Effect of Different N2 Partial Pressures on the Corrosion Properties and Conductivity of NbNx Coated Titanium Bipolar Plates for PEMFCs
Abstract
1. Introduction
2. Experiments
2.1. Coating Deposition
2.2. Coating Characterization
2.3. Electrochemical Measurements
3. Results and Discussion
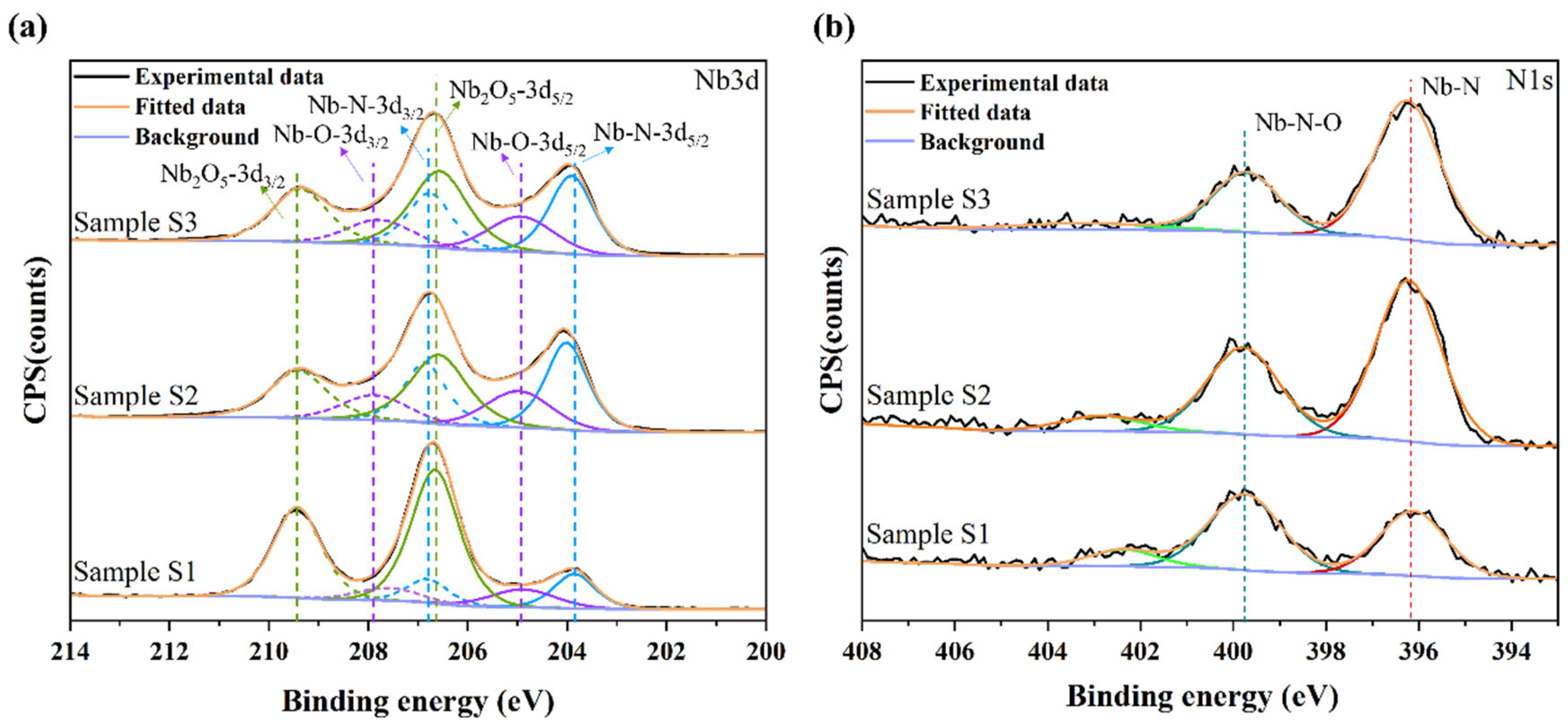
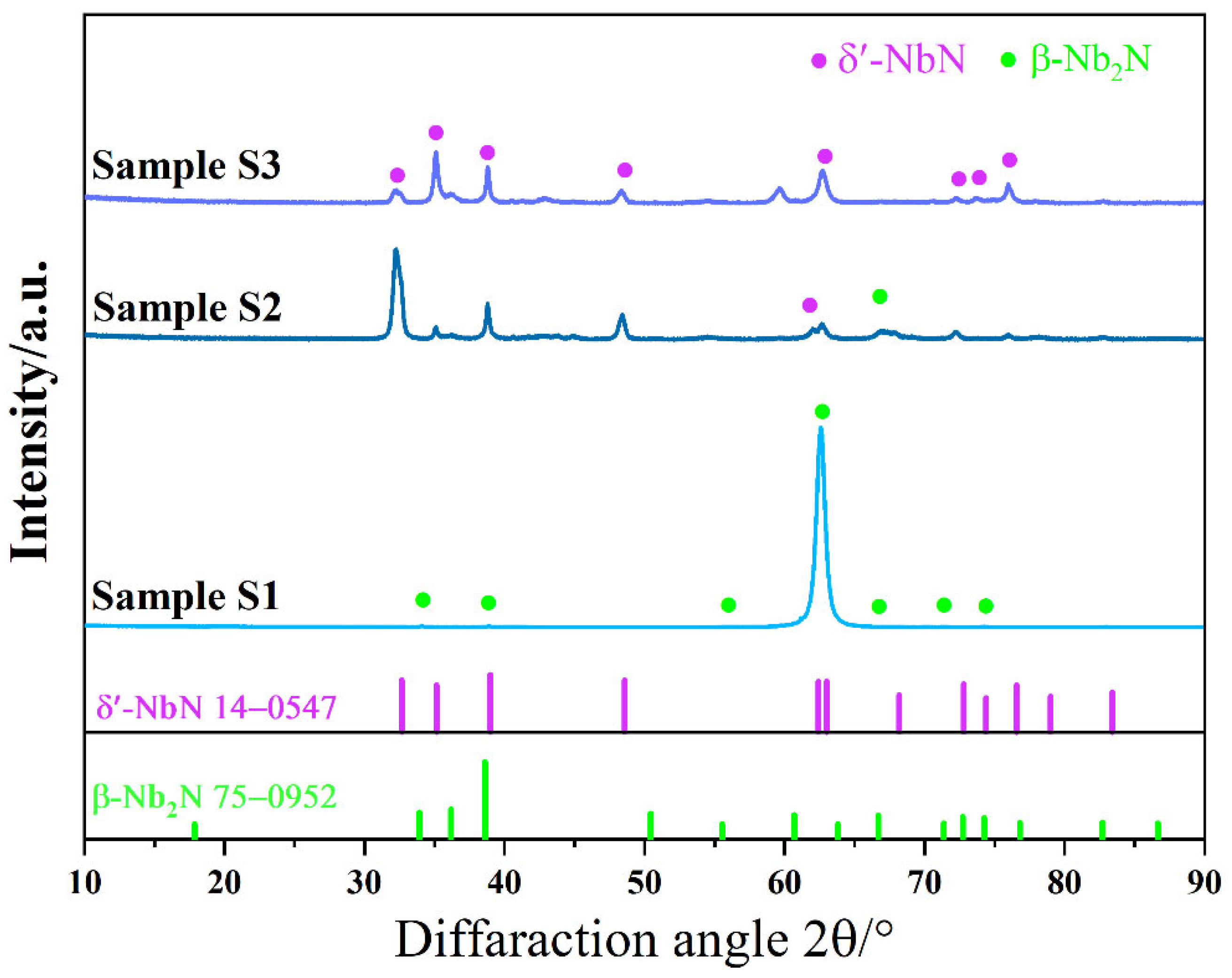
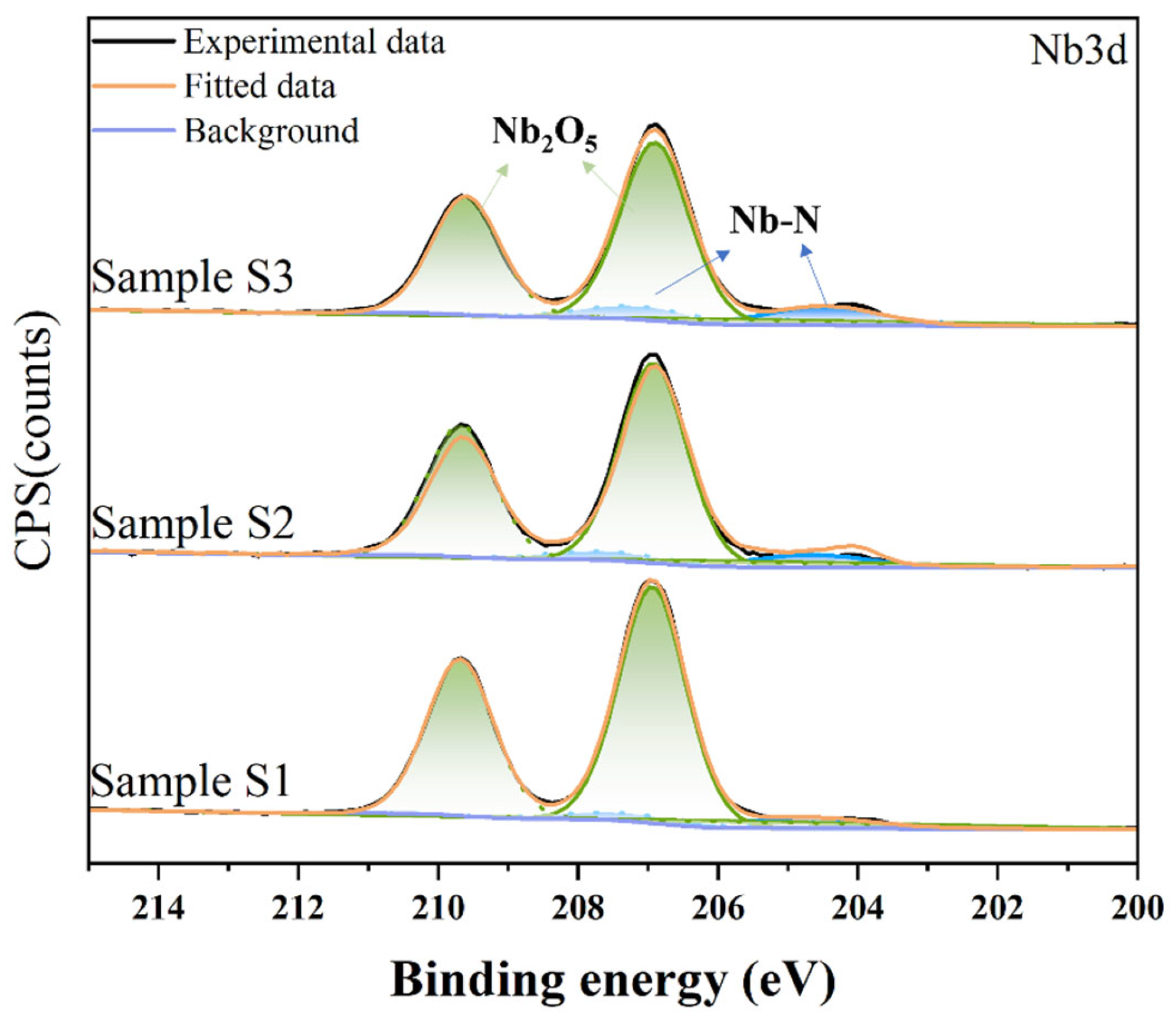
3.1. Microstructure and Characterization
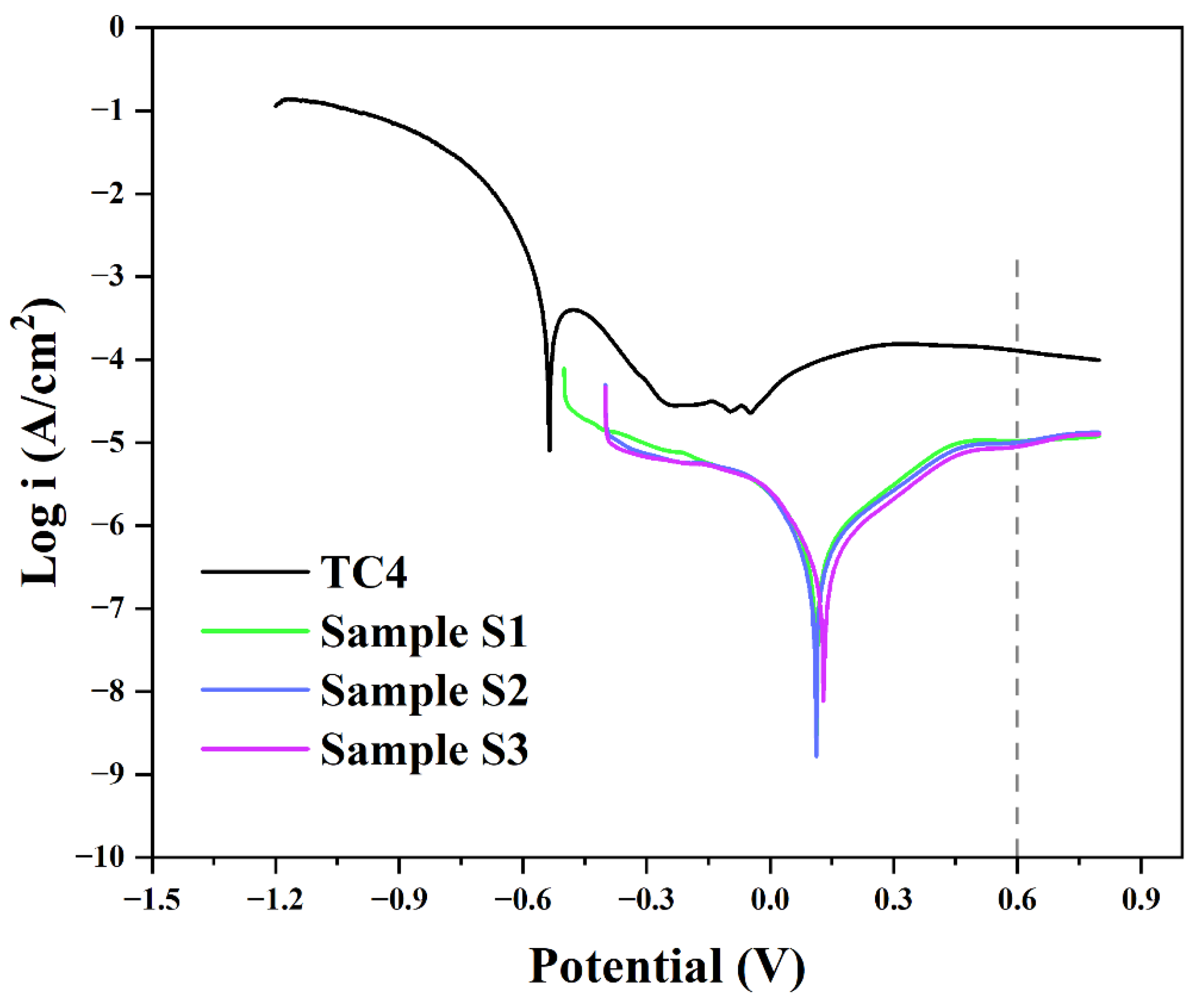
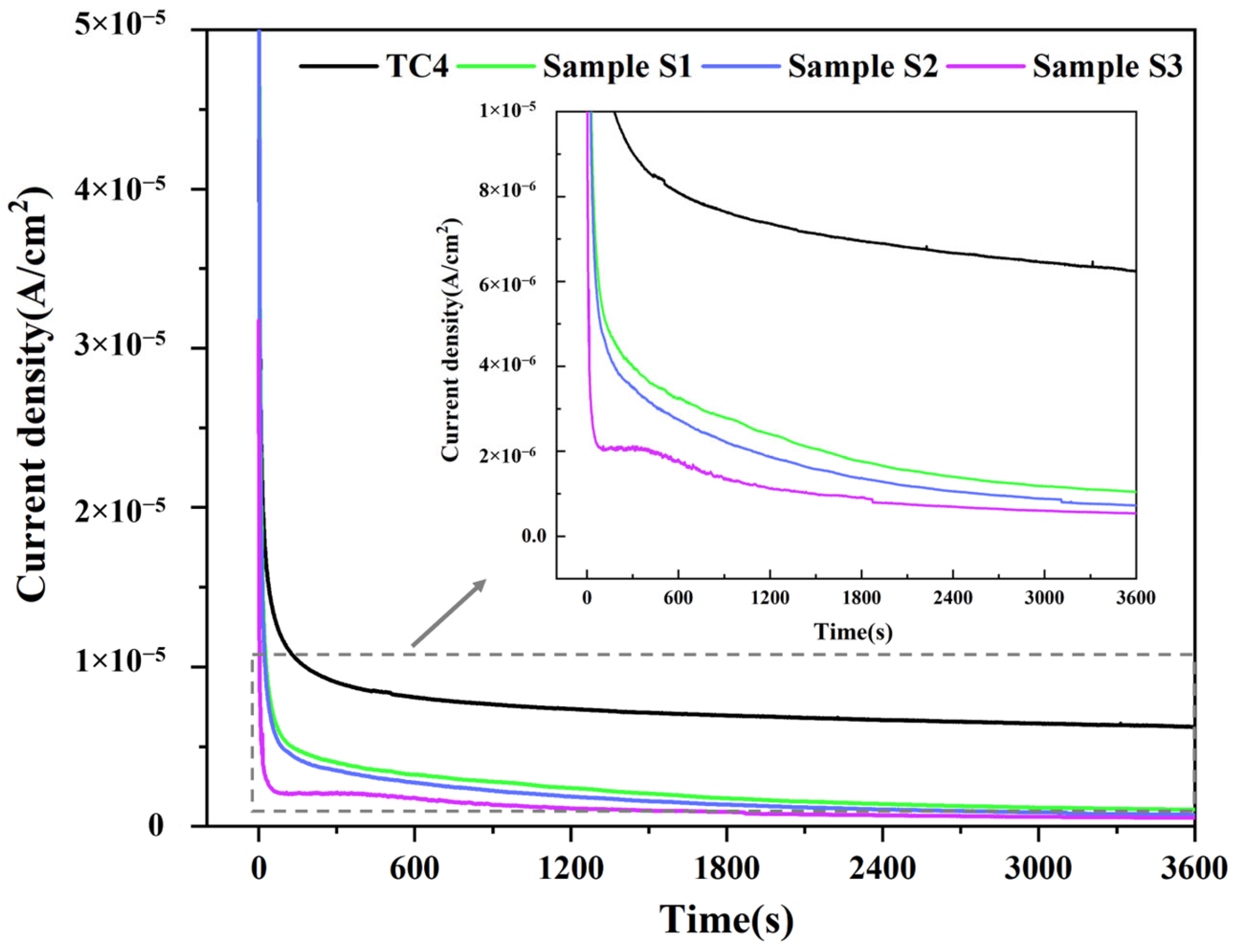
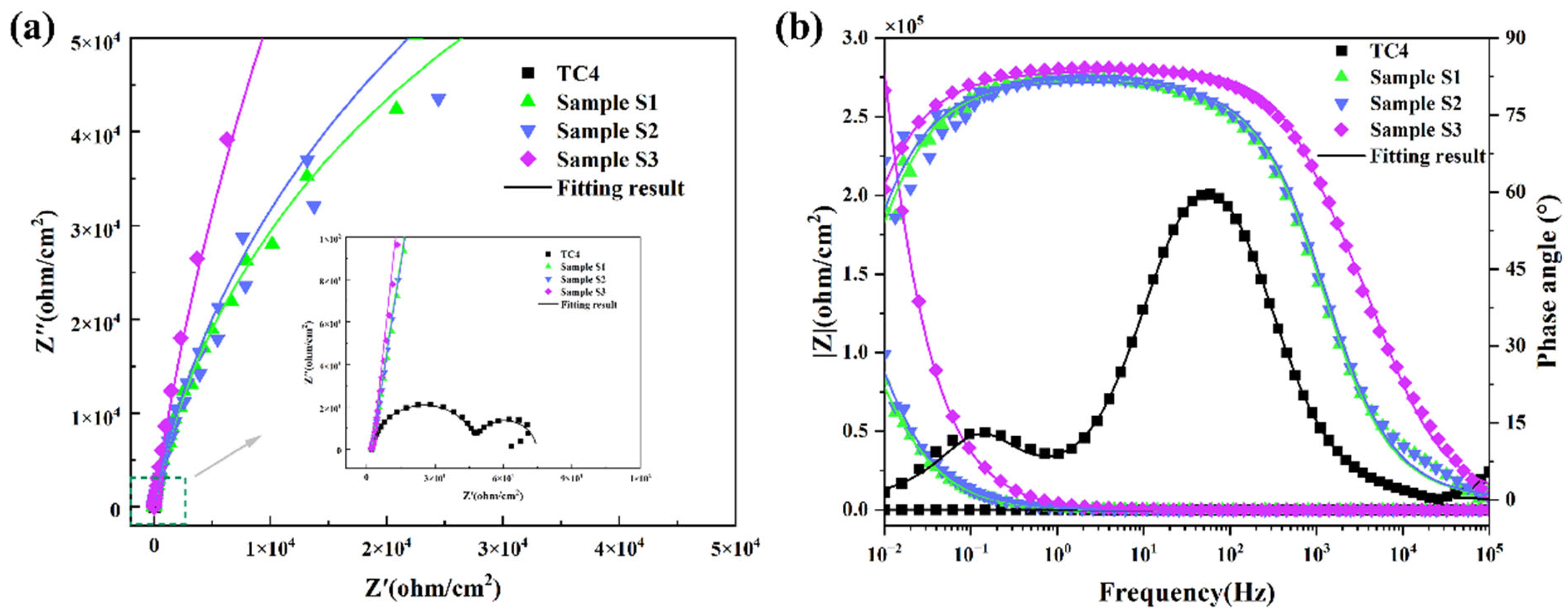
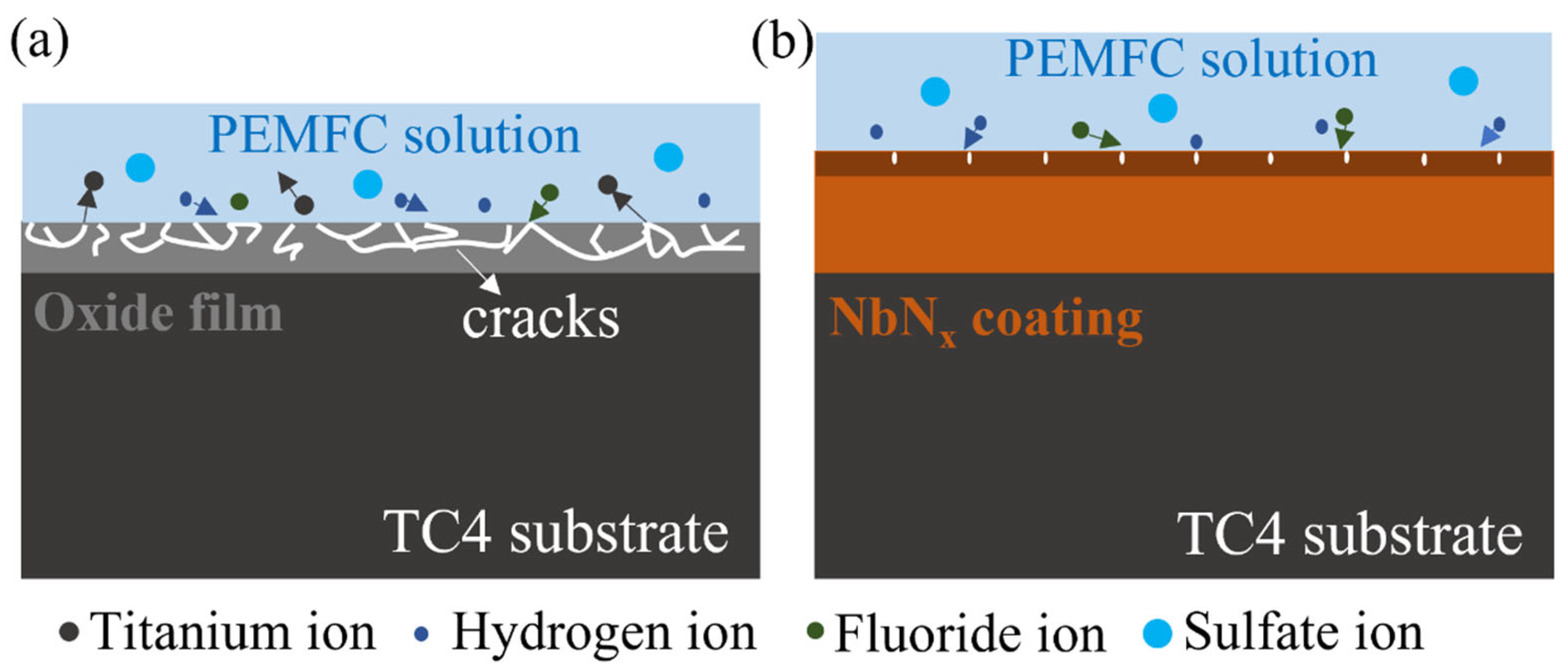
3.2. Corrosion Resistance
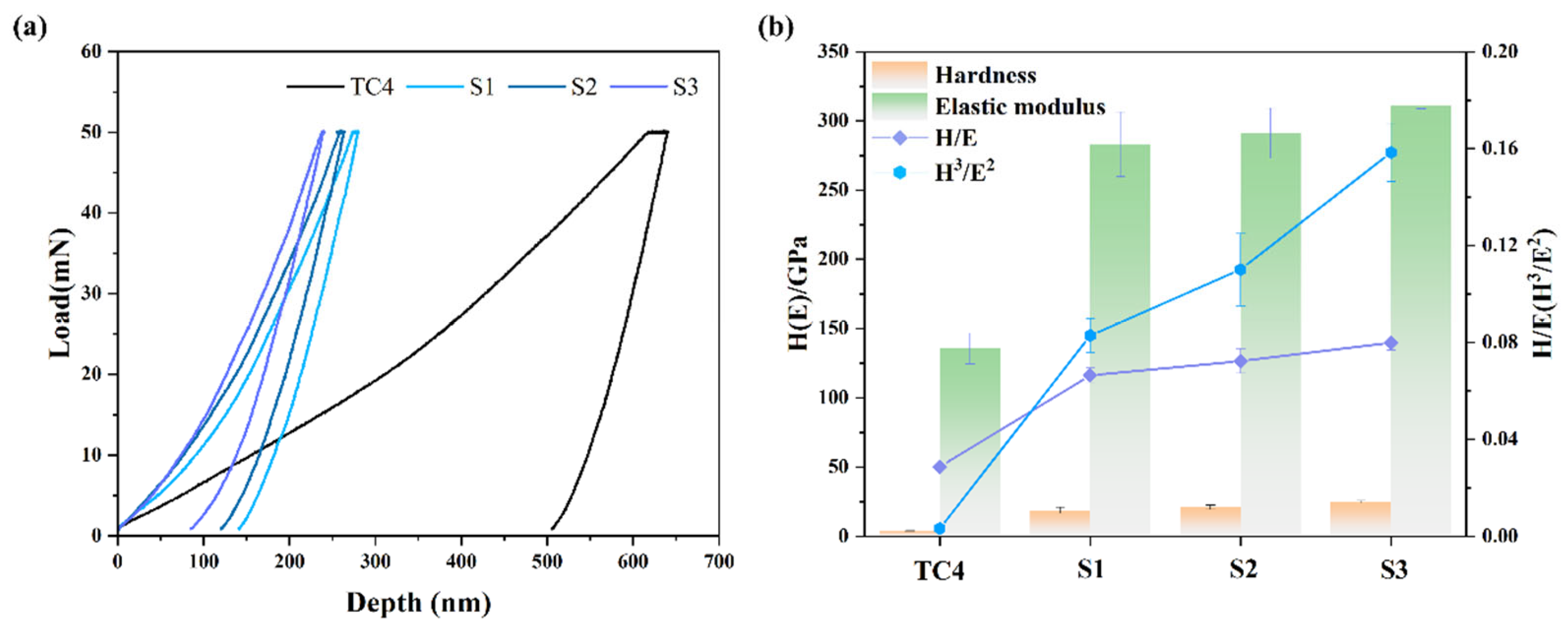
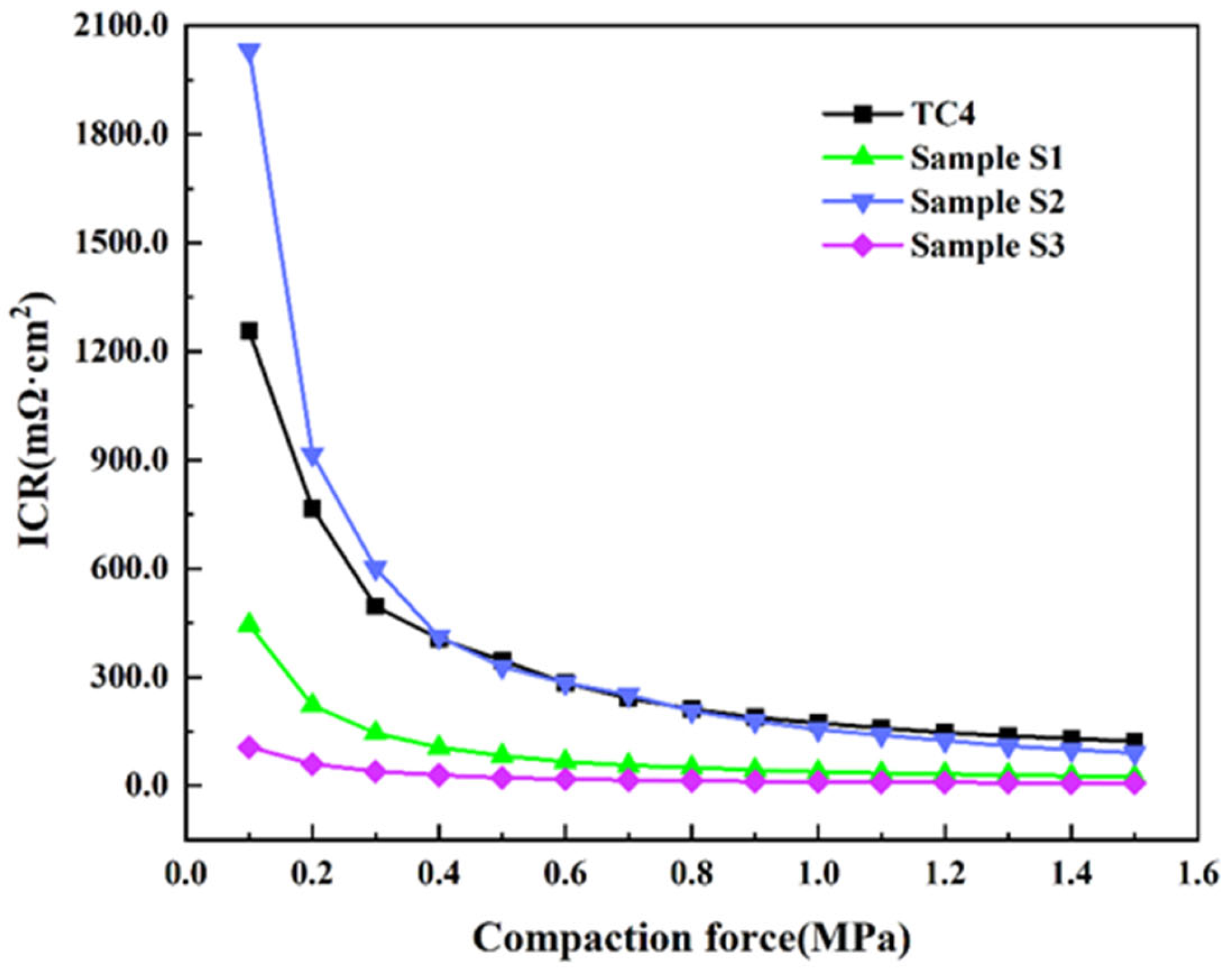
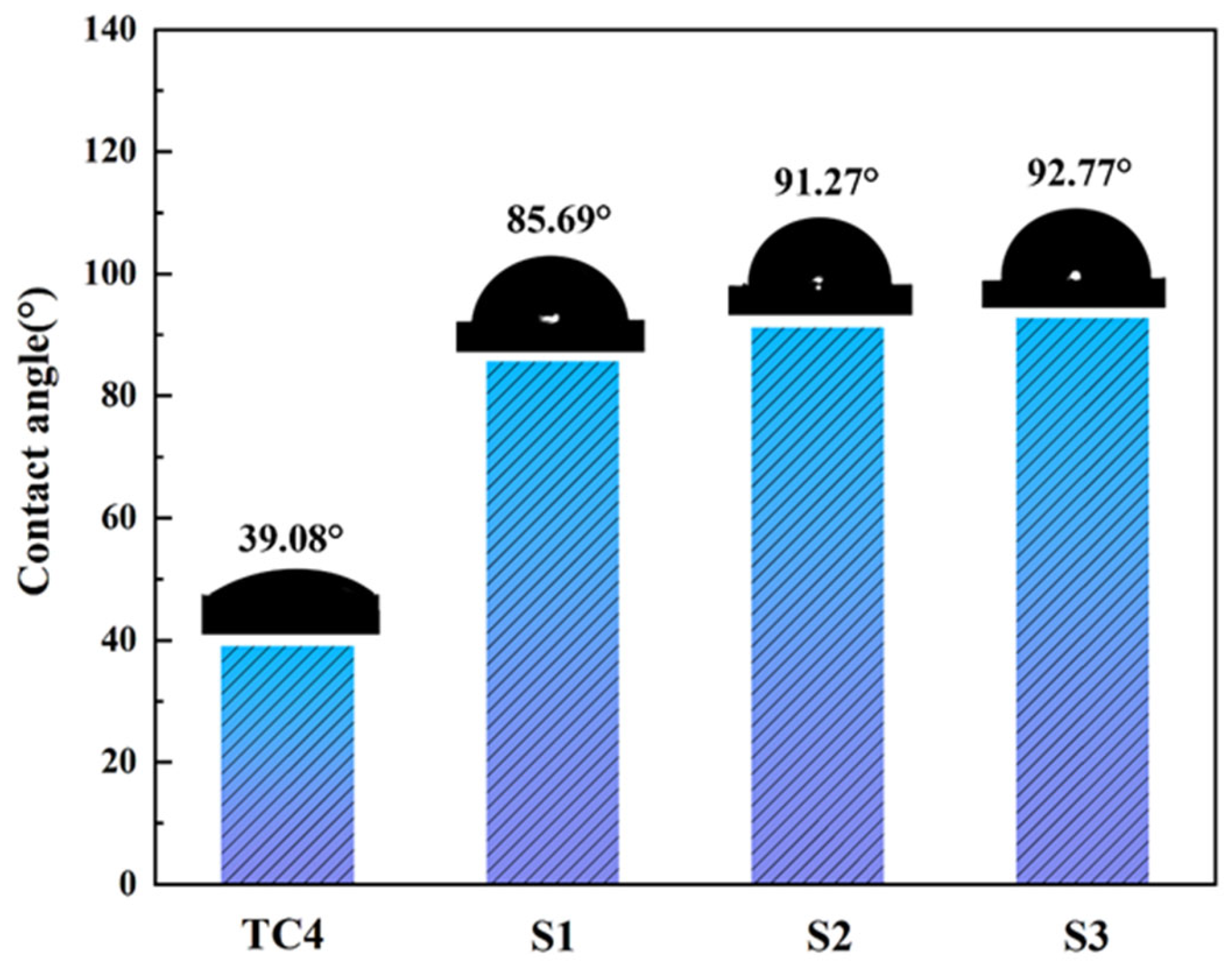
3.3. ICR and Hydrophobicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pan, T.; Zhang, B.; Li, J.; He, Y.; Lin, F. An investigation on corrosion protection of chromium nitride coated Fe–Cr alloy as a bipolar plate material for proton exchange membrane fuel cells. J. Power Sources 2014, 269, 81–87. [Google Scholar] [CrossRef]
- Antunes, R.A.; Oliveira, M.C.L.; Ett, G.; Ett, V. Corrosion of metal bipolar plates for PEM fuel cells: A review. Int. J. Hydrogen Energy 2010, 35, 3632–3647. [Google Scholar] [CrossRef]
- Yang, L.X.; Liu, R.J.; Wang, Y.; Liu, H.J.; Zeng, C.L.; Fu, C. Corrosion and interfacial contact resistance of nanocrystalline β-Nb2N coating on 430 FSS bipolar plates in the simulated PEMFC anode environment. Int. J. Hydrogen Energy 2021, 46, 32206–32214. [Google Scholar] [CrossRef]
- Kumar, A.; Ibraheem, S.; Ali, S.; Maiyalagan, T.; Javed, M.S.; Gupta, R.K.; Saad, A.; Yasin, G. Polypyrrole and Polyaniline-Based Membranes for Fuel Cell Devices: A Review. Surf. Interfaces 2022, 29, 101738. [Google Scholar] [CrossRef]
