Promoted Mechanical Properties and LBE Corrosion Resistance of FeCrAlTi-ODS Coatings Deposited by Magnetron Sputtering
Abstract
1. Introduction
2. Materials and Methods
2.1. Deposition of FeCrAlTi-ODS Coating
2.2. LBE Corrosion Tests
2.3. Coating Characterization
3. Results and Discussions
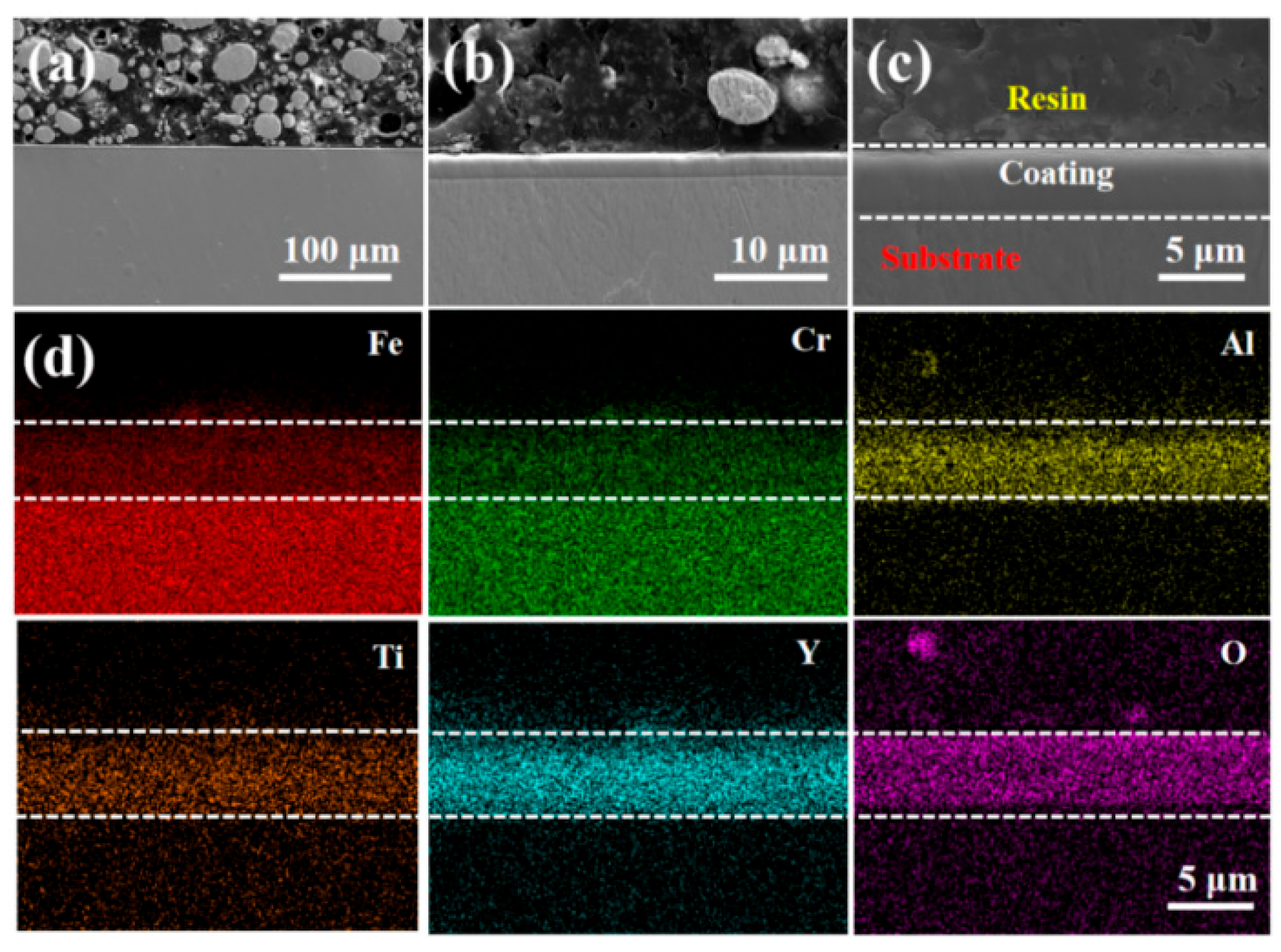
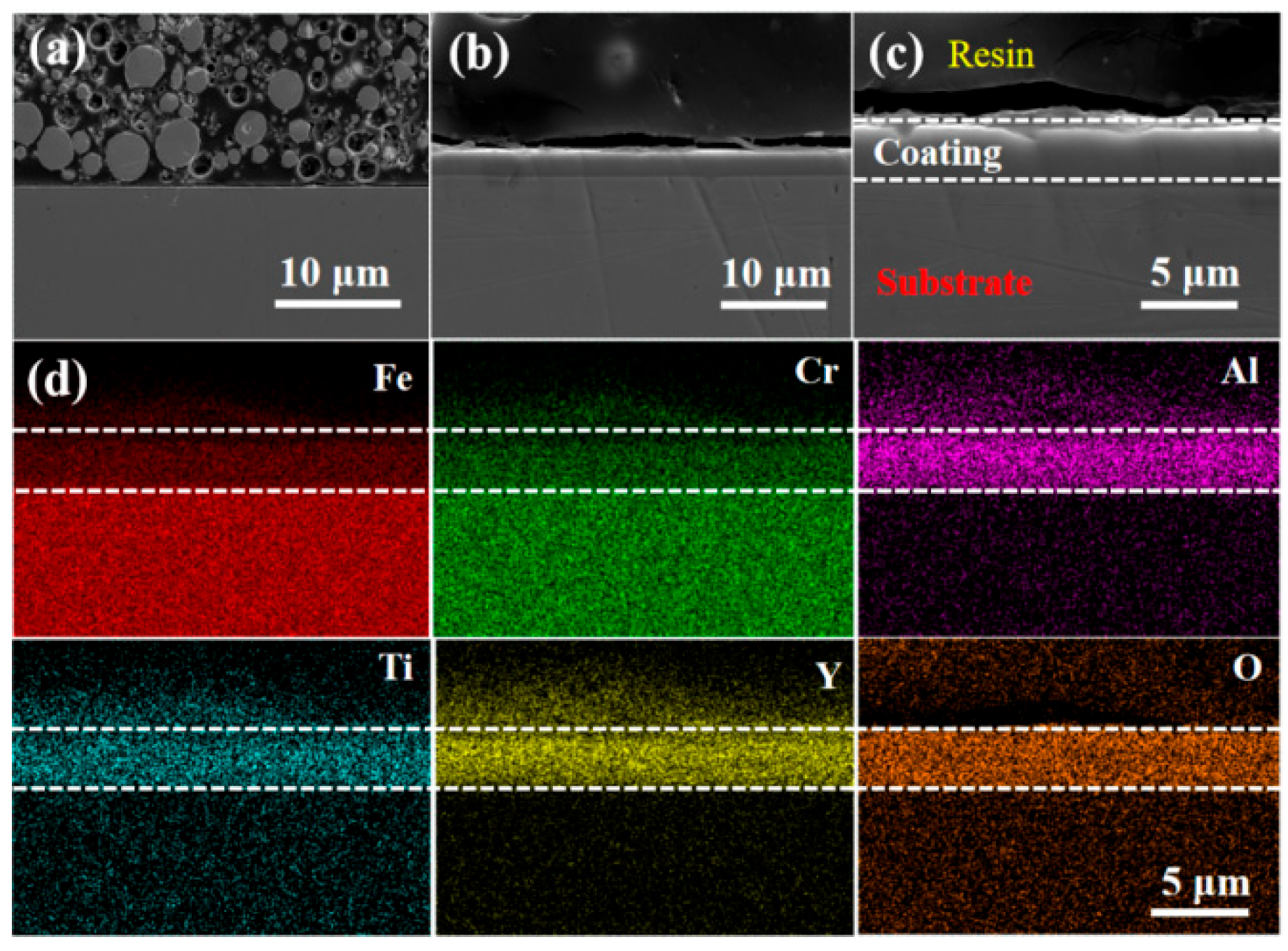
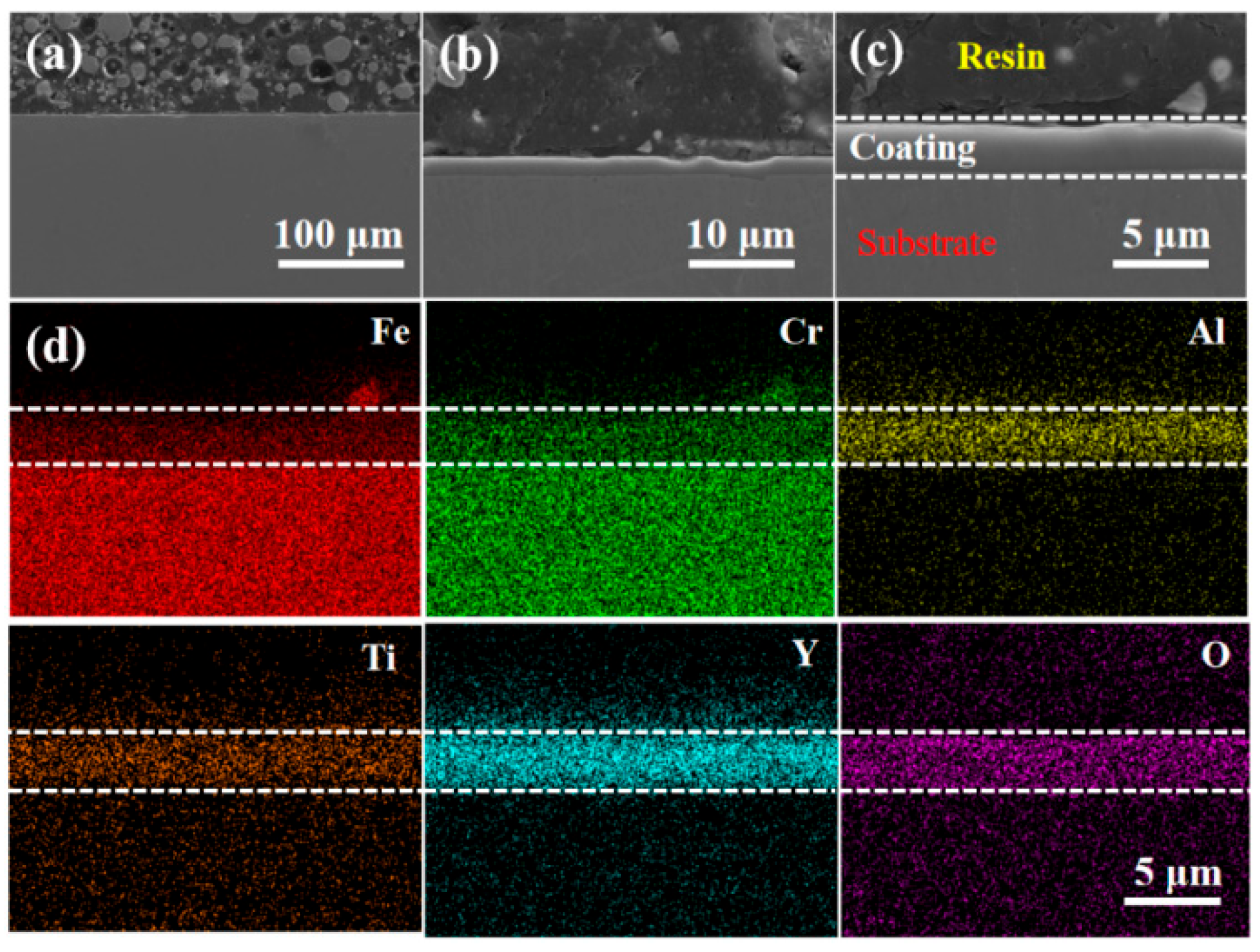
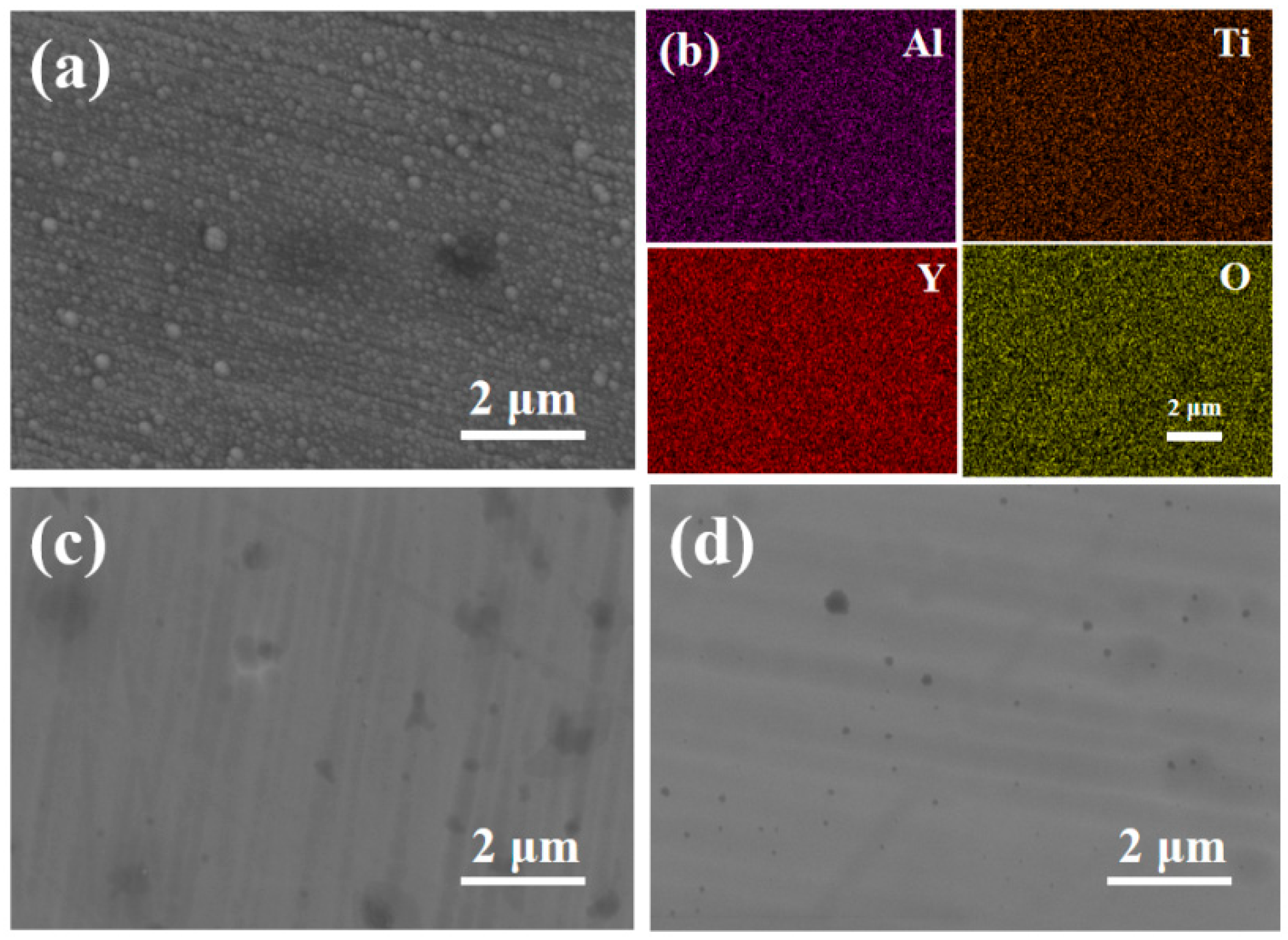
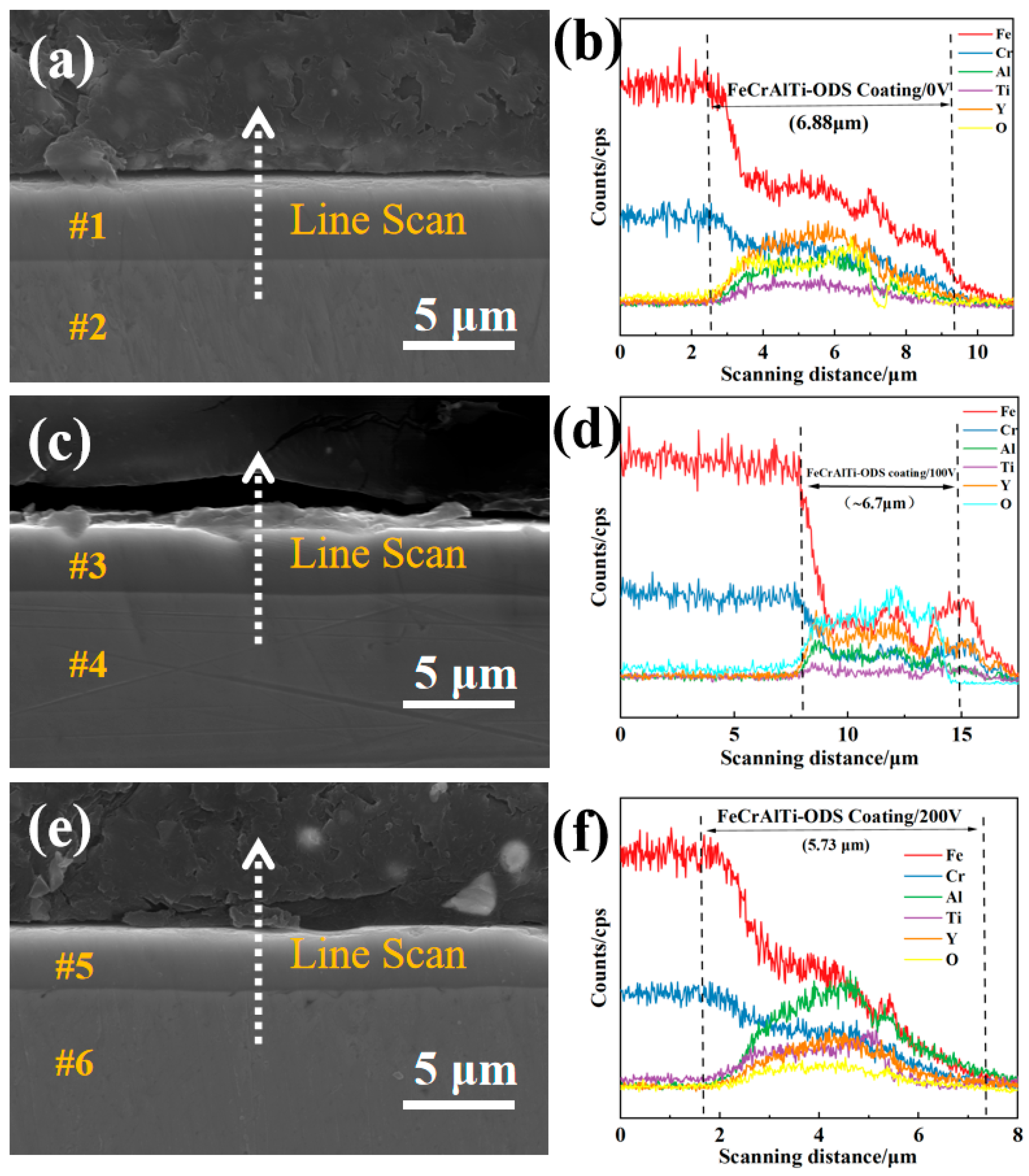
