Effect of Calcium Nitrate on Microstructure and Anti-Corrosion Properties of Zinc Phosphate Coatings on Stainless Steel
Abstract
1. Introduction
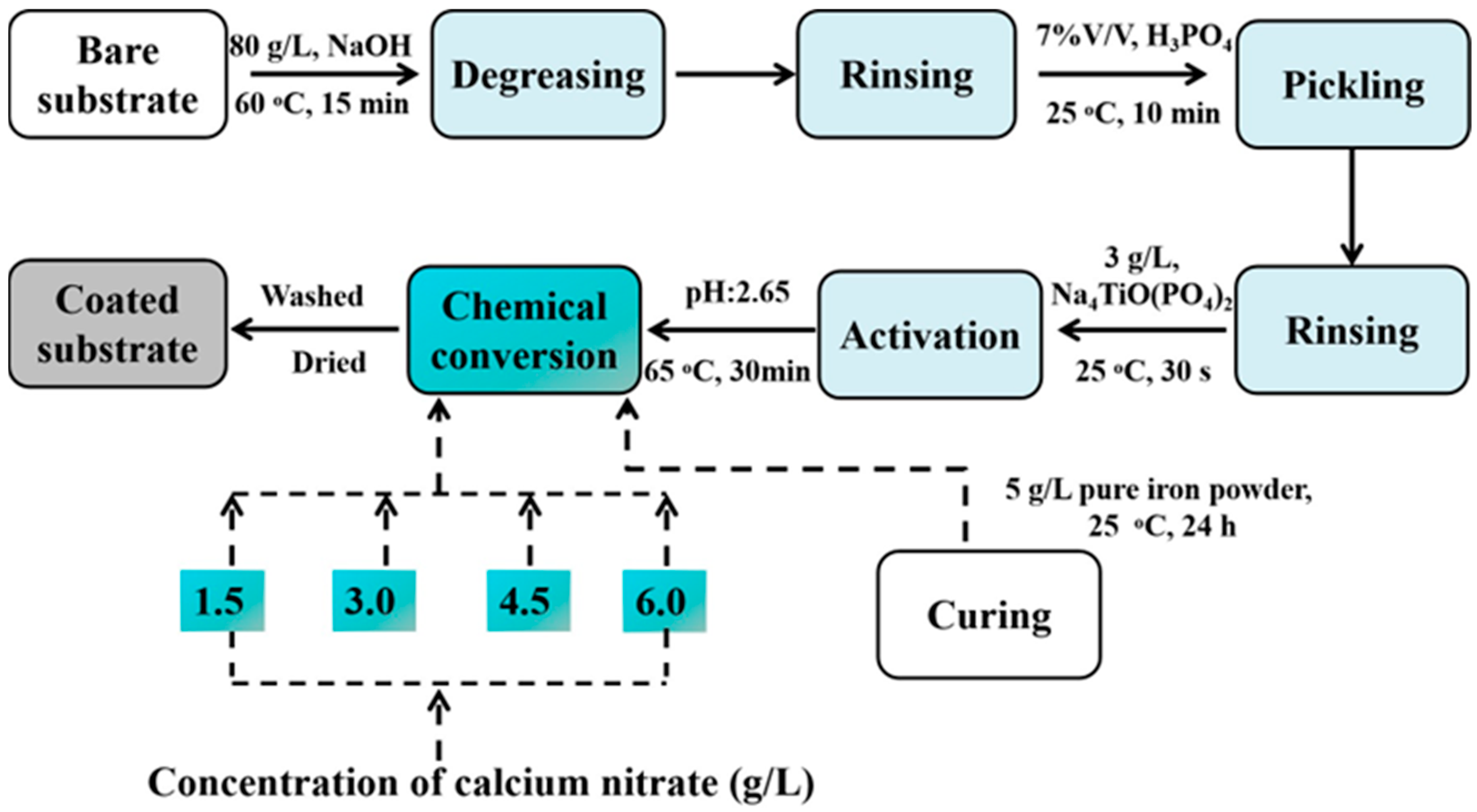
2. Materials and Methods