- Hermann, A.; Chaudhuri, T.; Spagnol, P. Bipolar plates for PEM fuel cells: A review. Int. J. Hydrogen Energy 2005, 30, 1297–1302. [Google Scholar] [CrossRef]
- Wang, Y.; Northwood, D. An investigation of the electrochemical properties of PVD TiN-coated SS410 in simulated PEM fuel cell environments. Int. J. Hydrogen Energy 2007, 32, 895–902. [Google Scholar] [CrossRef]
- Maistro, G.; Kante, S.; Nyborg, L.; Cao, Y. Low-temperature carburized high-alloyed austenitic stainless steels in PEMFC cathodic environment. Surf. Interfaces 2021, 24, 101093. [Google Scholar] [CrossRef]
- Asri, N.F.; Husaini, T.; Sulong, A.B.; Majlan, E.H.; Daud, W.R.W. Coating of stainless steel and titanium bipolar plates for anticorrosion in PEMFC: A review. Int. J. Hydrogen Energy 2017, 42, 9135–9148. [Google Scholar] [CrossRef]
- Wu, S.; Yang, W.; Yan, H.; Zuo, X.; Cao, Z.; Li, H.; Shi, M.; Chen, H. A review of modified metal bipolar plates for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2021, 46, 8672–8701. [Google Scholar] [CrossRef]
- Wind, J.; Späh, R.; Kaiser, W.; Böhm, G. Metallic bipolar plates for PEM fuel cells. J. Power Sources 2002, 105, 256–260. [Google Scholar] [CrossRef]
- Wang, H. Stainless steel as bipolar plate material for polymer electrolyte membrane fuel cells. J. Power Sources 2003, 115, 243–251. [Google Scholar] [CrossRef]
- Garzon-Fontecha, A.; Castillo, H.; Restrepo-Parra, E.; De La Cruz, W. The role of the nitrogen flow rate on the transport properties of CrN thin films produced by DC magnetron sputtering. Surf. Coat. Technol. 2018, 334, 98–104. [Google Scholar] [CrossRef]
