The Synergistic Influence of Trace Impurities and Temperature on the Corrosion Behavior of Tubing in Supercritical CO2 Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
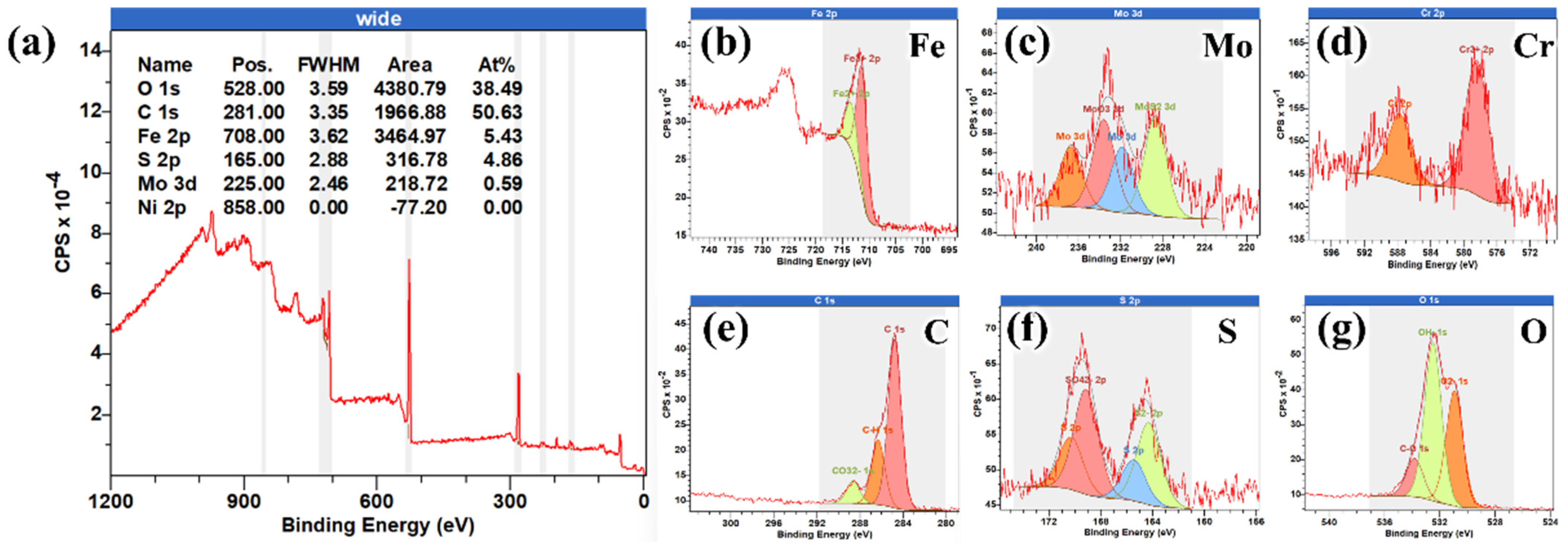
2.2. Characterization of Corrosion Products
3. Results and Discussions
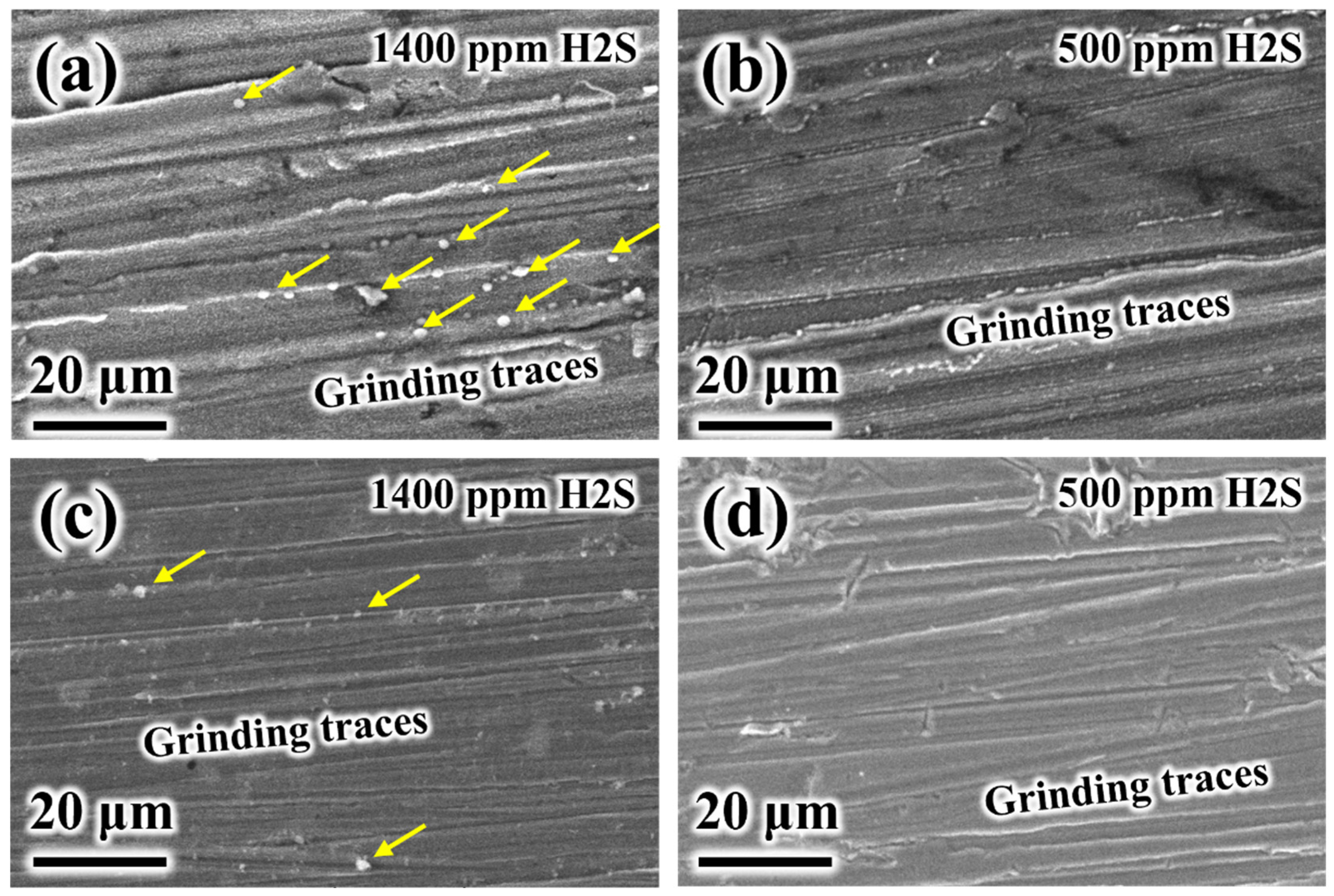
