Influence of B2O3 on Reactive and Non-Reactive Wetting Behavior of CaO-SiO2-MgO-Al2O3-B2O3 System
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Samples
2.2. Determination of Liquidus Temperatures
2.3. High-Temperature Wettability Test
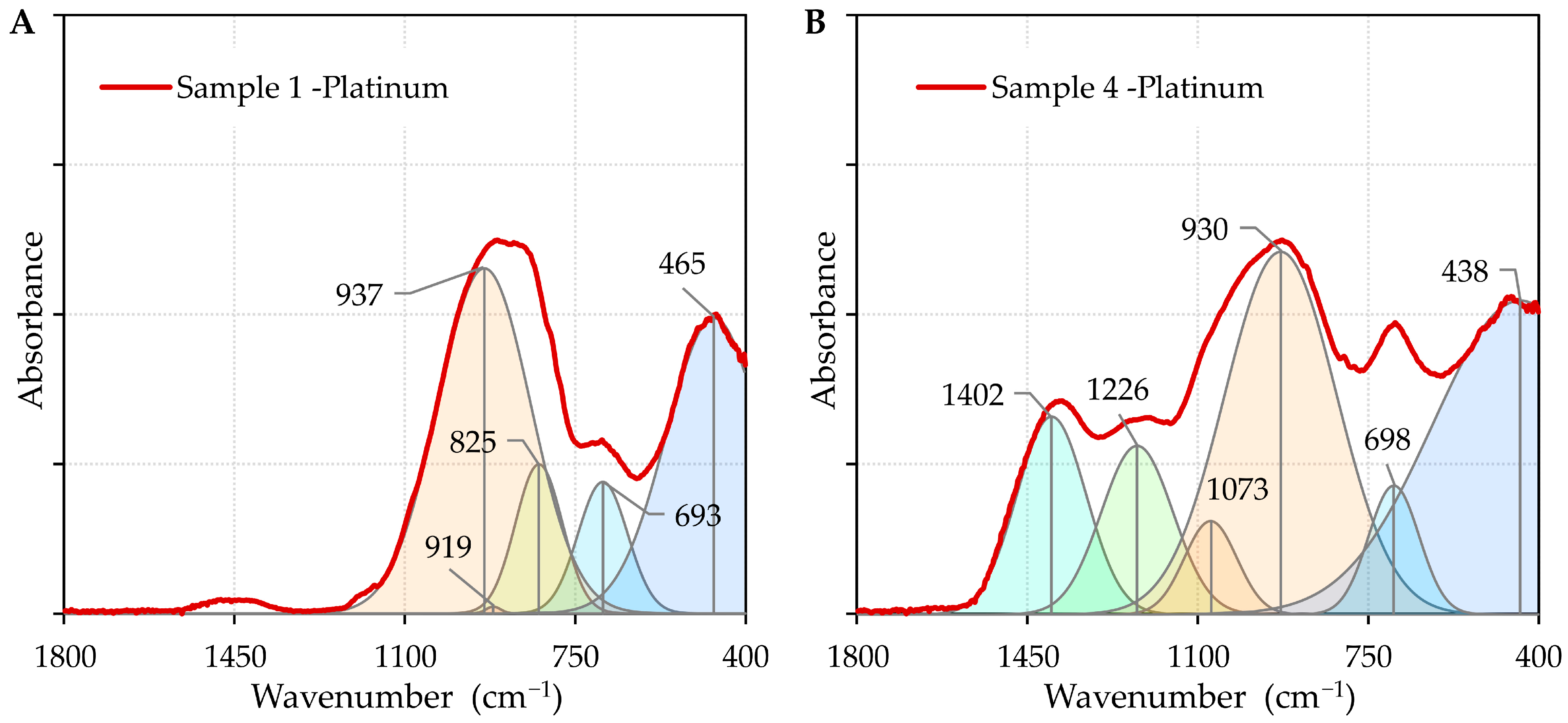
