dots. Angew. Chem. Int. Ed. 2024, 63, e202404454. [Google Scholar] [CrossRef]
- Luo, X.; Zhai, Y.; Wang, P.; Tian, B.; Liu, S.; Li, J.; Yang, C.; Strehmel, V.; Li, S.; Matyjaszewski, K.; et al. light-mediated polymerization catalyzed by carbon nanomaterials. Angew. Chem. Int. Ed. 2024, 63, e202316431. [Google Scholar] [CrossRef] [PubMed]
- Riad, K.B.; Kholghy, M.R.; Wood-Adams, P.M. Photo-polymerization using quantum dots for stable epoxy coatings. Ind. Chem. Mater. 2024, 2, 644–650. [Google Scholar] [CrossRef]
- Demina, P.A.; Khaydukov, K.V.; Sochilina, A.V.; Rocheva, V.V.; Ivanov, A.V.; Akasov, R.A.; Lin, Q.; Generalova, A.N.; Khaydukov, E.V. Role of energy transfer in a nanoinitiator complex for upconversion-driven polymerization. Mater. Today Adv. 2023, 19, 100388. [Google Scholar] [CrossRef]
- Zhu, Y.; Egap, E. Light-mediated polymerization induced by semiconducting nanomaterials: State-of-the-art and future perspectives. ACS Polym. Au 2021, 1, 76–99. [Google Scholar] [CrossRef] [PubMed]
- Breloy, L.; Alcay, Y.; Yilmaz, I.; Breza, M.; Bourgon, J.; Brezová, V.; Yagci, Y.; Versace, D.-L. Dimethyl amino phenyl substituted silver phthalocyanine as a UV- and visible-light absorbing photoinitiator: In situ preparation of silver/polymer nanocomposites. Polym. Chem. 2021, 12, 1273–1285. [Google Scholar] [CrossRef]
- Yi, B.; Ai, L.; Hou, C.; Lv, D.; Cao, C.; Yao, X. Liquid metal nanoparticles as a highly efficient photoinitiator to develop multifunctional hydrogel composites. ACS Appl. Mater. Interfaces 2022, 14, 29315–29323. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Y.; Launay, V.; Le Dot, M.; Liu, S.; Lalevée, J. How to overcome the light penetration issue in photopolymerization? An example for the preparation of high content iron-containing opaque composites and application in 3D printing. Eur. Polym. J. 2022, 165, 111011. [Google Scholar] [CrossRef]
- Li, B.; Lalevée, J.; Mazur, L.M.; Matczyszyn, K.; Ravaine, S.; Jradi, S. Copper complex-based photoinitiator for high resolution two-photon polymerization. Addit. Manuf. 2023, 75, 103741. [Google Scholar]
- Ma, G.; Qu, J. Synthesis and properties of acyldiphenylphosphine oxides photoinitiators carrying ferrocene group for free radical photopolymerization. Mater. Today Commun. 2023, 37, 107173. [Google Scholar] [CrossRef]
- Dumur, F. Recent advances on ferrocene-based photoinitiating systems. Eur. Polym. J. 2021, 147, 110328. [Google Scholar] [CrossRef]
- Schoumacker, M.; Subervie, D.; Dugas, P.-Y.; Lalevée, J.; Montarnal, D.; Lansalot, M.; Lacôte, E.; Bourgeat-Lami, E. Cerium oxide-armored composite latex particles by visible light emulsion photopolymerization: From synthesis to film properties. Adv. Funct. Mater. 2025, 35, 2407914. [Google Scholar]
- Pinkas, A.; Waiskopf, N.; Gigi, S.; Naor, T.; Layani, A.; Banin, U. Morphology effect on zinc oxide quantum photoinitiators for radical polymerization. Nanoscale 2021, 13, 7152–7160. [Google Scholar] [CrossRef]
- Tomal, W.; Świergosz, T.; Pilch, M.; Kasprzyk, W.; Ortyl, J. New horizons for carbon dots: Quantum nano-photoinitiating catalysts for cationic photopolymerization and three-dimensional (3D) printing under visible light. Polym. Chem. 2021, 12, 3661–3676. [Google Scholar]
- Liu, S.; Giacoletto, N.; Graff, B.; Morlet-Savary, F.; Nechab, M.; Xiao, P.; Dumur, F.; Lalevée, J. N-naphthalimide ester derivatives as Type Ⅰ photoinitiators for LED photopolymerization. Mater. Today Chem. 2022, 26, 101137. [Google Scholar] [CrossRef]
- Borjigin, T.; Feng, J.; Giacoletto, N.; Schmitt, M.; Morlet-Savary, F.; Graff, B.; Xiao, P.; Nechab, M.; Gigmes, D.; Dumur, F.; et al. Naphthoquinone-imidazolyl derivatives-based oxime esters as photoinitiators for blue LED-induced free radical photopolymerization. Eur. Polym. J. 2024, 206, 112801. [Google Scholar] [CrossRef]
- Liu, Z.; Song, B.; Zhang, Y.; Dietlin, C.; Morlet-Savary, F.; Schmitt, M.; Gigmes, D.; Dumur, F.; Lalevée, J. Phenothiazine-carbazole-based bis oxime esters (PCBOEs) for visible light polymerization. Eur. Polym. J. 2024, 219, 113381. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, Y.; Graff, B.; Dietlin, C.; Schmitt, M.; Morlet-Savary, F.; Petithory, T.; Pieuchot, L.; Zhang, J.; Xu, Y.; et al. 2,4,6-Trimethylbenzoyldiphenylphosphine oxide (TPO) analog: A non-cytotoxic type-I photoinitiator for free radical photopolymerization. Green Chem. 2025, 27, 1451–1461. [Google Scholar] [CrossRef]
- Gao, T.; Zhang, Y.; Morlet-Savary, F.; Graff, B.; Zhang, J.; Xiao, P.; Dumur, F.; Lalevée, J. Novel high-performance glyoxylate derivative-based photoinitiators for free radical photopolymerization and 3D printing with visible LED. Small 2024, 20, 2400234. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Qu, J. Synthesis and properties of novel bis-chalcone-based photoinitiators for LED polymerization with photobleaching and low migration. Prog. Org. Coat. 2023, 174, 107240. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Sun, K.; Graff, B.; Xiao, P.; Dumur, F.; Lalevée, J. Design of photoinitiating systems based on the chalcone-anthracene scaffold for LED cationic photopolymerization and application in 3D printing. Eur. Polym. J. 2021, 147, 110300. [Google Scholar] [CrossRef]
