The Effect of Laser Cleaning on the Cr Coating on the Surface of Steel Tyre Moulds
Abstract
1. Introduction
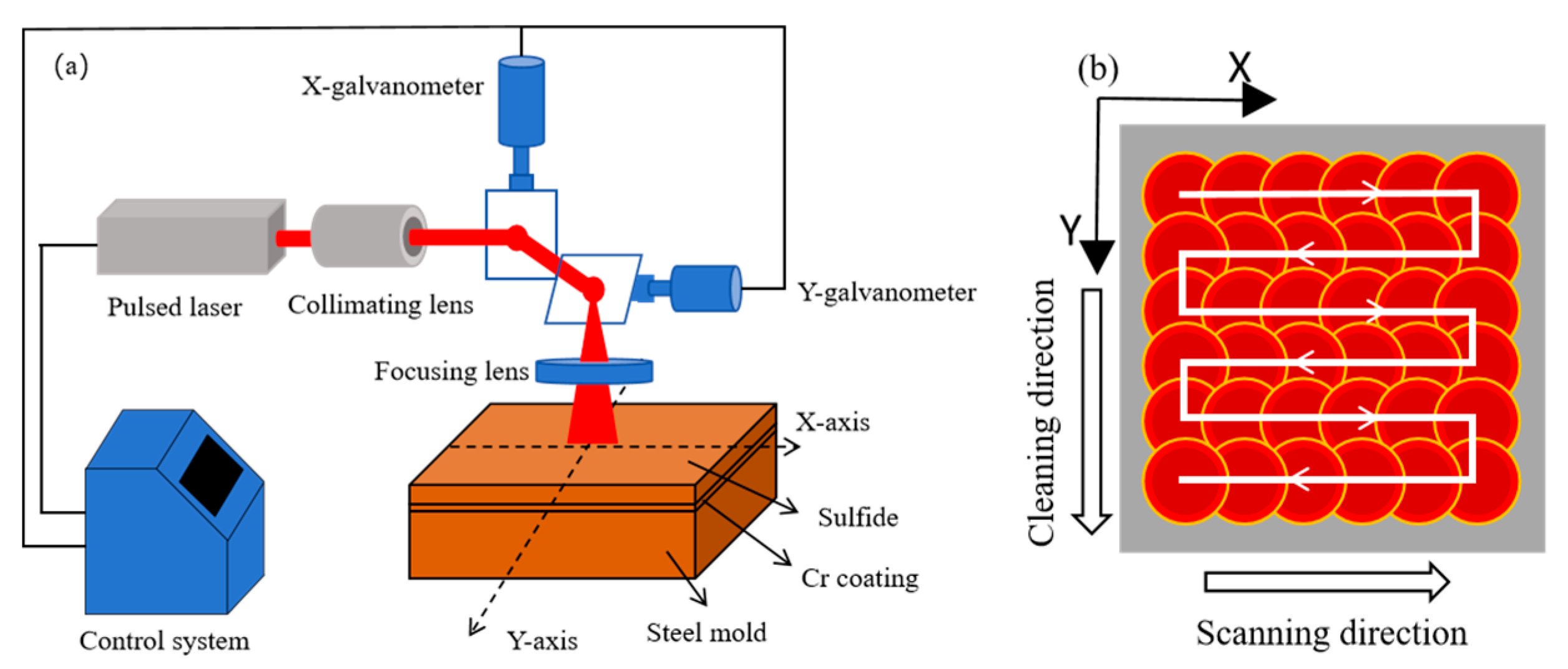
2. Experimental Procedure
2.1. Experimental Materials
2.2. Test Methods
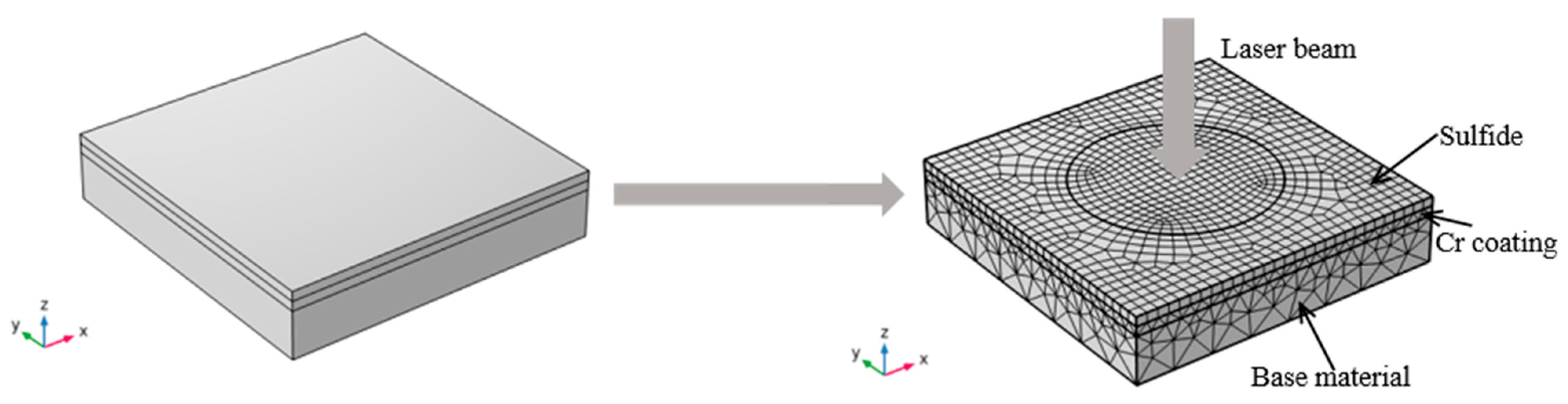