- Pei, C.; Deng, L.; Xiang, C.; Zhang, S.; Sun, D. Effect of the varied nitrogen vacancy concentration on mechanical and electrical properties of ZrNx thin films. Thin Solid Film. 2019, 683, 57–66. [Google Scholar] [CrossRef]
- Li, T.; Yan, Z.; Liu, Z.; Yan, Y.; Chen, Y. Surface microstructure and performance of TiN monolayer film on titanium bipolar plate for PEMFC. Int. J. Hydrogen Energy 2021, 46, 31382–31390. [Google Scholar] [CrossRef]
- Alishahi, M.; Mahboubi, F.; Khoie, S.M.; Aparicio, M.; Hübner, R.; Soldera, F.; Gago, R. Electrochemical behavior of nanocrystalline Ta/TaN multilayer on 316L stainless steel: Novel bipolar plates for proton exchange membrane fuel-cells. J. Power Sources 2016, 322, 1–9. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, S.; Li, J.; Chen, Z.; Sun, D. Effects of phase transformation on the corrosion resistance and conductivity of NbN coatings for metal bipolar plates. Surf. Coat. Technol. 2025, 497, 131800. [Google Scholar] [CrossRef]
- Mamun, M.; Farha, A.; Er, A.; Ufuktepe, Y.; Gu, D.; Elsayed-Ali, H.; Elmustafa, A. Nanomechanical properties of NbN films prepared by pulsed laser deposition using nanoindendation. Appl. Surf. Sci. 2012, 258, 4308–4313. [Google Scholar] [CrossRef]
- Gao, C.; Ran, Y.; Guo, Q.; Wang, T.; Lu, H.; Jiang, Z.; Wang, Z. Plasmonic characteristics of niobium nitride thin films modulated by assisting ions. Surf. Interfaces 2021, 24, 101024. [Google Scholar] [CrossRef]
- Sun, W.; Lv, Y.; Gao, J.; Feng, Q.; Jia, B.; Ma, F. Highly conductive and corrosion-resistant NbN coatings on Ti bipolar plate for proton exchange membrane water electrolysis. J. Mater. Sci. Technol. 2024, 210, 86–96. [Google Scholar] [CrossRef]