- Elian, C.; Sanosa, N.; Bogliotti, N.; Herrero, C.; Sampedro, D.; Versace, D.-L. An anthraquinone-based oxime ester as a visible-light photoinitiator for 3D photoprinting applications. Polym. Chem. 2023, 14, 3262–3269. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, B.; Liu, Z.; Noon, A.; Dietlin, C.; Morlet-Savary, F.; Schmitt, M.; Gigmes, D.; Dumur, F.; Lalevée, J. High photoinitiating efficiency of benzothioxanthene-based oxime esters in photopolymerization via photocleavage and/or single electron transfer under visible light and sunlight. Angew. Chem. Int. Ed. 2024, 63, e202405337. [Google Scholar] [CrossRef]
- Chi, T.; Somers, P.; Wilcox, D.A.; Schuman, A.J.; Johnson, J.E.; Liang, Z.; Pan, L.; Xu, X.; Boudouris, B.W. Substituted thioxanthone-based photoinitiators for efficient two-photon direct laser writing polymerization with two-color resolution. ACS Appl. Polym. Mater. 2021, 3, 1426–1435. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, R.; Liu, D.; Peng, C.; Wang, J.; Dong, X. New functionalized thioxanthone derivatives as type I photoinitiators for polymerization under UV-Vis LEDs. New J. Chem. 2023, 47, 5330–5337. [Google Scholar] [CrossRef]
- Ma, G.; Qu, J. Triphenylamine based oxime ester photoinitiator for LED-induced free radical photopolymerization and 3D printing. Eur. Polym. J. 2024, 215, 113254. [Google Scholar] [CrossRef]
- Hammoud, F.; Lee, Z.-H.; Graff, B.; Hijazi, A.; Lalevée, J.; Chen, Y.-C. Novel phenylamine-based oxime ester photoinitiators for LED-induced free radical, cationic, and hybrid polymerization. J. Polym. Sci. 2021, 59, 1711–1723. [Google Scholar] [CrossRef]
- Lee, Z.-H.; Hammoud, F.; Hijazi, A.; Graff, B.; Lalevée, J.; Chen, Y.-C. Synthesis and free radical photopolymerization of triphenylamine-based oxime ester photoinitiators. Polym. Chem. 2021, 12, 1286–1297. [Google Scholar] [CrossRef]
- Wu, Y.-H.; Graff, B.; Lalevée, J.; Chen, Y.-C. Visible light-induced free radical polymerization utilizing halogen-containing triphenylamine oxime esters as Type I photoinitiators. Eur. Polym. J. 2024, 217, 113300. [Google Scholar] [CrossRef]
- Lee, Z.-H.; Huang, T.-L.; Hammoud, F.; Chen, C.-C.; Hijazi, A.; Graff, B.; Lalevée, J.; Chen, Y.-C. Effect of the steric hindrance and branched substituents on visible phenylamine oxime ester photoinitiators: Photopolymerization kinetics investigation through photo-DSC experiments. Photochem. Photobiol. 2022, 98, 773–782. [Google Scholar] [CrossRef]
- Hsieh, J.-B.; Yen, S.-C.; Hammoud, F.; Lalevée, J.; Chen, Y.-C. Effect of terminal alkyl chains for free radical photopolymerization based on triphenylamine oxime ester visible-light absorbing Type I photoinitiators. ChemistrySelect 2023, 8, e202301297. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, Y.; Zhu, D.; Morlet-Savary, F.; Schmitt, M.; Graff, B.; Xiao, P.; Dumur, F.; Lalevée, J. Study on the photoinitiation mechanism of carbazole-based oxime esters (OXEs) as novel photoinitiators for free radical photopolymerization under near UV/visible-light irradiation exposure and the application of 3D printing. Eur. Polym. J. 2024, 202, 112662. [Google Scholar] [CrossRef]
- Liu, S.; Graff, B.; Xiao, P.; Dumur, F.; Lalevée, J. Nitro-carbazole based oxime esters as dual photo/thermal initiators for 3D printing and composite preparation. Macromol. Rapid Commun. 2021, 42, 2100207. [Google Scholar] [CrossRef]
- Dumur, F. Recent advances on carbazole-based oxime esters as photoinitiators of polymerization. Eur. Polym. J. 2022, 175, 111330. [Google Scholar] [CrossRef]
- Zhou, R.; Pan, H.; Wan, D.; Malval, J.-P.; Jin, M. Bicarbazole-based oxime esters as novel efficient photoinitiators for photopolymerization under UV-Vis LEDs. Prog. Org. Coat. 2021, 157, 106306. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, T.; Zhang, Y.; Dietlin, C.; Morlet-Savary, F.; Schmitt, M.; Gigmes, D.; Dumur, F.; Lalevée, J. Carbazole-fused coumarin bis oxime esters (CCOBOEs) as efficient photoinitiatorsof polymerization for 3D printing with 405 nm LED. Eur. Polym. J. 2024, 215, 113186. [Google Scholar] [CrossRef]
- Wu, X.; Gong, S.; Chen, Z.; Hou, J.; Liao, Q.; Xiong, Y.; Li, Z.; Tang, H. Photobleachable bis-chalcones-based oxime ester dyes for radical visible photopolymerization. Dye. Pigment. 2022, 205, 110556. [Google Scholar] [CrossRef]
- Chen, S.; Qin, C.; Jin, M.; Pan, H.; Wan, D. Novel chalcone derivatives with large conjugation structures as photosensitizers for versatile photopolymerization. J. Polym. Sci. 2021, 59, 578–593. [Google Scholar] [CrossRef]
- Gong, S.; Wu, X.; Liao, Q.; Deng, S.; Hou, J.; Tang, K.; Xiong, Y.; Li, Z.; Tang, H. Novel multifunctional unimolecular initiators built on natural indole featuring fast photobleaching and visible light/thermal double polymerization. Green Chem. 2023, 25, 2730–2744. [Google Scholar] [CrossRef]