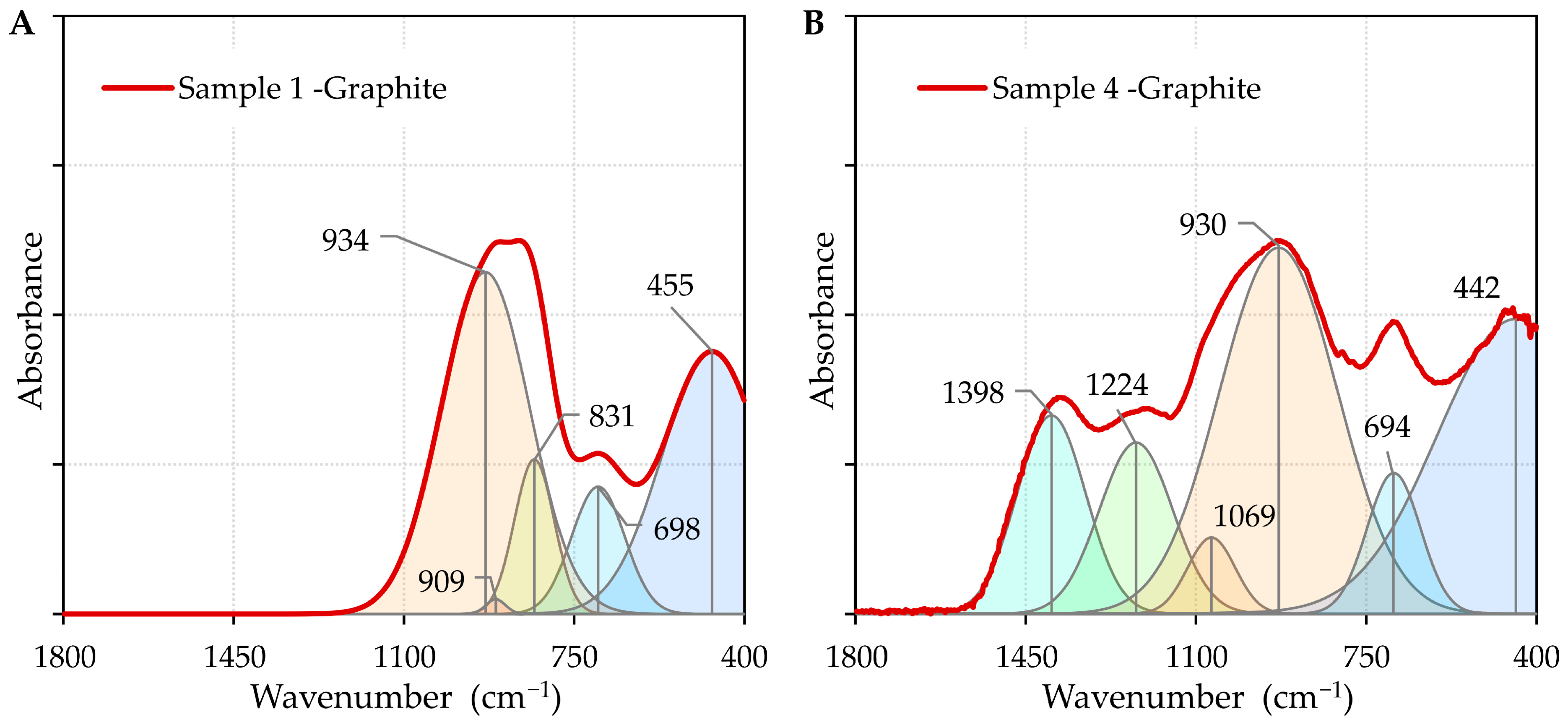
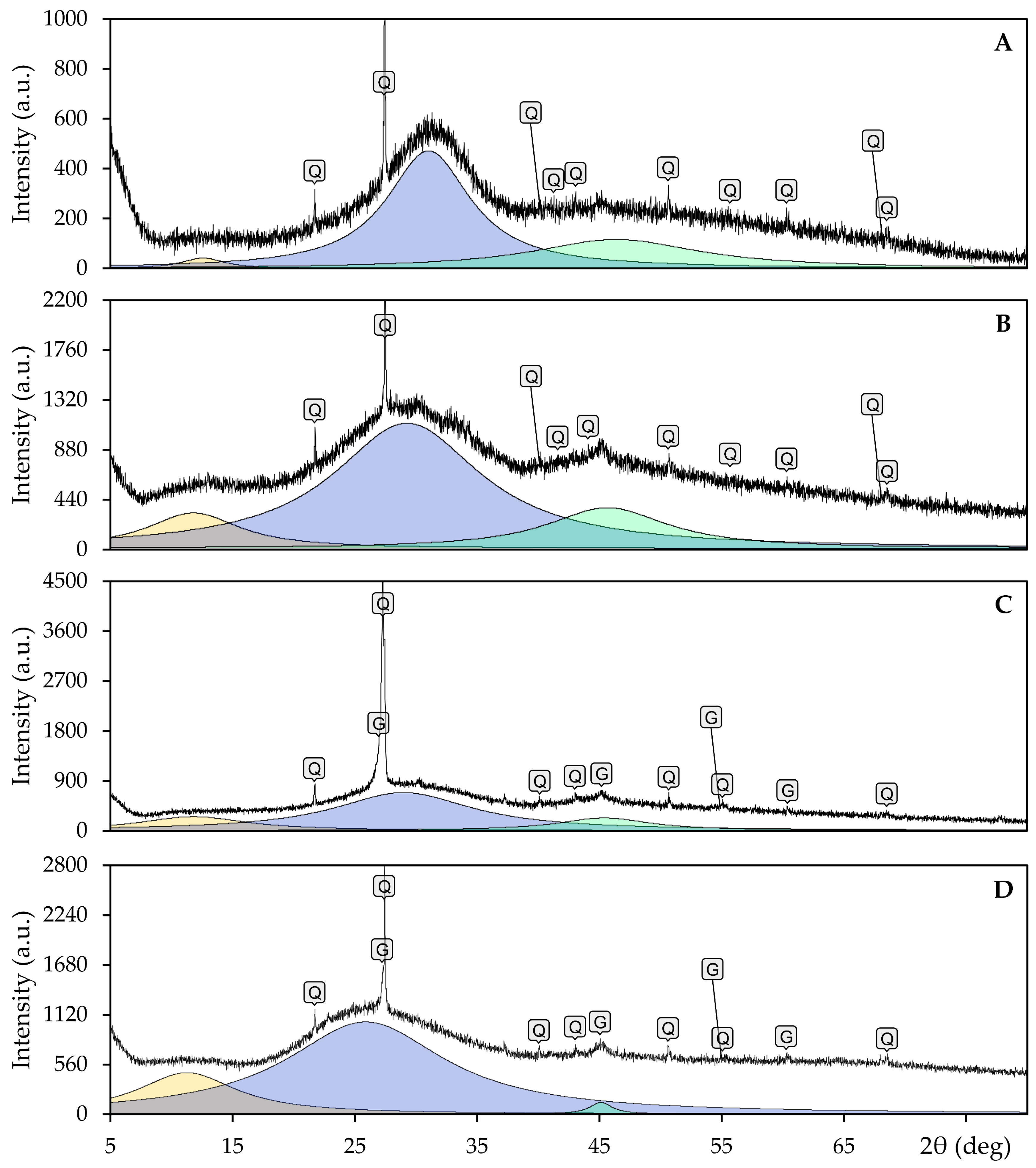
2.4. SEM, EDS, FTIR, and XRD Methods
3. Results and Discussion
3.1. Determination of Liquidus Temperatures
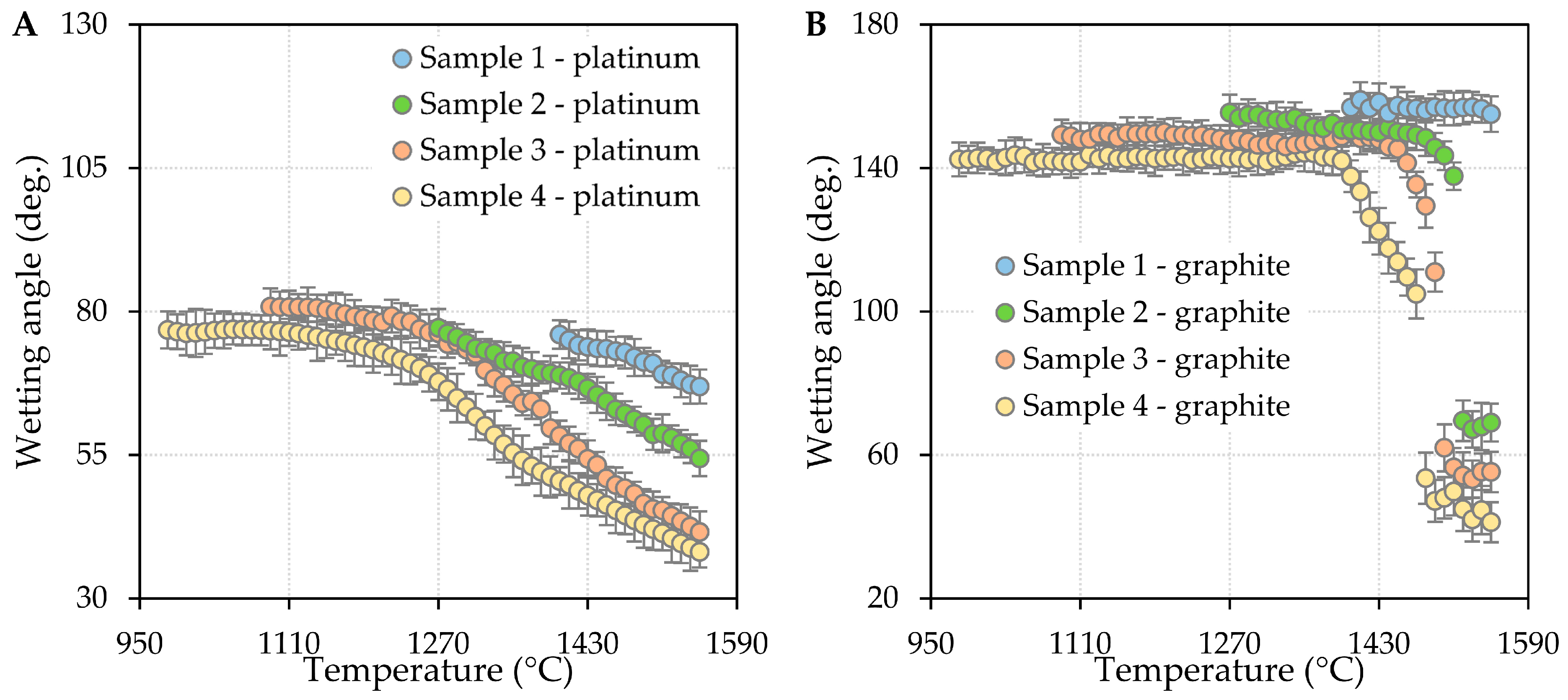
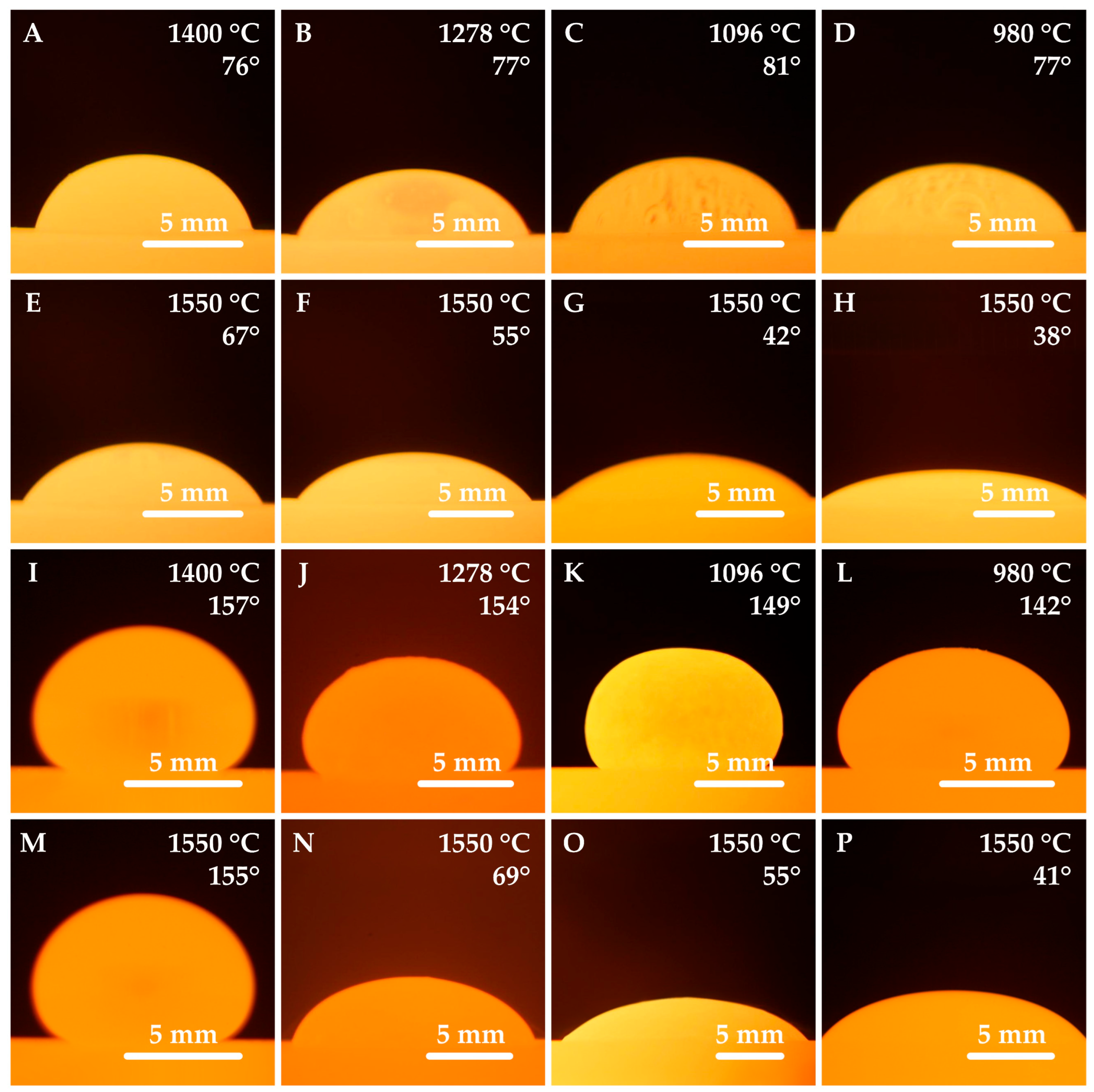