- Daudt, N.F.; Schneider, A.D.; Arnemann, E.R.; Scheuer, C.J.; Dorneles, L.S.; Schelp, L.F. Fabrication of NbN-Coated Porous Titanium Sheets for PEM Electrolyzers. J. Mater. Eng. Perform. 2020, 29, 5174–5183. [Google Scholar] [CrossRef]
- Yi, J.; Miao, Q.; Liang, W.; Ding, Z.; Qi, Y.; Lin, H.; Huang, C.; Li, Y. A study for pre-processing of Nb diffusion in Nb–N layer by double-glow plasma alloying. J. Alloy. Compd. 2020, 820, 153121. [Google Scholar] [CrossRef]
- Shen, H.; Wang, L. Characterization and properties of NbN–Nb bilayer formed on titanium for bipolar plates. Mater. Chem. Phys. 2022, 290, 126628. [Google Scholar] [CrossRef]
- Qi, Z.; Wu, Z.; Zhang, D.; Zuo, J.; Wang, Z. Microstructure, mechanical properties and oxidation behaviors of magnetron sputtered NbNx coatings. J. Alloy. Compd. 2016, 675, 22–30. [Google Scholar] [CrossRef]
- Cansever, N.; Danışman, M.; Kazmanlı, K. The effect of nitrogen pressure on cathodic arc deposited NbN thin films. Surf. Coat. Technol. 2008, 202, 5919–5923. [Google Scholar] [CrossRef]
- Alfonso, J.E.; Buitrago, J.; Torres, J.; Marco, J.F.; Santos, B. Influence of fabrication parameters on crystallization, microstructure, and surface composition of NbN thin films deposited by rf magnetron sputtering. J. Mater. Sci. 2010, 45, 5528–5533. [Google Scholar] [CrossRef]
- Chen, M.; Ding, J.C.; Kwon, S.-H.; Wang, Q.; Zhang, S. Corrosion resistance and conductivity of NbN-coated 316L stainless steel bipolar plates for proton exchange membrane fuel cells. Corros. Sci. 2022, 196, 110042. [Google Scholar] [CrossRef]
- Fenker, M.; Balzer, M.; Büchi, R.; Jehn, H.; Kappl, H.; Lee, J.-J. Deposition of NbN thin films onto high-speed steel using reactive magnetron sputtering for corrosion protective applications. Surf. Coat. Technol. 2002, 163–164, 169–175. [Google Scholar] [CrossRef]