3. Results and Discussion
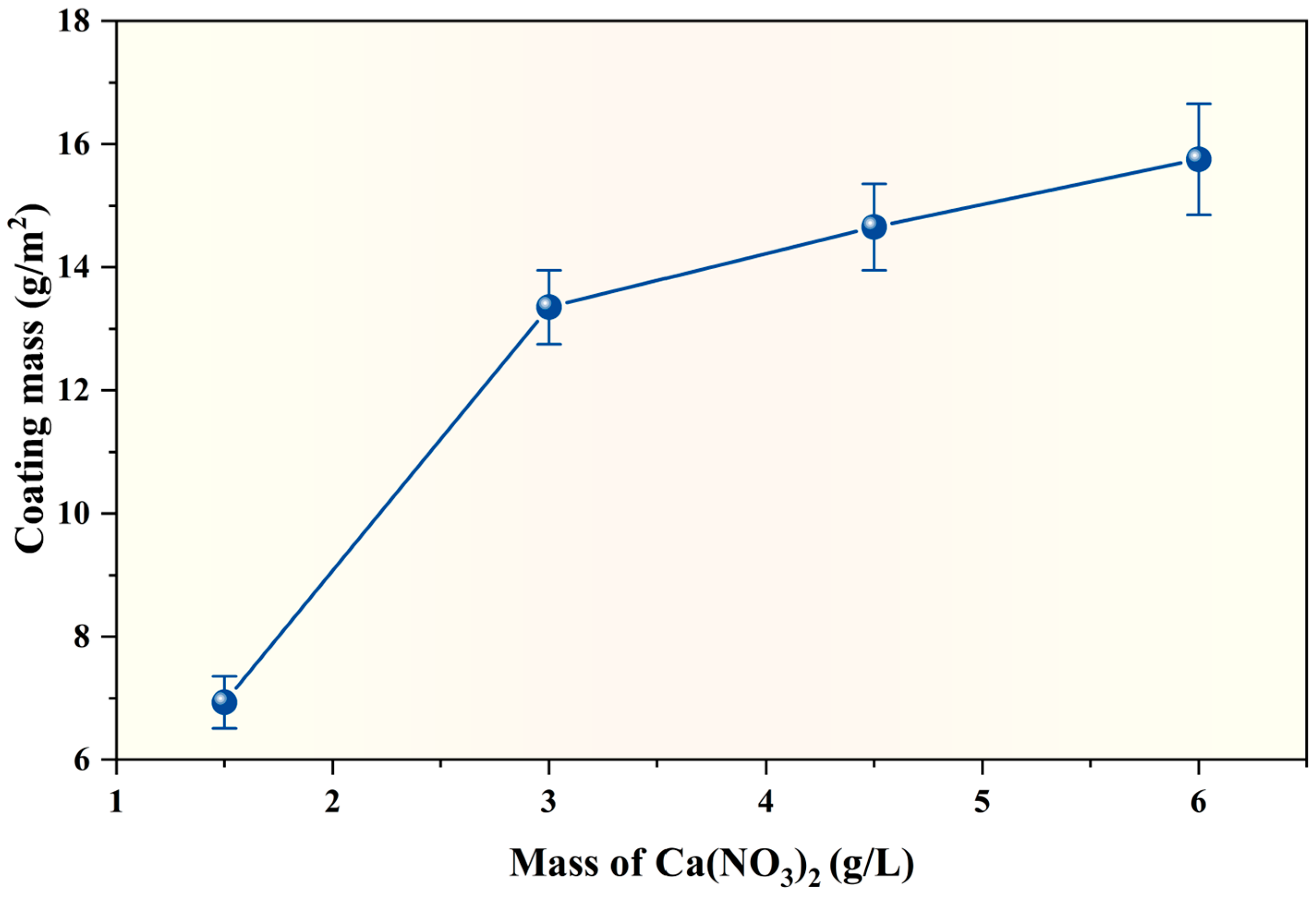
3.1. Coating Mass
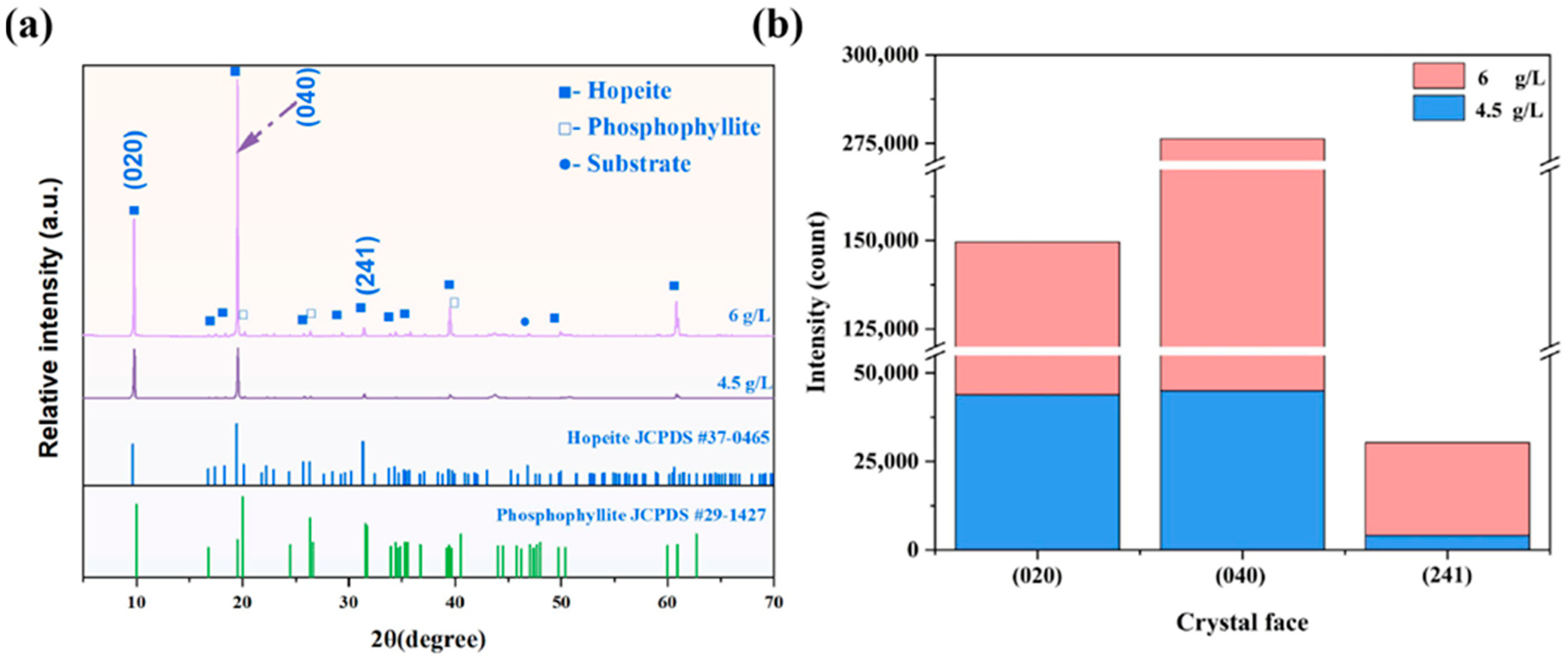
3.2. Phase Composition
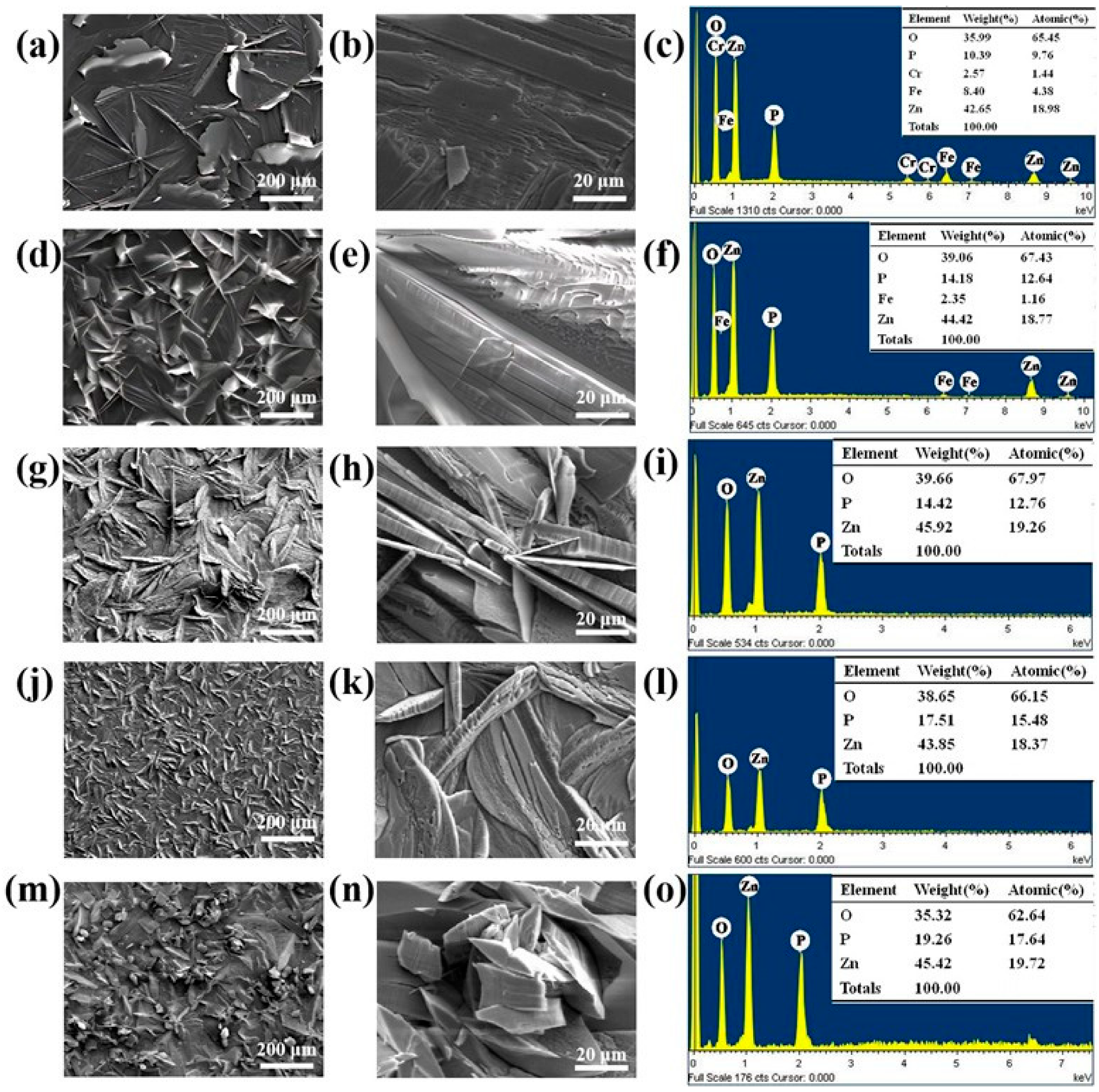
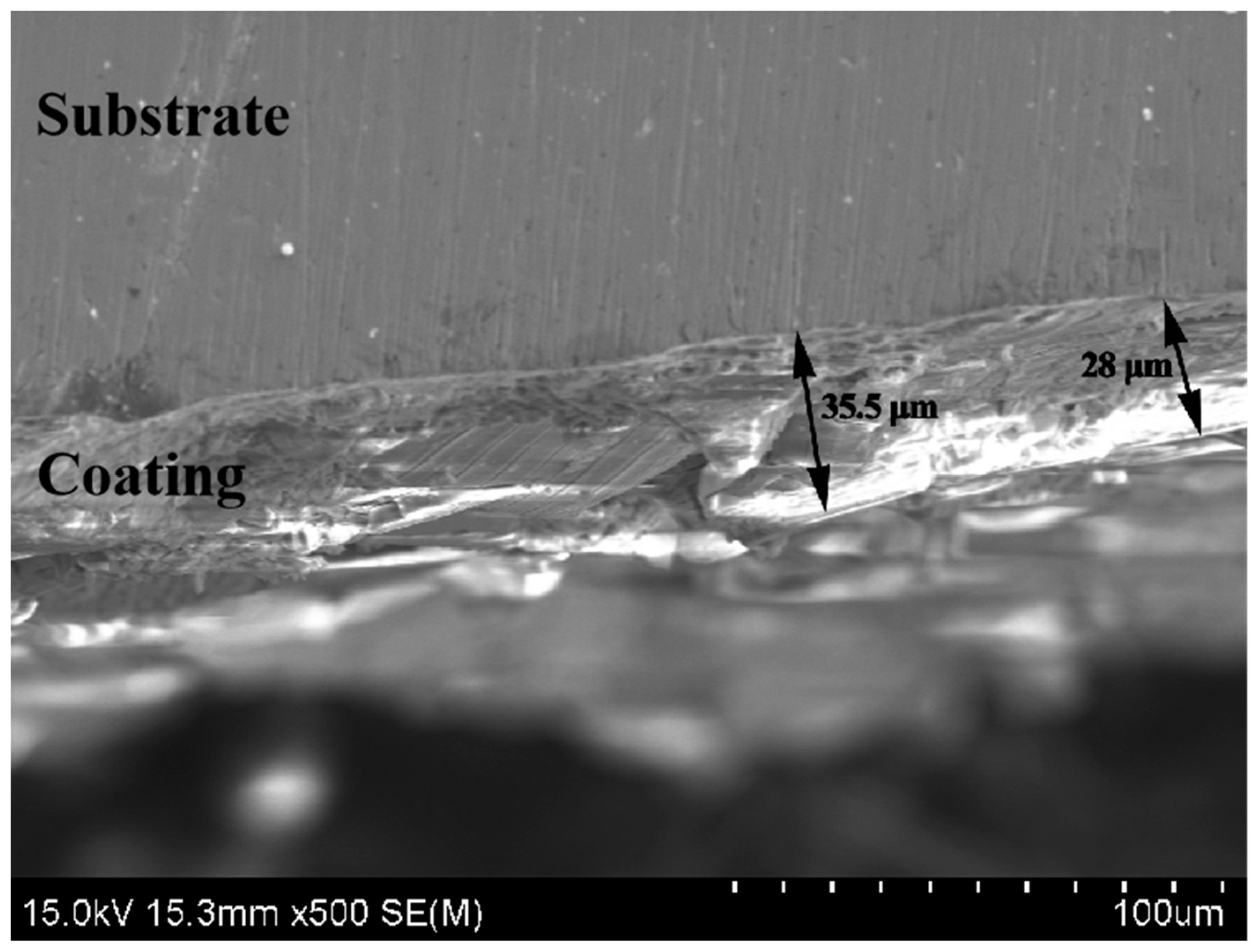
3.3. Microstructure Characterization
3.4. Electrochemical Investigation
3.5. Adhesion Strength Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Heydarian, A.; Najafi, A.; Khalaj, G. Enhancement of low-zinc phosphate coatings with addition Ni2+ and Mn2+ cations: Structure, corrosion resistance and paint adhesion. J. Mater. Res. Technol. 2024, 30, 7308–7327. [Google Scholar] [CrossRef]
- Narayanan, T.S.N.S. Surface pretreatment by phosphate conversion coatings—A review. Rev. Adv. Mater. Sci. 2005, 9, 130–177. Available online: https://www.ipme.ru/e-journals/RAMS/no_2905/narayanan.pdf (accessed on 10 September 2024).