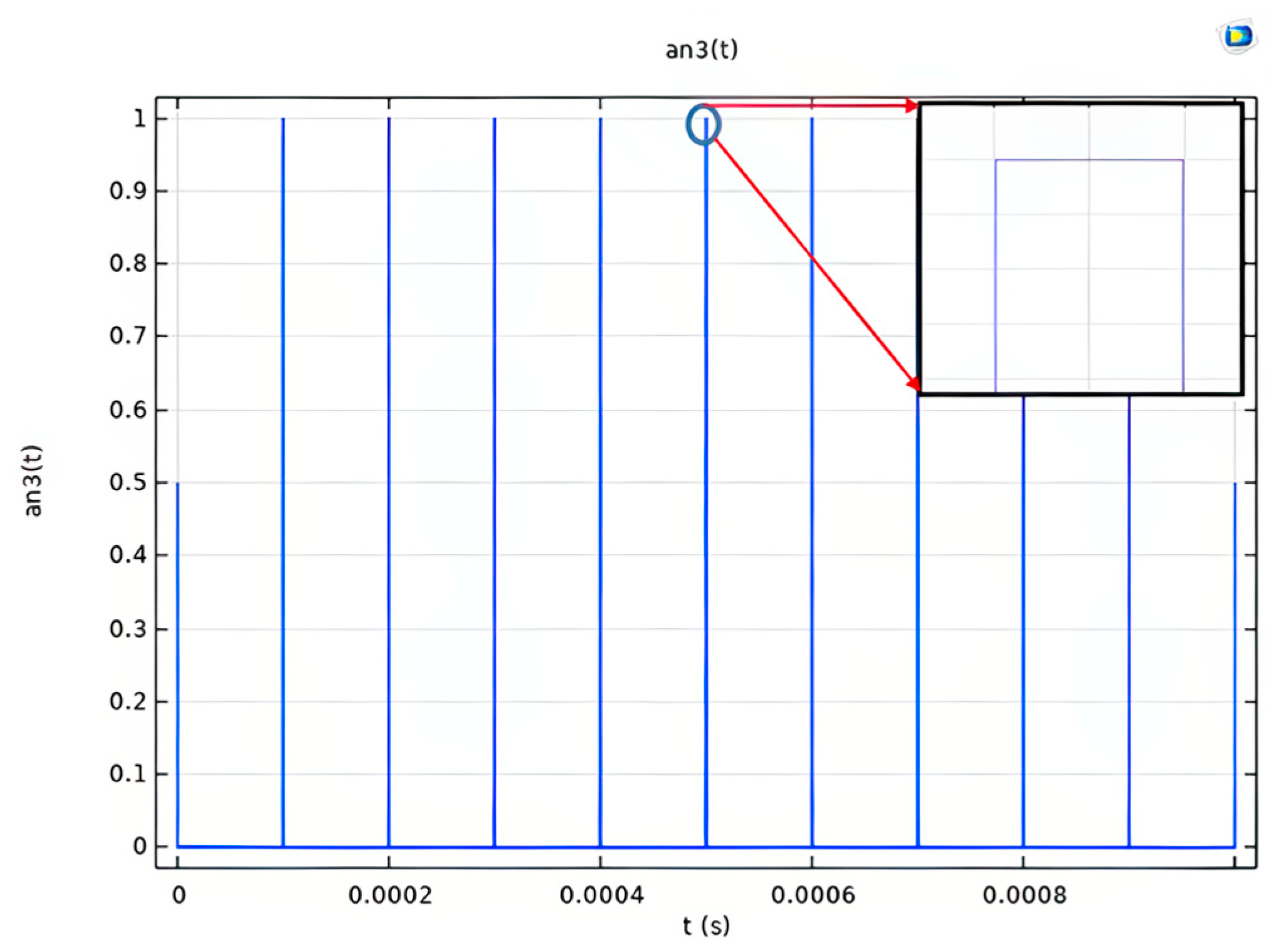
2.3. Numerical Simulation
- (1)
- Ideal heat conduction is achieved at the interface between the substrate and the material being cleaned;
- (2)
- Before laser cleaning begins, the initial temperature of the model and