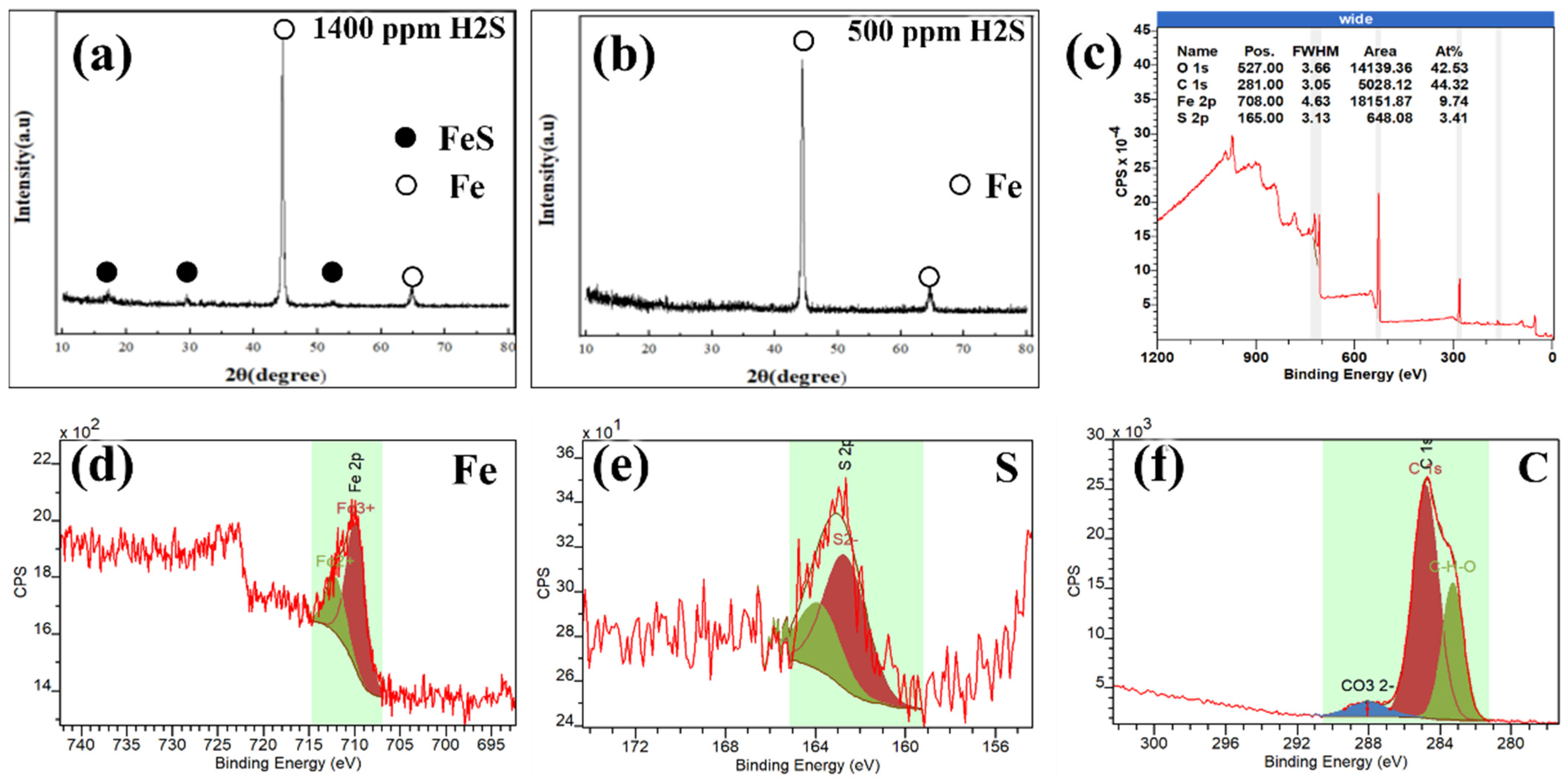
3.1. The Influence of H2S
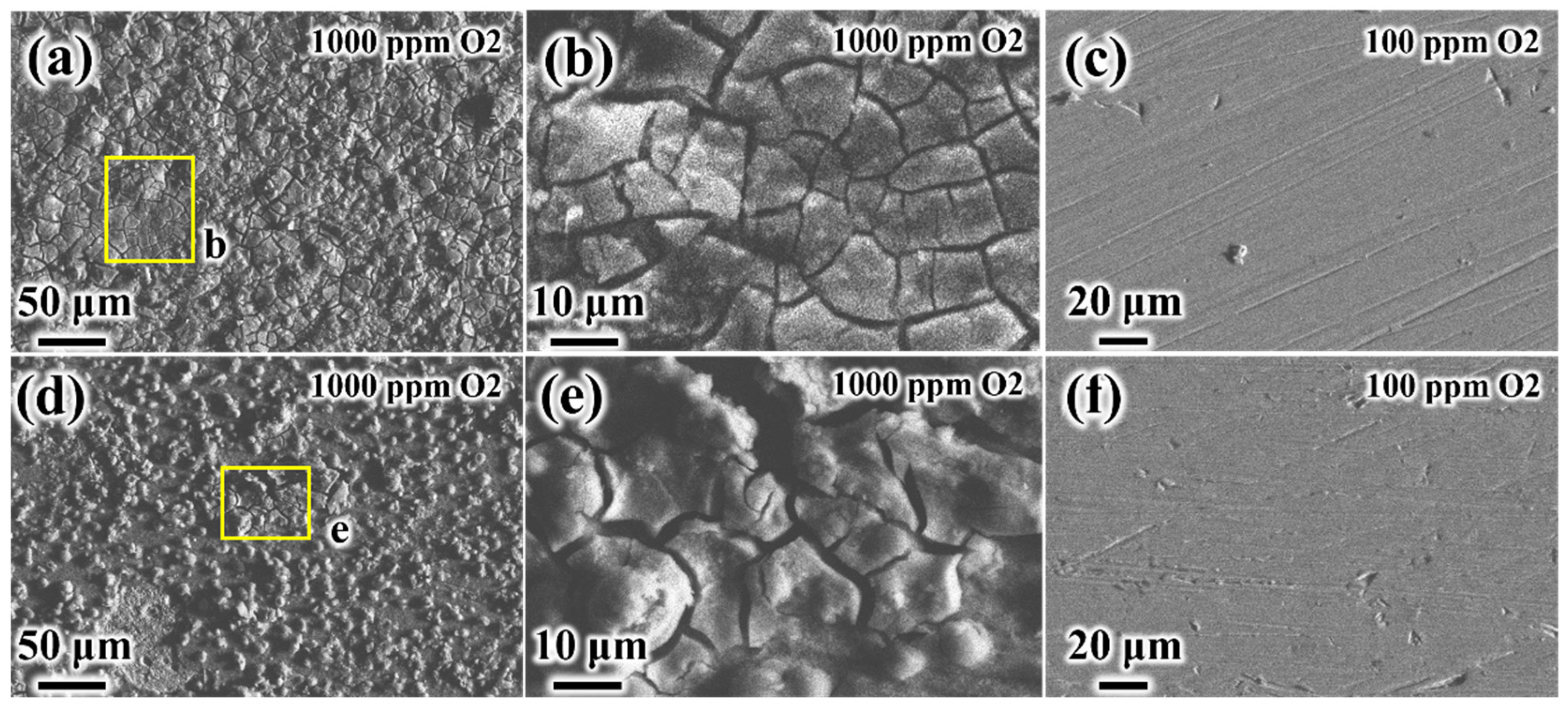
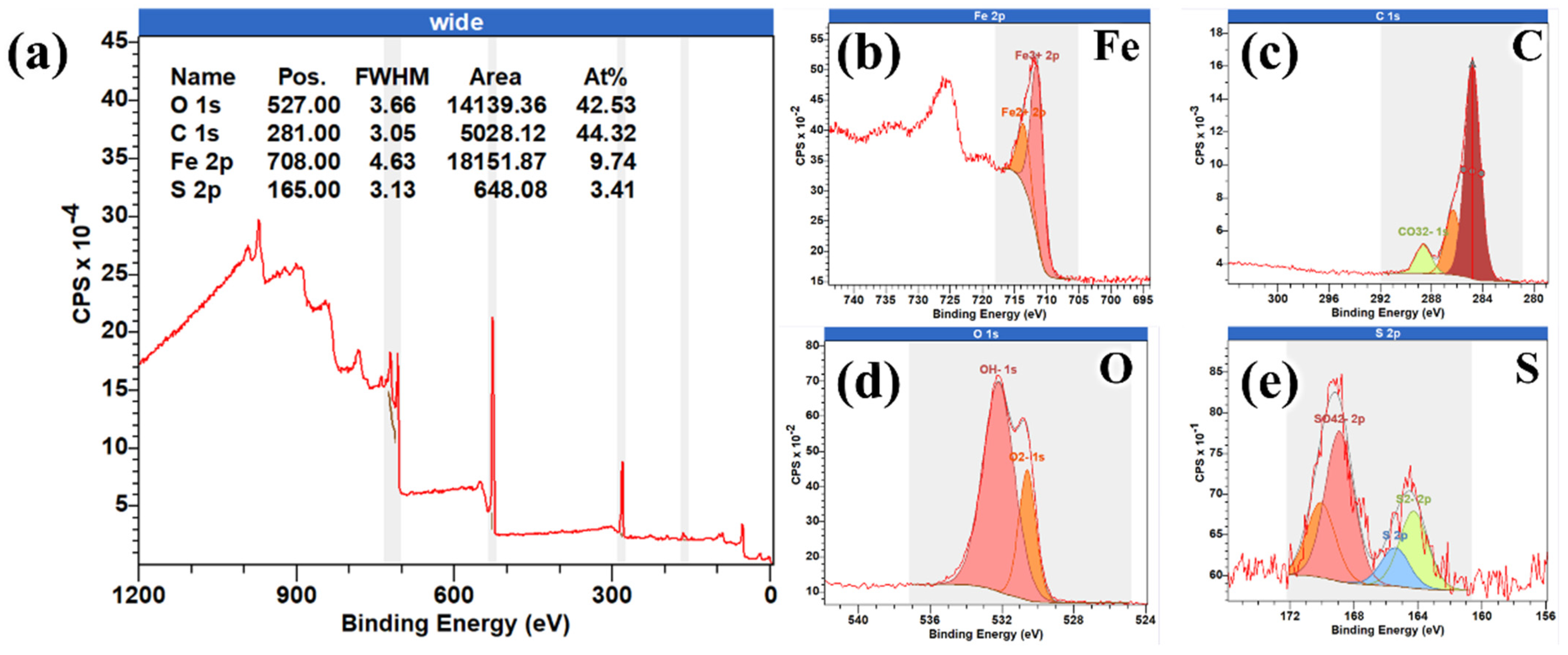
3.2. The Coupled Effect of H2S and O2
3.3. The Influence of Temperature
4. Conclusions
- In water-unsaturated (500 ppmv H2O) supercritical CO2-H2S environments, both P110 and S13Cr exhibited relatively low and stable general corrosion rates (approximately 0.03 mm/y and 0.01 mm/y, respectively). The corrosion product films were poorly developed and only partially obscured the grinding marks. However, S13Cr showed susceptibility to pitting corrosion when the H2S concentration exceeded 500 ppm. Therefore, a supercritical CO2 environment containing 500 ppm H2S was identified as the threshold condition, below which the corrosion behavior of both materials remained within acceptable limits.
- Upon the introduction of O2, both steels exhibited markedly accelerated corrosion, characterized by elevated general corrosion rates and the presence of deep pits. Although relatively thick product films formed on the specimen surfaces, these films displayed severe cracking, compromising their protective ability and exacerbating localized corrosion. When the O2 concentration was reduced to 100 ppm, the corrosion product films became thinner yet continuous, correlating with stable corrosion rates and the complete suppression of pitting in both materials.
