Synergistic Photoelectrocatalytic Degradation of Tetracycline Using Phosphate-Grafted Mo:BiVO4 Photoanode Coupled with Pd/CMK-3 Cathode for Dual-Functional Activation of Water and Molecular Oxygen
Abstract
1. Introduction
2. Experimental Section
2.1. Chemical Reagents
2.2. Synthesis of the Surface Phosphate-Treated Mo:BiVO4 Photoanode (PO43−-Mo:BiVO4)
2.3. Synthesis of Pd/CMK-3 Cathode
2.4. Catalyst Characterization
2.5. PEC Degradation Experimental Setup
3. Results and Discussion
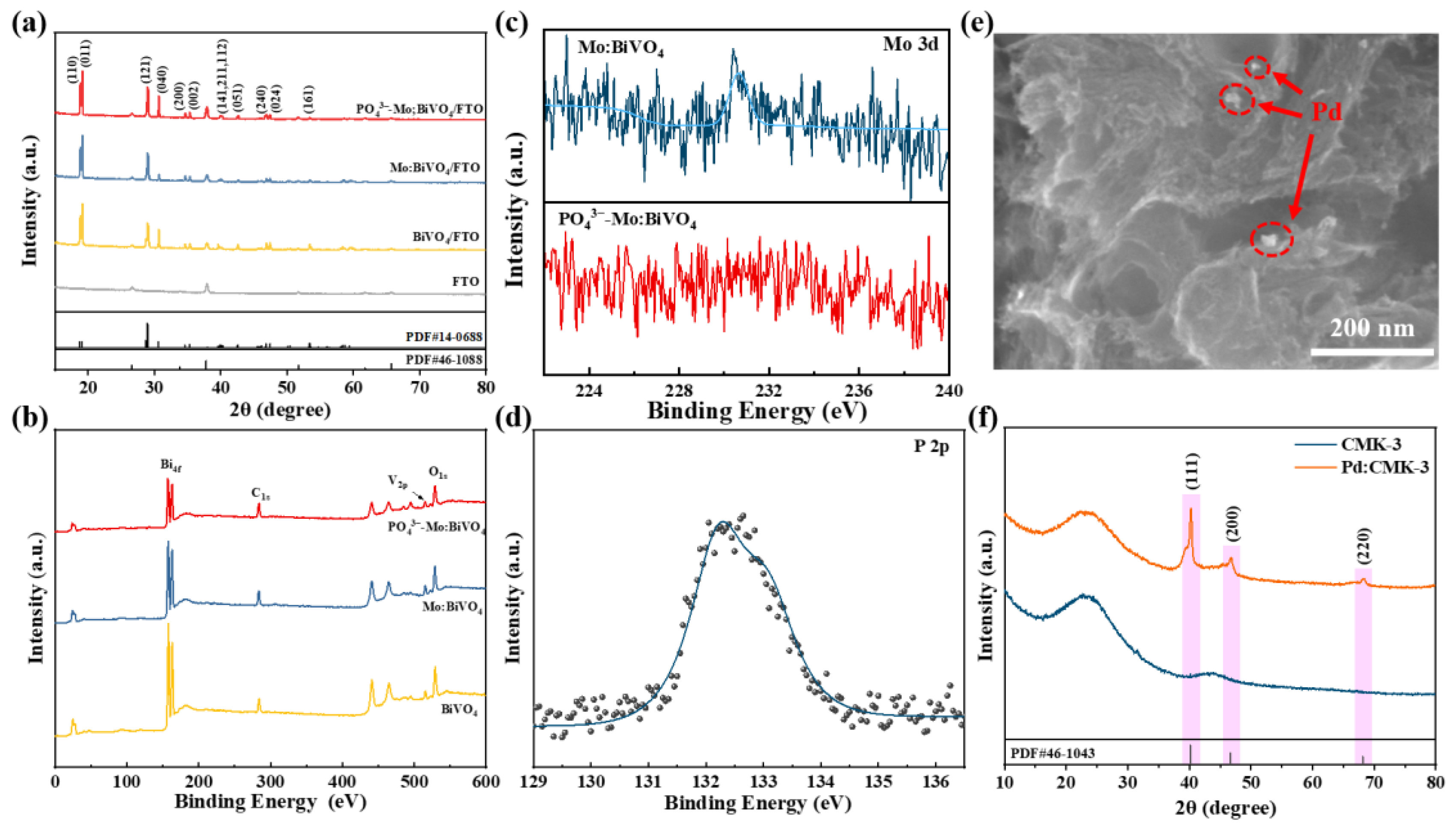
3.1. Characterization of PO43−-Mo:BiVO4 Photoanode and Pd/CMK-3 Cathode
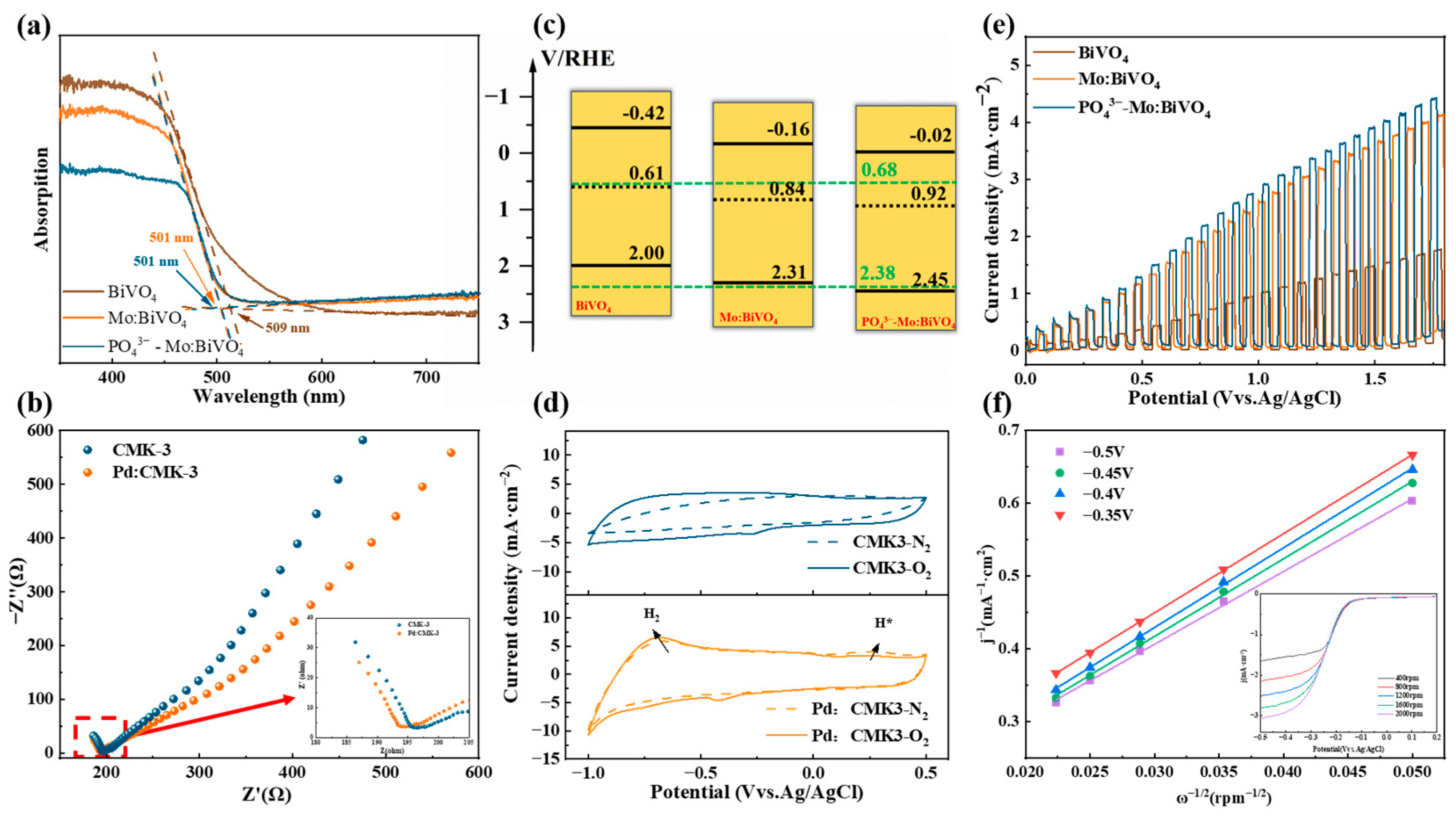
3.2. Photoelectric Properties of PO43−-Mo:BiVO4 Photoanode and Pd/CMK-3 Cathode
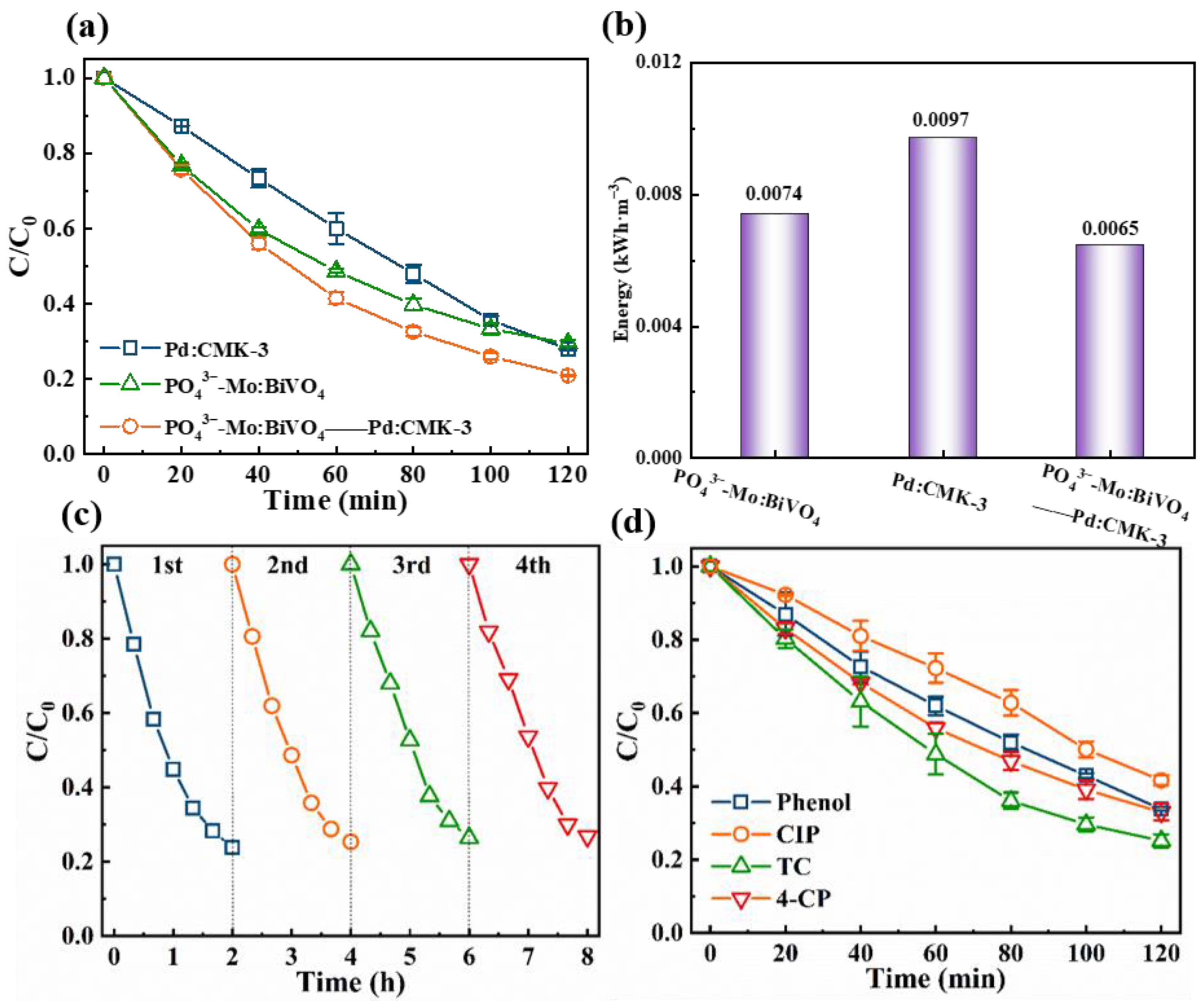
3.3. Degradation of TC by PO43−Mo:BiVO4 Photoanode & Pd/CMK-3 Cathode Synergistic System
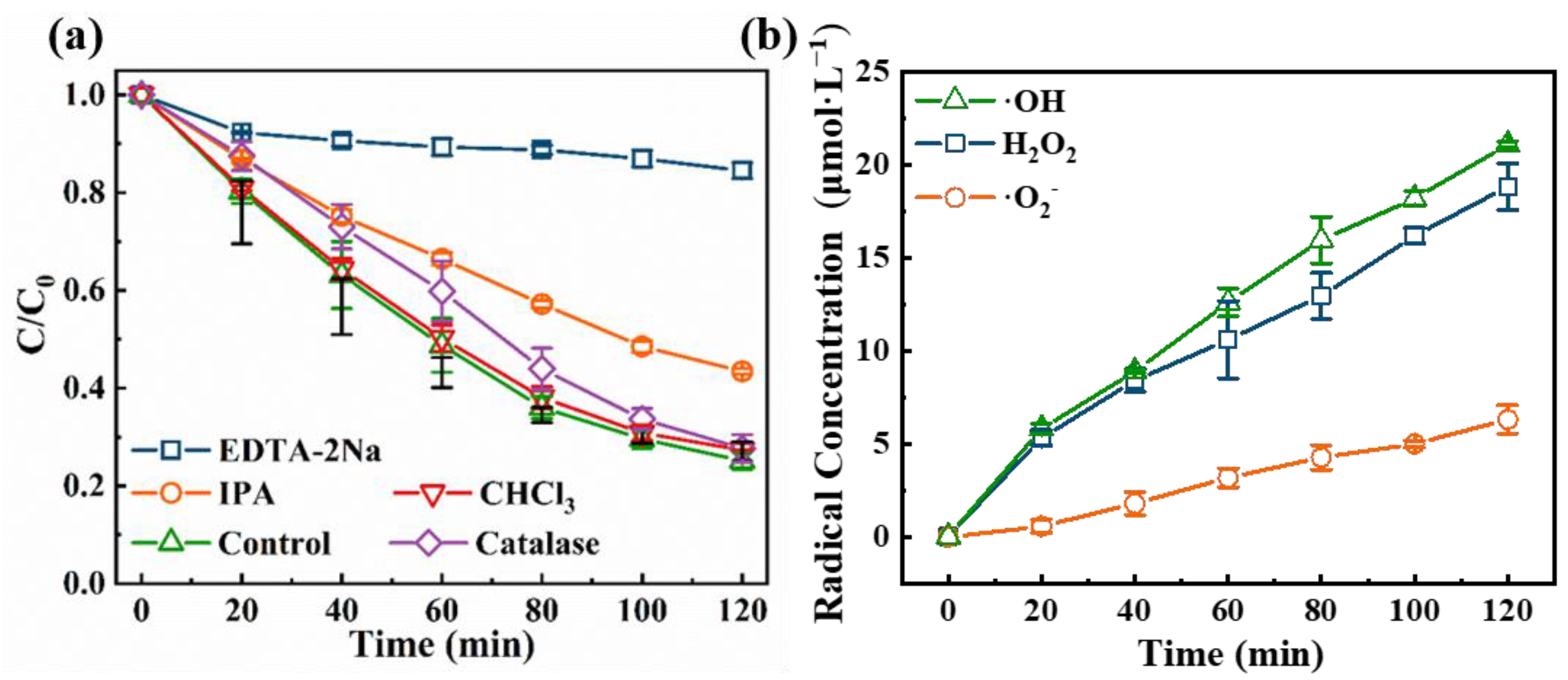
3.4. TC Degradation Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajapaksha, A.U.; Vithanage, M.; Lim, J.E.; Ahmed, M.B.M.; Zhang, M.; Lee, S.S.; Ok, Y.S. Invasive plant-derived biochar inhibits sulfamethazine uptake by lettuce in soil. Chemosphere 2014, 111, 500–504. [Google Scholar] [CrossRef]
- Jiménez-Tototzintle, M.; Ferreira, I.J.; da Silva Duque, S.; Guimarães Barrocas, P.R.; Saggioro, E.M. Removal of contaminants of emerging concern (CECs) and antibiotic resistant bacteria in urban wastewater using UVA/TiO2/H2O2 photocatalysis. Chemosphere 2018, 210, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, K.G.; Meyer, M.T. Occurrence of antibiotics in wastewater treatment facilities in Wisconsin, USA. Sci. Total Environ. 2006, 361, 196–207. [Google Scholar] [CrossRef]
- Dinh, Q.T.; Moreau-Guigon, E.; Labadie, P.; Alliot, F.; Teil, M.-J.; Blanchard, M.; Chevreuil, M. Occurrence of antibiotics in rural catchments. Chemosphere 2017, 168, 483–490. [Google Scholar] [CrossRef]