3.2. Results of High-Temperature Wettability Tests
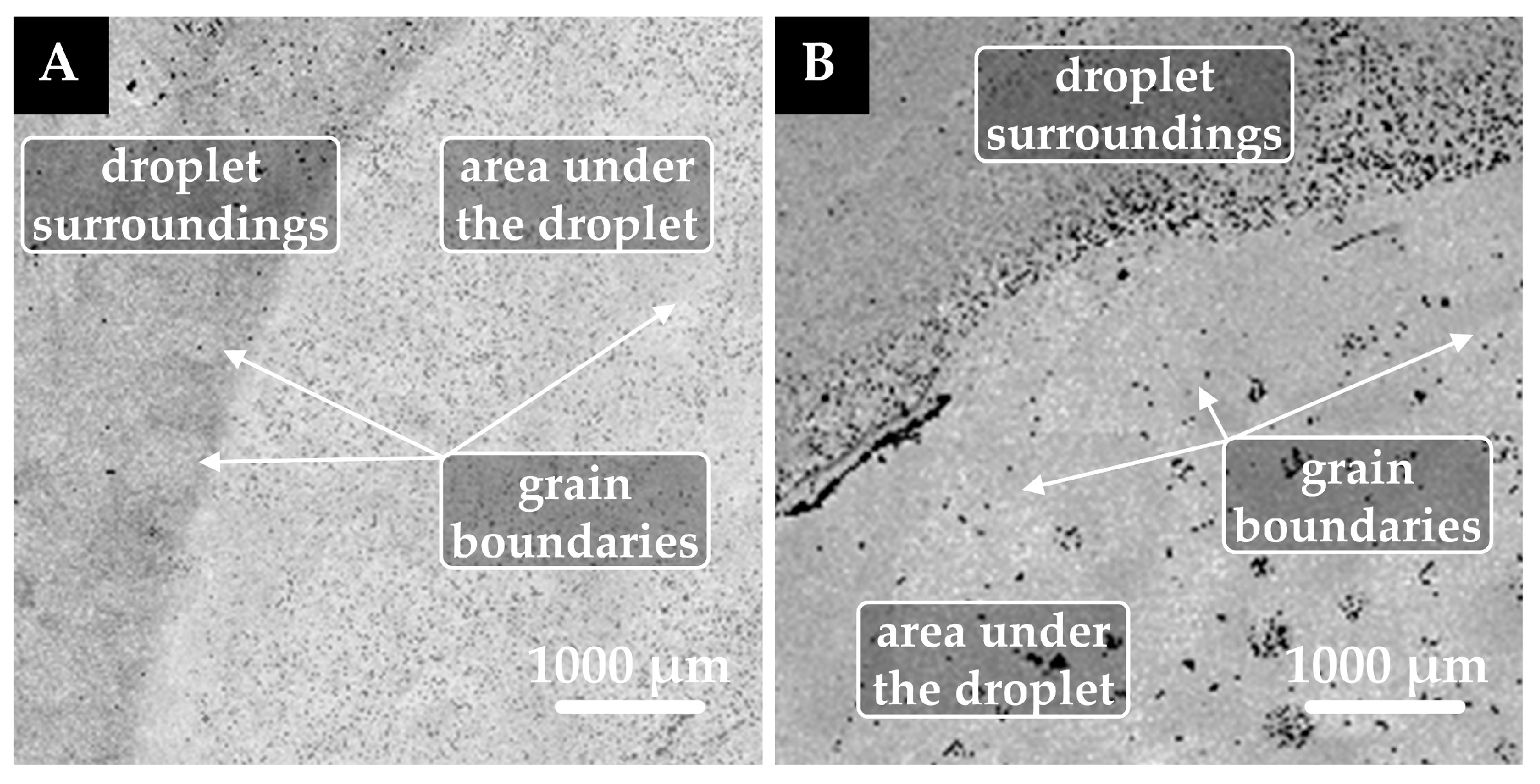
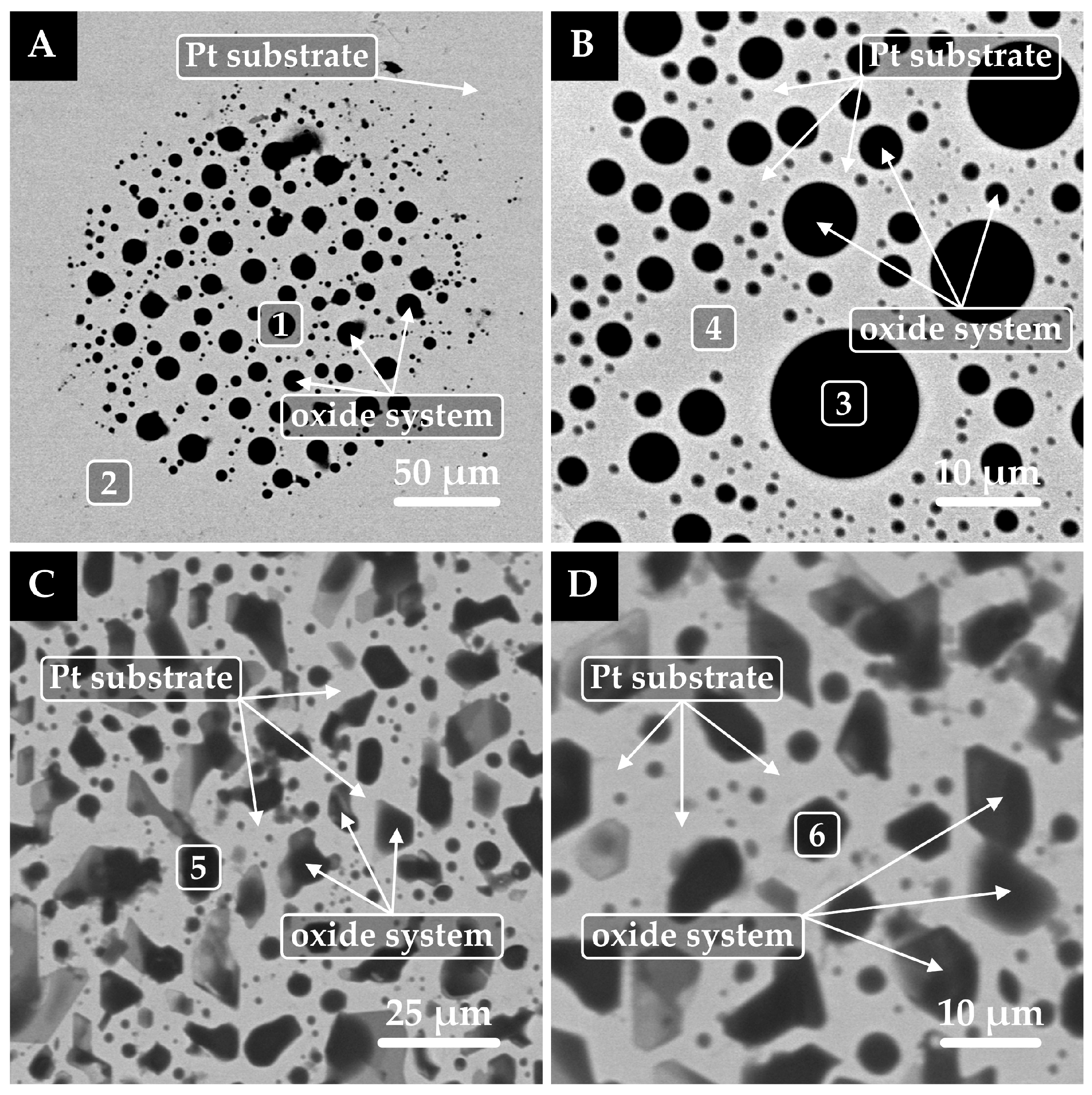
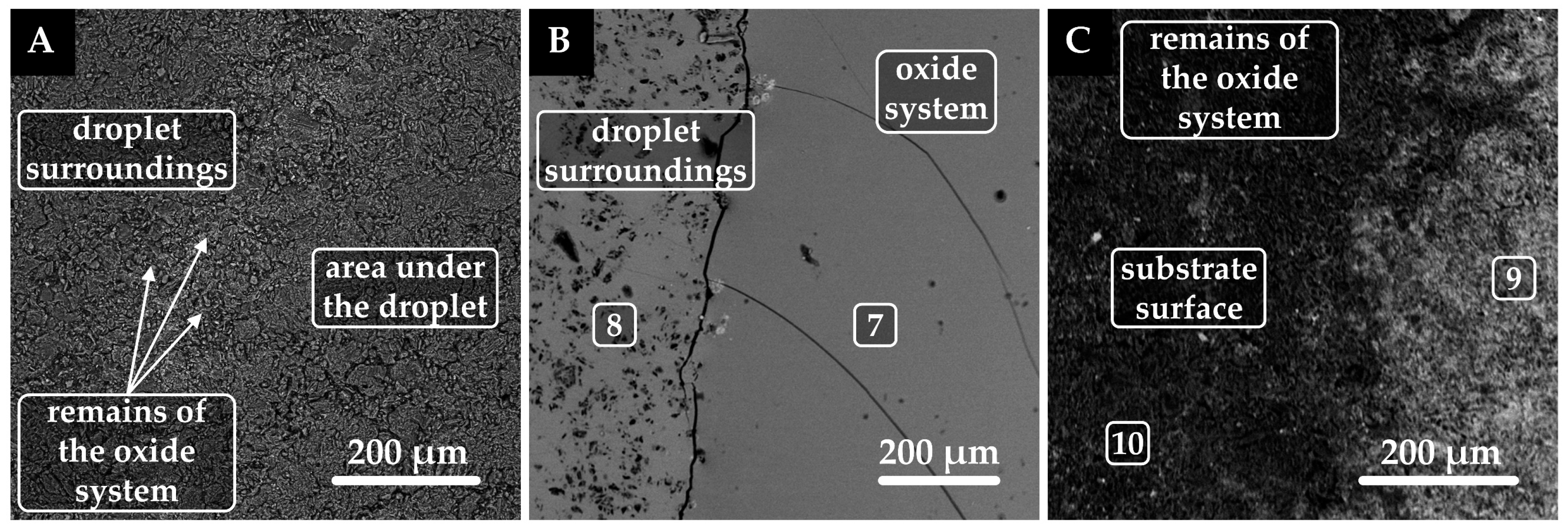
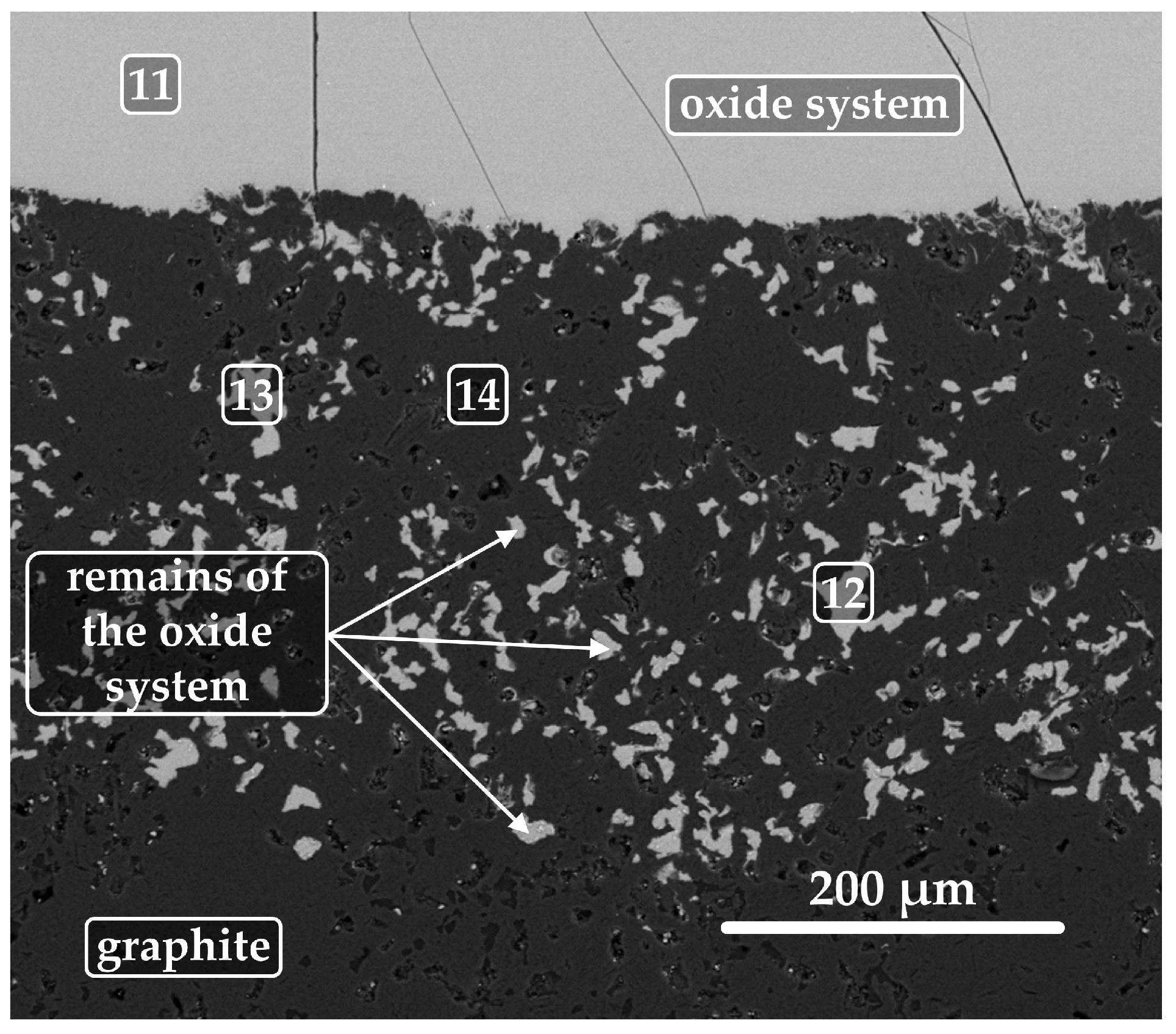
3.3. Analysis of Interaction at the Phase Interface
3.4. Wetting Mechanism
4. Conclusions
- The incorporation of boron oxide resulted in a reduction in contact angles on both platinum and graphite substrates. Furthermore, it was observed that contact angles decreased with an increase in temperature.