- Sandu, C.; Benkahoul, M.; Parlinska-Wojtan, M.; Sanjinés, R.; Lévy, F. Morphological, structural and mechanical properties of NbN thin films deposited by reactive magnetron sputtering. Surf. Coat. Technol. 2006, 200, 6544–6548. [Google Scholar] [CrossRef]
- Sanjinés, R.; Benkahoul, M.; Sandu, C.S.; Schmid, P.E.; Lévy, F. Electronic States and Physical Properties of Hexagonal β-Nb2N and δ′-NbN Nitrides. Thin Solid Film. 2006, 494, 190–195. [Google Scholar] [CrossRef]
- Wang, T.; Cao, H.; Ma, X.; Shen, X.; Min, Y.; Xu, Q. Electrodeposited Ti3C2Tx MXene composite coating toward superior surface protection on aluminum alloy in PEMFC environments. Corros. Sci. 2024, 232, 112044. [Google Scholar] [CrossRef]
- Chang, C.-L.; Huang, C.-H.; Lin, C.-Y.; Yang, F.-C.; Tang, J.-F. Mechanical properties of amorphous and crystalline CrN/CrAlSiN multilayer coating fabricated using HPPMS. Surf. Interfaces 2022, 31, 102064. [Google Scholar] [CrossRef]
- Chen, X.; Du, Y.; Chung, Y.-W. Commentary on using H/E and H/E as proxies for fracture toughness of hard coatings. Thin Solid Film 2019, 688, 137265. [Google Scholar] [CrossRef]
- Johar, M.; Moradizadeh, L.; Gupta, A.; Chellehbari, Y.M.; Li, X.; Shahgaldi, S. Development of novel Nb and Ta multilayer coatings for corrosion protection of Ti-based bipolar plates for proton exchange membrane fuel cells. Corros. Sci. 2025, 245, 112707. [Google Scholar] [CrossRef]
- Heo, H.-S.; Kim, S.-J. Effects of PVD deposition method on electrochemical properties and interfacial contact resistance of CrN coated titanium as PEMFC bipolar plate. Trans. IMF 2023, 101, 261–268. [Google Scholar] [CrossRef]