- Wang, W.; Jin, M.; Pan, H.; Wan, D. Phenylthioether thiophene-based oxime esters as novel photoinitiators for free radical photopolymerization under LED irradiation wavelength exposure. Prog. Org. Coat. 2021, 151, 106019. [Google Scholar] [CrossRef]
- Wang, W.; Jin, M.; Pan, H.; Wan, D. Remote effect of substituents on the properties of phenyl thienyl thioether-based oxime esters as LED-sensitive photoinitiators. Dye. Pigment. 2021, 192, 109435. [Google Scholar] [CrossRef]
- Zhang, Y.; Morlet-Savary, F.; Schmitt, M.; Graff, B.; Rico, A.; Ibrahim-Ouali, M.; Dumur, F.; Lalevée, J. Photoinitiation behavior of phenothiazines containing two oxime ester functionalities (OXEs) in free radical photopolymerization and 3D printing application. Dye. Pigment. 2023, 215, 111202. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, X.; Zhu, D.; Zhang, Y.; Le Dot, M.; Ozen, S.; Schmitt, M.; Morlet-Savary, F.; Xiao, P.; Dumur, F.; et al. Preparation of nitro-phenothiazine-based oxime esters as dual photo/thermal initiators for 3D printing. J. Polym. Sci. 2024, 62, 2597–2604. [Google Scholar] [CrossRef]
- Hammoud, F.; Giacoletto, N.; Noirbent, G.; Graff, B.; Hijazi, A.; Nechab, M.; Gigmes, D.; Dumur, F.; Lalevée, J. Substituent effects on the photoinitiation ability of coumarin-based oxime-ester photoinitiators for free radical photopolymerization. Mater. Chem. Front. 2021, 5, 8361–8370. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, M.; Ma, T.; Yan, S.; Li, J.; Jiang, X. Chalcone-derived oxime esters with efficient photoinitiation properties under LED irradiation. Chin. Chem. Lett. 2025, 36, 110427. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Borjigin, T.; Graff, B.; Morlet-Savary, F.; Schmitt, M.; Gigmes, D.; Dumur, F.; Lalevée, J. Carbazole-fused coumarin based oxime esters (OXEs): Efficient photoinitiators for sunlight driven free radical photopolymerization. Green Chem. 2023, 25, 6881–6891. [Google Scholar] [CrossRef]
- Liao, W.; Hou, J.; Fan, X.; Zhu, Q.; Jin, M. New ketoxime ester-amine photoinitiator based on charge transfer complex for free radical LED polymerization. Eur. Polym. J. 2024, 209, 112909. [Google Scholar] [CrossRef]
- Liao, W.; Jin, M. New-generation ketoxime ester-type photoinitiator model molecules: Improving photoactivity by introducing o-allyloxy group. Prog. Org. Coat. 2024, 189, 108290. [Google Scholar] [CrossRef]
- Sandmeier, M.; Paunović, N.; Conti, R.; Hofmann, L.; Wang, J.; Luo, Z.; Masania, K.; Wu, N.; Kleger, N.; Coulter, F.B.; et al. Solvent-Free Three-Dimensional Printing of Biodegradable Elastomers Using Liquid Macrophotoinitiators. Macromolecules 2021, 54, 7830–7839. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, Y.; Wang, Q.; Zhang, Y.; Zhang, X.; Du, Y.; Wang, T. Acylphosphine oxide photoinitiators containing Si with sulfhydryl bridge linkages. Prog. Org. Coat. 2024, 187, 108110. [Google Scholar] [CrossRef]
- Ma, G.; Luo, J.; Qu, J. Long-wavelength absorbing photoinitiators based phenothiazine acyldiphenylphosphine oxides for free radical photopolymerization. Prog. Org. Coat. 2024, 189, 108296. [Google Scholar] [CrossRef]
- Wu, Y.; Li, R.; Wang, J.; Situ, Y.; Huang, H. A new carbazolyl-based acylphosphine oxide photoinitiator with high performance and low migration. J. Polym. Sci. 2022, 60, 52–61. [Google Scholar] [CrossRef]
- Wu, Y.; Li, R.; Ke, J.; Cheng, X.; Tang, R.; Situ, Y.; Huang, H. Study on bifunctional acyldiphenylphosphine oxides photoinitiator for free radical polymerization. Eur. Polym. J. 2022, 168, 111093. [Google Scholar] [CrossRef]
- Wu, Y.; Li, R.; Huang, C.; Wu, J.; Sun, X.; Situ, Y.; Huang, H. New acyl phosphine oxides as high-performance and low migration type I photoinitiators of radical polymerization. Prog. Org. Coat. 2022, 168, 106876. [Google Scholar] [CrossRef]
- Dietlin, C.; Trinh, T.T.; Schweizer, S.; Graff, B.; Morlet-Savary, F.; Noirot, P.-A.; Lalevée, J. Rational design of acyldiphenylphosphine oxides as photoinitiators of radical polymerization. Macromolecules 2019, 52, 7886–7893. [Google Scholar] [CrossRef]
- Wu, Y.; Ke, J.; Dai, C.; Wang, J.; Huang, C.; Situ, Y.; Huang, H. Large-molecular-weight acyldiphenylphosphine oxides as low-mobility type I photoinitiator for radical polymerization. Eur. Polym. J. 2022, 175, 111380. [Google Scholar] [CrossRef]
- Kati, M.; Cakir, Y.B.; Kaya, K.; Kiliclar, H.C.; Kiskan, B. Phenacyl bromide as Norrish type I photoinitiator for the facile synthesis of chain-end functional PMMA and polystyrene. Front. Mater. 2024, 11, 1490070. [Google Scholar] [CrossRef]
- Wan, M.; Liu, X.; Yagci, Y.; Li, Z. Coumarinacyl bromides as unimolecular photoinitiators for conjugated polymer synthesis under visible and NIR light irradiation. ChemPhotoChem 2024, 8, e202400196. [Google Scholar] [CrossRef]
- He, X.; Gao, Y.; Nie, J.; Sun, F. Methyl benzoylformate derivative Norrish Type I photoinitiators for deep-layer photocuring under near-UV or visible LED. Macromolecules 2021, 54, 3854–3864. [Google Scholar] [CrossRef]