- The findings from scanning electron microscopy (SEM) microanalysis revealed that the reactive wetting of the graphite substrate was influenced by the concentration of boron oxide, with a marked increase in intensity corresponding to higher concentrations of boron oxide. Conversely, no evidence of reactive wetting was observed in the case of platinum.
- FTIR analysis confirmed that the addition of boron oxide altered the structural network of the oxide system, weakening the intermolecular forces at the surface and resulting in a decrease in the contact angles.
- The X-ray diffraction (XRD) analysis results demonstrated the amorphous characteristics of all samples within the oxide system. Quartz was identified as the predominant crystalline phase, accompanied by graphite in cases where the graphite substrate was wet.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADSA | Axisymmetric Drop Shape Analysis |
| ATR | Attenuated Total Reflectance |
| CA | Calcium Aluminate |
| CA2 | Calcium Dialuminate |
| CA6 | Calcium Hexaaluminate |
| EDS | Energy Dispersive X-ray Spectroscopy |
| FWHM | Full Width at Half Maximum |
| FTIR | Fourier Transform Infrared Spectroscopy |
| NBO | Non-Bridging Oxygens |
| SEM | Scanning Electron Microscopy |
| XRD | X-Ray Powder Diffraction |
References
- Morizet, Y.; Paris, M.; Hamon, J.; La, C.; Grolleau, S.; Suzuki-Muresan, T. Predicting iodine solubility at high pressure in borosilicate nuclear waste glasses using optical basicity: An experimental study. J. Mater. Sci. 2022, 57, 16600–16618. [Google Scholar] [CrossRef]
- Bengisu, M. Borate glasses for scientific and industrial applications: A review. J. Mater. Sci. 2016, 51, 2199–2242. [Google Scholar] [CrossRef]
- Zu, Q.; Song, W.; Zeng, H.; Yu, X.; Huang, S.; Huang, S.; Li, H. Glass resistance to radiation–part I: Preliminary investigation of three commercial glass fibers. Int. J. Appl. Glass Sci. 2020, 11, 522–536. [Google Scholar] [CrossRef]
- Kim, K.; Kim, K. Valuable Recycling of waste glass generated from the liquid crystal display panel industry. J. Clean. Prod. 2018, 174, 191–198. [Google Scholar] [CrossRef]
- Zhang, L.; Kang, J.; Wang, J.; Khater, G.A.; Shi, Q.; Li, S.; Zhao, J.; Teng, J.; Yue, Y. Effects of Y2O3 on structure and dielectric properties of aluminoborosilicate glasses. J. Non-Cryst. Solids 2019, 503–504, 110–114. [Google Scholar] [CrossRef]
- Li, H.; Richards, C.; Watson, J. High-performance glass fiber development for composite applications. Int. J. Appl. Glass Sci. 2014, 5, 65–81. [Google Scholar] [CrossRef]
- Ellison, A.; Cornejo, I.A. Glass substrates for liquid crystal displays. Int. J. Appl. Glass Sci. 2010, 1, 87–103. [Google Scholar] [CrossRef]
- Wang, J.; Wu, M.; Tang, H.; Han, J.; Liu, C.; Cao, X.; Kang, J. Correlation between viscosity, electrical resistivity and network connectivity of alkali-free boroalumiosilicate glasses. J. Non-Cryst. Solids 2019, 509, 88–94. [Google Scholar] [CrossRef]
- Hao, N.; Zhang, G.; Yang, Z.; Qin, G.; Jin, H.; Gao, S. Improving the dielectric properties of aluminoborosilicate glasses for packaging ceramics by optimizing structure. J. Non-Cryst. Solids 2023, 601, 122042. [Google Scholar] [CrossRef]
- Yue, Y.; Zhang, X.; Xu, Y.; Huang, S.; Chen, P. Structural, dielectric and melting properties of aluminosilicate glasses based on blast furnace slag for printed circuit board applications. Mater. Lett. 2014, 136, 356–358. [Google Scholar] [CrossRef]
- Mills, K.C. The Influence of Structure on the Physico-chemical Properties of Slags. ISIJ Int. 1993, 33, 148–155. [Google Scholar] [CrossRef]
- Ma, J.; Li, W.; Fu, G.; Zhu, M. Effect of B2O3 on the Melting Temperature and Viscosity of CaO–SiO2–MgO–Al2O3–TiO2–Cr2O3 Slag. J. Sustain. Metall. 2021, 7, 1190–1199. [Google Scholar] [CrossRef]
- Babenko, A.A.; Smetannikov, A.N.; Zhuchkov, V.I.; Upolovnikova, A.G. Influence of B2O3 and Basicity of CaO–SiO2–B2O3–Al2O3 Slag on the Saturation Concentration of Magnesium Oxide. Steel Transl. 2019, 49, 87–90. [Google Scholar] [CrossRef]
- Salina, V.A.; Sychev, A.V.; Zhuchkov, V.I.; Leont’ev, L.I.; Babenko, A.A. Thermodynamic Simulation of the Influence of the Temperature and the Basicity of a Boron-Containing Slag on Steel Desulfurization. Russ. Metall. 2018, 2018, 427–431. [Google Scholar] [CrossRef]
- Tayeb, M.A.; Assis, A.N.; Sridhar, S.; Fruehan, R.J. MgO Solubility in Steelmaking Slags. Metall. Mater. Trans. B 2015, 46, 1112–1114. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, T.; Zhu, H.; Li, G.; Yan, Y.; Wang, J. Effect of B2O3 on melting temperature, viscosity and desulfurization capacity of CaO-based refining flux. ISIJ Int. 2011, 51, 702–706. [Google Scholar] [CrossRef]
- Li, S.; Kong, L.; Xu, Z. Effect of refining slag compositions on its melting property and desulphurization. High Temp. Mater. Process. 2023, 42, 20220293. [Google Scholar] [CrossRef]
- Babenko, A.A.; Shartdinov, R.R.; Upolovnikova, A.G.; Smetannikov, A.N.; Gulyakov, V.S. Physical Properties of CaO–SiO2–B2O3 Slags Containing 15% Al2O3 and 8% MgO. Steel Transl. 2019, 49, 667–670. [Google Scholar] [CrossRef]
- Qin, X.; Wei, Z.; Fan, Z.; Xiong, D.; Wang, Y.; Teng, Z.; Zhang, J.; Xie, J. Effect of B2O3 substitution for Al2O3 on the structure and properties of calcium aluminosilicate glass. J. Mater. Sci. Mater. Electron. 2024, 35, 2220. [Google Scholar] [CrossRef]
- Chen, J.B.; Che, H.J.; Zhao, M.H.; Pan, W.B.; Chen, Z.Y.; Liu, H.D. Thermodynamic Activity of B2O3 in CaO–SiO2–Al2O3–B2O3–MnO–MgO Molten Slags at 1723 K. Metall. Mater. Trans. B 2023, 54, 2737–2746. [Google Scholar] [CrossRef]