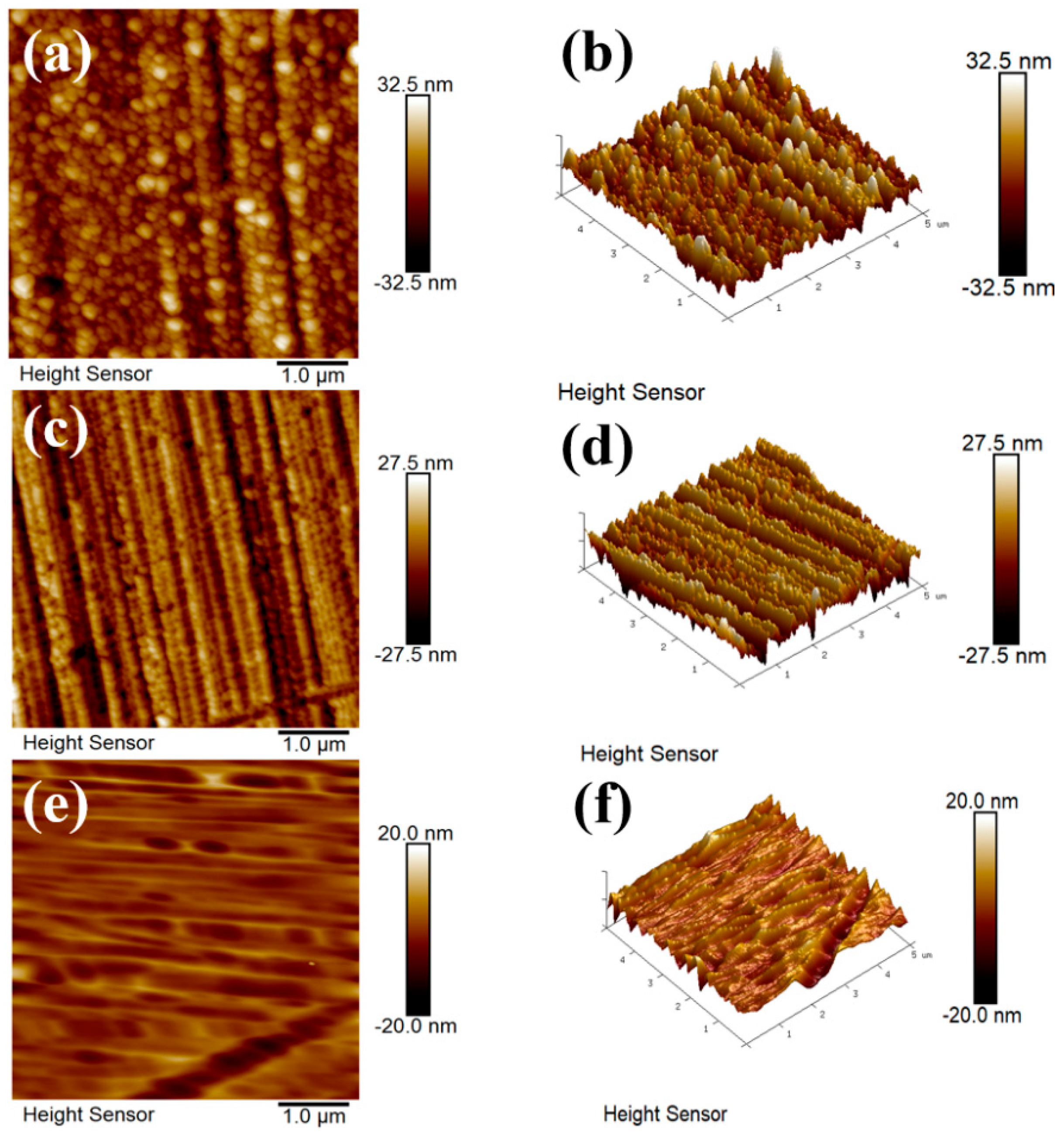
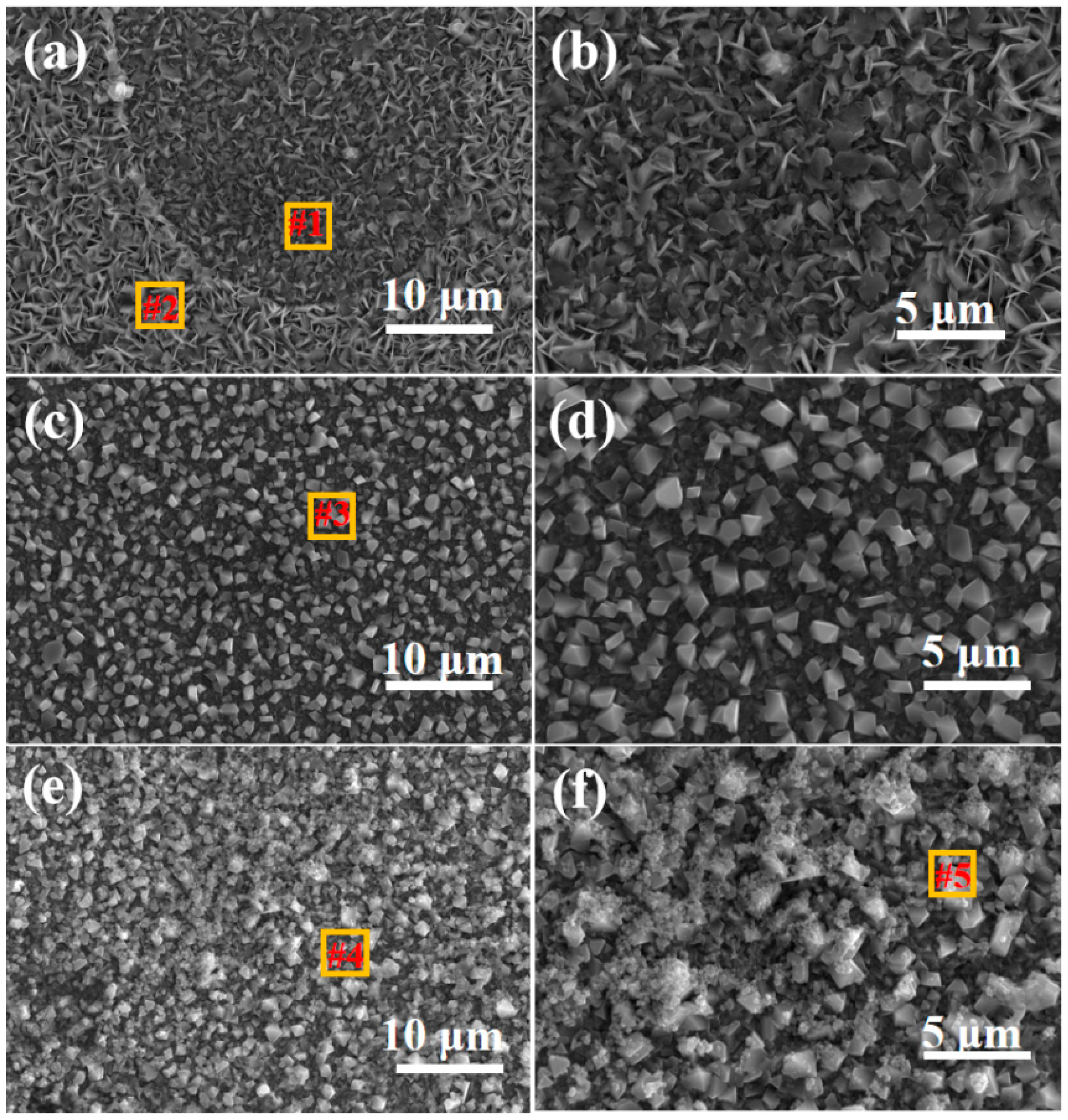
3.1. Morphology and Microstructure of FeCrAlTi-ODS Alloy Coating
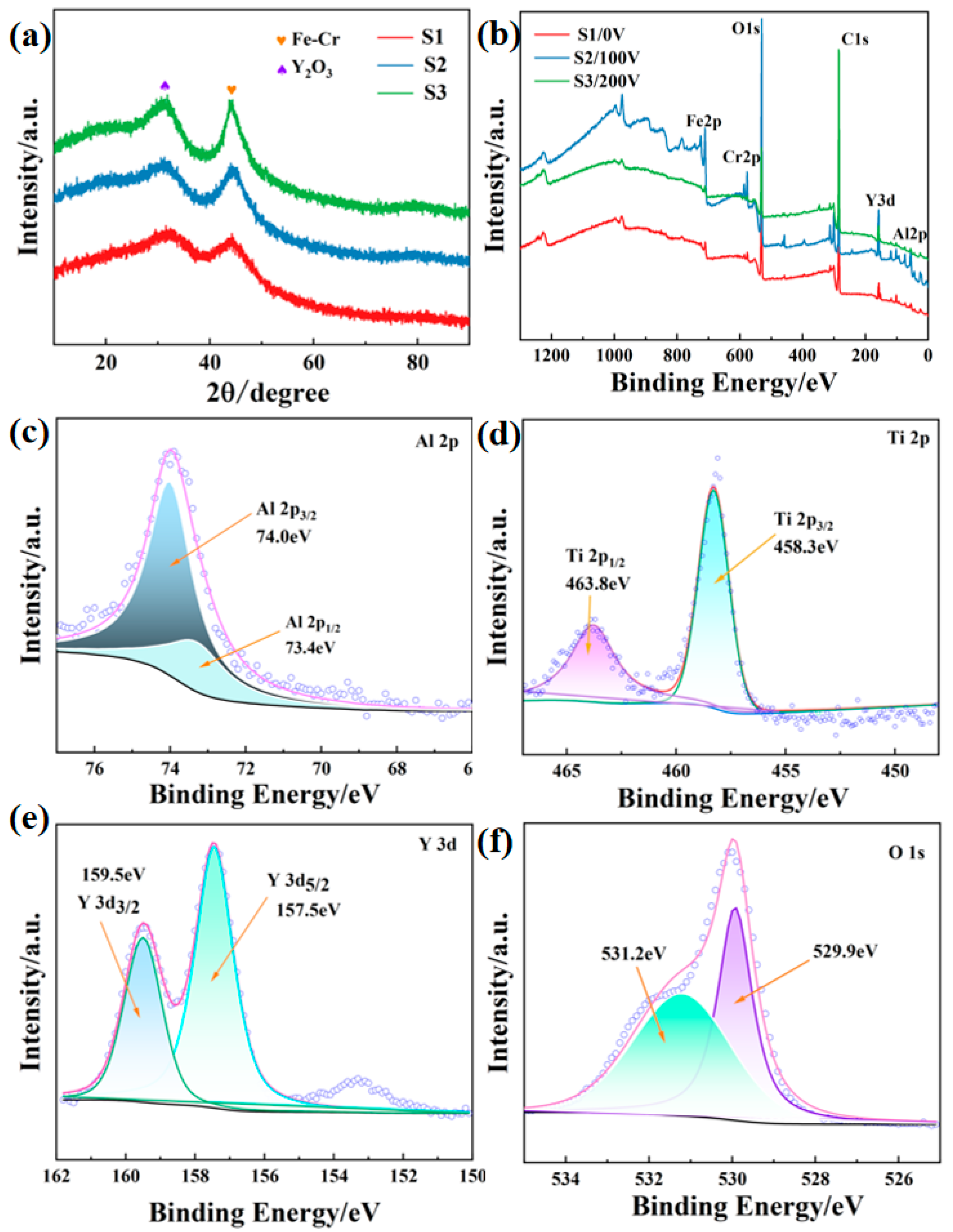
3.2. Mechanical Properties
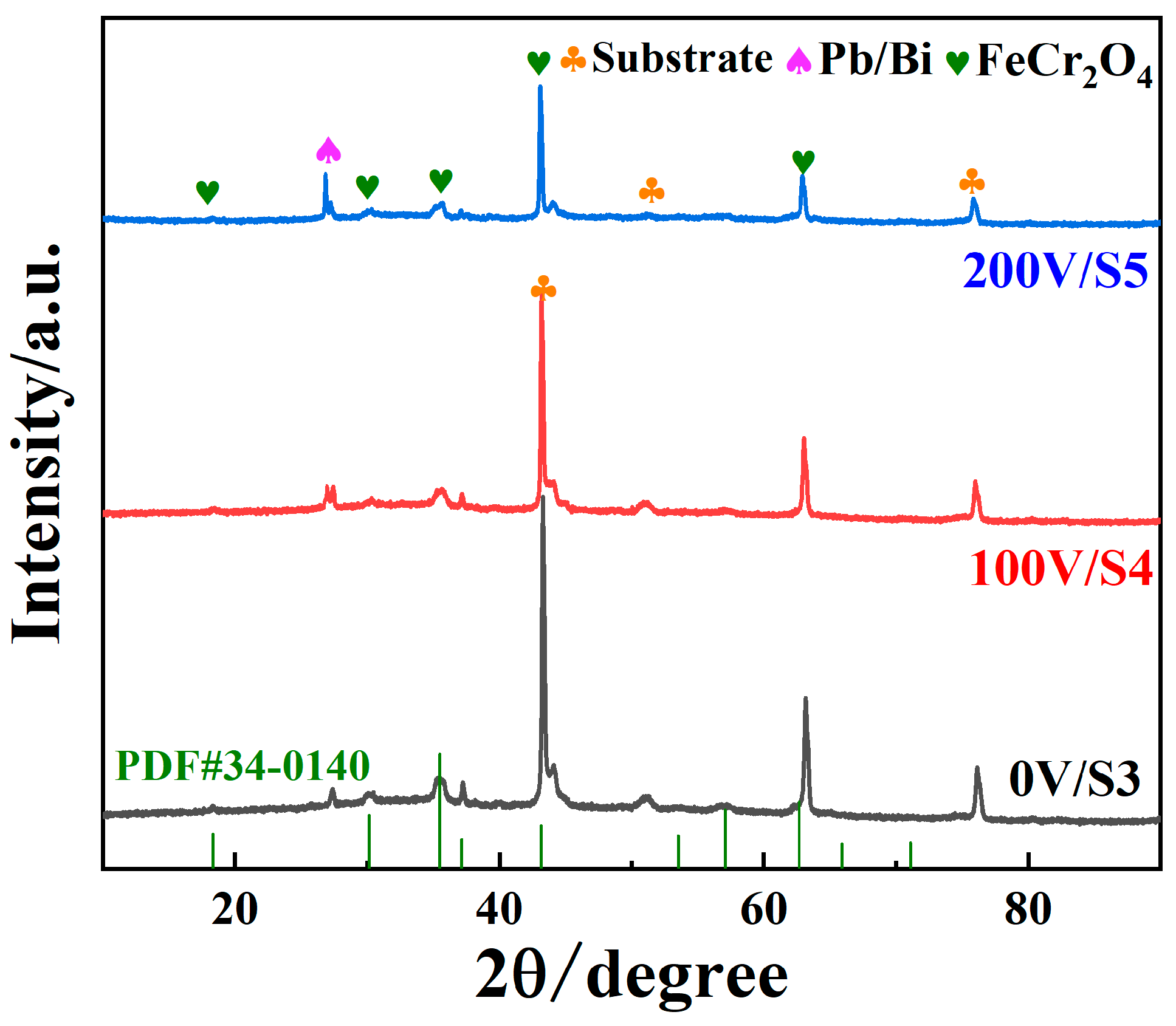