- Sun, X.; He, X.; Yi, M.; Fan, S.; Xiang, B.; Yuan, B.; Zhu, J.; Luo, P.; Zou, Y.; Pang, Y. Coumarin based glyoxylate photoinitiators for free radical and cationic Photopolymerizations with UV-Visible LED irradiation. Eur. Polym. J. 2024, 211, 113025. [Google Scholar] [CrossRef]
- Wang, A.; Zhao, X.; Shi, Z.; Lv, C.; Li, P.; Yu, J.; Rao, H.; Chen, H.; Liu, H.; Li, W.; et al. Developing high-efficiency photoinitiation systems by Leveraging highly Matched Interplays between photosensitizer and photoinitiator for enhanced energy transfer. Eur. Polym. J. 2025, 223, 113669. [Google Scholar] [CrossRef]
- Zhu, Y.; Pi, J.; Zhang, Y.; Xu, D.; Yagci, Y.; Liu, R. A new anthraquinone derivative as a near UV and visible light photoinitiator for free-radical, thiol–ene and cationic polymerizations. Polym. Chem. 2021, 12, 3299–3306. [Google Scholar] [CrossRef]
- Breloy, L.; Mhanna, R.; Malval, J.-P.; Brezová, V.; Jacquemin, D.; Pascal, S.; Siri, O.; Versace, D.-L. Azacalixphyrins as an innovative alternative for the free-radical photopolymerization under visible and NIR irradiation without the need of co-initiators. Chem. Commun. 2021, 57, 8973–8976. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, L.; Zhang, Y.; Ou, Y.; Zhang, J.; Yagci, Y.; Liu, R. Broad wavelength sensitive coumarin sulfonium salts as photoinitiators for cationic, free radical and hybrid photopolymerizations. Prog. Org. Coat. 2023, 174, 107272. [Google Scholar] [CrossRef]
- Calvez, I.; Szczepanski, C.R.; Landry, V. Hybrid free-radical/cationic phase-separated UV-curable system: Impact of photoinitiator content and monomer fraction on surface morphologies and gloss appearance. Macromolecules 2022, 55, 3129–3139. [Google Scholar] [CrossRef]
- Li, S.; Ma, G.; Qu, J. Novel benzophenone derivatives photoinitiators based on carbazole group for deep curing and fast 3D printing. J. Photochem. Photobiol. A Chem. 2025, 460, 116144. [Google Scholar] [CrossRef]
- Lu, W.; Qu, J. Water-soluble bis-chalcone-based photoinitiators with long-wavelength absorption for radical polymerization and 3D printing. Polym. Chem. 2024, 15, 796–806. [Google Scholar] [CrossRef]
- Tang, L.; Nie, J.; Zhu, X. A high performance phenyl-free LED photoinitiator for cationic or hybrid photopolymerization and its application in LED cationic 3D printing. Polym. Chem. 2020, 11, 2855–2863. [Google Scholar] [CrossRef]
- Deng, L.; Tang, L.; Qu, J. Novel chalcone-based phenothiazine derivative photoinitiators for visible light induced photopolymerization with photobleaching and good biocompatibility. Prog. Org. Coat. 2022, 167, 106859. [Google Scholar] [CrossRef]
- Gao, Y.; Qu, J. New long-wavelength D–π-A–π-D chalcone photoinitiator for visible light polymerization with photobleaching and biocompatibility properties. Polym. Chem. 2023, 14, 952–962. [Google Scholar] [CrossRef]
- Li, Y.; Shaukat, U.; Schlögl, S.; Xue, T.; Li, J.; Nie, J.; Zhu, X. A pyrrole–carbazole photoinitiator for radical and cationic visible light LED photopolymerization. Eur. Polym. J. 2023, 182, 111700. [Google Scholar] [CrossRef]
- Li, S.; Ma, G.; Lu, W.; Qu, J. Novel di-2-thienyl ketone derivatives photoinitiators enable fast visible light polymerization and 3D printing. Prog. Org. Coat. 2025, 198, 108920. [Google Scholar] [CrossRef]
- Liu, S.; Brunel, D.; Noirbent, G.; Mau, A.; Chen, H.; Morlet-Savary, F.; Graff, B.; Gigmes, D.; Xiao, P.; Dumur, F.; et al. New multifunctional benzophenone-based photoinitiators with high migration stability and their applications in 3D printing. Mater. Chem. Front. 2021, 5, 1982–1994. [Google Scholar] [CrossRef]
- Jia, X.; Zhao, D.; You, J.; Hao, T.; Li, X.; Nie, J.; Wang, T. Acetylene bridged D-(π-A)2 type dyes containing benzophenone moieties: Photophysical properties, and the potential application as photoinitiators. Dye. Pigment. 2021, 184, 108583. [Google Scholar] [CrossRef]
- Hola, E.; Fiedor, P.; Dzienia, A.; Ortyl, J. Visible-Light Amine Thioxanthone Derivatives as Photoredox Catalysts for Photopolymerization Processes. ACS Appl. Polym. Mater. 2021, 3, 5547–5558. [Google Scholar] [CrossRef]
- Wu, Q.; Guo, J.; Song, K.; Xu, S.; Li, F.; Deng, M. Photoinitiation mechanism and ability of thioxanthone-based versatile visible photoinitiators. Macromol. Chem. Phys. 2022, 223, 2200242. [Google Scholar] [CrossRef]
- Rahal, M.; Mokbel, H.; Graff, B.; Pertici, V.; Gigmes, D.; Toufaily, J.; Hamieh, T.; Dumur, F.; Lalevée, J. Naphthalimide-based dyes as photoinitiators under visible light irradiation and their applications: Photocomposite synthesis, 3D printing and polymerization in water. ChemPhotoChem 2021, 5, 476–490. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, B.; Zhou, Q.; Yu, Y.; Hao, E.; Guo, X.; Xu, Y.; Yu, C.; Sun, K.; Lalevée, J. riphenylamine-substituted BODIPY dye based photoinitiators for free radical and cationic photopolymerization under visible LED irradiation and application in 3D printing. Dye. Pigment. 2024, 222, 111880. [Google Scholar] [CrossRef]