the ambient temperature are both 293.15 K;
- (3)
- The material is isotropic, with physical properties that are the same in all directions.
3. Experimental Results and Analysis
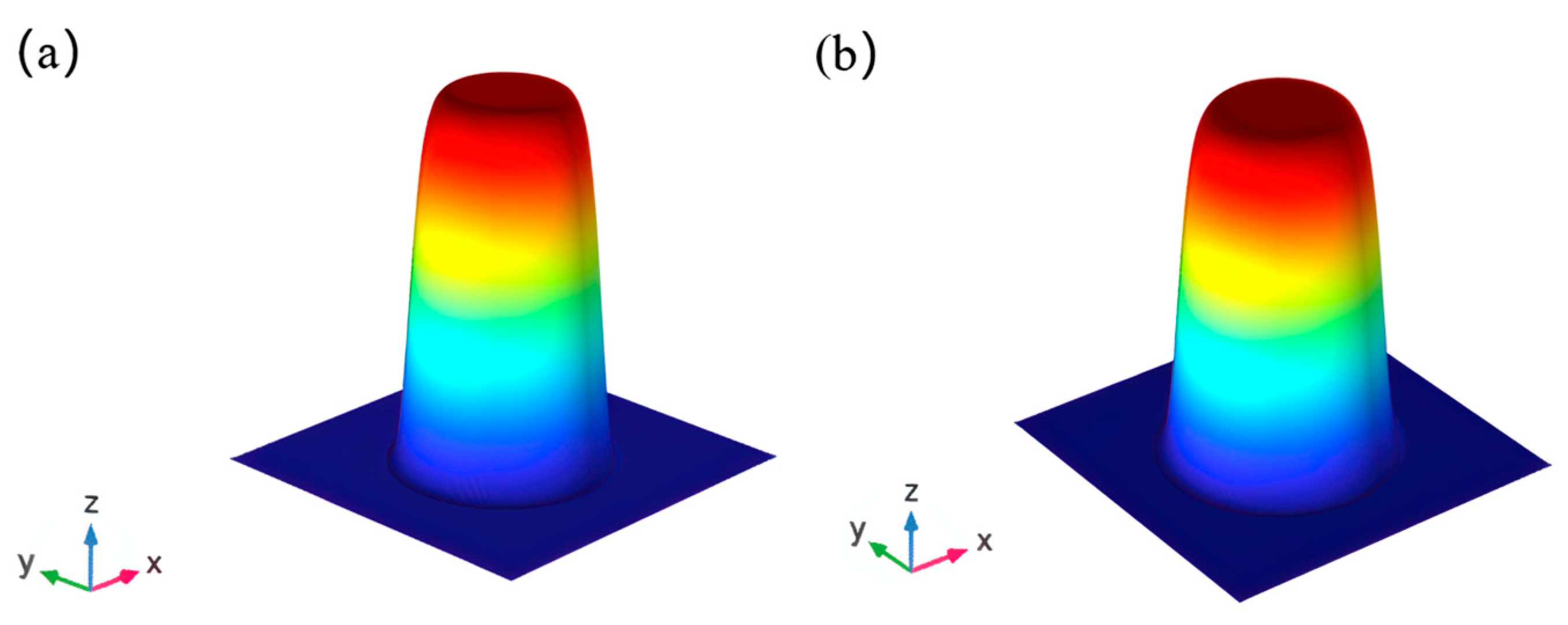
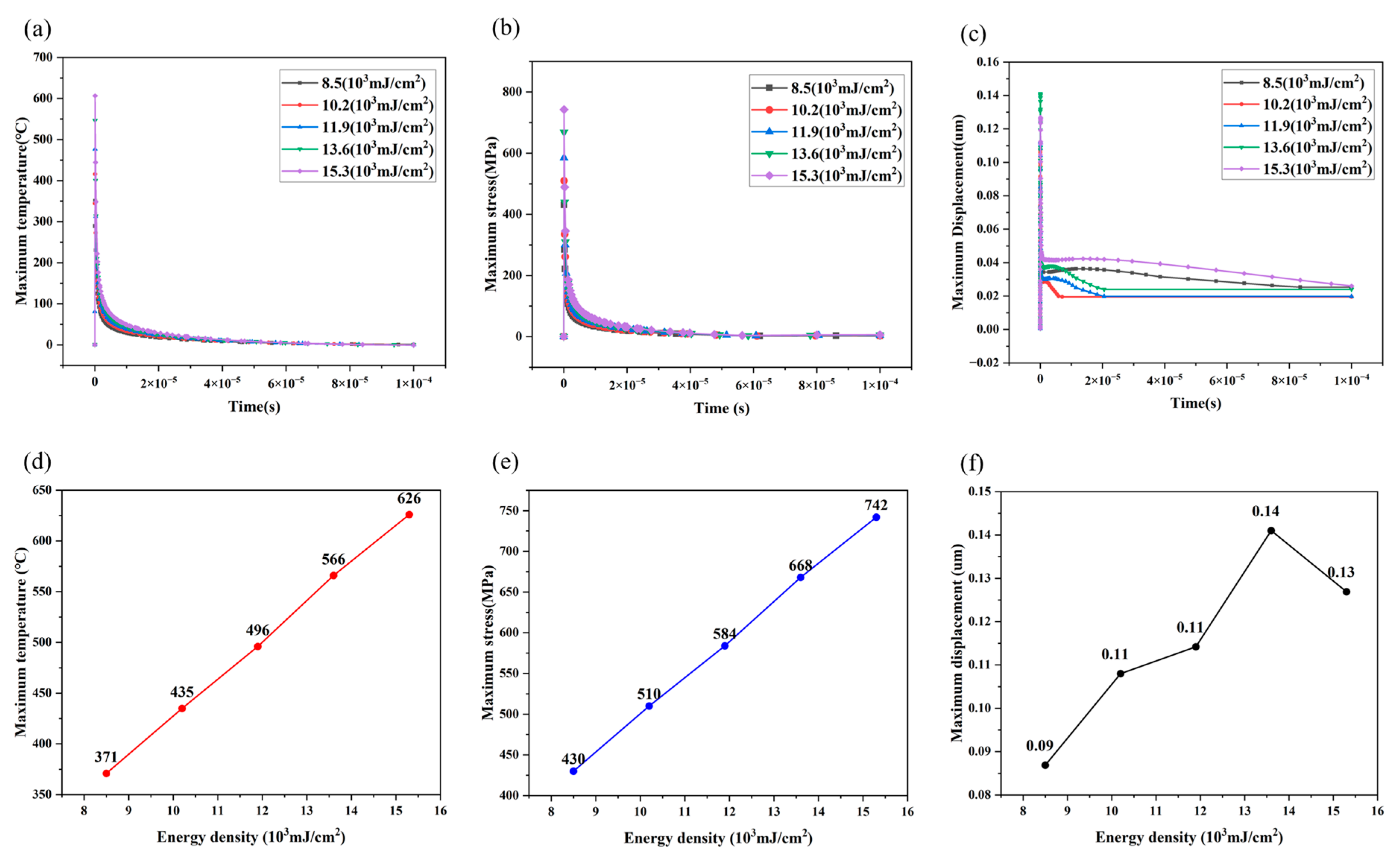