- Wang, X.; Ma, Y.; Jiang, J.; Li, M.; Li, T.; Li, C.; Dong, S. Cl-based functional group modification MIL-53(Fe) as efficient photocatalysts for degradation of tetracycline hydrochloride. J. Hazard. Mater. 2022, 434, 128864. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.; Que, M.; Wu, Q.; Wang, X.; Zhou, Y.; Ma, Y.; Li, Y.; Yang, X. MXene-derived Ti3C2Tx/Bi4Ti3O12 heterojunction photocatalyst for enhanced degradation of tetracycline hydrochloride, rhodamine B, and methyl orange under visible-light irradiation. Appl. Surf. Sci. 2023, 639, 158270. [Google Scholar] [CrossRef]
- Dehghan, A.; Dehghani, M.H.; Nabizadeh, R.; Ramezanian, N.; Alimohammadi, M.; Najafpoor, A.A. Adsorption and visible-light photocatalytic degradation of tetracycline hydrochloride from aqueous solutions using 3D hierarchical mesoporous BiOI: Synthesis and characterization, process optimization, adsorption and degradation modeling. Chem. Eng. Res. Des. 2018, 129, 217–230. [Google Scholar] [CrossRef]
- Yin, F.; Lin, S.; Zhou, X.; Dong, H.; Zhan, Y. Fate of antibiotics during membrane separation followed by physical-chemical treatment processes. Sci. Total Environ. 2021, 759, 143520. [Google Scholar] [CrossRef]
- Mao, J.; Yin, K.; Zhang, Y.; Shang, Y.; Li, Q.; Li, Y.; Gao, B.; Xu, X. Ultrafast oxidation of refractory organics via PMS activation by Si-O doped biomimetic montmorillonite: Simultaneous enhanced radical/electron transfer pathways and efficient catalytic membrane system. Appl. Catal. B Environ. 2024, 342, 123428. [Google Scholar] [CrossRef]
- Han, C.-H.; Park, H.-D.; Kim, S.-B.; Yargeau, V.; Choi, J.-W.; Lee, S.-H.; Park, J.-A. Oxidation of tetracycline and oxytetracycline for the photo-Fenton process: Their transformation products and toxicity assessment. Water Res. 2020, 172, 115514. [Google Scholar] [CrossRef]