- Xu, Y.; Tang, W.; Deng, H.; Zhang, J.; Zhu, Z.; Yu, C.; Zhu, H.; Sun, K.; Li, J.; Lalevée, J. Bisbenzothieno[b]-fused BODIPYs in panchromatic photoinitiation for free radical and cationic photopolymerization and application in 3D printing. Polym. Chem. 2024, 15, 4875–4887. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, Y.; Zhang, Y.; Liu, S.; Zhang, X.; Wang, T. Silicone-modified flavonols for organosilicon photopolymerization. ACS Appl. Polym. Mater. 2024, 6, 9260–9271. [Google Scholar] [CrossRef]
- Xu, D.; Zou, X.; Zhu, Y.; Yu, Z.; Jin, M.; Liu, R. Phenothiazine-based charge-transfer complexes as visible-light photoinitiating systems for acrylate and thiol-ene photopolymerization. Prog. Org. Coat. 2022, 166, 106772. [Google Scholar] [CrossRef]
- Li, M.; Bao, B.; You, J.; Du, Y.; Li, D.; Zhan, H.; Zhang, L.; Wang, T. Phenothiazine derivatives based on double benzylidene ketone structure: Application to red light photopolymerization. Prog. Org. Coat. 2022, 173, 107217. [Google Scholar] [CrossRef]
- Fan, S.; Lai, P.; Pang, Y.; Zou, Y.; Wei, J. Design of oxime ester photoinitiators based on methoxy-substituted coumarin chromophores for LED photopolymerization and 3D printing. ACS Appl. Polym. Mater. 2024, 6, 12866–12874. [Google Scholar] [CrossRef]
- Rahal, M.; Graff, B.; Toufaily, J.; Hamieh, T.; Dumur, F.; Lalevée, J. Design of keto-coumarin based photoinitiator for Free Radical Photopolymerization: Towards 3D printing and photocomposites applications. Eur. Polym. J. 2021, 154, 110559. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Wan, D.; Jin, M. Effect of substituents on 1,3,5-triphenylpyrazoline as light-emitting diode-sensitive initiators in photopolymerization. Eur. Polym. J. 2023, 184, 111783. [Google Scholar] [CrossRef]
- Xue, T.; Tang, L.; Tang, R.; Li, Y.; Nie, J.; Zhu, X. Color evolution of a pyrrole-based enone dye in radical photopolymerization formulations. Dye. Pigment. 2021, 188, 109212. [Google Scholar] [CrossRef]
- Wen, Y.; Xiang, H.; Luo, X.; Ji, S.; Zhao, J.; Zhao, J.; Zhang, H.-L.; Chen, W.-C.; Huo, Y. Colorless phenanthroimidazole photoinitiators featuring tunable D-π-A configuration by frontier molecular orbital engineering. Dye. Pigment. 2022, 205, 110551. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Blue-to-red light sensitive push–pull structured pphotoinitiators: Indanedione derivatives for radical and cationic photopolymerization reactions. Macromolecules 2013, 46, 3332–3341. [Google Scholar] [CrossRef]
- Breloy, L.; Brezová, V.; Richeter, S.; Clément, S.; Malval, J.-P.; Andaloussi, S.A.; Versace, D.-L. Bio-based porphyrins pyropheophorbide a and its Zn-complex as visible-light photosensitizers for free-radical photopolymerization. Polym. Chem. 2022, 13, 1658–1671. [Google Scholar] [CrossRef]
- Liao, W.; Liao, Q.; Xu, C.; Wu, X.; Xiong, Y.; Li, Z.; Tang, H. Structural effects of cinnamoyl-indanone-based photobleachable free radical visible initiators. ACS Appl. Polym. Mater. 2022, 4, 6466–6476. [Google Scholar] [CrossRef]
- Petko, F.; Jankowska, M.; Galek, M.; Noworyta, M.; Popielarz, R.; Ortyl, J. One-Component Stilbene-Based Iodonium Photoinitiators with increased photoacid quantum yield for cationic Vat 3D printing. Macromolecules 2024, 57, 11639–11657. [Google Scholar] [CrossRef]
- Wang, Y.; Sheng, G.; Xie, J.; Wan, D.; Jin, M. Push–pull biphenyl–based iodonium salts: Highly sensitive one-component photoinitiators for photopolymerization under UV–visible LEDs. Prog. Org. Coat. 2024, 188, 108209. [Google Scholar] [CrossRef]
- Hammoud, F.; Hijazi, A.; Duval, S.; Lalevée, J.; Dumur, F. Dihydroindolo [3, 2-a] carbazole: A promising scaffold for the design of visible light photoinitiators of polymerization. Eur. Polym. J. 2022, 162, 110880. [Google Scholar] [CrossRef]
- Taschner, R.; Koch, T.; Wolff, R.; Stampfl, J.; Liska, R.; Knaack, P. Evaluation of sulfonium borate initiators for cationic photopolymerization and their application in hot lithography. ACS Appl. Polym. Mater. 2023, 5, 3023–3033. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, D.; Zhang, Y.; Zhou, Y.; Yagci, Y.; Liu, R. Phenacyl phenothiazinium salt as a new broad-wavelength-absorbing photoinitiator for cationic and free radical polymerizations. Angew. Chem. Int. Ed. 2021, 60, 16917–16921. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Gong, S.; Wu, X.; Huang, M.; Wei, T.; Xiong, Y.; Tang, H. Low-migration, deep photocuring thiobarbituric acid-based one-component visible photoinitiators for high-efficiency photopolymerization. Prog. Org. Coat. 2024, 194, 108567. [Google Scholar] [CrossRef]
- Liao, W.; Jin, M. Strategies to develop α-aminoketone derivatives photoinitiators with low migration ability for UV–vis LED photopolymerization. Eur. Polym. J. 2023, 187, 111899. [Google Scholar] [CrossRef]
- Xue, T.; Li, Y.; Li, X.; Huang, B.; Song, Q.; Nie, J.; Zhu, X. Benzylidene ketones as visible light radical photoinitiator: The effects of electron-donating group and co-initiator. J. Photochem. Photobiol. A Chem. 2021, 418, 113395. [Google Scholar] [CrossRef]
- Zheng, H.; Lu, H.; Li, J.; Nie, J.; Zhu, X. The photoinitiator of bifunctional α-hydroxy ketone with long-wavelength in photopolymerization under UV-LEDs. Prog. Org. Coat. 2023, 185, 107936. [Google Scholar] [CrossRef]
