Temperature-Dependent Performance of Thermally Oxidized Zr2.5Nb Alloy for Orthopedic Implants: Mechanical Properties, Wear Resistance, and Biocompatibility
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
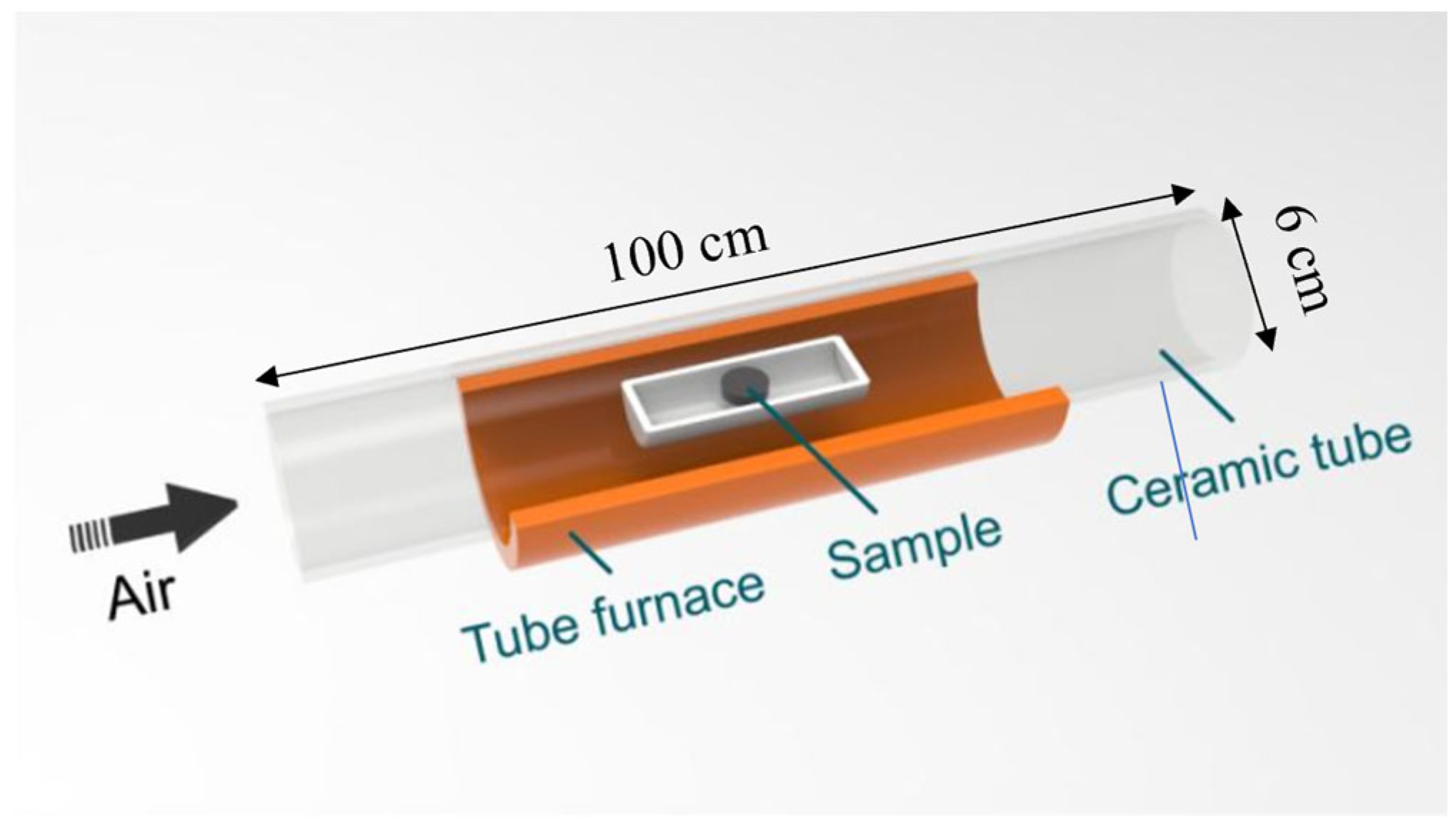
2.2. Thermal Oxidation Treatment
2.3. Microstructure and Phase