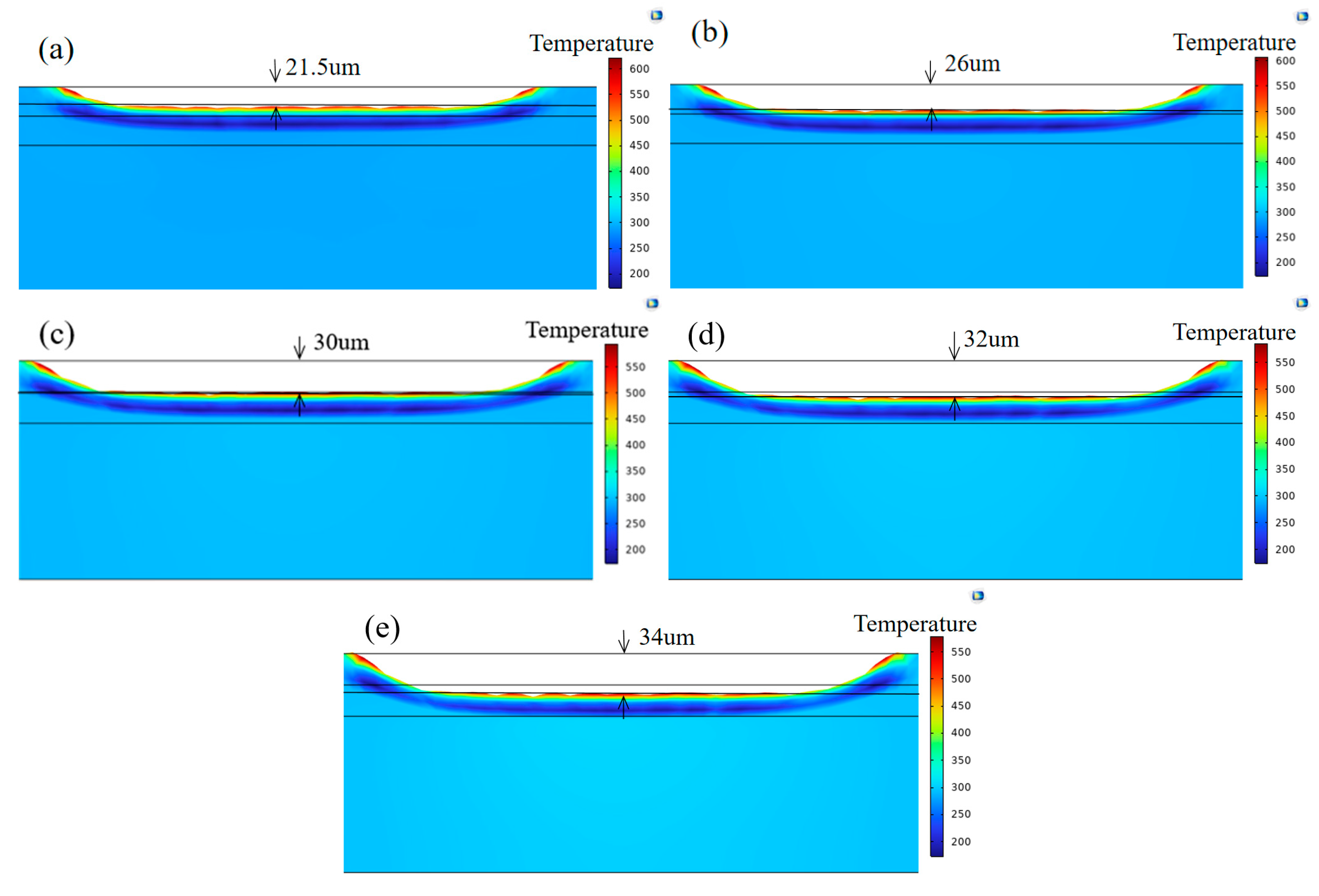
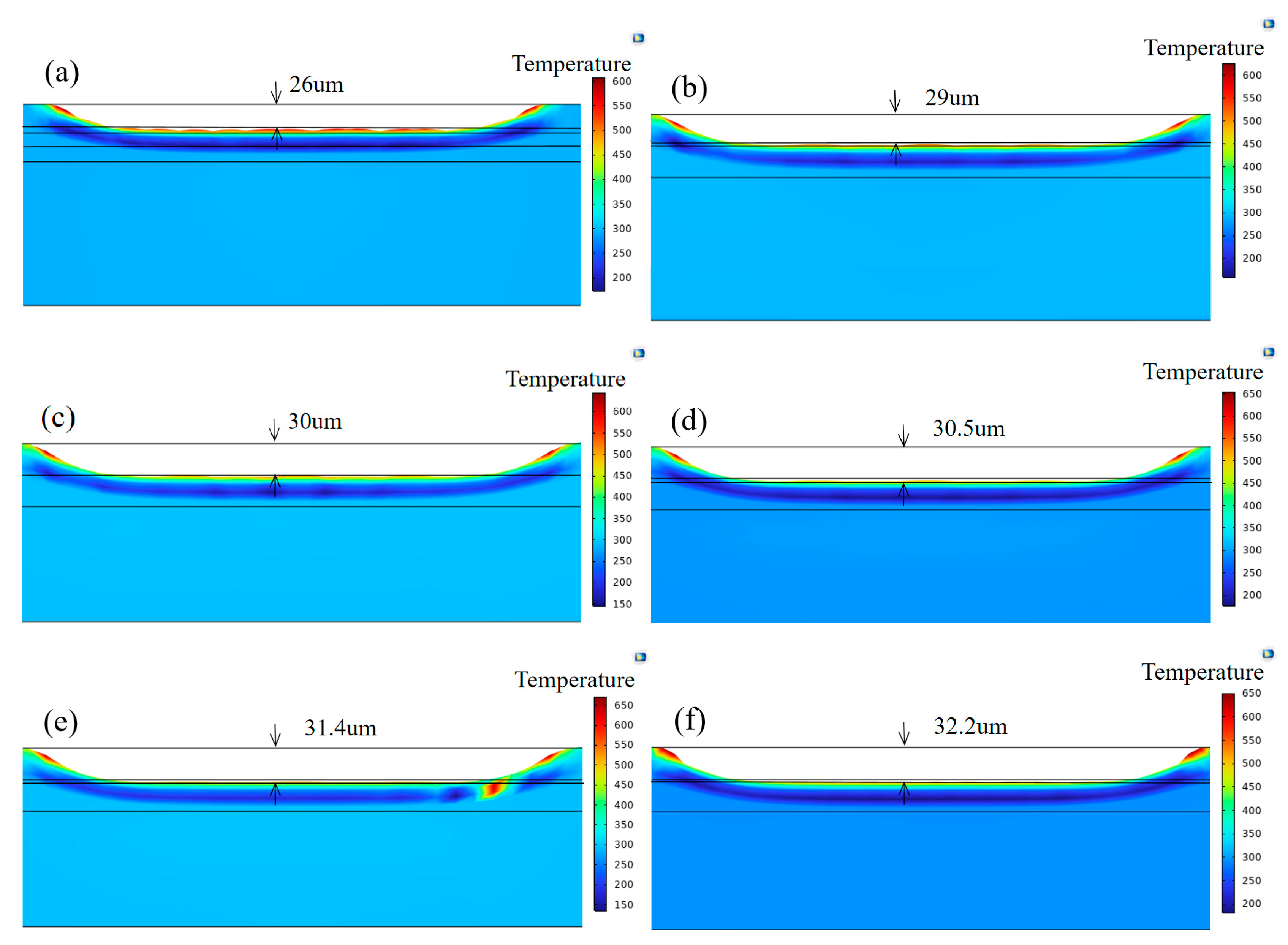
3.1. Simulation Results
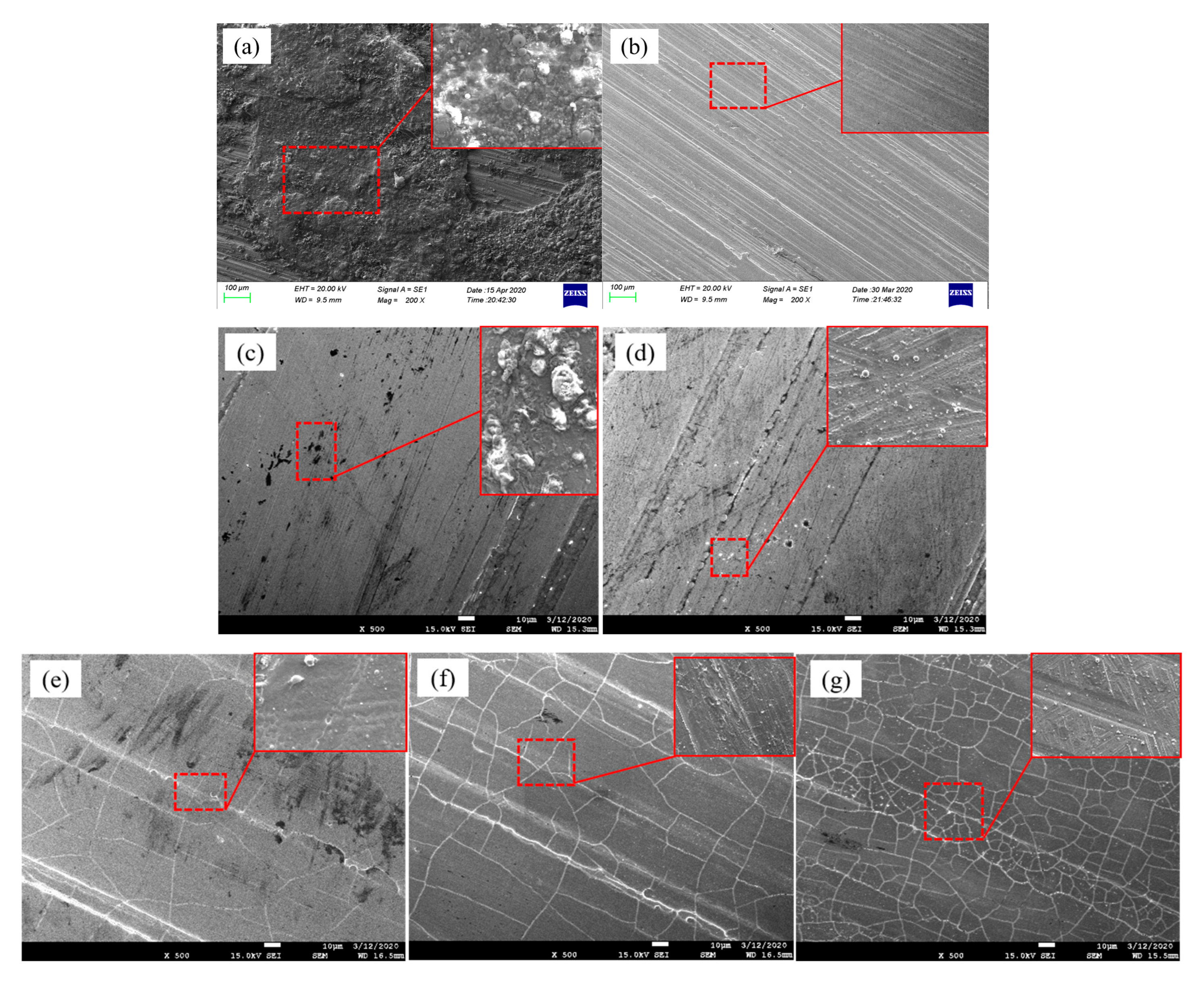
3.2. Microstructure
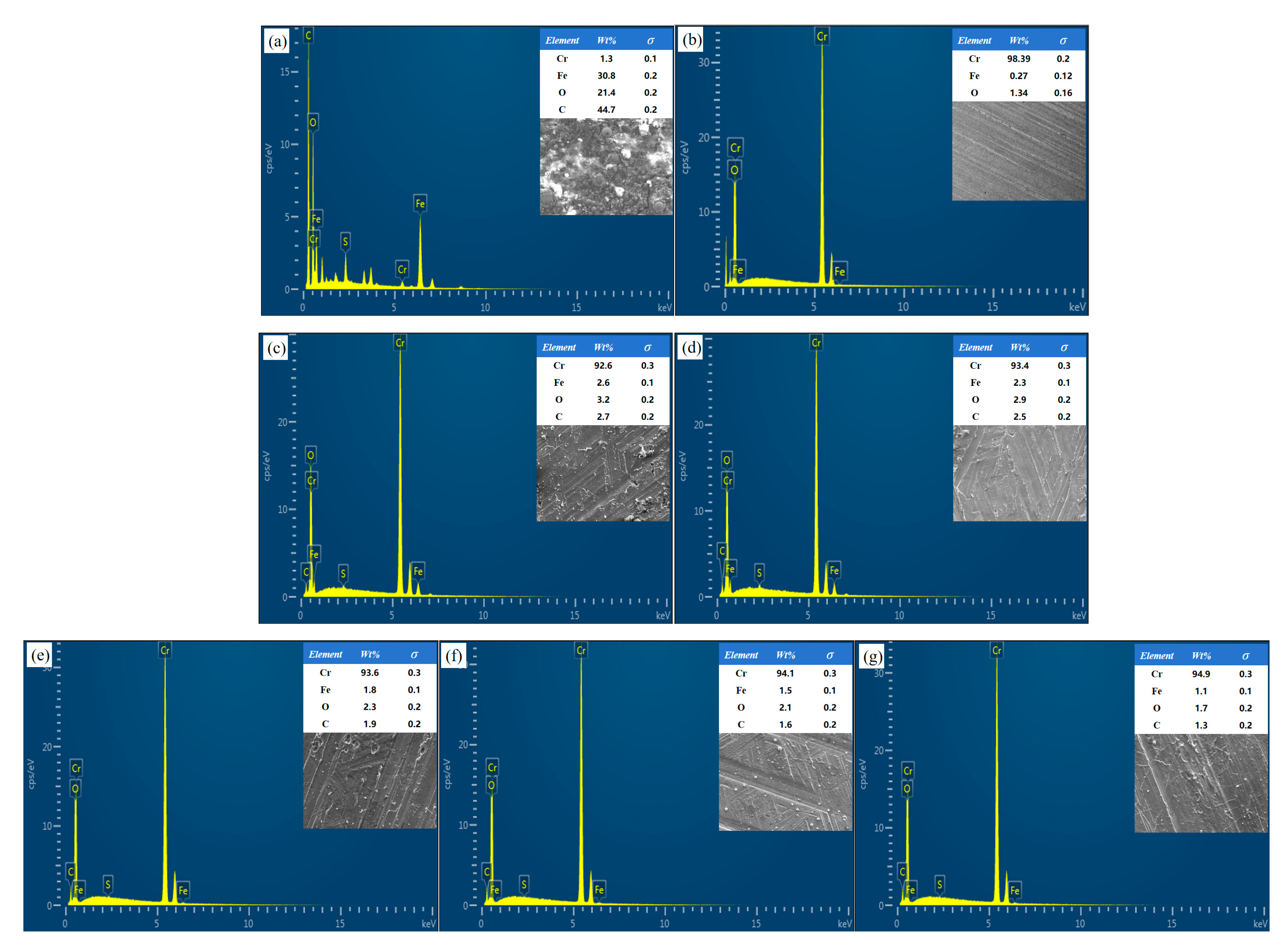
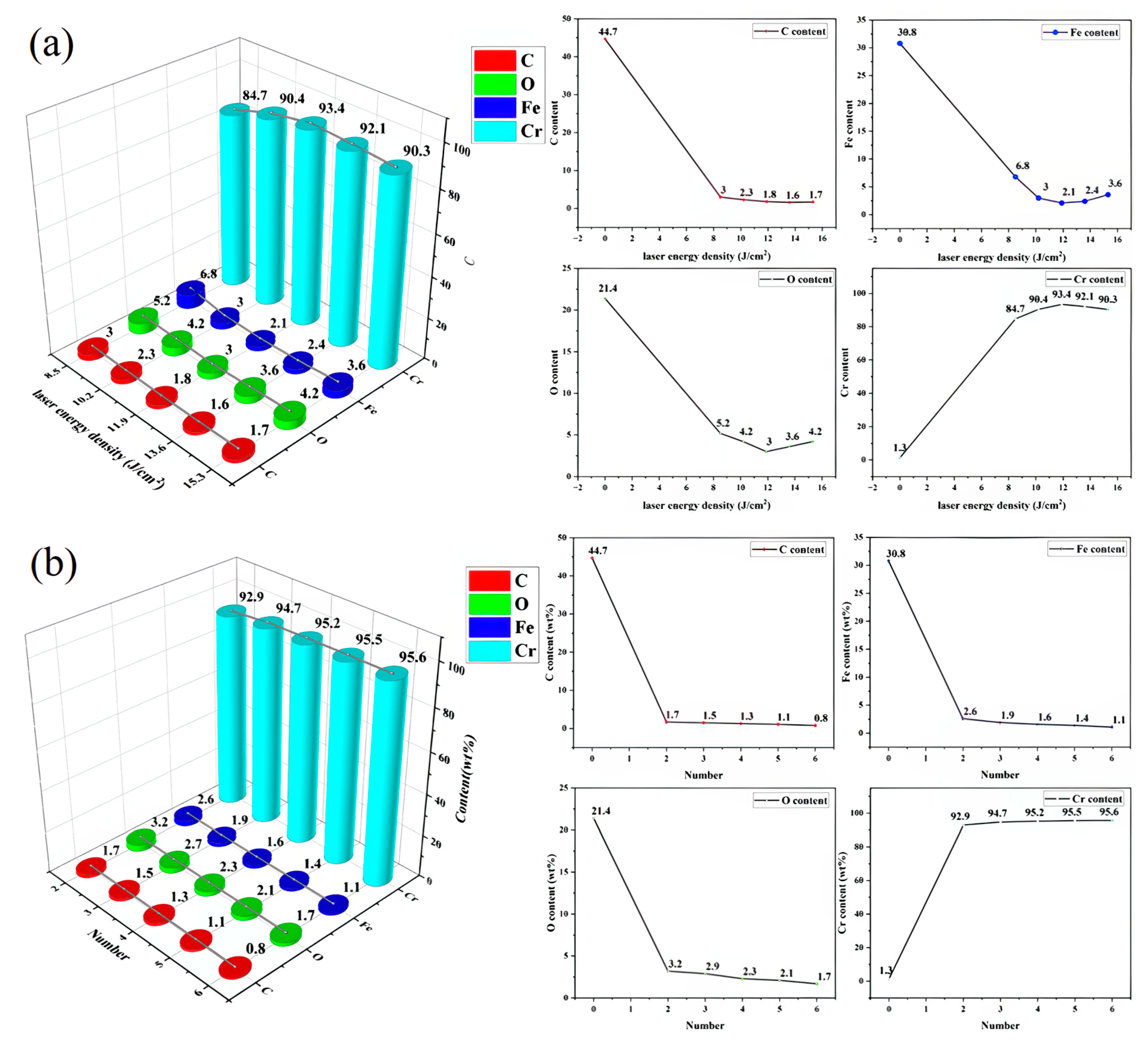
3.3. Element Distribution