- Feng, X.; Yao, W.; Li, J. Effect of B2O3 on the structure of CaO-Al2O3-B2O3 ternary melts: A molecular dynamics simulation. J. Non-Cryst. Solids 2021, 574, 121141. [Google Scholar] [CrossRef]
- Mehta, A.S.; Sahajwalla, V. Coal-char/Slag Interactions during Pulverised Coal Injection in a Blast Furnace: Reaction Kinetics and Wetting Investigations. ISIJ Int. 2003, 43, 1512–1518. [Google Scholar] [CrossRef]
- Mehta, A.S.; Sahajwalla, V. Influence of composition of slag and carbonaceous materials on the wettability at the slag/carbon interface during pulverised coal injection in a blast furnace. Scand. J. Metall. 2000, 29, 17–29. [Google Scholar] [CrossRef]
- Mehta, A.S.; Sahajwalla, V. Influence of temperature on the wettability at the slag/carbon interface during pulverised coal injection in a blast furnace. Scand. J. Metall. 2001, 30, 370–378. [Google Scholar] [CrossRef]
- Siddiqi, N.; Bhoi, B.; Paramguru, R.K.; Sahajwalla, V.; Ostrovski, O. Slag-graphite wettability and reaction kinetics. Part 1. Kinetics and mechanism of molten FeO reduction reaction. Ironmak. Steelmak. 2000, 27, 367–372. [Google Scholar] [CrossRef]
- Siddiqi, N.; Bhoi, B.; Paramguru, R.K.; Sahajwalla, V.; Ostrovski, O. Slag-graphite wettability and reaction kinetics. Part 2. Wettability influenced by reduction kinetics. Ironmak. Steelmak. 2000, 27, 437–441. [Google Scholar] [CrossRef]
- Shen, P.; Fujii, H.; Nogi, K. Wettability of some refractory materials by molten SiO2-MnO-TiO2-FeOx slag. Mater. Chem. Phys. 2009, 114, 681–686. [Google Scholar] [CrossRef]
- Parry, G.; Ostrovski, O. Wettability of solid metals by molten CaO-SiO2-Al2O3 slag. Metall. Mater. Trans. B 2008, 39, 681–689. [Google Scholar] [CrossRef]
- Parry, G.; Ostrovski, O. Wetting of solid iron, nickel and platinum by liquid MnO-SiO2 and CaO-AI2O3-SiO2. ISIJ Int. 2009, 49, 788–795. [Google Scholar] [CrossRef]
- Eustathopoulos, N.; Nicholas, M.G.; Drevet, B. Wettability at High Temperatures; Pergamon, Elsevier Science Ltd.: Oxford, UK, 1999; pp. 339–347. [Google Scholar]
- Oh, J.S.; Lee, J. Composition-dependent reactive wetting of molten slag on coke substrates. J. Mater. Sci. 2016, 51, 1813–1819. [Google Scholar] [CrossRef]
- Siddiqi, N.; Sahajwalla, V.; Ostrovski, O.; Belton, G.R. Wettability of graphite by CaO-SiO2-Al2O3-FeO-MgO slag. High Temp. Mater. Proc. 1997, 16, 213–225. [Google Scholar] [CrossRef]
- Duchesne, M.A.; Hughes, R.W. Slag density and surface tension measurements by the constrained sessile drop method. Fuel 2017, 188, 173–181. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, X.; Bai, C.; Li, B. Wettability of pyrolytic graphite by molten blast furnace slag bearing TiO2. Charact. Miner. Met. Mater. 2016, 91–98. [Google Scholar] [CrossRef]
- Řeháčková, L.; Novák, V.; Tokarský, J.; Heger, M.; Zimný, O.; Matýsek, D.; Peikertová, P.; Ritz, M.; Walek, J.; Leinweberová, S. Rheological behaviour of CaO–MgO–SiO2–Al2O3–B2O3 system with varying B2O3 content up to 30 wt% at basicity of 0.4. Ceram. Int. 2024, 50, 1389–1397. [Google Scholar] [CrossRef]
- Sagadin, C.; Luidold, S.; Wagner, C.; Wenzl, C. Melting behaviour of ferronickel slags. JOM-J. Min. Met. Mat. S 2016, 68, 3022–3028. [Google Scholar] [CrossRef]
- Ueda, S.; Kon, T.; Miki, T.; Kim, S.J.; Nogami, H. Softening, melting, and permeation phenomena of CaO-FeO-SiO2 oxide on a coke bed. ISIJ Int. 2015, 55, 2098–2104. [Google Scholar] [CrossRef]
- Chuang, H.C.; Hwang, W.S.; Liu, S.H. Effects of basicity and FeO content on the softening and melting temperatures of the CaO-SiO2-MgO-Al2O3 slag system. Mater. Trans. 2009, 50, 1448–1456. [Google Scholar] [CrossRef]
- Řeháčková, L.; Novák, V.; Váňová, P.; Matýsek, D.; Tkadlečková, M.; Konečná, K.; Sniegoň, M.; Smetana, B.; Rosypalová, S.; Kawuloková, M.; et al. Interfacial phenomena between alumina substrate and nickel containing low-alloy steel. J. Alloys Compd. 2022, 900, 163376. [Google Scholar] [CrossRef]
- Novák, V.; Řeháčková, L.; Rosypalová, S.; Matýsek, D. Wetting of Refractory Ceramics with High-Manganese and Structural Steel and Description of Interfacial Interaction. Crystals 2022, 12, 1782. [Google Scholar] [CrossRef]
- Jak, E.; Zhao, B.; Hayes, P. Phase equilibria in the system FeO-Fe2O3-Al2O3-CaO-SiO2 with applications to non-ferrous smelting slags. Trans. Inst. Min. Metall. Sect. C Miner. Process. Extr. Metall. 2008, 117, 147–152. [Google Scholar] [CrossRef]
- Wallenberger, F.T.; Smrček, A. The Liquidus Temperature; Its Critical Role in Glass Manufacturing. Int. J. Appl. Glass Sci. 2010, 1, 151–163. [Google Scholar] [CrossRef]
- Yeo, T.M.; Cho, J.W.; Alloni, M.; Casagrande, S.; Carli, R. Structure and its effect on viscosity of fluorine-free mold flux: Substituting CaF2 with B2O3 and Na2O. J. Non-Cryst. Solids 2020, 529, 119756. [Google Scholar] [CrossRef]
- Yan, W.; Chen, W.; Yang, Y.; McLean, A. Viscous characteristics and modelling of CaO–Al2O3-based mould flux with B2O3 as a substitute for CaF2. Ironmak. Steelmak. 2019, 46, 347–352. [Google Scholar] [CrossRef]
- Kilinc, E.; Bell, A.M.T.; Bingham, P.A. Dynamic high-temperature crystallization and processing properties of industrial soda–lime–silica glasses. J. Am. Ceram. Soc. 2024, 107, 2242–2259. [Google Scholar] [CrossRef]
- Wang, H.M.; Li, G.R.; Li, B.; Zhang, X.J.; Yan, Y.Q. Effect of B2O3 on melting temperature of CaO-based ladle refining slag. J. Iron Steel Res. Int. 2010, 17, 18–22. [Google Scholar] [CrossRef]
- Wang, Z.; Shu, Q.; Chou, K. Viscosity of fluoride-free mold fluxes containing B2O3 and TiO2. Steel Res. Int. 2013, 84, 766–776. [Google Scholar] [CrossRef]
- Kumar, G.; Prabhu, K.N. Review of non-reactive and reactive wetting of liquids on surfaces. Adv. Colloid Interface Sci. 2007, 133, 61–89. [Google Scholar] [CrossRef]