- The corrosion behavior of S13Cr was found to be highly sensitive to temperature. As the test temperature decreased from 120 °C to 60 °C, the general corrosion rate of S13Cr increased substantially from 0.0031 mm/y to 0.08 mm/y. This trend is attributed to the influence of temperature on water solubility in supercritical CO2. At lower temperatures, water is more likely to precipitate on the steel surface, enhancing the dissolution of H2S into the aqueous phase and thus intensifying its corrosive effect.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shiyi, Y.; Desheng, M.A.; Junshi, L.I.; Tiyao, Z.; Zemin, J.I.; Haishui, H.A.N. Progress and Prospects of Carbon Dioxide Capture, EOR-Utilization and Storage Industrialization. Pet. Explor. Dev. 2022, 49, 955–962. [Google Scholar] [CrossRef]
- Yang, J.; Yang, C.; Gu, Q.; Zhu, C.; Luo, M. Economic Evaluation and Influencing Factors of CCUS-EOR Technology: A Case Study from a High Water-Bearing Oilfield in Xinjiang, China. Energy Rep. 2023, 10, 153–160. [Google Scholar] [CrossRef]
- Fang, W.; Guangzhi, L.; Chunmei, S.U.; Feng, W.; Jianguo, M.A.; Yongzhi, Y. Carbon Emission Reduction Accounting Method for a CCUS-EOR Project. Pet. Explor. Dev. 2023, 50, 989–1000. [Google Scholar] [CrossRef]
- Kang, H.; Li, G.; Zhou, X.; Gao, J. Challenge and Strategy for the Successful Application of CCUS-EOR in China. Front. Energy Res. 2023, 11, 1073402. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Nesic, S. Corrosion Behvaior of Carbon Steel in Supercritical CO2-Water Environment. In Proceedings of the Corrosion Conference and Expo 2009, Atlanta, GA, USA, 22–26 March 2009; pp. 1–20. [Google Scholar] [CrossRef]
- Wang, Z.M.; Song, G.; Zhang, J. Corrosion Control in CO2 Enhanced Oil Recovery From a Perspective of Multiphase Fluids. Front. Mater. 2019, 6, 272. [Google Scholar] [CrossRef]
- Dai, Z.; Viswanathan, H.; Middleton, R.; Pan, F.; Ampomah, W.; Yang, C.; Jia, W.; Xiao, T.; Lee, S.; Mcpherson, B.; et al. CO2 Accounting and Risk Analysis for CO2 Sequestration at Enhanced Oil Recovery Sites. Environ. Sci. Technol. 2016, 50, 7546–7554. [Google Scholar] [CrossRef] [PubMed]
- Morland, B.H.; Dugstad, A.; Svenningsen, G. Corrosion of Carbon Steel in Dense Phase CO2 with Water above and below the Solubility Limit. Energy Procedia 2017, 114, 6752–6765. [Google Scholar] [CrossRef]
- Morland, B.H.; Norby, T.; Tjelta, M.; Svenningsen, G. Effect of SO2, O2, NO2, and H2O Concentrations on Chemical Reactions and Corrosion of Carbon Steel in Dense Phase CO2. Corrosion 2019, 75, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Sun, J.; Luo, J.L. Unlocking the Impurity-Induced Pipeline Corrosion Based on Phase Behavior of Impure CO2 Streams. Corros. Sci. 2020, 165, 108367. [Google Scholar] [CrossRef]
- Kladkaew, N.; Idem, R.; Tontiwachwuthikul, P.; Saiwan, C. Corrosion Behavior of Carbon Steel in the Monoethanolamine-H2O-CO2-O2-SO2 System. Ind. Eng. Chem. Res. 2009, 48, 8913–8919. [Google Scholar] [CrossRef]
- Kairy, S.K.; Zhou, S.; Turnbull, A.; Hinds, G. Corrosion of Pipeline Steel in Dense Phase CO2 Containing Impurities: A Critical Review of Test Methodologies. Corros. Sci. 2023, 214, 110986. [Google Scholar] [CrossRef]
- Hua, Y.; Barker, R.; Neville, A. Comparison of Corrosion Behaviour for X-65 Carbon Steel in Supercritical CO2-Saturated Water and Water-Saturated/Unsaturated Supercritical CO2. J. Supercrit. Fluids 2015, 97, 224–237. [Google Scholar] [CrossRef]
- Sun, C.; Sun, J.; Wang, Y.; Lin, X.; Li, X.; Cheng, X.; Liu, H. Synergistic Effect of O2, H2S and SO2 Impurities on the Corrosion Behavior of X65 Steel in Water-Saturated Supercritical CO2 System. Corros. Sci. 2016, 107, 193–203. [Google Scholar] [CrossRef]
- Ji, X.; Zhu, C. Predicting Possible Effects of H2S Impurity on CO2 Transportation and Geological Storage. Environ. Sci. Technol. 2013, 47, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Ding, T.; Sun, J.; Lin, X.; Zhao, W.; Chen, H. Insights into the Effect of H2S on the Corrosion Behavior of N80 Steel in Supercritical CO2 Environment. J. Mater. Res. Technol. 2023, 26, 5462–5477. [Google Scholar] [CrossRef]
- Deshmukh, S.; Brown, B.; Young, D. Investigating Oxygen Ingress in the Oil and Gas Industry. Proceedings of the AMPP Annual Conference + Expo 2024 1–17.