3.4. Chromium Coating Thickness Analysis
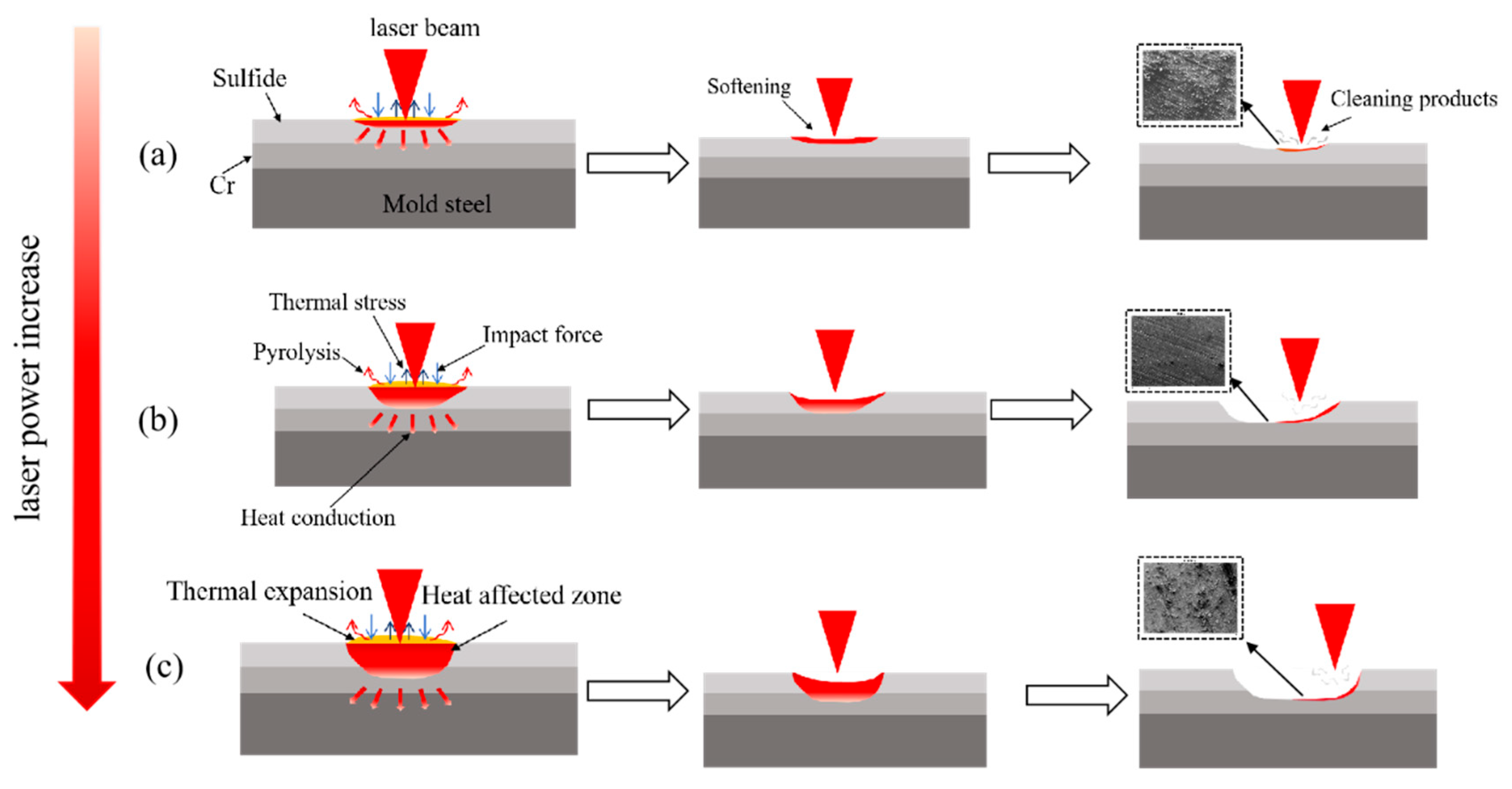
3.5. Removal Mechanism Analysis
4. Conclusions
- (1)
- The COMSOL thermal–mechanical coupling model established is highly consistent with experimental results, precisely defining the energy density and pulse frequency process window for laser cleaning of Cr-plated moulds. This provides critical theoretical and practical guidance for optimising cleaning processes, extending mould lifespan, and enhancing tyre production quality.