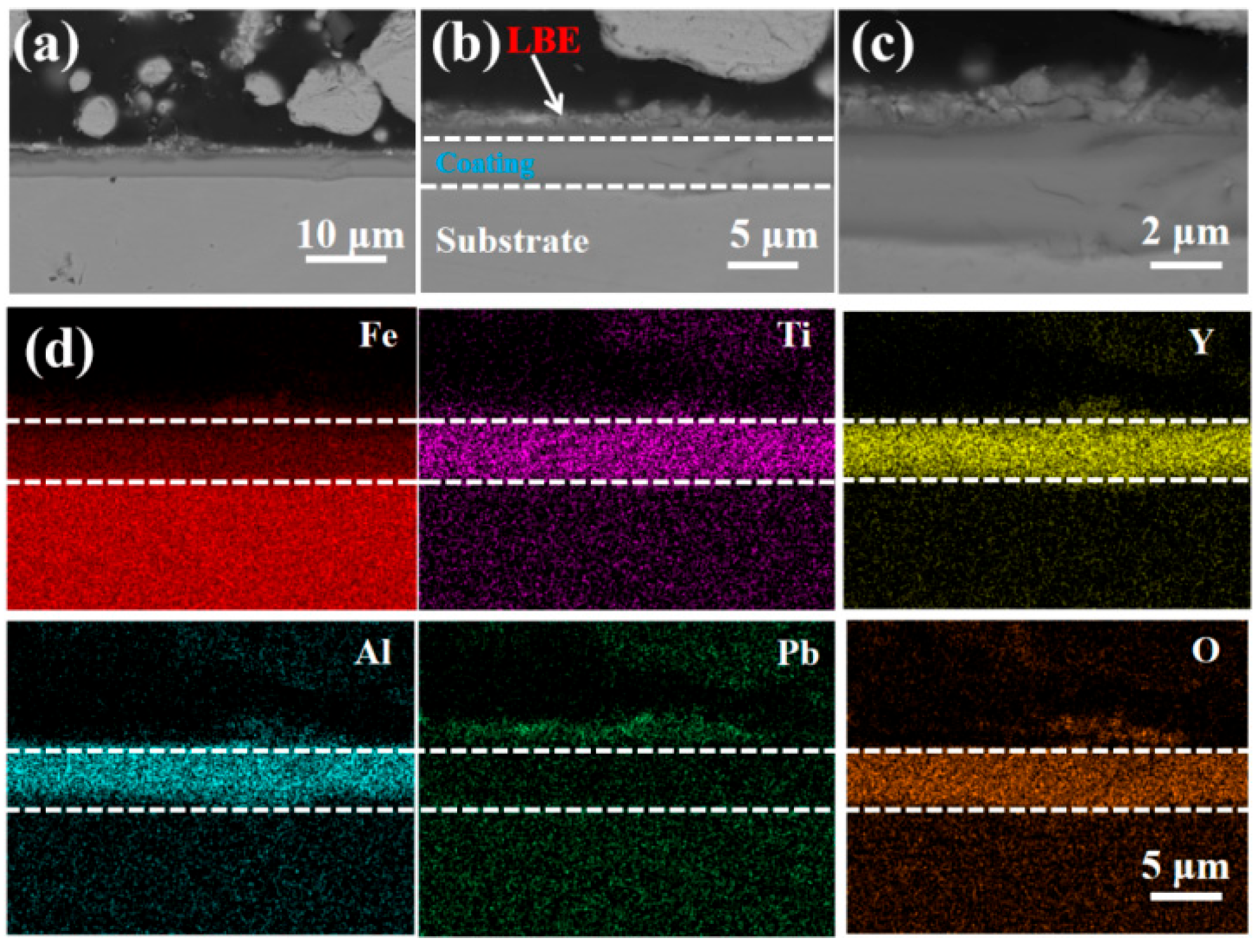
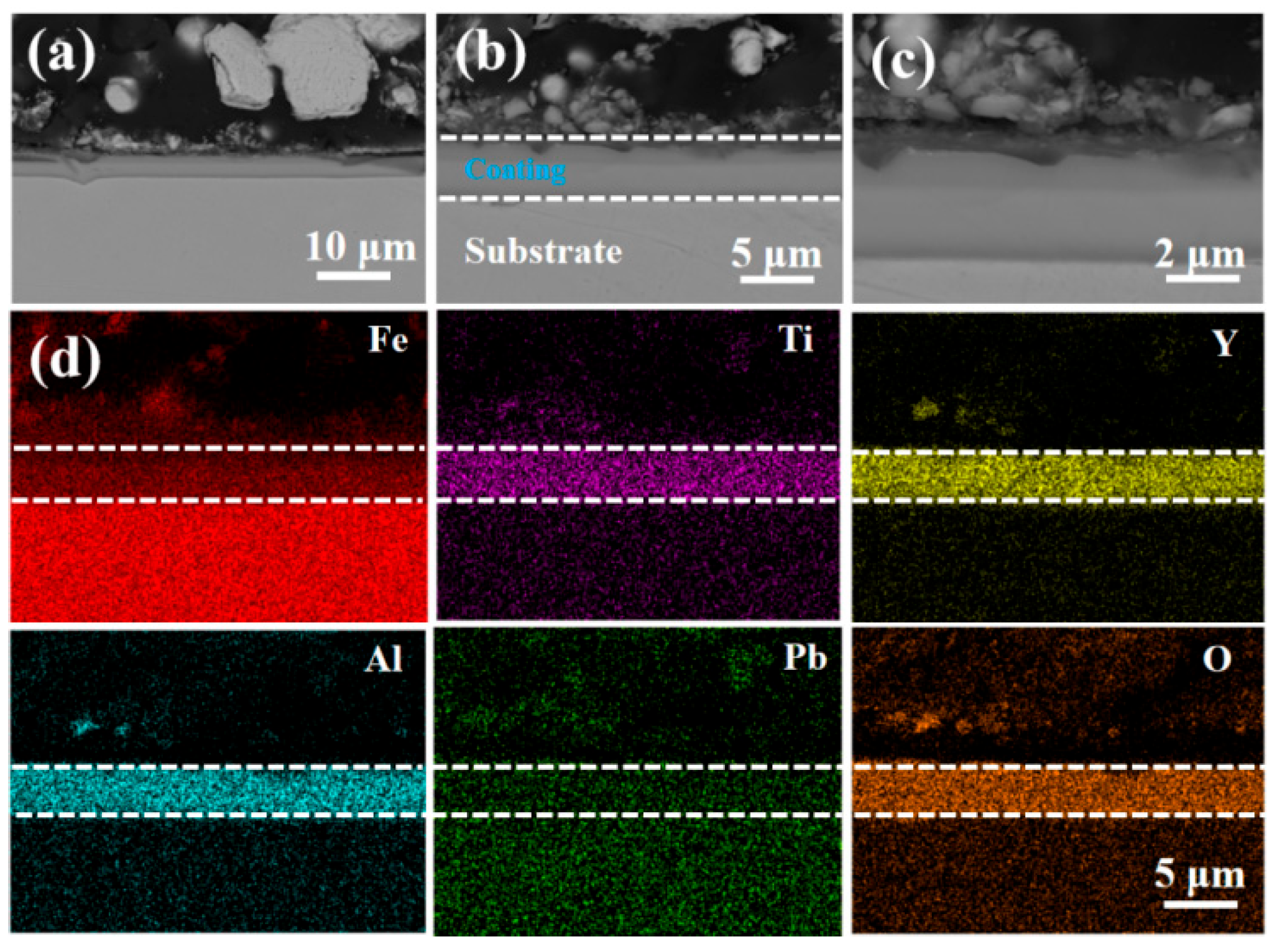
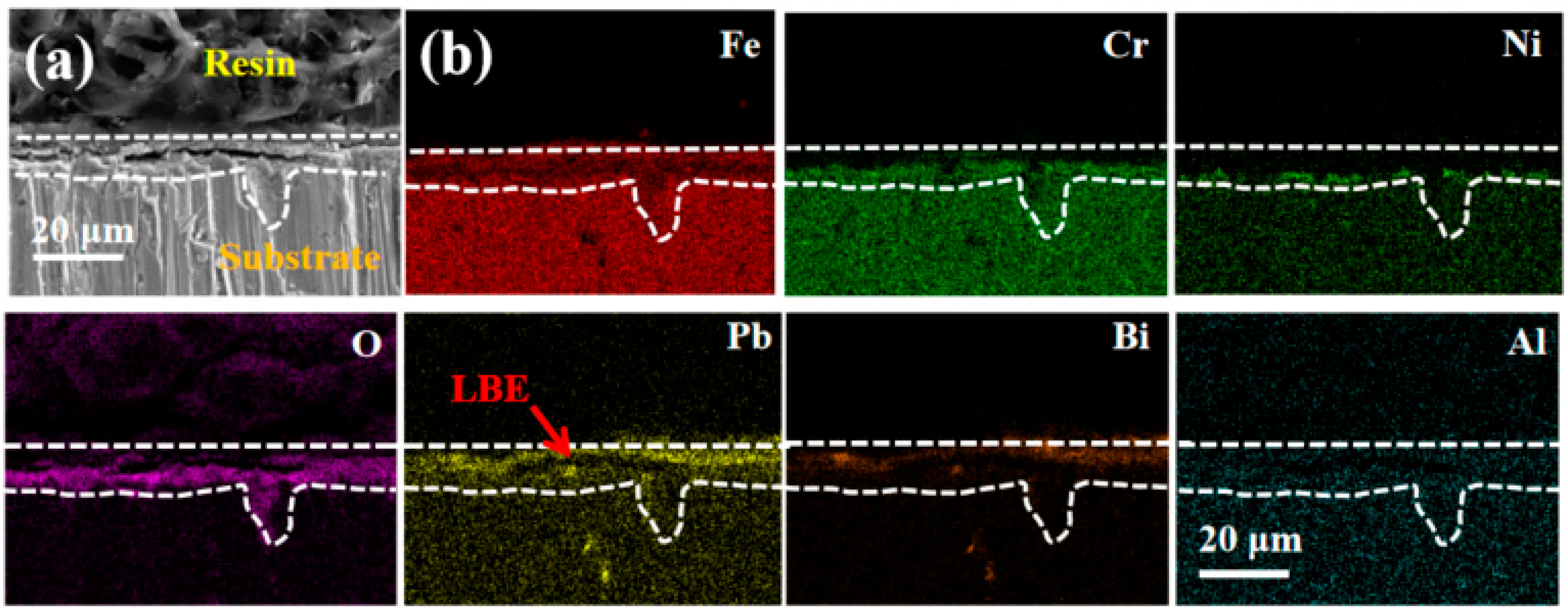
3.3. Resistance to LBE Corrosion Properties
4. Conclusions
- According to SEM and GIXRD, the surface of FeCrAlTi-ODS coating prepared at different bias voltages is relatively uniform, without bad pores and defects. The surface EDS shows that the coating surface elements are evenly distributed, and the atom ratio of the surface elements is similar.