- Xiang, Y.; Wang, Z.; Li, Z.; Ni, W.D. Effect of Temperature on Corrosion Behaviour of X70 Steel in High Pressure CO2/SO2/O2/H2O Environments. Corros. Eng. Sci. Technol. 2013, 48, 121–130. [Google Scholar] [CrossRef]
- Hua, Y.; Barker, R.; Neville, A. International Journal of Greenhouse Gas Control Effect of Temperature on the Critical Water Content for General and Localised Corrosion of X65 Carbon Steel in the Transport of Supercritical CO2. Int. J. Greenh. Gas Control 2014, 31, 48–60. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Nesic, S.; Young, D. Effect of Impurities on the Corrosion Behavior of CO2 Transmission Pipeline Steel in Supercritical CO2-Water Environments. Environ. Sci. Technol. 2010, 44, 9233–9238. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhang, L.; Lu, M.; Wang, J.; Wen, Z.; Hao, W. The Electrochemical Behaviour of 316L Austenitic Stainless Steel in Cl-Containing Environment under Different H2S Partial Pressures. Appl. Surf. Sci. 2014, 289, 33–41. [Google Scholar] [CrossRef]
- NACE SP0775-2013; Standard Practice: Preparation, Installation, Analysis, and Interpretation of Corrosion Coupons in Oilfield Operations. NACE INTER.: Houston, TX, USA, 2012.
- Li, C.; Xiang, Y.; Song, C.; Ji, Z. Assessing the Corrosion Product Scale Formation Characteristics of X80 Steel in Supercritical CO2-H2O Binary Systems with Flue Gas and NaCl Impurities Relevant to CCUS Technology. J. Supercrit. Fluids 2019, 146, 107–119. [Google Scholar] [CrossRef]
- Heuer, J.K.; Stubbins, J.F. An XPS Characterization of FeCO3 Films from CO2 Corrosion. Corros. Sci. 1999, 41, 1231–1243. [Google Scholar] [CrossRef]
- Bukhtiyarova, G.A.; Bukhtiyarov, V.I.; Sakaeva, N.S.; Kaichev, V.V.; Zolotovskii, B.P. XPS Study of the Silica-Supported Fe-Containing Catalysts for Deep or Partial H2S Oxidation. J. Mol. Catal. A Chem. 2000, 158, 251–255. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Xing, X.; He, S.; Zhang, L.; Lu, M. Effects of Flow Velocity on the Corrosion Behaviour of Super 13Cr Stainless Steel in Ultra-HTHP CO2-H2S Coexistence Environment. Corros. Sci. 2022, 200, 110235. [Google Scholar] [CrossRef]
- Ueda, M. 2006 F.N. Speller Award Lecture: Development of Corrosion-Resistance Alloys for the Oil and Gas Industry-Based on Spontaneous Passivity Mechanism. Corrosion 2006, 62, 856–867. [Google Scholar] [CrossRef]
- Shuttleworth, D. Preparation of Metal-Polymer Dlspersions by Plasma Techniques. J. Phys. Chem. 1980, 84, 1629–1634. [Google Scholar] [CrossRef]
- Guo, S.; Xu, L.; Zhang, L.; Chang, W.; Lu, M. Corrosion of Alloy Steels Containing 2% Chromium in CO2 Environments. Corros. Sci. 2012, 63, 246–258. [Google Scholar] [CrossRef]
- Fierro, G.; Ingo, G.M.; Mancia, F. XPS Investigation on the Corrosion Behavior of 13Cr-Martensitic Stainless Steel in CO2-H2S-Cl− Environments. Corrosion 1989, 45, 814–823. [Google Scholar] [CrossRef]