- Yoon, S.W.; Choi, W.K.; Lee, H.M. Calculation of surface tension and wetting properties of Sn-based solder alloys. Scr. Mater. 1999, 40, 297–302. [Google Scholar] [CrossRef]
- Luo, C.; Min, Y.; Chen, F.; Guo, P.; Jiao, S.; Liu, C. Molecular dynamics simulation of microstructure and surface tension in Fe-C-N-Ti quaternary molten steel system. J. Mol. Liq. 2025, 435, 128099. [Google Scholar] [CrossRef]
- Peng, M.; Wang, Q.; Zhang, M.; Xi, X.; Liu, G.; Wang, L.; Chen, L. Optimization of boron depletion for boron-doped emitter of N-type TOPCon solar cells. Mater. Sci. Semicond. 2024, 178, 108424. [Google Scholar] [CrossRef]
- Shi, S.; Guo, X.; An, G.; Jiang, D.; Qin, S.; Meng, J.; Li, P.; Tan, Y.; Noor ul Huda Khan Asghar, H.M. Separation of boron from silicon by steam-added electron beam melting. Sep. Purif. Technol. 2019, 215, 242–248. [Google Scholar] [CrossRef]
- Xu, R.Z.; Zhang, J.L.; Wang, Z.Y.; Jiao, K.X. Influence of Cr2O3 and B2O3 on viscosity and structure of high alumina slag. Steel Res. Int. 2017, 88, 1600241. [Google Scholar] [CrossRef]
- Wang, L.; Cui, Y.; Yang, J.; Zhang, C.; Cai, D.; Zhang, J.; Sasaki, Y.; Ostrovski, O. Melting properties and viscosity of SiO2-CaO-Al2O3-B2O3 system. Steel Res. Int. 2015, 86, 670–677. [Google Scholar] [CrossRef]
- Eustathopoulos, N.; Drevet, B. Determination of the nature of metal-oxide interfacial interactions from sessile drop data. Mater. Sci. Eng. A 1998, 249, 176–183. [Google Scholar] [CrossRef]
- Pech, J.; Braccini, M.; Mortensen, A.; Eustathopoulos, N. Wetting, interfacial interactions and sticking in glass/steel systems. Mater. Sci. Eng. A 2004, 384, 117–128. [Google Scholar] [CrossRef]
- Lamoreaux, R.H.; Hildenbrand, D.L.; Brewer, L. High-Temperature Vaporization Behavior of Oxides II. Oxides of Be, Mg, Ca, Sr, Ba, B, Al, Ga, In, Tl, Si, Ge, Sn, Pb, Zn, Cd, and Hg. J. Phys. Chem. Ref. Data. 1987, 16, 419–443. [Google Scholar] [CrossRef]
- Bögels, T.F.J.; Caracas, R. Comparison of liquid-vapor relations and the critical points of CaO and MgO. Phys. Rev. B 2025, 111, 144106. [Google Scholar] [CrossRef]
- Huang, S.; Li, S.; Wu, F.; Yue, Y. Effect of B2O3 on Structure and Properties of CaO–MgO–B2O3–Al2O3–SiO2 Glasses. J. Inorg. Organomet. Polym. 2015, 25, 816–822. [Google Scholar] [CrossRef]
- Hamuyuni, J.; Daramola, M.O.; Oluwasina, O.O. Energy-Dispersive X-Ray Spectroscopy: Theory and Application in Engineering and Science. In Encyclopedia of Physical Organic Chemistry; Wang, Z., Wille, U., Juaristi, E., Eds.; Wiley: New York, NY, USA, 2017; pp. 1–23. [Google Scholar]
- Wang, W.; Dai, S.; Zhou, L.; Zhang, J.; Tian, W.; Xu, J. Viscosity and structure of MgO–SiO2-based slag melt with varying B2O3 content. Ceram. Int. 2020, 46, 3631–3636. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Z. Structural roles of boron and silicon in the CaO-SiO2-B2O3 glasses using FTIR, Raman, and NMR spectroscopy. Metall. Mater. Trans. B 2015, 46, 1549–1554. [Google Scholar] [CrossRef]
- Baroni, A.; Pacaud, F.; Salanne, M.; Micoulaut, M.; Delaye, J.M.; Salmon, P.S.; Ferlat, G. Many-body effects at the origin of structural transitions in B2O3. J. Chem. Phys. 2019, 151, 224508. [Google Scholar] [CrossRef]
- Park, J.H. The effect of boron oxide on the crystallization behavior of MgAl2O4 spinel phase during the cooling of the CaO-SiO 2-10 mass.% MgO-30 mass.% Al2O3 systems. Met. Mater. Int. 2010, 16, 987–992. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Sasaki, Y.; Ostrovski, O.; Zhang, C.; Cai, D.; Kashiwaya, Y. Effect of B2O3 on Crystallization Behavior, Structure, and Heat Transfer of CaO-SiO2-B2O3-Na2O-TiO2-Al2O3-MgO-Li2O Mold Fluxes. Metall. Mater. Trans. B 2017, 48, 2077–2091. [Google Scholar] [CrossRef]
- White, J.F.; Ma, L.; Forwald, K.; Sichen, D. Reactions between silicon and graphite substrates at high temperature: In situ observations. Metall. Mater. Trans. B 2014, 45, 150–160. [Google Scholar] [CrossRef]
- Tran, A.T.; Tran, V.T.; Nguyet, N.T.M.; Luong, A.T.Q.; Le, T.V.; Phuc, N.H.H. Solid-State Reaction Synthesis of MgAl2O4 Spinel from MgO-Al2O3 Composite Particles Prepared via Electrostatic Adsorption. ACS Omega 2023, 8, 36253–36260. [Google Scholar] [CrossRef] [PubMed]
- Rumiantseva, Y.; Lysovenko, S.; Grzechnik, A.; Wachnicky, L.; Polczyk, T.; Pienizek, A.; Gawron, M.; Klimczyk, P.; Zhydachevskyy, Y. Synthesis of MgAl2O4 Spinel in MgO-Al2O3 and MgO-Al2O3-Al Systems via HPHT Sintering. Acta Phys. Pol. A. 2025, 147, 456–462. [Google Scholar] [CrossRef]
- Ganesh, I. A review on magnesium aluminate (MgAl2O4) spinel: Synthesis, processing and applications. Int. Mater. Rev. 2013, 58, 63–112. [Google Scholar] [CrossRef]
- Abdeyazdan, H.; Dogan, N.; Rhamdhani, M.A.; Chapman, M.W.; Monaghan, B.J. Dynamic Wetting of CaO-Al2O3-SiO2-MgO Liquid Oxide on MgAl2O4 Spinel. Metall. Mater. Trans. B 2015, 46, 208–219. [Google Scholar] [CrossRef]
- Wang, H.; Glaser, B.; Sichen, D. Improvement of Resistance of MgO-Based Refractory to Slag Penetration by In Situ Spinel Formation. Metall. Mater. Trans. B 2015, 46, 749–757. [Google Scholar] [CrossRef]
- Wei, X.; Yehorov, A.; Volkova, O. Corrosion of MgO–C Refractory with Ladle Slags. Steel Res. Int. 2025, 96, 2400147. [Google Scholar] [CrossRef]
- Bavand-Vandchali, M.; Sarpoolaky, H.; Golestani-Fard, F.; Rezaie, H.R. Atmosphere and carbon effects on microstructure and phase analysis of in situ spinel formation in MgO-C refractories matrix. Ceram. Int. 2009, 35, 861–868. [Google Scholar] [CrossRef]
- White, J.F.; Lee, J.; Hessling, O.; Glaser, B. Reactions Between Liquid CaO-SiO2 Slags and Graphite Substrates. Metall. Mater. Trans. B 2017, 48, 506–515. [Google Scholar] [CrossRef]