- Li, X.; Ye, F.; Zhang, H.; Ahmad, M.; Zeng, Z.; Wang, S.; Wang, S.; Gao, D.; Zhang, Q. Ternary rGO decorated W18O49 @g-C3N4 composite as a full-spectrum-responded Z-scheme photocatalyst for efficient photocatalytic H2O2 production and water disinfection. J. Environ. Chem. Eng. 2023, 11, 110329. [Google Scholar] [CrossRef]
- Tong, J.; Tan, P.; Feng, N.; Liao, H.; Liu, F.; He, B.; Xie, J.; Pan, J. Atomic hydrogen induced single-electron activation of peroxydisulfate on silver-doped MoS2-x/TiO2-y for efficient photoelectrocatalytic decontamination. Appl. Catal. B Environ. Energy 2025, 366, 125072. [Google Scholar] [CrossRef]
- Sipuka, D.S.; Sebokolodi, T.I.; Jayeola, K.D.; Bechelany, M.; Cretin, M.; Arotiba, O.A. A ZnO/Bi3TaO7 S-scheme heterojunction for photoelectrocatalytic degradation of ciprofloxacin in synthetic and real wastewater. J. Water Process Eng. 2025, 69, 106771. [Google Scholar] [CrossRef]
- Chi, J.; Jiang, Z.; Yan, J.; Larimi, A.; Wang, Z.; Wang, L.; Shangguan, W. Recent advancements in bismuth vanadate photoanodes for photoelectrochemical water splitting. Mater. Today Chem. 2022, 26, 101060. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, W.; Guo, Y.; Li, S.; Chen, Y.; Wang, H.; Bian, Z. Recent advances in photoelectrocatalytic advanced oxidation processes: From mechanism understanding to catalyst design and actual applications. Chem. Eng. J. 2023, 455, 140801. [Google Scholar] [CrossRef]
- Dang, Q.; Wang, L.; Liu, J.; Wang, D.; Chai, J.; Wu, M.; Tang, L. Recent progress of photoelectrocatalysis systems for wastewater treatment. J. Water Process Eng. 2023, 53, 103609. [Google Scholar] [CrossRef]
- Fei, W.; Gao, J.; Li, N.; Chen, D.; Xu, Q.; Li, H.; He, J.; Lu, J. A visible-light active p-n heterojunction NiFe-LDH/Co3O4 supported on Ni foam as photoanode for photoelectrocatalytic removal of contaminants. J. Hazard. Mater. 2021, 402, 123515. [Google Scholar] [CrossRef]
- Jeon, T.H.; Koo, M.S.; Kim, H.; Choi, W. Dual-Functional Photocatalytic and Photoelectrocatalytic Systems for Energy- and Resource-Recovering Water Treatment. ACS Catal. 2018, 8, 11542–11563. [Google Scholar] [CrossRef]