- Yao, M.; Liu, S.; Huang, C.; Nie, J.; He, Y. Significantly improve the photoinitiation ability of hydroxyalkyl-derived polymerizable α-hydroxyalkylacetophenone photoinitiators by blocking hyperconjugation. J. Photochem. Photobiol. A Chem. 2021, 419, 113451. [Google Scholar] [CrossRef]
- Li, J.; Deng, Q.; Xing, Z.; Yu, J.; Li, W.; Zhou, X.; Zhu, X.; Nie, J. Precision synthesis of diblock copolymers via free radical photopolymerization. Chem. Commun. 2024, 60, 11072–11075. [Google Scholar] [CrossRef]
- Lu, H.; Zhu, X. Synthesis of D-π-A-π-D photoinitiator with high-photoinitiation efficiency by restricting photoinduced isomerization process. J. Appl. Polym. Sci. 2022, 139, 51569. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, Y.; Li, M.-D.; Li, Z.A.; Peng, H.; Xie, T.; Xie, X. Efficient 3D printing via photooxidation of ketocoumarin based photopolymerization. Nat. Commun. 2021, 12, 2873. [Google Scholar] [CrossRef]
- Dumur, F. Recent advances on benzylidene ketones as photoinitiators of polymerization. Eur. Polym. J. 2022, 178, 111500. [Google Scholar] [CrossRef]
- Xu, Y.; Noirbent, G.; Brunel, D.; Ding, Z.; Gigmes, D.; Graff, B.; Xiao, P.; Dumur, F.; Lalevée, J. Allyloxy ketones as efficient photoinitiators with high migration stability in free radical polymerization and 3D printing. Dye. Pigment. 2021, 185, 108900. [Google Scholar] [CrossRef]
- Eren, T.N.; Kariksiz, N.; Demirci, G.; Tuncel, D.; Okte, N.; Acar, H.Y.; Avci, D. Irgacure 2959-functionalized poly(ethyleneimine)s as improved photoinitiators: Enhanced water solubility, migration stability and visible-light operation. Polym. Chem. 2021, 12, 2772–2785. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Chen, D.; Ma, Y.; Wang, Q.; Wang, J.; Yang, W. Synthesis and characterization of a novel kind of water-soluble macromolecular photoinitiators and their application for the preparation of water-soluble branched polymers. Ind. Eng. Chem. Res. 2021, 60, 7755–7763. [Google Scholar] [CrossRef]
- Xue, T.; Huang, B.; Li, Y.; Li, X.; Nie, J.; Zhu, X. Enone dyes as visible photoinitiator in radical polymerization: The influence of peripheral N-alkylated (hetero)aromatic amine group. J. Photochem. Photobiol. A Chem. 2021, 419, 113449. [Google Scholar] [CrossRef]
- Singh, A.K.; Bhasikuttan, A.C.; Palit, D.K.; Mittal, J.P. Excited-state dynamics and photophysical properties of para-aminobenzophenone. J. Phys. Chem. A 2000, 104, 7002–7009. [Google Scholar] [CrossRef]
- Xue, T.; Li, Y.; Si, Z.; Li, C.; Nie, J.; Zhu, X. Benzophenone based salicylaldimine and its boron complex as radical photoinitiator: A comparative study. J. Photochem. Photobiol. A Chem. 2022, 424, 113625. [Google Scholar] [CrossRef]
- Xue, T.; Li, Y.; Zhao, X.; Nie, J.; Zhu, X. A facile synthesized benzophenone Schiff-base ligand as efficient type II visible light photoinitiator. Prog. Org. Coat. 2021, 157, 106329. [Google Scholar] [CrossRef]
- Regehly, M.; Garmshausen, Y.; Reuter, M.; König, N.F.; Israel, E.; Kelly, D.P.; Chou, C.-Y.; Koch, K.; Asfari, B.; Hecht, S. Xolography for linear volumetric 3D printing. Nature 2020, 588, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.M.; Fernandes, J.R.; Coelho, P.J. Naphthopyrans as efficient dual color photoinitiators for volumetric 3D printing. Eur. Polym. J. 2023, 196, 112312. [Google Scholar] [CrossRef]
- Yang, F.; Zhong, M.; Zhao, X.; Li, J.; Sun, F.; Nie, J.; Zhu, X. High efficiency photoinitiators with extremely low concentration based on furans derivative. J. Photochem. Photobiol. A Chem. 2021, 406, 112994. [Google Scholar] [CrossRef]
- Li, J.; Deng, Q.; Zhou, X.; Yu, J.; Zhu, X.; Nie, J. Ketone constructed by alkyl bridge strategy for LED-sensitive free radical photopolymerization. Eur. Polym. J. 2025, 224, 113681. [Google Scholar] [CrossRef]
- Hu, T.; Fu, H.; Xiong, J.; Wang, T. Benzylidene piperidones as photosensitizers for visible light photopolymerization. J. Photochem. Photobiol. A Chem. 2021, 405, 112968. [Google Scholar] [CrossRef]
- Yen, S.-C.; Ni, J.-S.; Chen, Y.-C. Triphenylamine-functionalized chalcones as one-component type II visible-light-absorbing photoinitiators for free radical photopolymerization. Eur. Polym. J. 2023, 187, 111885. [Google Scholar] [CrossRef]
- Wang, S.-C.; Wu, Y.-H.; Hsieh, J.-B.; Ni, J.-S.; Chen, Y.-C. Triphenylamine based benzylidene ketones as visible-light-absorbing Type II photoinitiators for free radical photopolymerization. J. Photochem. Photobiol. A Chem. 2023, 443, 114870. [Google Scholar] [CrossRef]
- Ibrahim-Ouali, M.; Dumur, F. Recent advances on chalcone-based photoinitiators of polymerization. Eur. Polym. J. 2021, 158, 110688. [Google Scholar] [CrossRef]
- Chen, H.; Noirbent, G.; Sun, K.; Brunel, D.; Gigmes, D.; Morlet-Savary, F.; Zhang, Y.; Liu, S.; Xiao, P.; Dumur, F.; et al. Photoinitiators derived from natural product scaffolds: Monochalcones in three-component photoinitiating systems and their applications in 3D printing. Polym. Chem. 2020, 11, 4647–4659. [Google Scholar] [CrossRef]