- XPS analysis of the alloy coating surface shows the existence of diffusion oxide Y2O3 in the FeCrAlTi-ODS coating. Nanoindentation experiment shows that the applied bias voltage can effectively improve the binding force and mechanical properties of the alloy coating membrane, and its nano-hardness and elastic modulus can reach 11.52 GPa and 172.89 GPa. With the increase of the applied negative bias voltage, the thickness of the prepared coating decreases to a certain extent, and the coating thickness can affect the performance of the coating.
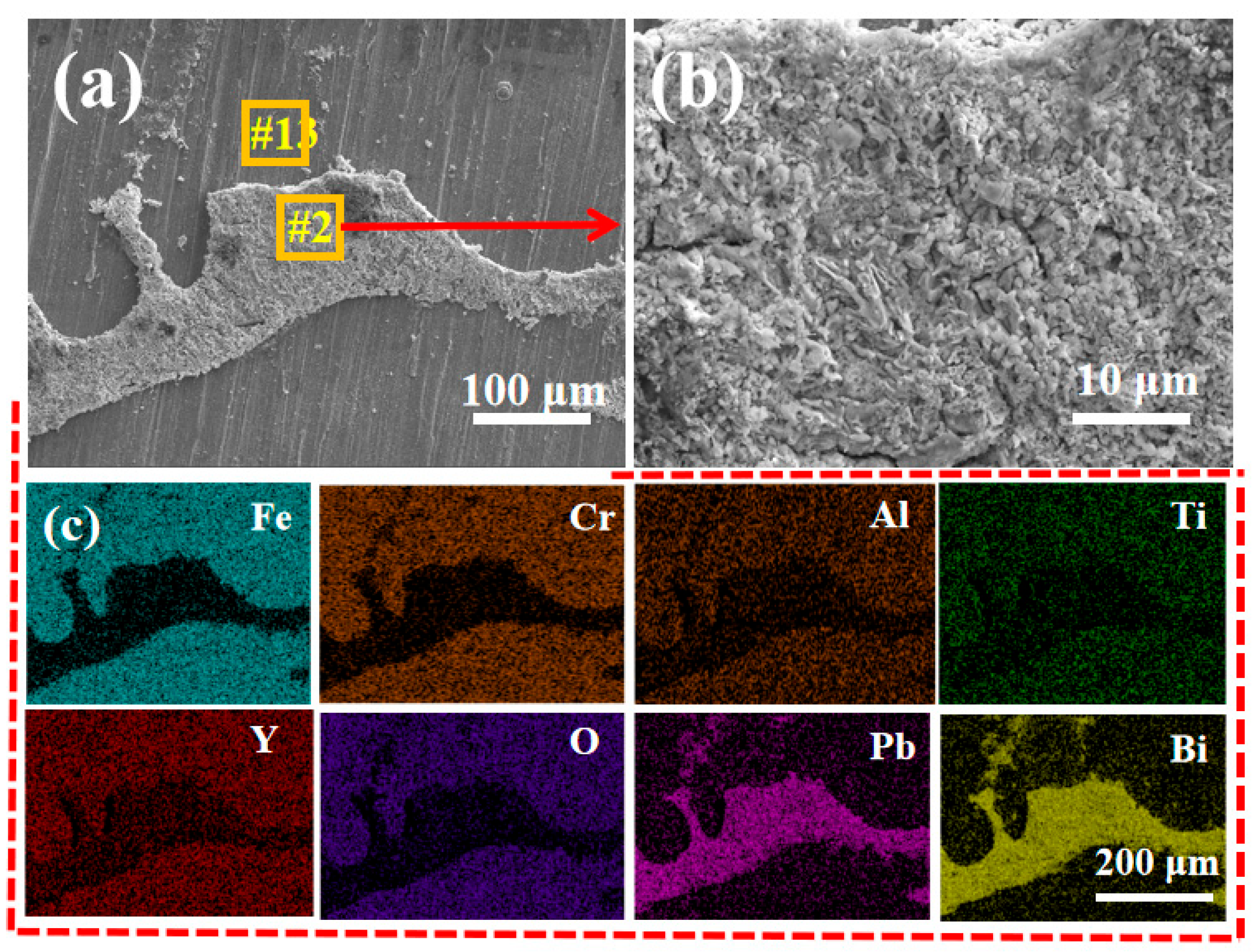
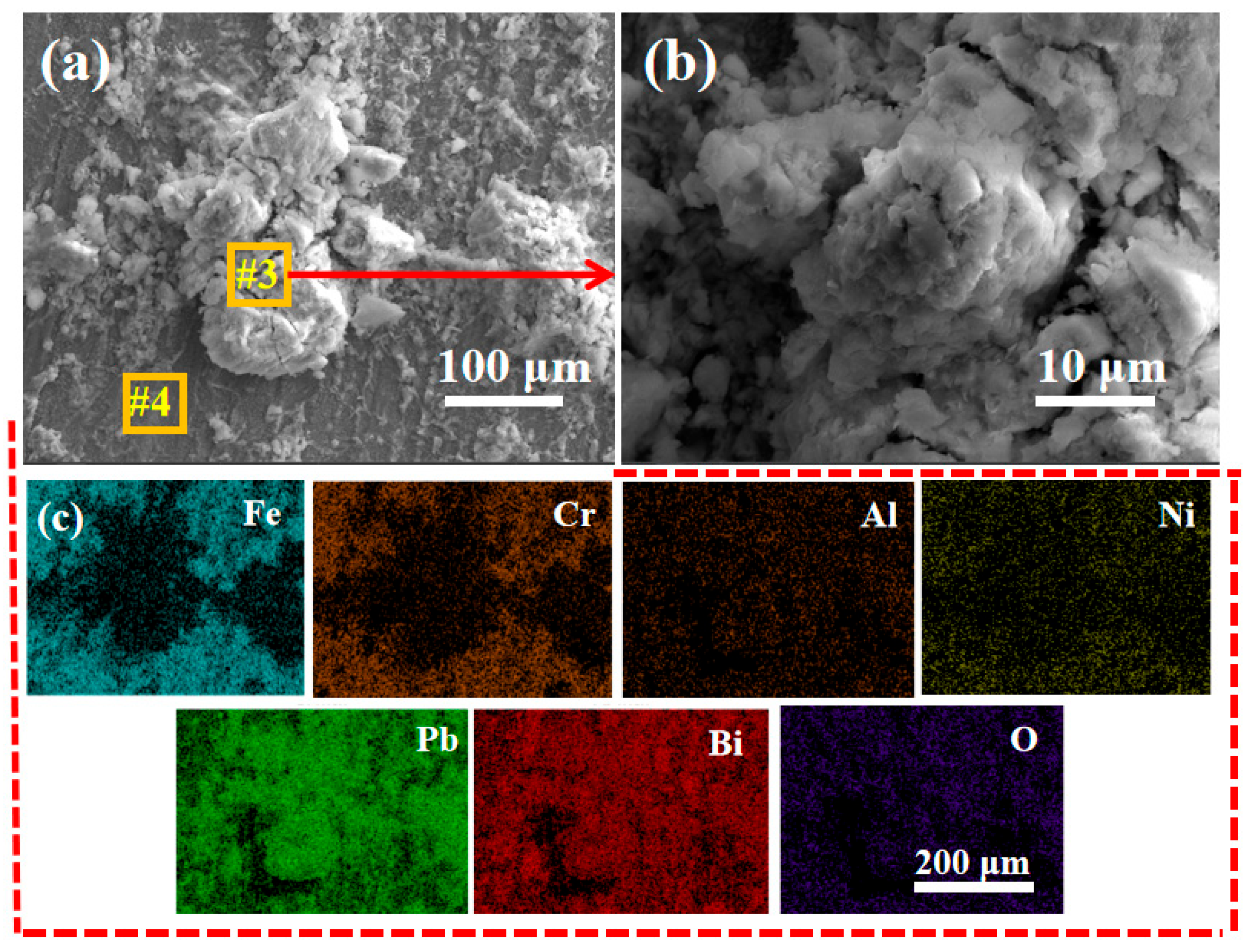
- The LBE corrosion experiment at saturated oxygen concentration (550 °C, 100 h) shows that the S2 alloy coating has the least residual LBE on the surface, and the composite oxide FeCr2O4 of Fe and Cr is mainly formed on the surface. After the LBE corrosion, the coating can remain intact, indicating that it has good corrosion resistance effect on the substrate 316L steel. Compared with the uncoated 316L substrate material, the preparation of the alloy coating can effectively improve its LBE corrosion resistance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grasso, G.; Petrovich, C.; Mattioli, D.; Artioli, C.; Sciora, P.; Gugiu, D.; Bandini, G.; Bubelis, E.; Mikityuk, K. The core design of ALFRED, a demonstrator for the European lead-cooled reactors. Nucl. Eng. Des. 2014, 278, 287–301. [Google Scholar] [CrossRef]
- Kelly, J.E. Generation IV International Forum: A decade of progress through international cooperation. Prog. Nucl. Energy 2014, 77, 240–246. [Google Scholar] [CrossRef]
- Murty, K.L.; Charit, I. Structural materials for Gen-IV nuclear reactors: Challenges and opportunities. J. Nucl. Mater. 2008, 383, 189–195. [Google Scholar] [CrossRef]
- Legkikh, A.Y.; Askhadullin, R.S.; Martynov, P.N.; Melnikov, V.P.; Storozhenko, A.N. Conceptual aspects of melting unit vessel cooling by heavy liquid metal coolant. Nucl. Energy Technol. 2016, 2, 161–165. [Google Scholar] [CrossRef]
- Heinzel, A.; Weisenburger, A.; Müller, G. Corrosion behavior of austenitic steels in liquid lead bismuth containing 10−6 wt% and 10−8 wt% oxygen at 400–500 °C. J. Nucl. Mater. 2014, 448, 163–171. [Google Scholar] [CrossRef]
- Wang, H.; Xiao, J.; Wang, H.; Chen, Y.; Yin, X.; Guo, N. Corrosion Behavior and Surface Treatment of Cladding Materials Used in High-Temperature Lead-Bismuth Eutectic Alloy: A Review. Coatings 2021, 11, 364. [Google Scholar] [CrossRef]
- He, L.; Liu, C.; Lin, J.; Chen, Q.; Yang, J.; Zhang, R.; Yang, H.; Wang, Y.; Wang, J.; Long, J.; et al. Microstructure, oxidation and corrosion properties of FeCrAl coatings with low Al content prepared by magnetron sputtering for accident tolerant fuel cladding. J. Nucl. Mater. 2021, 551, 152966. [Google Scholar] [CrossRef]
- Zhang, W.; Zhong, Y.; Qiu, X.; Li, Q.; Yue, H.; Zhou, Y.; Deng, J.; Yang, J.; Liu, H.; Li, Q.; et al. Screening of the FeCrAl LBE corrosion-resistant coatings: The effect of Cr and Al contents. Surf. Coat. Technol. 2023, 462, 111054. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Liang, Z.; Bai, P.; Nie, J.; Liu, S.; Chen, B.; Wei, S.; Guan, Q.; Cai, J. Continuous hot corrosion behaviour of an FeCrAlSi coating prepared by laser cladding. Surf. Coat. Technol. 2021, 421, 127424. [Google Scholar] [CrossRef]
- Dai, Y.; Boutellier, V.; Gavillet, D.; Glasbrenner, H.; Weisenburger, A.; Wagner, W. FeCrAlY and TiN coatings on T91 steel after irradiation with 72MeV protons in flowing LBE. J. Nucl. Mater. 2012, 431, 66–76. [Google Scholar] [CrossRef]
- Zhang, W.; Deng, J.; Zhou, M.; Zhong, Y.; Wu, L.; Mao, J.; Xu, X.; Zhou, Y.; Yang, J. Synergistic effect of simultaneous proton irradiation and LBE corrosion on the microstructure of the FeCrAl(Y) coatings. Corros. Sci. 2024, 229, 111874. [Google Scholar] [CrossRef]
- Zhang, P.; Yao, Z.; Wang, X.; Zheng, Y.; Cui, K.; Yao, R.; Lin, S.; Liu, Y.; Lu, S.; Wu, X. A novel FeCrAlW high entropy alloy coating for enhancing lead-bismuth eutectic corrosion resistance. J. Nucl. Mater. 2024, 589, 154844. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, Y.; Ye, Q.; Yang, B. Enhanced Corrosion−Resistance of AlTiCrFeMoSi High−Entropy Alloy Coating by Magnetron Sputtering. Coatings 2023, 13, 332. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, W.; Qiu, X.; Li, Q.; Chen, Q.; Yang, J.; Zhong, Y.; Zhu, C.; Liu, H.; Zhao, S.; et al. Effect of Au-ion irradiation on the morphology, microstructure and lead-bismuth eutectic corrosion behavior of refractory TiNbZrMoV high-entropy alloy coating. J. Nucl. Mater. 2023, 584, 154592. [Google Scholar] [CrossRef]
- Deng, J.; Yang, J.; Lv, L.; Zhang, W.; Chen, Q.; Zhou, M.; Zhu, C.; Liu, N.; Yang, J. Corrosion behavior of refractory TiNbZrMoV high-entropy alloy coating in static lead-bismuth eutectic alloy: A novel design strategy of LBE corrosion-resistant coating? Surf. Coat. Technol. 2022, 448, 128884. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, K.; Wang, G.; Deng, C.; Liu, N.; Zhang, W.; Yang, J. Influence of coating thickness on microstructure, mechanical and LBE corrosion performance of amorphous AlCrFeTiNb high-entropy alloy coatings. Surf. Coat. Technol. 2022, 441, 128502. [Google Scholar] [CrossRef]