- Ye, F.; Wang, T.; Quan, X.; Yu, H.; Chen, S. Constructing efficient WO3-FPC system for photoelectrochemical H2O2 production and organic pollutants degradation. Chem. Eng. J. 2020, 389, 123427. [Google Scholar] [CrossRef]
- Yang, Y.; Bian, Z. Oxygen doping through oxidation causes the main active substance in g-C3N4 photocatalysis to change from holes to singlet oxygen. Sci. Total Environ. 2021, 753, 141908. [Google Scholar] [CrossRef]
- Zhang, X.; Nengzi, L.; Li, B.; Liu, L.; Cheng, X. Design and construction of a highly efficient photoelectrocatalytic system based on dual-Pd/TNAs photoelectrodes for elimination of triclosan. Sep. Purif. Technol. 2020, 235, 116232. [Google Scholar] [CrossRef]
- McDonald, K.J.; Choi, K.-S. A new electrochemical synthesis route for a BiOI electrode and its conversion to a highly efficient porous BiVO4 photoanode for solar water oxidation. Energy Environ. Sci. 2012, 5, 8553. [Google Scholar] [CrossRef]
- Kim, T.W.; Choi, K.-S. Nanoporous BiVO4 Photoanodes with Dual-Layer Oxygen Evolution Catalysts for Solar Water Splitting. Science 2014, 343, 990–994. [Google Scholar] [CrossRef]
- Pan, Q.; Yang, K.; Wang, G.; Li, D.; Sun, J.; Yang, B.; Zou, Z.; Hu, W.; Wen, K.; Yang, H. BiVO4 nanocrystals with controllable oxygen vacancies induced by Zn-doping coupled with graphene quantum dots for enhanced photoelectrochemical water splitting. Chem. Eng. J. 2019, 372, 399–407. [Google Scholar] [CrossRef]
- Wang, L.; Gu, X.; Zhao, Y.; Wei, M.; Qiang, Y.; Zhao, Y. Enhanced photoelectrochemical performance by doping Mo into BiVO4 lattice. J. Mater. Sci. Mater. Electron. 2018, 29, 19278–19286. [Google Scholar] [CrossRef]