- Li, J.; Zheng, H.; Lu, H.; Li, J.; Yao, L.; Wang, Y.; Zhou, X.; Nie, J.; Zhu, X.; Fu, Z. Study on pyrrole chalcone derivatives used for blue LED free radical photopolymerization: Controllable initiating activity achieved through photoisomerization property. Eur. Polym. J. 2022, 176, 111393. [Google Scholar] [CrossRef]
- Lu, W.; Ma, G.; Qu, J. Novel bis-chalcone-based carbazole derivative photoinitiators for visible light polymerization with good photobleaching and biocompatibility. Prog. Org. Coat. 2024, 187, 108102. [Google Scholar] [CrossRef]
- Li, J.; Deng, Q.; Zhou, X.; Li, W.; Zhu, X.; Nie, J. Enhancement in the initiating activity of chalcones via a long-alkyl chain strategy for free radical photopolymerization and 3D printing. Polym. Chem. 2024, 15, 2840–2848. [Google Scholar] [CrossRef]
- Li, J.; Nie, J.; Zhu, X. Hydrogen bond complex used as visible light photoinitiating system for free radical photopolymerization: Photobleaching, water solubility. Prog. Org. Coat. 2021, 151, 106099. [Google Scholar] [CrossRef]
- Li, J.; Lu, H.; Zheng, H.; Zhou, X.; Nie, J.; Zhu, X. Thermally activated pyrrole chalcone free radical photoinitiator with excellent stability to sunlight. Eur. Polym. J. 2022, 162, 110884. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, W.; Chen, X.; Lin, S.; Li, X.; He, L.; Liao, X.; Ye, G. A comparison of hydrogen abstraction reaction between allyl-type monomers with thioxanthone-based photoinitiators without amine synergists. Front. Chem. 2022, 10, 967836. [Google Scholar] [CrossRef]
- Dumur, F. Recent advances on crosslinkable thioxanthones for photopolymerization. Eur. Polym. J. 2024, 216, 113299. [Google Scholar] [CrossRef]
- Dumur, F. Recent advances on core-extended thioxanthones as efficient photoinitiators of polymerization. Eur. Polym. J. 2024, 206, 112794. [Google Scholar] [CrossRef]
- Dumur, F. Recent advances on naphthoquinone-based photoinitiators of polymerization. Eur. Polym. J. 2023, 193, 112120. [Google Scholar] [CrossRef]
- Pyszka, I.; Jędrzejewska, B. Photoinitiation abilities of indeno- and indoloquinoxaline derivatives and mechanical properties of dental fillings based on multifunctional acrylic monomers and glass ionomer. Polymer 2023, 266, 125625. [Google Scholar] [CrossRef]
- Dogruyol, S.K.; Dogruyol, Z.; Kazancioglu, E.O.; Gokcek, H.; Arsu, N. Thioxanthonation of 2-naphthalene-thioacetic acid as a visible-light photoinitiator: The investigation of photophysical and photochemical properties. Eur. Polym. J. 2023, 198, 112440. [Google Scholar] [CrossRef]
- Yang, S.; Chen, P.; Fu, H.; Li, Y.; Li, L.; Li, W.; Song, S.; Xu, R.; Pan, Q.; Xin, Y.; et al. Single-component thioxanthone-based photoinitiator for free-radical photopolymerization of acrylates via UV-LED irradiation. Dye. Pigment. 2025, 235, 112640. [Google Scholar] [CrossRef]
- Strzelczyk, R.; Podsiadły, R. Derivatives of,4-naphthoquinone as visible-light-absorbing one-component photoinitiators for radical polymerisation. Color. Technol. 2015, 131, 229–235. [Google Scholar] [CrossRef]
- Koyuncu, U.; Metin, E.; Ocal, N.; Arsu, N. Synthesis of one-component type II dithioxanthone-disulfide photoinitiator and investigation of photophysical and photochemical properties. Eur. Polym. J. 2021, 153, 110510. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, J.; Zhang, Y.; Chen, Y.; An, G.; Liu, R. Designing unimolecular photoinitiator by installing NHPI esters along the TX backbone for acrylate photopolymerization and their applications in coatings and 3D printing. Chin. Chem. Lett. 2024, 35, 109573. [Google Scholar] [CrossRef]
- Banerjee, P.; Kar, M.; Dinda, P.; Mandal, T.K. Ionic liquid-based unconventional photoinitiators for aqueous polymerization. Eur. Polym. J. 2022, 162, 110870. [Google Scholar] [CrossRef]
- Begantsova, Y.E.; Zvagelsky, R.; Baranov, E.V.; Chubich, D.A.; Chechet, Y.V.; Kolymagin, D.A.; Pisarenko, A.V.; Vitukhnovsky, A.G.; Chesnokov, S.A. Imidazole-containing photoinitiators for fabrication of sub-micron structures by 3D two-photon polymerization. Eur. Polym. J. 2021, 145, 110209. [Google Scholar] [CrossRef]
- Chen, S.; Pan, H.; Wan, D.; Jin, M. High-performance LED induces cationic photopolymerization using novel 1, 3, 5-triaryl-2-pyrazoline as photosensitizer. Prog. Org. Coat. 2021, 161, 106460. [Google Scholar] [CrossRef]
- Tu, W.-H.; Hsieh, J.-B.; Wu, Y.-H.; Graff, B.; Lalevée, J.; Chen, Y.-C. Influence of alkyl chain lengths and positions on visible-light absorbing triphenylamine oxime esters for free radical photopolymerization. Eur. Polym. J. 2025, 223, 113625. [Google Scholar] [CrossRef]
- Chung, K.-Y.; Page, Z.A. Boron-Methylated Dipyrromethene as a Green Light Activated Type I Photoinitiator for Rapid Radical Polymerizations. J. Am. Chem. Soc. 2023, 145, 17912–17918. [Google Scholar] [CrossRef]
- Zhou, J.; Pitzer, L.; Ley, C.; Rölle, T.; Allonas, X. A highly sensitive photoinitiating system based on pre-associated ion-pairs for NIR radical photopolymerization of optically clear materials. Polym. Chem. 2022, 13, 6475–6483. [Google Scholar] [CrossRef]