- Liu, B.; Xiao, G.Y.; Lu, Y.P. Effect of pH on the phase composition and corrosion characteristics of calcium zinc phosphate conversion coatings on titanium. J. Electrochem. Soc. 2016, 163, C477. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, G.Y.; Liu, B.; Jiang, C.C.; Li, N.B.; Lu, Y.P. The formation of hydroxyapatite layer onto hopeite coating on stainless steel substrate. Corros. Sci. 2016, 111, 216–229. [Google Scholar] [CrossRef]
- Zuo, K.; Yin, Y.; Yao, L.; Wang, K.; Yan, Y.; Ma, Z.; Liu, B.; Lu, Y.; Li, X.; Xiao, G. Antibacterial activities and cytocompatibility of zinc-contained strontium phosphate coating on titanium. Mater. Res. Express 2020, 7, 75402. [Google Scholar] [CrossRef]
- Li, Y.B.; Lu, Y.P.; Du, C.M.; Zuo, K.Q.; Wang, Y.Y.; Tang, K.L.; Xiao, G.Y. Effect of reaction temperature on the microstructure and properties of magnesium phosphate chemical conversion coatings on titanium. Molecules 2023, 28, 4495. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, L.; Lei, Q.; Zhang, T.; Bing, J.; Dong, J. A nano-CeO2/Zn–Mn composite conversion coatings on AZ91D magnesium alloy surface of corrosion resistance research. Coatings 2023, 13, 929. [Google Scholar] [CrossRef]
- He, W.; Yan, R.; Gao, X.; Wang, Y.; Sun, X.; Ma, H. Effect of Al3+ on the surface microstructure and film formation mechanism of Zr-based conversion coatings with improved corrosion resistance performance on cold rolled steel. Electrochim. Acta 2024, 487, 144196. [Google Scholar] [CrossRef]
- Hafeez, M.; Farooq, A.; Zang, A.; Saleem, A.; Deen, K. Phosphate chemical conversion coatings for magnesium alloys: A review. J. Coat. Technol. Res. 2020, 17, 827–849. [Google Scholar] [CrossRef]
- Leng, B.; Xue, Y.; Li, J.; Qi, J.; Yi, A.; Zhao, Q. A critical review of anti-corrosion chemical surface treatment of aluminum alloys used for sports equipment. Crystals 2024, 14, 101. [Google Scholar] [CrossRef]
- Zuo, K.; Lei, W.; Cao, Q.; Si, T.; Zhang, L.; Zhang, T.; Li, J.; Xiao, G.; Lu, Y.; Li, N. Structural evolution of sr-phosphate chemical conversion coatings induced by in-situ ca doping to achieve efficient osteogenic activity. Appl. Surf. Sci. 2025, 689, 162591. [Google Scholar] [CrossRef]