- Lyu, L.; Yang, J.; Zhou, M.; Yan, M.; Yang, J. Microstructure, mechanical properties and lead-bismuth eutectic corrosion behavior of (AlCrFeTiMo)NO and (AlCrFeTiNb)NO high entropy metal sublattice ceramic coatings. Vacuum 2023, 209, 111774. [Google Scholar] [CrossRef]
- Devia, D.M.; Restrepo-Parra, E.; Arango, P.J.; Tschiptschin, A.P.; Velez, J.M. TiAlN coatings deposited by triode magnetron sputtering varying the bias voltage. Appl. Surf. Sci. 2011, 257, 6181–6185. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Zhao, X.; Liu, Y.; Cai, Y.; Li, J.Y.; Chen, H.; Wan, Q.; Yang, D.; Tan, J.; Liu, H.D.; et al. Lead-bismuth eutectic (LBE) corrosion behavior of AlTiN coatings at 550 and 600 °C. J. Nucl. Mater. 2020, 539, 152280. [Google Scholar] [CrossRef]
- Wan, Q.; Wu, Z.Y.; Liu, Y.; Yang, B.; Liu, H.D.; Ren, F.; Wang, P.; Xiao, Y.Y.; Zhang, J.; Zhang, G.D. Lead-bismuth eutectic (LBE) corrosion mechanism of nano-amorphous composite TiSiN coatings synthesized by cathodic arc ion plating. Corros. Sci. 2021, 183, 109264. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, W.; Chen, Q.; Yang, J.; Zhu, C.; Li, Q.; Yang, J.; Liu, N.; Yang, J. Effect of LBE corrosion on microstructure of amorphous Al2O3 coating by magnetron sputtering. Surf. Coat. Technol. 2022, 443, 128598. [Google Scholar] [CrossRef]
- Yin, X.; Li, X.; Wang, H.; Zhao, K.; Wang, J.; Chen, L.; Wu, Z.; Chen, Y. The Corrosion Behavior of Al/Al2O3 Composite Films with Ultra-Dense Structure Exposed to Lead-Bismuth Eutectic at 450 to 650 °C. Coatings 2023, 13, 1274. [Google Scholar] [CrossRef]
- Li, H.; Bai, P.; Lin, Z.; Zhang, J.; Tang, Q.; Pan, Y. Corrosion resistance in Pb-Bi alloy of 15-15Ti steel coated with Al2O3/SiC bilayer thin films by magnetron sputtering. Fusion Eng. Des. 2017, 125, 384–390. [Google Scholar] [CrossRef]
- Heinzel, A.; Weisenburger, A.; Müller, G. Long-term corrosion tests of Ti3SiC2 and Ti2AlC in oxygen containing LBE at temperatures up to 700 °C. J. Nucl. Mater. 2016, 482, 114–123. [Google Scholar] [CrossRef]
- Bentzel, G.W.; Ghidiu, M.; Griggs, J.; Lang, A.; Barsoum, M.W. On the interactions of Ti2AlC, Ti3AlC2, Ti3SiC2 and Cr2AlC with pure sodium at 550 °C and 750 °C. Corros. Sci. 2016, 111, 568–573. [Google Scholar] [CrossRef]
- Jiang, H.; Zhao, X.; Cao, S.; Wang, D.; Zhu, Q.; Lei, Y. Improvement of the mechanical properties and LBE cavitation erosion resistance of laser cladded FeCrAlTiC coating by Y2O3 addition. Mater. Lett. 2022, 326, 132882. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, W.; Zhou, M.; Lv, L.; Zhong, Y.; Li, Q.; Zhou, Y.; Yang, J. Effect of Y2O3 content on microstructure and corrosion behavior of ZrO2 coatings in liquid Lead-Bismuth eutectic. Mater. Chem. Phys. 2023, 309, 128392. [Google Scholar] [CrossRef]
- Zhao, B.-L.; Wang, L.; Zhang, L.-F.; Ke, J.-G.; Xie, Z.-M.; Yang, J.-F.; Wang, X.-P.; Hao, T.; Liu, C.-S.; Wu, X.-B. Effect of Nano-Y2O3 Content on Microstructure and Mechanical Properties of Fe18Cr Films Fabricated by RF Magnetron Sputtering. Nanomaterials 2021, 11, 1754. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Wang, X.; Wang, L.; Zhang, Y.; Liu, W.; Jiang, W.; Zhang, T.; Fang, Q.; Liu, C. Fabrication and characterization of He-charged ODS-FeCrNi films deposited by a radio-frequency plasma magnetron sputtering technique. Plasma Sci. Technol. 2017, 19, 045502. [Google Scholar] [CrossRef]
- Kurata, Y. Corrosion behavior of cold-worked austenitic stainless steels in liquid lead–bismuth eutectic. J. Nucl. Mater. 2014, 448, 239–249. [Google Scholar] [CrossRef]
- Yamaki, E.; Ginestar, K.; Martinelli, L. Dissolution mechanism of 316L in lead–bismuth eutectic at 500 °C. Corros. Sci. 2011, 53, 3075–3085. [Google Scholar] [CrossRef]
- Gromov, B.F.; Orlov, Y.I.; Martynov, P.N.; Ivanov, K.D.; Gulevsky, V.A. Physical-Chemical Principles of Lead-Bismuth Coolant Technology. In Liquid Metal Systems: Material Behavior and Physical Chemistry in Liquid Metal Systems 2; Borgstedt, H.U., Frees, G., Eds.; Springer: Boston, MA, USA, 1995; pp. 339–343. [Google Scholar]
- Yang, J.; Shi, K.; Zhang, W.; Chen, Q.; Ning, Z.; Zhu, C.; Liao, J.; Yang, Y.; Liu, N.; Zhang, W.; et al. A novel AlCrFeMoTi high-entropy alloy coating with a high corrosion-resistance in lead-bismuth eutectic alloy. Corros. Sci. 2021, 187, 109524. [Google Scholar] [CrossRef]
- Xu, Y.; Li, G.; Xia, Y. Synthesis and characterization of super-hard AlCrTiVZr high-entropy alloy nitride films deposited by HiPIMS. Appl. Surf. Sci. 2020, 523, 146529. [Google Scholar] [CrossRef]
- Bradley, R.M.; Harper, J.M.E.; Smith, D. Theory of thin-film orientation by ion bombardment during deposition. J. Appl. Phys. 1986, 60, 4160–4164. [Google Scholar] [CrossRef]