- Conde, J.J.; Ferreira-Aparicio, P.; Chaparro, A.M. Anti-corrosion coating for metal surfaces based on superhydrophobic electrosprayed carbon layers. Appl. Mater. Today 2018, 13, 100–106. [Google Scholar] [CrossRef]
- Bocchetta, P.; Chen, L.-Y.; Tardelli, J.D.C.; dos Reis, A.C.; Almeraya-Calderón, F.; Leo, P. Passive Layers and Corrosion Resistance of Biomedical Ti-6Al-4V and β-Ti Alloys. Coatings 2021, 11, 487. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, Q.-X.; Chen, G.-Q.; Cao, X.; Zhang, S.; Pan, J.-L.; Zhang, G.; Shi, Q.-Y. Enhanced corrosion resistance of AZ91 magnesium alloy through refinement and homogenization of surface microstructure by friction stir processing. Corros. Sci. 2018, 138, 284–296. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, S.; Zheng, J.; Dong, Y.; Zhang, C.; Li, J.; Chen, Z.; Zhang, J.; Sun, D. Excellent anti-corrosion and conductivity of NbN coated on Ti bipolar plate by controlling N2 flow rates. J. Alloy. Compd. 2023, 976, 173033. [Google Scholar] [CrossRef]
- Jin, J.; Zhang, J.; Hu, M.; Li, X. Investigation of high potential corrosion protection with titanium carbonitride coating on 316L stainless steel bipolar plates. Corros. Sci. 2021, 191, 109757. [Google Scholar] [CrossRef]
- Jorcin, J.-B.; Orazem, M.E.; Pébère, N.; Tribollet, B. CPE analysis by local electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 1473–1479. [Google Scholar] [CrossRef]
- Jin, J.; Zhao, X.; Liu, H. Durability and degradation of CrMoN coated SS316L in simulated PEMFCs environment: High potential polarization and electrochemical impedance spectroscopy (EIS). Int. J. Hydrogen Energy 2019, 44, 20293–20303. [Google Scholar] [CrossRef]