- Elian, C.; Mourot, B.; Benbouziyane, C.; Malval, J.-P.; Lajnef, S.; Peyrot, F.; Massuyeau, F.; Siri, O.; Jacquemin, D.; Pascal, S.; et al. Tris-benzo[cd]indole Cyanine Enables the NIR-photosensitized Radical and Thiol-ene Polymerizations at 940 nm. Angew. Chem. Int. Ed. 2023, 62, e202305963. [Google Scholar] [CrossRef]
- Song, Q.; Shang, K.; Xue, T.; Wang, Z.; Pei, D.; Zhao, S.; Nie, J.; Chang, Y. Macrocyclic photoinitiator based on prism [5]arene matching LEDs light with low mmigration. Macromol. Rapid Commun. 2021, 42, 2100299. [Google Scholar] [CrossRef] [PubMed]
- Bureš, F. Fundamental aspects of property tuning in push–pull molecules. RSC Adv. 2014, 4, 58826–58851. [Google Scholar] [CrossRef]
- Sun, K.; Xiao, P.; Dumur, F.; Lalevée, J. Organic dye-based photoinitiating systems for visible-light-induced photopolymerization. J. Polym. Sci. 2021, 59, 1338–1389. [Google Scholar] [CrossRef]
- Pigot, C.; Noirbent, G.; Bui, T.-T.; Péralta, S.; Gigmes, D.; Nechab, M.; Dumur, F. Push-pull chromophores based on the naphthalene scaffold: Potential candidates for optoelectronic applications. Materials 2019, 12, 1342. [Google Scholar] [CrossRef]
- Gong, S.; Hou, J.; Wu, X.; Huang, M.; Wei, T.; Deng, S.; Xiong, Y.; Tang, H. Fast photobleachable and low migration one-component green indole visible photoinitiators. Chem. Eng. J. 2023, 477, 146904. [Google Scholar] [CrossRef]
- Noirbent, G.; Pigot, C.; Bui, T.-T.; Péralta, S.; Nechab, M.; Gigmes, D.; Dumur, F. Synthesis, optical and electrochemical properties of a series of push-pull dyes based on the 2-(3-cyano-4, 5, 5-trimethylfuran-2 (5H)-ylidene) malononitrile (TCF) acceptor. Dye. Pigment. 2021, 184, 108807. [Google Scholar] [CrossRef]
- Guo, X.D.; Zhou, H.Y.; Wang, J.X. A novel thioxanthone-hydroxyalkylphenone bifunctional photoinitiator: Synthesis, characterization and mechanism of photopolymerization. Prog. Org. Coat. 2021, 154, 106214. [Google Scholar] [CrossRef]
- Xu, C.; Gong, S.; Wu, X.; Wu, Y.; Liao, Q.; Xiong, Y.; Li, Z.; Tang, H. High-efficient carbazole-based photo-bleachable dyes as free radical initiators for visible light polymerization. Dye. Pigment. 2022, 198, 110039. [Google Scholar] [CrossRef]
- Deng, L.; Qu, J. Design of novel phenothiazine-based push-pull photoinitiators for visible light with high activity, good solubility and low migration. Prog. Org. Coat. 2023, 183, 107766. [Google Scholar] [CrossRef]
- Sun, K.; Liu, S.; Chen, H.; Morlet-Savary, F.; Graff, B.; Pigot, C.; Nechab, M.; Xiao, P.; Dumur, F.; Lalevée, J. N-ethyl carbazole-1-allylidene-based push-pull dyes as efficient light harvesting photoinitiators for sunlight induced polymerization. Eur. Polym. J. 2021, 147, 110331. [Google Scholar] [CrossRef]
- Tang, Z.; Gao, Y.; Jiang, S.; Nie, J.; Sun, F. Cinnamoylformate derivatives photoinitiators with excellent photobleaching ability and cytocompatibility for visible LED photopolymerization. Prog. Org. Coat. 2022, 170, 106969. [Google Scholar] [CrossRef]
- Zhiganshina, E.R.; Arsenyev, M.V.; Chubich, D.A.; Kolymagin, D.A.; Pisarenko, A.V.; Burkatovsky, D.S.; Baranov, E.V.; Vitukhnovsky, A.G.; Lobanov, A.N.; Matital, R.P. Tetramethacrylic benzylidene cyclopentanone dye for one-and two-photon photopolymerization. Eur. Polym. J. 2022, 162, 110917. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, X.; Huang, X.; Zhang, Y.; Shi, M.; Zhao, Y. High-efficient lignin-based polymerizable macromolecular photoinitiator with UV-blocking property for visible light polymerization. Int. J. Biol. Macromol. 2022, 204, 234–244. [Google Scholar] [CrossRef]
- Wu, Q.; Mo, Y.; Zhang, Y.; Li, F.; Deng, M. Photophysical/photochemical; kinetic, and migration stability studies of one-component polymerizable thioxanthone-based photoinitiators. Macromol. Chem. Phys. 2021, 222, 2100221. [Google Scholar] [CrossRef]
- Gencoglu, T.; Eren, T.N.; Lalevée, J.; Avci, D. A water soluble; low migration, and visible light photoinitiator by thioxanthone-functionalization of poly (ethylene glycol)-containing poly (β-amino ester). Macromol. Chem. Phys. 2022, 223, 2100450. [Google Scholar] [CrossRef]
- Kariksiz, N.; Balaban, B.; Gencoglu, T.; Morlet-Savary, F.; Lalevee, J.; Avci, D. A cyclopolymerizable; doubly Irgacure 2959 functionalized quaternary ammonium salt photoinitiator and its water-soluble copolymer with diallyldimethylammonium chloride. Polym. Chem. 2023, 14, 1195–1205. [Google Scholar] [CrossRef]
- Huang, Y.; Liao, H.; Huang, R.; Huang, L.; Xie, G.; Hu, H.; Yang, J. Bis(trimethylsilyl)amino modified Type Ⅱ photoinitiators can alleviate oxygen inhibition of near-UV LED photopolymerization due to moisture induced decomposition of initiators. Eur. Polym. J. 2022, 172, 111236. [Google Scholar] [CrossRef]
- Sheehan, A.; Mikulchyk, T.; De Castro, C.S.P.; Karuthedath, S.; Althobaiti, W.; Dvoracek, M.; Sabad, E.G.; Byrne, H.J.; Laquai, F.; Naydenova, I.; et al. Diethoxycarbonyl-BODIPYs as heavy-atom-free photosensitizers for holographic recording in cellulose acetate photopolymer. J. Mater. Chem. C 2023, 11, 15084–15096. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).