- Zhao, G.; Sun, Y.; Zhou, W.; Wang, X.; Chang, K.; Liu, G.; Liu, H.; Kako, T.; Ye, J. Superior Photocatalytic H2 Production with Cocatalytic Co/Ni Species Anchored on Sulfide Semiconductor. Adv. Mater. 2017, 29, 1703258. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.; Yu, S.; Wen, T.; Zhu, X.; Yang, F.; Sun, X.; Wang, X.; Hu, W. Ternary NiCo2Px Nanowires as pH-Universal Electrocatalysts for Highly Efficient Hydrogen Evolution Reaction. Adv. Mater. 2017, 29, 1605502. [Google Scholar] [CrossRef]
- Wang, F.; Yang, J.; Li, S.; Liang, J.; Wang, Y.; Du, R.; Zhou, B.; Liu, Q.; Li, C. Constructing ultralow-loading Cu single atoms/Fe2O3 particles on Nb2C MXenes for efficient utilization of atomic H to boost electrochemical debromination. Chem. Eng. J. 2024, 498, 155550. [Google Scholar] [CrossRef]
- Zhou, G.-N.; Li, W.-Q.; He, C.-S.; Liu, X.-C.; Ding, R.-R.; Wang, Y.-X.; Mu, Y. Enhanced hydrodeiodination of iodinated contrast medium by sulfide-modified nano-sized zero-valent iron: Kinetics, mechanisms and application prospects. Chem. Eng. J. 2020, 401, 126050. [Google Scholar] [CrossRef]
- Chen, X.; Fu, W.; Yang, Y.; Li, Y.; Yang, K.; Xu, X.; Zhang, X. Essential role of atomic H* in activating hydrogen peroxide to produce singlet oxygen for emerging organic contaminants degradation. J. Environ. Chem. Eng. 2023, 11, 109442. [Google Scholar] [CrossRef]
- Wang, C.; Chen, H.; Zhou, Y.; Wen, T.; Shao, J.; Zhang, D.; Pan, X. Humic acid promotes pathway of chloronitrobenzene cathodic reduction shunting from atomic H* to direct electron transfer. Water Res. 2025, 272, 122918. [Google Scholar] [CrossRef]
- Zhou, H.; Lu, D.; Fang, S.; Liu, C.; Chen, Y.; Hu, Y.; Luo, Q. Prompting direct single electron transfer to produce non-radical 1O2/H* by electro-activating peroxydisulfate process with core-shell cathode. J. Environ. Manag. 2021, 287, 112294. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Liu, Y.; Lv, Q.; Gao, B.; Xia, J.; Li, X.; Dou, M.; Zhao, K.; Ahmad, M.; Xiao, Z.; et al. Unveiling the mechanism of efficient detoxification by Pd species in chlorinated pollutant degradation. Chin. Chem. Lett. 2025, 111136. [Google Scholar] [CrossRef]