- Hosseinirad, R.; Toorani, M.; Rouhaghdam, A.S. Corrosion resistance and cathodic disbondment of epoxy coating applied on zinc phosphate conversion coating containing different amounts of cobalt ions. Surf. Eng. Appl. Electrochem. 2020, 56, 112–125. [Google Scholar] [CrossRef]
- Du, B.; Zhou, X.; Li, Q.; Liu, J.; Liu, Y.; Zeng, X.; Cheng, X.; Hu, H. Surface treat method to improve the adhesion between stainless steel and resin: A review. ACS Omega 2023, 8, 39984–40004. [Google Scholar] [CrossRef] [PubMed]
- Zaborowska, A.; Kurpaska, Ł.; Wyszkowska, E.; Clozel, M.; Vanazzi, M.; Di Fonzo, F.; Turek, M.; Jóźwik, I.; Kosińska, A.; Jagielski, J. Influence of ion irradiation on the nanomechanical properties of thin alumina coatings deposited on 316l ss by PLD. Surf. Coat. Tech. 2020, 386, 125491. [Google Scholar] [CrossRef]
- Olumide Olugbade, T. Corrosion resistance, evaluation methods, and surface treatments of stainless steels. In Stainless Steels; Singh, A., Ed.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Zanca, C.; Milazzo, A.; Campora, S.; Capuana, E.; Pavia, F.C.; Patella, B.; Lopresti, F.; Brucato, V.; La Carrubba, V.; Inguanta, R. Galvanic deposition of calcium phosphate/bioglass composite coating on AISI 316L. Coatings 2023, 13, 1006. [Google Scholar] [CrossRef]
- Rasheed, B.G.; Ibrahem, M.A.; Ibrahim, M.H. Surface modification of nobel metals and stainless steel by pulsed nd: YAG laser. Lasers Manuf. Mater. Process. 2021, 8, 45–59. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, G.Y.; Jiang, C.C.; Liu, B.; Li, N.B.; Zhu, R.F.; Lu, Y.P. Influence of process parameters on microstructure and corrosion properties of hopeite coating on stainless steel. Corros. Sci. 2015, 94, 428–437. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, G.Y.; Liu, B.; Jiang, C.C.; Lu, Y.P. Influence of processing time on the phase, microstructure and electrochemical properties of hopeite coating on stainless steel by chemical conversion method. New J. Chem. 2015, 39, 5813–5822. [Google Scholar] [CrossRef]
- Zhao, X.C.; Xiao, G.Y.; Zhang, X.; Wang, H.Y.; Lu, Y.P. Ultrasonic induced rapid formation and crystal refinement of chemical conversed hopeite coating on titanium. J. Phys. Chem. C 2014, 118, 1910–1918. [Google Scholar] [CrossRef]