- Rauschenbach, B.; Helming, K. Implantation-induced texture. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1989, 42, 216–223. [Google Scholar] [CrossRef]
- Zeng, X.K.; Li, Y.T.; Zhang, X.D.; Liu, M.; Ye, J.Z.; Qiu, X.L.; Jiang, X.; Leng, Y.X. Effect of bias voltage on the structure and properties of CuNiTiNbCr dual-phase high entropy alloy films. J. Alloys Compd. 2023, 931, 167371. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Wang, Y.; Liu, S. Synthesis and characterization of Y2O3 nanoparticles and nanorods in magnetron sputtered Ti-Y alloy films. Appl. Phys. A 2012, 110, 465–470. [Google Scholar] [CrossRef]
- Chen, X.; Du, Y.; Chung, Y.-W. Commentary on using H/E and H3/E2 as proxies for fracture toughness of hard coatings. Thin Solid Film. 2019, 688, 137265. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, L.; Qiu, C.; He, B.; Zhou, L.; Zhao, J.; Li, Y. Influence of LBE Temperatures on the Microstructure and Properties of Crystalline and Amorphous Multiphase Ceramic Coatings. Coatings 2019, 9, 543. [Google Scholar] [CrossRef]
- Peng, X.; Tang, Y.; Ding, X.; Lu, Z.; Hou, S.; Zhou, J.; Han, S.; Lü, Z.; Lu, G.; Wu, Y. Fe-based amorphous coating prepared using high-velocity oxygen fuel and its corrosion behavior in static lead-bismuth eutectic alloy. Int. J. Miner. Metall. Mater. 2022, 29, 2032–2040. [Google Scholar] [CrossRef]
- Zhang, J.; Li, N. Review of the studies on fundamental issues in LBE corrosion. J. Nucl. Mater. 2008, 373, 351–377. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, J.; Ma, S.; Xiong, Y.; Huang, S.; Kai, J.J.; Zhao, S. Revealing the crucial role of rough energy landscape on self-diffusion in high-entropy alloys based on machine learning and kinetic Monte Carlo. Acta Mater. 2022, 234, 118051. [Google Scholar] [CrossRef]
- Lambrinou, K.; Charalampopoulou, E.; Van der Donck, T.; Delville, R.; Schryvers, D. Dissolution corrosion of 316L austenitic stainless steels in contact with static liquid lead-bismuth eutectic (LBE) at 500 °C. J. Nucl. Mater. 2017, 490, 9–27. [Google Scholar] [CrossRef]
- Chen, Q.; Bai, F.; Wang, P.; Yang, J.; Zhu, C.; Zhang, W.; Liu, H.; Zhong, Y.; Deng, J.; Liu, N.; et al. Microstructure response and lead-bismuth eutectic corrosion behavior of 11Cr1Si ferritic/martensitic steel after Au-ion irradiation. Corros. Sci. 2022, 198, 110101. [Google Scholar] [CrossRef]
- Wu, W.; Ran, G.; Li, Y.; Cong, S.; Ye, C.; Zhang, R.; Sun, Y. Early corrosion behaviour of irradiated FeCrAl alloy in a simulated pressurized water reactor environment. Corros. Sci. 2020, 174, 108824. [Google Scholar] [CrossRef]


| Fe | Cr | Ni | Mo | Mn | Si |
|---|---|---|---|---|---|
| 68.8 | 17.9 | 9.5 | 1.2 | 1.8 | 1.3 |
| Parameters | Value |
|---|---|
| Base pressure | 3 × 10−4 Pa |
| Substrate temperature | 150 °C |
| Deposition pressure | 0.6 Pa |
| Air flow rate | 120 Sccm |
| Deposition time | 4 h |
| Target-substrate distance | 4 cm |
| Negative substrate bias | 0 V/100 V/200 V (S1/S2/S3) |
| Voltage | Fe | Cr | Al | Ti | Y | O |
|---|---|---|---|---|---|---|
| 0 V | Bal. | 6.97 | 5.16 | 1.21 | 5.91 | 50.85 |
| 100 V | Bal. | 7.15 | 6.68 | 1.94 | 5.08 | 55.89 |
| 200 V | Bal. | 9.62 | 5.21 | 1.50 | 4.55 | 49.11 |
| Fe | Cr | Al | Ti | Y | O | |
|---|---|---|---|---|---|---|
| #1 | 23.77 | 8.80 | 7.09 | 2.62 | 6.49 | 51.23 |
| #2 | 76.00 | 21.64 | 0 | 0 | 0 | 2.36 |
| #3 | 22.95 | 8.79 | 8.61 | 2.99 | 8.50 | 48.16 |
| #4 | 76.64 | 21.65 | 0 | 0 | 0 | 1.71 |
| #5 | 21.49 | 8.13 | 8.24 | 2.85 | 9.40 | 49.89 |
| #6 | 75.76 | 21.12 | 0 | 0 | 0 | 3.12 |
| Fe | Cr | Al | Ti | Y | O | Pb | Bi | |
|---|---|---|---|---|---|---|---|---|
| #1 | 19.32 | 4.45 | 2.88 | 1.44 | 3.06 | 66.52 | 1.97 | 0.36 |
| #2 | 24.61 | 8.74 | 4.71 | 2.81 | 5.93 | 51.09 | 1.73 | 0.38 |
| #3 | 19.24 | 3.87 | 2.94 | 1.29 | 3.16 | 69.36 | 0.14 | 0 |
| #4 | 24.71 | 7.48 | 2.97 | 2.54 | 5.50 | 56.49 | 0.31 | 0 |
| #5 | 23.64 | 3.19 | 1.75 | 0.98 | 2.58 | 67.01 | 0.75 | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Li, J.; Zhang, B.; Zhang, J.; Li, Z.; Zhao, H. Promoted Mechanical Properties and LBE Corrosion Resistance of FeCrAlTi-ODS Coatings Deposited by Magnetron Sputtering. Coatings 2025, 15, 942. https://doi.org/10.3390/coatings15080942
Huang H, Li J, Zhang B, Zhang J, Li Z, Zhao H. Promoted Mechanical Properties and LBE Corrosion Resistance of FeCrAlTi-ODS Coatings Deposited by Magnetron Sputtering. Coatings. 2025; 15(8):942. https://doi.org/10.3390/coatings15080942
Chicago/Turabian StyleHuang, Hongtao, Jinfeng Li, Bao Zhang, Jianwei Zhang, Zhigang Li, and Hongtao Zhao. 2025. "Promoted Mechanical Properties and LBE Corrosion Resistance of FeCrAlTi-ODS Coatings Deposited by Magnetron Sputtering" Coatings 15, no. 8: 942. https://doi.org/10.3390/coatings15080942
APA StyleHuang, H., Li, J., Zhang, B., Zhang, J., Li, Z., & Zhao, H. (2025). Promoted Mechanical Properties and LBE Corrosion Resistance of FeCrAlTi-ODS Coatings Deposited by Magnetron Sputtering. Coatings, 15(8), 942. https://doi.org/10.3390/coatings15080942