- Guo, Y.; Li, Y.; Shi, W.; Yuan, J.; Wang, L.; Wang, Z. In situ redox growth of Pd/CuOx for enhanced electrocatalytic degradation of organic chlorides via indirect atomic H* reduction. Appl. Catal. B Environ. Energy 2024, 356, 124252. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Z.; Feng, Z.; Zhao, H.; Zhao, G. Fast Generation of Hydroxyl Radicals by Rerouting the Electron Transfer Pathway via Constructed Chemical Channels during the Photo-Electro-Reduction of Oxygen. Environ. Sci. Technol. 2022, 56, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Li, M.; Zhao, Y.; Zhang, H.; Shang, J.; Zhong, J.; Sheng, H.; Chen, C.; Zhao, J. The vital role of surface Brönsted acid/base sites for the photocatalytic formation of free ·OH radicals. Appl. Catal. B Environ. 2020, 266, 118634. [Google Scholar] [CrossRef]
- Sheng, H.; Li, Q.; Ma, W.; Ji, H.; Chen, C.; Zhao, J. Photocatalytic degradation of organic pollutants on surface anionized TiO2: Common effect of anions for high hole-availability by water. Appl. Catal. B Environ. 2013, 138–139, 212–218. [Google Scholar] [CrossRef]
- Zeng, H.; Zhang, G.; Ji, Q.; Liu, H.; Hua, X.; Xia, H.; Sillanpää, M.; Qu, J. pH-Independent Production of Hydroxyl Radical from Atomic H*-Mediated Electrocatalytic H2O2 Reduction: A Green Fenton Process without Byproducts. Environ. Sci. Technol. 2020, 54, 14725–14731. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Rafiq, M.; Alamgir Qadir, A.; Ahmed, A.; Fayaz, M.; Adnan, M.; Ullah, N.; Yu, B.; Cong, H. Hollow cavity engineering of MOF-derived hierarchical nitrogen-doped In2O3@carbon for efficient photocatalytic degradation of tetracycline hydrochloride. J. Water Process Eng. 2025, 76, 108311. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Shi, H.; Xie, S.; Zhang, C.; Zhao, G. Enhanced Photoelectrocatalytic Reduction and Removal of Atrazine: Effect of Co-Catalyst and Cathode Potential. ACS Appl. Mater. Interfaces 2019, 11, 38663–38673. [Google Scholar] [CrossRef]