- Arunima, S.R.; Deepa, M.J.; Elias, L.; Aju Thara, T.R.; Geethanjali, C.V.; Shibli, S.M.A. Tuning of surface characteristics of composite (WO3/BiVO4) zinc phosphate coatings for industrial applications. Appl. Surf. Sci. 2021, 543, 148822. [Google Scholar] [CrossRef]
- Nair, U.B. Calcium as a phosphating additive: An overview. Met. Finish. 1995, 93, 40–41. [Google Scholar] [CrossRef]
- Mehdi, S.M.Z.; Ali, M.; Maqsood, M.F.; Abbas, N.; Lee, N. Synthesis of nickel phosphate nanowires as a bifunctional catalyst for water electrolysis in alkaline media. J. Alloys Compd. 2024, 988, 174250. [Google Scholar] [CrossRef]
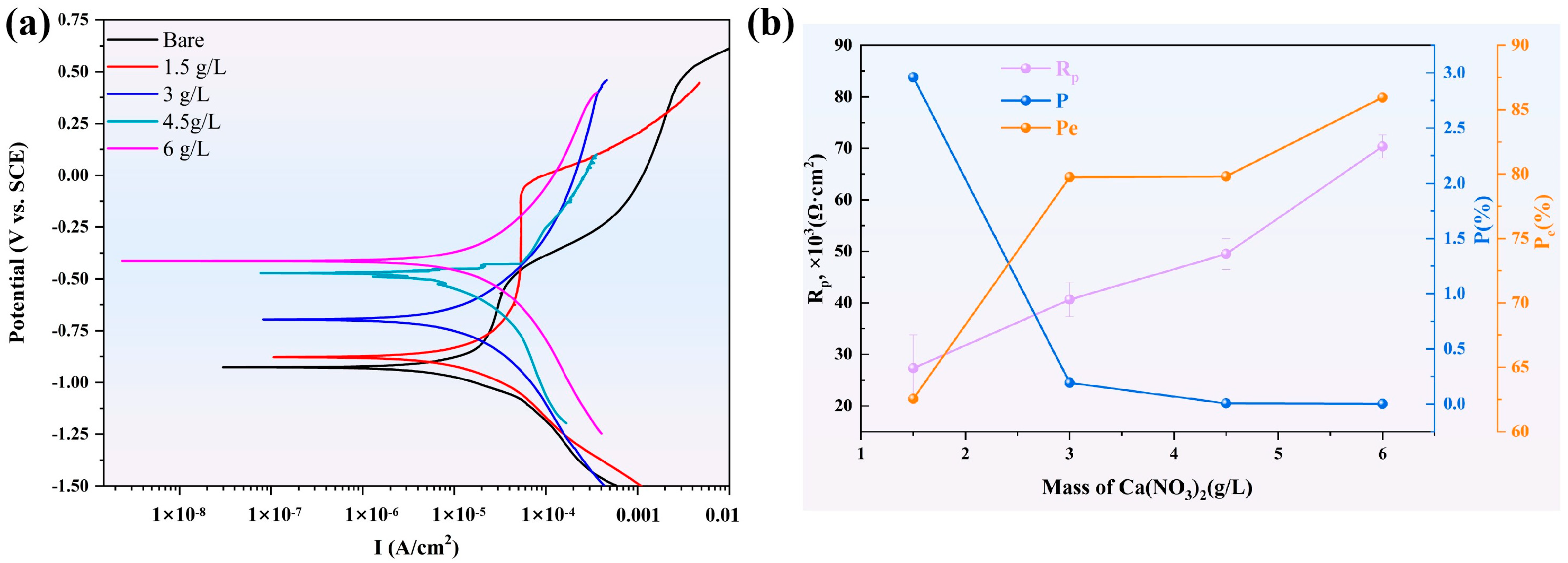
| Introduction | Ecorr (VSCE) | Icorr (μA/cm2) | Rp, × 103 (Ω·cm2) | P (%) b | Pe (%) c |
|---|---|---|---|---|---|
| Bare | −0.922 ± 0.18 | 1.68 ± 0.46 | 2.20 ± 1.64 | -- | -- |
| 1.5 g/L CN | −0.851 ± 0.009 | 0.629 ± 0.25 | 27.3 ± 6.46 | 2.96 | 62.56 |
| 3 g/L CN | −0.686 ± 0.04 | 0.340 ± 0.34 | 40.65 ± 3.35 | 0.193 | 79.76 |
| 4.5 g/L CN | −0.465 ± 0.008 | 0.356 ± 0.046 | 49.5 ± 2.97 | 0.007 | 79.81 |
| 6 g/L CN | −0.414 ± 0.011 | 0.236 ± 0.026 | 70.36 ± 2.23 | 0.0024 | 85.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhang, H.-H.; Wu, K.; Zhang, Y.; Yang, Z.-N.; Chen, Y. Effect of Calcium Nitrate on Microstructure and Anti-Corrosion Properties of Zinc Phosphate Coatings on Stainless Steel. Coatings 2025, 15, 1018. https://doi.org/10.3390/coatings15091018
Zhang X, Zhang H-H, Wu K, Zhang Y, Yang Z-N, Chen Y. Effect of Calcium Nitrate on Microstructure and Anti-Corrosion Properties of Zinc Phosphate Coatings on Stainless Steel. Coatings. 2025; 15(9):1018. https://doi.org/10.3390/coatings15091018
Chicago/Turabian StyleZhang, Xian, Hong-Hong Zhang, Kang Wu, Yan Zhang, Zhong-Nian Yang, and Yu Chen. 2025. "Effect of Calcium Nitrate on Microstructure and Anti-Corrosion Properties of Zinc Phosphate Coatings on Stainless Steel" Coatings 15, no. 9: 1018. https://doi.org/10.3390/coatings15091018
APA StyleZhang, X., Zhang, H.-H., Wu, K., Zhang, Y., Yang, Z.-N., & Chen, Y. (2025). Effect of Calcium Nitrate on Microstructure and Anti-Corrosion Properties of Zinc Phosphate Coatings on Stainless Steel. Coatings, 15(9), 1018. https://doi.org/10.3390/coatings15091018