- (2)
- Within this energy density range, sulphides can be completely removed (EDS analysis shows Cr content increased to over 90%, with significant decreases in C and O), and the surface microstructure is smooth with no cracks or melt pits. Below mJ/cm2, cleaning is incomplete, while above mJ/cm2, thermal stress and excessive melting cause cracks and ablation in the Cr coating.
- (3)
- At mJ/cm2, only two to three pulses are required to achieve efficient cleaning (smooth surface, EDS element levels restored to original levels); more than three pulses result in increased thermal accumulation (simulated stress up to 742 MPa), causing microcracks in the Cr layer accompanied by local melting. After the fourth pulse, the coating thickness suddenly decreases from 2.6 μm to 2.0 μm and further thins to 1.6 μm after the fifth and sixth pulses.
- (4)
- Laser photothermal conversion causes the sulphide layer to pyrolyse, vaporise, and expand instantaneously. The pulse shock wave synergistically overcomes the adsorption force to achieve peeling. Under optimal parameters, the thermal expansion and thermal stress generated by the shock wave are insufficient to damage the Cr layer. However, when the energy density or pulse count is too high, the thermal stress combined with the shock pressure exceeds the fracture toughness of the Cr layer, leading to crack initiation, propagation, and local melting and ablation.
- (5)
- The thermal expansion stress shown in Figure 7 and Figure 8 is shear. Due to the significant differences in thermal properties (thermal expansion coefficient, elastic modulus, thermal conductivity, etc.) between the contaminant layer and the substrate, thermal expansion mismatch occurs when they are heated simultaneously, resulting in stress within the two materials and at their interface.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- D’Avico, L.; Beltrami, R.; Pargoletti, E.; Trasatti, S.; Cappelletti, G. Insight into the Release Agents/PVD Coatings Interaction for Plastic Mold Technology. Coatings 2020, 10, 281. [Google Scholar] [CrossRef]
- Hanulikova, B.; Shejbalova, D.; Dvorak, Z. Infrared analysis of fouling during EPDM curing studied on molds made of steel and aluminium alloys. Rubber Chem. Technol. 2018, 91, 390–400. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z.; Wang, X.; Xu, X.; Wu, Y.; Wu, D. Comparative study of anti-sticking properties of coatings for tire molds. Coatings 2022, 12, 1740. [Google Scholar] [CrossRef]
- Fragassa, C.; Ippoliti, M. Technology Assessment of Tire Mould Cleaning Systems and Quality Finishing. Int. J. Qual. Res. 2016, 10, 523–546. [Google Scholar]
- Vítězslav, M.; David, H.; Dalimil, P. Industrial use of dry ice blasting in surface cleaning. J. Clean. Prod. 2021, 329, 129630. [Google Scholar] [CrossRef]
- Tai, X.; Liu, C.; Chen, W.; Mai, Y.; Zhang, L.; Wu, X.; Jie, X. Electroplated wear and corrosion–resistant Co-Mo/CeO2 composite coatings for reducing mold fouling application. J. Solid State Electrochem. 2023, 27, 679–694. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, W.; Wu, J.; Chen, H.; Zhang, F.; Wang, S. The Fundamental Mechanisms of Laser Cleaning Technology and Its Typical Applications in Industry. Processes 2023, 11, 1445. [Google Scholar] [CrossRef]
- Deng, J.; Zhao, G.; Lei, J.; Zhong, L.; Lei, Z. Research Progress and Challenges in Laser-Controlled Cleaning of Aluminum Alloy Surfaces. Materials 2022, 15, 5469. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, L.; Wang, M.; Wang, Y. Simulation of nanosecond laser cleaning the paint based on the thermal stress. Optik 2021, 227, 165589. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, W.; Wang, S.; Wang, Y.; Pan, Y. Progress of laser cleaning technology from the perspective of Chinese patents. Front. Mech. Eng. 2024, 19, 44. [Google Scholar] [CrossRef]
- Zhu, G.; Xu, Z.; Jin, Y.; Chen, X.; Yang, L. Mechanism and application of laser cleaning: A review. Opt. Lasers Eng. 2022, 157. [Google Scholar] [CrossRef]
- Kong, D.J.; Feng, A.X.; Zhang, Y.K.; Lu, J.Z.; Tang, C.P. Experiment study on CO2 laser cleaning rubber sulfuring mould. ICO20 Lasers Laser Technol. 2005, 6028, 371–376. [Google Scholar]
- Ye, Y.; Jia, B.; Chen, J.; Jiang, Y.; Tang, H.; Wang, H.; Luan, X.; Liao, W.; Zhang, C.; Yao, C. Laser cleaning of the contaminations on the surface of tire mould. Int. J. Mod. Phys. B 2017, 31, 1744100. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, Y.; Chen, Y.; Wang, H.; Zhu, G.; Zhu, X. Laser cleaning of slots of chrome-plated die. Opt. Laser Technol. 2019, 119, 105659. [Google Scholar] [CrossRef]
- Cai, Y.; Song, X.; Chen, C.; Yang, J.; Gao, J.; Zhang, K. Effect of energy distribution on laser cleaning quality of 30Cr3 ultra-high strength steel. J. Mater. Res. Technol. 2024, 30, 4868–4880. [Google Scholar] [CrossRef]