- Sahajwalla, V.; Mehta, A.S.; Khanna, R. Influence of Chemical Compositions of Slag and Graphite on the Phenomena Occurring in the Graphite/Slag Interfacial Region. Metall. Mater. Trans. B 2004, 35, 75–83. [Google Scholar] [CrossRef]
- Akberdin, A.A.; Kim, A.S.; Sultangaziev, R.B. Surface Tension of Melts of CaO–SiO2–Al2O3–B2O3 System. Steel Transl. 2021, 51, 15–21. [Google Scholar] [CrossRef]
- Lee, S.; Chung, Y. The effect of C content in MgO–C on dissolution behavior in CaO–SiO2–Al2O3 slag. Ceram. Int. 2022, 48, 26984–26991. [Google Scholar] [CrossRef]
- Abolpour, B.; Shamsoddini, R. Mechanism of reaction of silica and carbon for producing silicon carbide. Prog. React. Kinet. Mec. 2019, 45, 1468678319891416. [Google Scholar] [CrossRef]
- Jiang, S.; Gao, S.; Kong, J.; Jin, X.; Wei, D.; Li, D.; Xing, P. Study on the synthesis of β-SiC nanoparticles from diamond-wire silicon cutting waste. RSC Adv. 2019, 9, 23785–23790. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.E.; Zhang, S. Melt corrosion of oxide and oxide-carbon refractories. Int. Mater. Rev. 1999, 44, 77–104. [Google Scholar] [CrossRef]
- Jeon, J.; Lindberg, D. Interfacial reaction between different MgO-based refractories and the CaO-FeOx-SiO2 slag system at 1400 °C. J. Eur. Ceram. Soc. 2025, 45, 117099. [Google Scholar] [CrossRef]
- Song, J.; Liu, Y.; Lv, X.; You, Z. Corrosion behavior of Al2O3 substrate by SiO2-MgO-FeO-CaO-Al2O3 slag. J. Mater. Res. Technol. 2020, 9, 314–321. [Google Scholar] [CrossRef]
- Lao, Y.; Li, G.; Gao, Y.; Yuan, C. Wetting and corrosion behavior of MgO substrates by CaO–Al2O3–SiO2–(MgO) molten slags. Ceram. Int. 2022, 48, 14799–14812. [Google Scholar] [CrossRef]
- Berjonneau, J.; Prigent, P.; Poirier, J. The development of a thermodynamic model for Al2O3-MgO refractory castable corrosion by secondary metallurgy steel ladle slags. Ceram. Int. 2009, 35, 623–635. [Google Scholar] [CrossRef]
- Chong, J.; Shen, Y.; Yang, P.; Tian, J.; Zhang, W.; Tang, X.; Du, X. Effects of B2O3 on melting characteristics and temperature-dependent viscosity of high-basicity CaO-SiO2-FeOx-MgO slag. Materials 2020, 13, 1214. [Google Scholar] [CrossRef] [PubMed]
| Properties | Value | Unit |
|---|---|---|
| Bulk density | 1.78 | g·cm−3 |
| Resistivity | 16 | µΩ·m |
| Flexural strength | 52 | MPa |
| Compressive strength | 110 | MPa |
| Thermal conductivity | 82 | W·(m·K)−1 |
| Coefficient of thermal expansion (20–200 °C) | 5 | 10−6 K−1 |
| Hardness | 66 | shore hardness scale |
| Porosity | 14 | % |
| Ash content | 50 | ppm |
| Sample | CaO | SiO2 | MgO | Al2O3 | B2O3 |
|---|---|---|---|---|---|
| 1 | 42.8 | 37.7 | 10 | 9.5 | 0 |
| 2 | 39.9 | 35.6 | 10 | 9.5 | 5 |
| 3 | 34.0 | 31.5 | 10 | 9.5 | 15 |
| 4 | 25.3 | 25.2 | 10 | 9.5 | 30 |
| Sample | Rheometer | Heating Microscope |
|---|---|---|
| 1 | 1399 | 1400 |
| 2 | 1275 | 1278 |
| 3 | 1095 | 1096 |
| 4 | 977 | 980 |
| Point | Caption | Ca | Si | Mg | Al | C | O | Pt |
|---|---|---|---|---|---|---|---|---|
| (wt%) | ||||||||
| 1 | Remains of the oxide system | 25.1 | 18.2 | 6.0 | 5.9 | ― | 44.8 | ― |
| 2 | Platinum substrate | ― | ― | ― | ― | ― | ― | 100.0 |
| 3 | Remains of the oxide system | 21.6 | 17.9 | 5.8 | 10.4 | ― | 44.3 | ― |
| 4 | Platinum substrate | ― | ― | ― | ― | ― | ― | 100.0 |
| 5 | Oxide system after evaporation | ― | 4.9 | ― | 16.5 | ― | 30.1 | 48.5 |
| 6 | Oxide system after evaporation | ― | 5.5 | ― | 19.6 | ― | 30.1 | 44.8 |
| 7 | Oxide system | 17.6 | 12.8 | 7.1 | 6.2 | ― | 56.3 | ― |
| 8 | Remains of the oxide system | 39.6 | 4.3 | 1.4 | 2.1 | ― | 52.6 | ― |
| 9 | Graphite substrate, Si, SiC, SiO2 | ― | 39.3 | ― | ― | 44.6 | 16.1 | ― |
| 10 | Graphite substrate, Si, SiC, SiO2 | ― | 4.9 | ― | ― | 92.1 | 3.0 | ― |
| 11 | Oxide system | 19.7 | 17.1 | 8.3 | 8.0 | ― | 46.9 | ― |
| 12 | Remains of the oxide system | 19.7 | 14.7 | 6.8 | 11.3 | ― | 47.5 | ― |
| 13 | Remains of the oxide system | 19.9 | 15.8 | 7.8 | 8.9 | ― | 47.6 | ― |
| 14 | Graphite with oxide remains | 1.1 | 0.8 | 0.3 | 0.5 | 91.3 | 6.0 | ― |
| IR Assignment | Wavenumber (cm−1) | Ref. |
|---|---|---|
| T–O–T bond bending vibrations; T denotes a Si or Al atom | 400–600 | [61] |
| stretching vibrations of fourfold coordinated Al3+ ions, Al–O–Si bending modes | 600–800 | [61] |
| stretching vibrations of [SiO4] tetrahedra | 800–1200 | [62] |
| stretching vibration of Si-O-B in [SiO4] | 1070 | [59] |
| stretching vibrations of boroxol rings | 1225 | [59] |
| B–O stretching vibration of varied borate groups in [BO3] units | 1400 | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novák, D.; Řeháčková, L.; Novák, V.; Matýsek, D.; Peikertová, P. Influence of B2O3 on Reactive and Non-Reactive Wetting Behavior of CaO-SiO2-MgO-Al2O3-B2O3 System. Coatings 2025, 15, 967. https://doi.org/10.3390/coatings15080967
Novák D, Řeháčková L, Novák V, Matýsek D, Peikertová P. Influence of B2O3 on Reactive and Non-Reactive Wetting Behavior of CaO-SiO2-MgO-Al2O3-B2O3 System. Coatings. 2025; 15(8):967. https://doi.org/10.3390/coatings15080967
Chicago/Turabian StyleNovák, Dalibor, Lenka Řeháčková, Vlastimil Novák, Dalibor Matýsek, and Pavlína Peikertová. 2025. "Influence of B2O3 on Reactive and Non-Reactive Wetting Behavior of CaO-SiO2-MgO-Al2O3-B2O3 System" Coatings 15, no. 8: 967. https://doi.org/10.3390/coatings15080967
APA StyleNovák, D., Řeháčková, L., Novák, V., Matýsek, D., & Peikertová, P. (2025). Influence of B2O3 on Reactive and Non-Reactive Wetting Behavior of CaO-SiO2-MgO-Al2O3-B2O3 System. Coatings, 15(8), 967. https://doi.org/10.3390/coatings15080967