- Chen, N.; Xia, J.; Li, L.; Lv, Q.; Zhao, K.; Ahmad, M.; Xiao, Z.; Wang, S.; Ye, F.; Zhang, Q. Comprehensive enhancement of photocatalytic H2O2 generation and antibacterial efficacy on carbon nitride through a straightforward polydopamine coating strategy. Surf. Interfaces 2025, 56, 105566. [Google Scholar] [CrossRef]
- Ye, F.; Qian, J.; Xia, J.; Li, L.; Wang, S.; Zeng, Z.; Mao, J.; Ahamad, M.; Xiao, Z.; Zhang, Q. Efficient photoelectrocatalytic degradation of pollutants over hydrophobic carbon felt loaded with Fe-doped porous carbon nitride via direct activation of molecular oxygen. Environ. Res. 2024, 249, 118497. [Google Scholar] [CrossRef]
- He, C.; Luo, Z.; Zhang, L.; Zhang, Q.; He, C.; Ren, X. Progress and challenges for electrocatalytic production of hydrogen peroxide. Appl. Catal. A Gen. 2024, 681, 119803. [Google Scholar] [CrossRef]
- Shi, X.; Chen, S.; Zhao, K.; Wu, S.; Ye, F.; Yu, H.; Zhang, Y.; Chen, X.; Liang, Y.; Niu, J. Nanoconfinement-mediated non-radical enhanced pollutant degradation on Fe single-atom electrocatalyst. J. Hazard. Mater. 2025, 490, 137764. [Google Scholar] [CrossRef]
- Chen, M.; Hu, C.; Liu, H.; Qu, J. Model Unraveling the Ultra-Efficient Multi-Stage Flow-Through Anodes for Water Purification Application: Development of Design and Evaluation. ACS EST Eng. 2023, 3, 787–797. [Google Scholar] [CrossRef]
- Qin, X.; Cao, P.; Quan, X.; Zhao, K.; Chen, S.; Yu, H.; Su, Y. Highly Efficient Hydroxyl Radicals Production Boosted by the Atomically Dispersed Fe and Co Sites for Heterogeneous Electro-Fenton Oxidation. Environ. Sci. Technol. 2023, 57, 2907–2917. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tang, Y.; Liu, H.; Wang, Z.; Liu, X.; Tang, H.; Zhang, H.; Wang, D.; Long, Y.; Liu, C. Electrocatalytic Deep Dehalogenation and Mineralization of Florfenicol: Synergy of Atomic Hydrogen Reduction and Hydroxyl Radical Oxidation over Bifunctional Cathode Catalyst. Environ. Sci. Technol. 2023, 57, 20315–20325. [Google Scholar] [CrossRef]