- Jeon, W.-S.; Kim, J.-G.; Kim, Y.-J.; Han, J.-G. Electrochemical properties of TiN coatings on 316L stainless steel separator for polymer electrolyte membrane fuel cell. Thin Solid Film. 2008, 516, 3669–3672. [Google Scholar] [CrossRef]
- Luo, X.; Chang, L.; Ren, C.; Ding, Y.; Zhang, J.; Zhang, D.; Yao, J.; Deng, Z.; Dong, C. Dynamic response to fluctuating input of Nb:Ti:N film modified Ti bipolar plates for proton exchange membrane water electrolyser. Corros. Sci. 2025, 249. [Google Scholar] [CrossRef]
- Lee, C.-H.; Lee, Y.-B.; Kim, K.-M.; Jeong, M.-G.; Lim, D.-S. Electrically conductive polymer composite coating on aluminum for PEM fuel cells bipolar plate. Renew. Energy 2013, 54, 46–50. [Google Scholar] [CrossRef]
- Zhang, P.; Hao, C.; Han, Y.; Du, F.; Wang, H.; Wang, X.; Sun, J. Electrochemical behavior and surface conductivity of NbC modified Ti bipolar plate for proton exchange membrane fuel cell. Surf. Coat. Technol. 2020, 397, 126064. [Google Scholar] [CrossRef]
- Xu, J.; Xu, S.; Munroe, P.; Xie, Z.-H. A ZrN nanocrystalline coating for polymer electrolyte membrane fuel cell metallic bipolar plates prepared by reactive sputter deposition. RSC Adv. 2015, 5, 67348–67356. [Google Scholar] [CrossRef]


| Target Voltage/V | Substrate Voltage/V | Working Pressure/Pa | Ratio of N2/Ar | Renamed Samples | |
|---|---|---|---|---|---|
| Nb pre-diffusion | 750 | 550 | 38 | / | / |
| Nb-N co-diffusion | 1050–1300 | 650–750 | 1:2 | S1 | |
| 1:1 | S2 | ||||
| 3:1 | S3 |
| Samples | OCP (V) | Ecorr (V) | Icorr (A/cm2) | I0.6V (A/cm2) | Pi (%) |
|---|---|---|---|---|---|
| TC4 | −0.670 | −0.536 | 1.28 × 10−4 | 1.28 × 10−4 | / |
| S1 | 0.140 | 0.112 | 4.14 × 10−6 | 1.05 × 10−5 | 96.77 |