- Jiang, J.; Zhai, H.; Gong, P.; Zhang, W.; He, X.; Ma, X.; Wang, B. In-situ study on the tensile behavior of Cr-coated zircaloy for accident tolerant fuel claddings. Surf. Coat. Technol. 2020, 394, 125747. [Google Scholar] [CrossRef]
- Lu, J.; Liu, H.; Chen, Z.; Liao, X. Experimental investigation into the post-fire mechanical properties of hot-rolled and cold-formed steels. J. Constr. Steel Res. 2016, 121, 291–310. [Google Scholar] [CrossRef]
- He, C.; Yang, C.; Yang, H.; Wang, J.; Liu, J.; Deng, L.; Fang, L.; Li, C. Effect of Different Laser Parameters on Surface Physical Characteristics and Corrosion Resistance of 20 Steel in Laser Cleaning. Appl. Sci. 2024, 14, 2058. [Google Scholar] [CrossRef]
- Harish, D.V.N.; Bharatish, A.; Murthy, H.N.; Anand, B.; Subramanya, K.N. Investigation of thermal residual stresses during laser ablation of tantalum carbide coated graphite substrates using micro-Raman spectroscopy and COMSOL multiphysics. Ceram. Int. 2020, 47, 3498–3513. [Google Scholar] [CrossRef]
- Zhou, H.; Zhou, H.; Zhao, Z.; Li, K.; Yin, J. Numerical Simulation and Verification of Laser-Polishing Free Surface of S136D Die Steel. Metals 2021, 11, 400. [Google Scholar] [CrossRef]
- Hu, P.; Yao, L.; Zhang, M.; Nie, Z.; Ji, E.; Lue, Q.; He, Z. Femtosecond laser micro-milling dental glass ceramics: An experimental analysis and COMSOL finite element simulation. Ceram. Int. 2020, 46, 22146–22153. [Google Scholar] [CrossRef]
- Zhang, G.; Hua, X.; Huang, Y.; Zhang, Y.; Li, F.; Shen, C.; Cheng, J. Investigation on mechanism of oxide removal and plasma behavior during laser cleaning on aluminum alloy. Appl. Surf. Sci. 2020, 506, 144666. [Google Scholar] [CrossRef]
- Raddadi, M.; Mohamed, M.S.; Mahdy, A.M.S.; EIBary, A.A.; Lotfy, K. Pulsed laser heating-induced generalized thermo-acoustic-elastic waves with two-temperature theory. Arch. Appl. Mech. 2024, 95, 3. [Google Scholar] [CrossRef]
- Tayel, M.I. Thermoelastic Response Induced by Volumetric Absorption of Uniform Laser Radiation in a Half-Space. Coatings 2020, 10, 228. [Google Scholar] [CrossRef]
- Zhao, W.; Gu, Y.; Yang, Z.; Zhang, Z. Thermoelastic Vibration of Nickel Film Irradiated by Femtosecond Laser: Molecular Dynamics Study. Coatings 2024, 15, 1. [Google Scholar] [CrossRef]
- Dong, H.; Li, J.; Li, Y.; Zhang, W.; Jin, G. Thermal stress analysis of laser cleaning of aluminum alloy oxide film. Appl. Opt. 2023, 62, 7805–7811. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar-Nouri, Z.; Matthews, D.; Bolt, H.; De-Rooij, M. Insights in the wettability of hard chromium coated steel for cold rolling applications. Results Eng. 2024, 23, 102815. [Google Scholar] [CrossRef]
- Che, L.; Yanling, G. Flat-Top Line-Shaped Beam Shaping and System Design. Sensors 2022, 22, 4199. [Google Scholar]
- Fabbro, R.; Fournier, J.; Ballard, P.; Devaux, D.; Virmont, J. Physical study of laser-produced plasma in confined geometry. J. Appl. Phys. 1990, 68, 775–784. [Google Scholar] [CrossRef]






| Element | C | Si | Mn | P | S | Cr | Fe |
|---|---|---|---|---|---|---|---|
| Content/wt% | 0.14–0.22 | ≤0.3 | 0.3–0.65 | 0.045 | 0.05 | 0.15–0.35 | Bal. |
| Parameter | Value |
|---|---|
| Wavelength/nm | 1064 |
| Average power/W | ≤500 |
| Pulse Width/ns | ≤500 |
| Frequency/kHz | ≤500 |
| Scan Speed/mm·s−1 | ≤20,000 |
| Spot Diameter/um | 130 |
| Parameter | Value |
|---|---|
| Energy density/103 mJ·cm−2 | 8.5, 10.2, 11.9, 13.6, 15.3 |
| Pulse Width/ns | 100 |
| Frequency/kHz | 10 |
| Pulse count | 1, 2, 3, 4, 5, 6 |
| Parameter | Value |
|---|---|
| Density | |
| Specific heat capacity | |
| Thermal conductivity coefficient | |
| Decomposition temperature | |
| Laser absorption rate | |
| Surface emissivity | |
| Stefan–Boltzmann constant | |
| Coefficient of thermal expansion | |
| Young’s modulus | |
| Poisonby | |
| Density | |
| Young’s modulus | |
| h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Li, J.; Xue, Y.; Wang, L.; Ma, X.; Zhu, Y.; Yao, X.; Lin, L.; Cheng, W. The Effect of Laser Cleaning on the Cr Coating on the Surface of Steel Tyre Moulds. Coatings 2025, 15, 978. https://doi.org/10.3390/coatings15080978
Ren Y, Li J, Xue Y, Wang L, Ma X, Zhu Y, Yao X, Lin L, Cheng W. The Effect of Laser Cleaning on the Cr Coating on the Surface of Steel Tyre Moulds. Coatings. 2025; 15(8):978. https://doi.org/10.3390/coatings15080978
Chicago/Turabian StyleRen, Yuan, Jianfeng Li, Yinghao Xue, Liming Wang, Xinqiang Ma, Yongmei Zhu, Xingwei Yao, Li Lin, and Wei Cheng. 2025. "The Effect of Laser Cleaning on the Cr Coating on the Surface of Steel Tyre Moulds" Coatings 15, no. 8: 978. https://doi.org/10.3390/coatings15080978
APA StyleRen, Y., Li, J., Xue, Y., Wang, L., Ma, X., Zhu, Y., Yao, X., Lin, L., & Cheng, W. (2025). The Effect of Laser Cleaning on the Cr Coating on the Surface of Steel Tyre Moulds. Coatings, 15(8), 978. https://doi.org/10.3390/coatings15080978