- Li, L.; Ye, F.; Lv, Q.; Xia, J.; Chen, N.; Wang, H.; Chen, L.; Zhao, K.; Zeng, Z.; Ahmad, M.; et al. Polydopamine-coated hollow carbon nitride as a full-spectral response photocatalyst for efficient H2O2 production via redox dual pathways. Appl. Catal. B Environ. Energy 2025, 363, 124802. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Xu, Z.; Tang, C.; Wang, S.; Xiao, Z.; Ye, F. Synergistic Photoelectrocatalytic Degradation of Tetracycline Using Phosphate-Grafted Mo:BiVO4 Photoanode Coupled with Pd/CMK-3 Cathode for Dual-Functional Activation of Water and Molecular Oxygen. Coatings 2025, 15, 1027. https://doi.org/10.3390/coatings15091027
Yang M, Xu Z, Tang C, Wang S, Xiao Z, Ye F. Synergistic Photoelectrocatalytic Degradation of Tetracycline Using Phosphate-Grafted Mo:BiVO4 Photoanode Coupled with Pd/CMK-3 Cathode for Dual-Functional Activation of Water and Molecular Oxygen. Coatings. 2025; 15(9):1027. https://doi.org/10.3390/coatings15091027
Chicago/Turabian StyleYang, Minglei, Zhenhong Xu, Chongjun Tang, Shuaijie Wang, Zhourong Xiao, and Fei Ye. 2025. "Synergistic Photoelectrocatalytic Degradation of Tetracycline Using Phosphate-Grafted Mo:BiVO4 Photoanode Coupled with Pd/CMK-3 Cathode for Dual-Functional Activation of Water and Molecular Oxygen" Coatings 15, no. 9: 1027. https://doi.org/10.3390/coatings15091027
APA StyleYang, M., Xu, Z., Tang, C., Wang, S., Xiao, Z., & Ye, F. (2025). Synergistic Photoelectrocatalytic Degradation of Tetracycline Using Phosphate-Grafted Mo:BiVO4 Photoanode Coupled with Pd/CMK-3 Cathode for Dual-Functional Activation of Water and Molecular Oxygen. Coatings, 15(9), 1027. https://doi.org/10.3390/coatings15091027