| S2 | 0.170 | 0.112 | 4.96 × 10−6 | 1.01 × 10−5 | 96.13 |
| S3 | 0.180 | 0.131 | 4.81 × 10−6 | 8.92 × 10−6 | 96.24 |
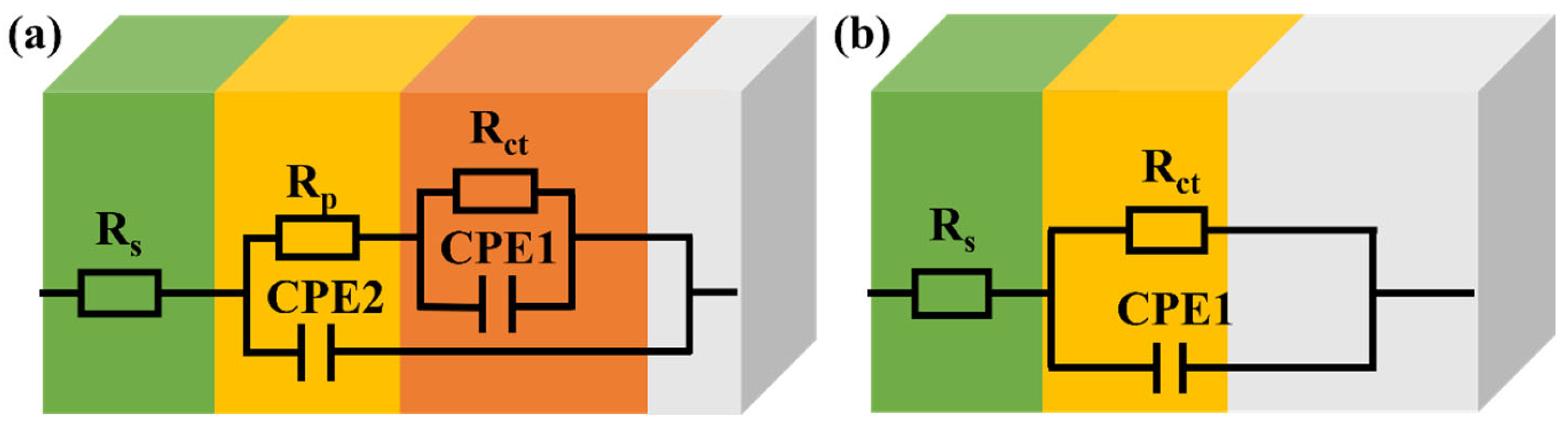
| Samples | Rs (Ω/cm2) | Rp (Ω/cm2) | CPE2 | Rct (Ω/cm2) | CPE1 | ||
|---|---|---|---|---|---|---|---|
| C2 (μF/cm2) | n2 | C1 (μF/cm2) | n1 | ||||
| TC4 | 2.39 | 26.7 | 412 | 0.935 | 45.5 | 58,300 | 0.882 |
| S1 | 2.30 | / | / | / | 166k | 130 | 0.910 |
| S2 | 2.50 | / | / | / | 203k | 119 | 0.909 |
| S3 | 2.32 | / | / | / | 737k | 42 | 0.924 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dang, B.; Han, Y.; Yang, K.; Chen, D.; Zhan, M.; Ding, F.; Li, S.; Zhang, P. Effect of Different N2 Partial Pressures on the Corrosion Properties and Conductivity of NbNx Coated Titanium Bipolar Plates for PEMFCs. Coatings 2025, 15, 973. https://doi.org/10.3390/coatings15080973
Dang B, Han Y, Yang K, Chen D, Zhan M, Ding F, Li S, Zhang P. Effect of Different N2 Partial Pressures on the Corrosion Properties and Conductivity of NbNx Coated Titanium Bipolar Plates for PEMFCs. Coatings. 2025; 15(8):973. https://doi.org/10.3390/coatings15080973
Chicago/Turabian StyleDang, Bo, Yu Han, Kai Yang, Dong Chen, Mengling Zhan, Feng Ding, Shuqin Li, and Pingze Zhang. 2025. "Effect of Different N2 Partial Pressures on the Corrosion Properties and Conductivity of NbNx Coated Titanium Bipolar Plates for PEMFCs" Coatings 15, no. 8: 973. https://doi.org/10.3390/coatings15080973
APA StyleDang, B., Han, Y., Yang, K., Chen, D., Zhan, M., Ding, F., Li, S., & Zhang, P. (2025). Effect of Different N2 Partial Pressures on the Corrosion Properties and Conductivity of NbNx Coated Titanium Bipolar Plates for PEMFCs. Coatings, 15(8), 973. https://doi.org/10.3390/coatings15080973