- Keshri, S.; Pandit, N.; Singh, P.; Grain, A.K.; Keshri, A.K. Synergistic Effect of Al2O3 and MoS2 on the Corrosion Behaviour of Plasma Sprayed Aluminium Matrix Composite Coating. J. Alloys Compd. 2024, 998, 174935. [Google Scholar] [CrossRef]
- Zhao, X.; Li, G.; Liu, J.; Li, M.; Du, Q.; Han, Y. Corrosion Performance Analysis of Tubing Materials with Different Cr Contents in the CO2 Flooding Injection–Production Environment. Coatings 2023, 13, 1812. [Google Scholar] [CrossRef]
- Naixin, L.; Anqing, F.; Hanwei, L.; Wei, L.; Yan, G.; Haitao, B.; Shaohua, S.; Chengxian, Y.; Xin, L.; Zhengyi, X. Influence of Oxygen Partial Pressure on the Passivation and Depassivation of Super 13Cr Stainless Steel in High Temperature and CO2 Rich Environment. Sci. Rep. 2024, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Barker, R.; Charpentier, T.; Ward, M.; Neville, A. Relating Iron Carbonate Morphology to Corrosion Characteristics for Water-Saturated Supercritical CO2 Systems. J. Supercrit. Fluids 2015, 98, 183–193. [Google Scholar] [CrossRef]
- Barker, R.; Hua, Y.; Neville, A. Internal Corrosion of Carbon Steel Pipelines for Dense-Phase CO2 Transport in Carbon Capture and Storage (CCS)—A Review. Int. Mater. Rev. 2017, 62, 1–31. [Google Scholar] [CrossRef]
- Sun, W.; Nešić, S. A Mechanistic Model of Uniform Hydrogen Sulfide/Carbon Dioxide Corrosion of Mild Steel. Corrosion 2009, 65, 291–307. [Google Scholar] [CrossRef]
- Spycher, N.; Ruess, K.A.P.; Ing, J.O.E.N. CO2-H2O Mixtures in the Geological Sequestration of CO2. I. Assessment and Calculation of Mutual Solubilities from 12 to 100 °C and up to 600 Bar. Geochim. Cosmochim. Acta 2003, 67, 3015–3031. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Zhao, Z.; Xie, J.; Li, X.; Song, W.; Zhou, J.; He, Q. The Synergistic Influence of Trace Impurities and Temperature on the Corrosion Behavior of Tubing in Supercritical CO2 Environment. Coatings 2025, 15, 944. https://doi.org/10.3390/coatings15080944
Zhao M, Zhao Z, Xie J, Li X, Song W, Zhou J, He Q. The Synergistic Influence of Trace Impurities and Temperature on the Corrosion Behavior of Tubing in Supercritical CO2 Environment. Coatings. 2025; 15(8):944. https://doi.org/10.3390/coatings15080944
Chicago/Turabian StyleZhao, Mifeng, Zaipeng Zhao, Junfeng Xie, Xuanpeng Li, Wenwen Song, Jinjie Zhou, and Qiyao He. 2025. "The Synergistic Influence of Trace Impurities and Temperature on the Corrosion Behavior of Tubing in Supercritical CO2 Environment" Coatings 15, no. 8: 944. https://doi.org/10.3390/coatings15080944
APA StyleZhao, M., Zhao, Z., Xie, J., Li, X., Song, W., Zhou, J., & He, Q. (2025). The Synergistic Influence of Trace Impurities and Temperature on the Corrosion Behavior of Tubing in Supercritical CO2 Environment. Coatings, 15(8), 944. https://doi.org/10.3390/coatings15080944