Identification Characterization
2.4. Mechanical and Tribological Properties Evaluations
2.5. Biocompatibility Testing
3. Results and Discussion
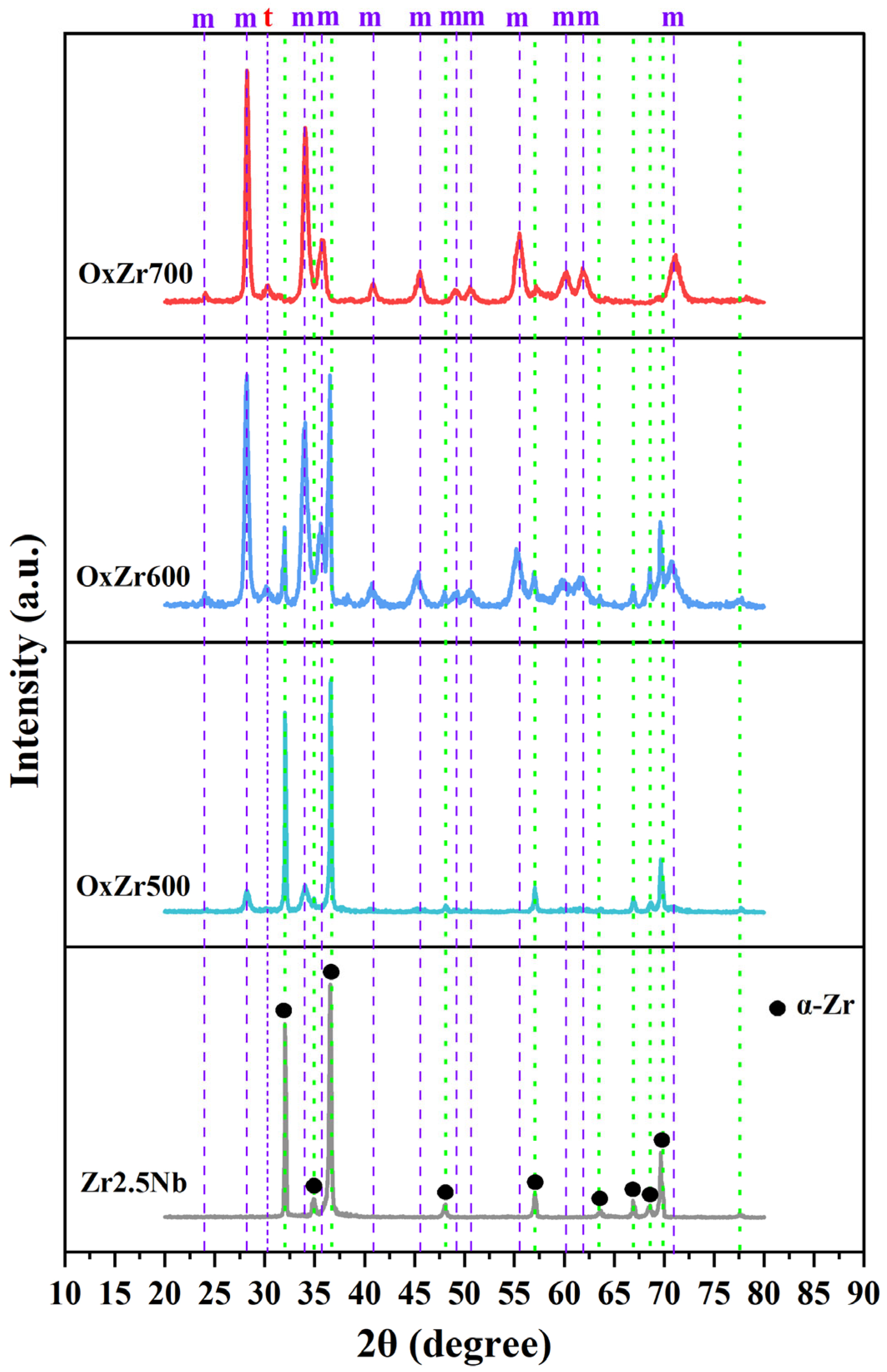
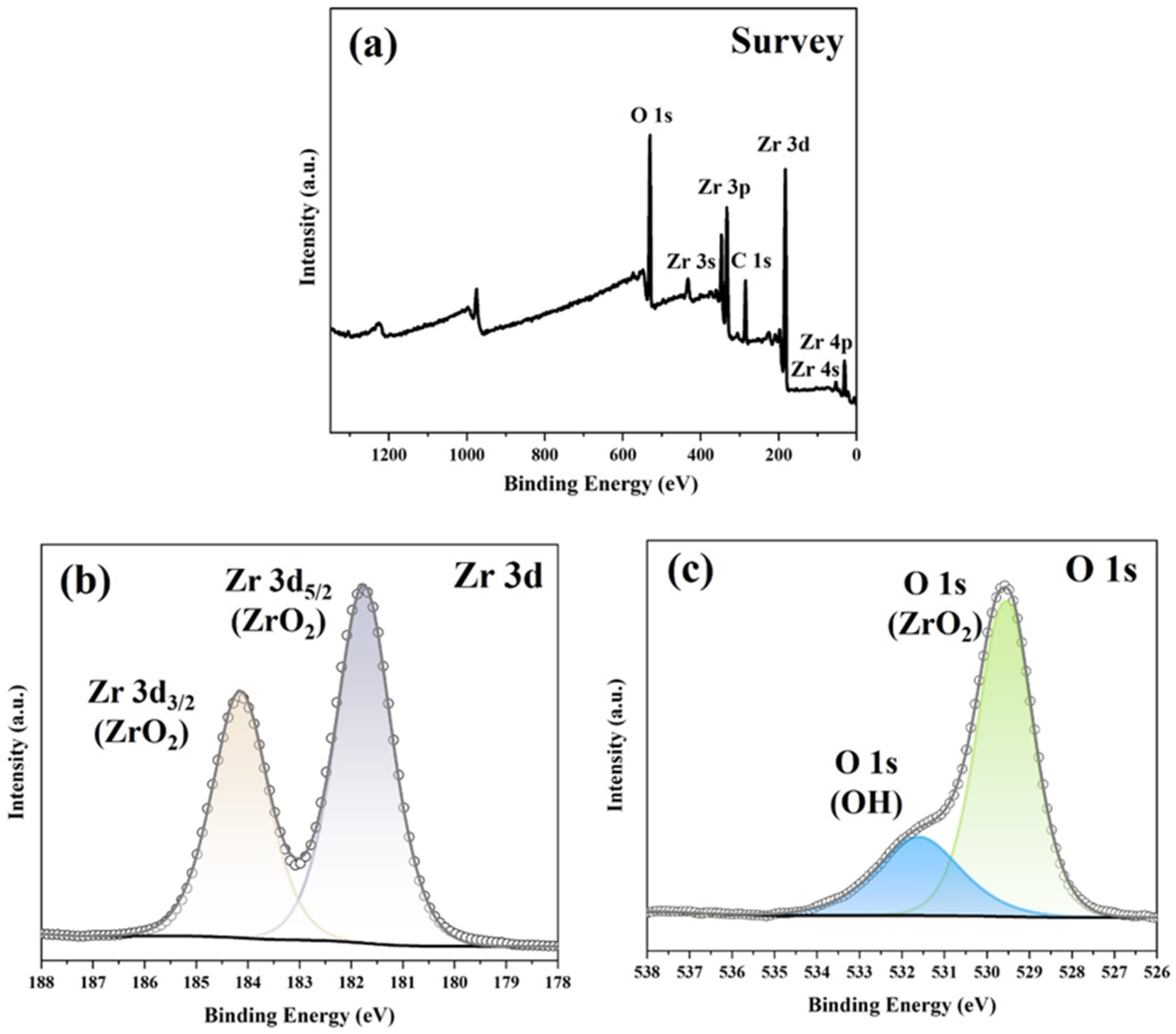
3.1. Microstructure and Phase Identification
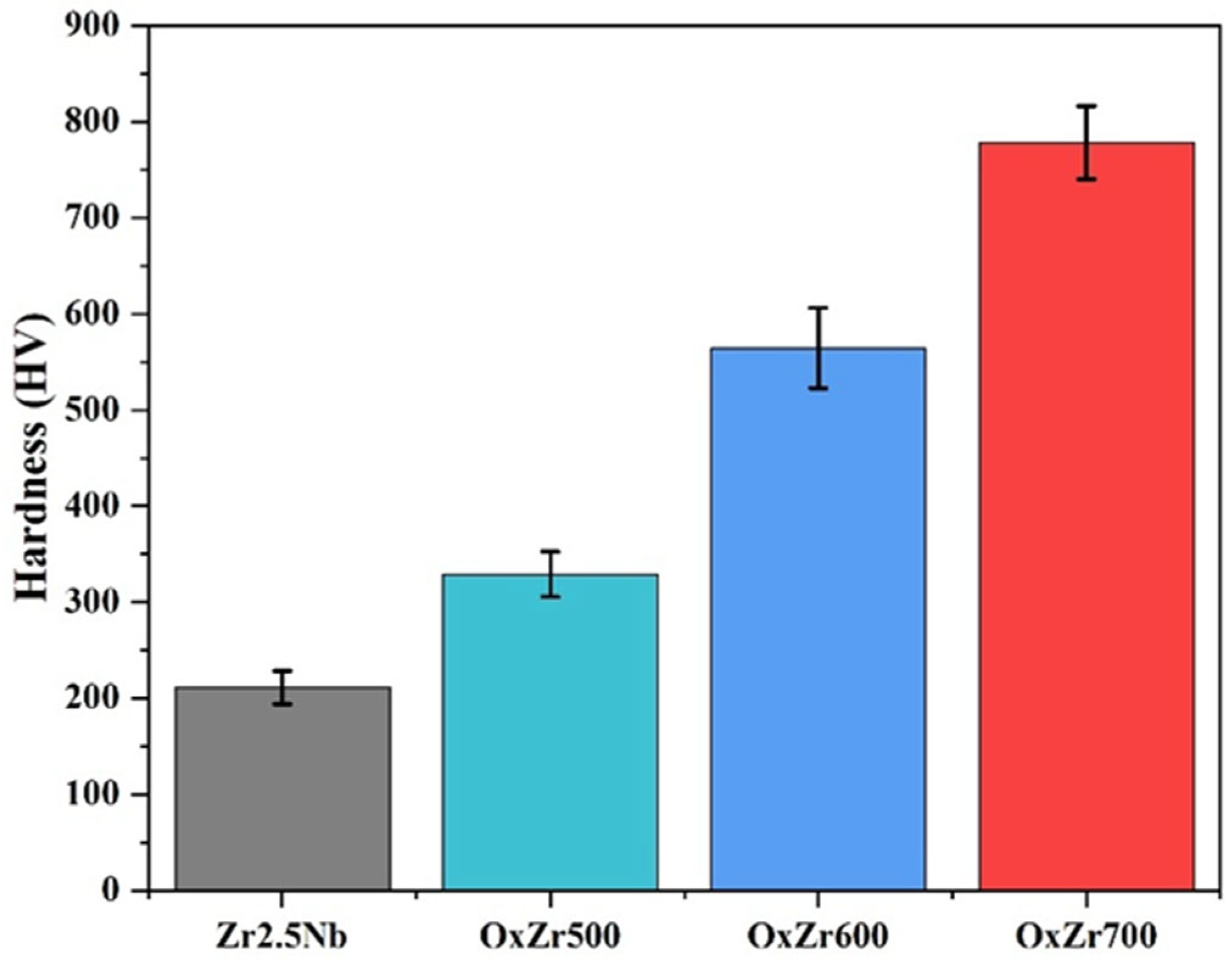
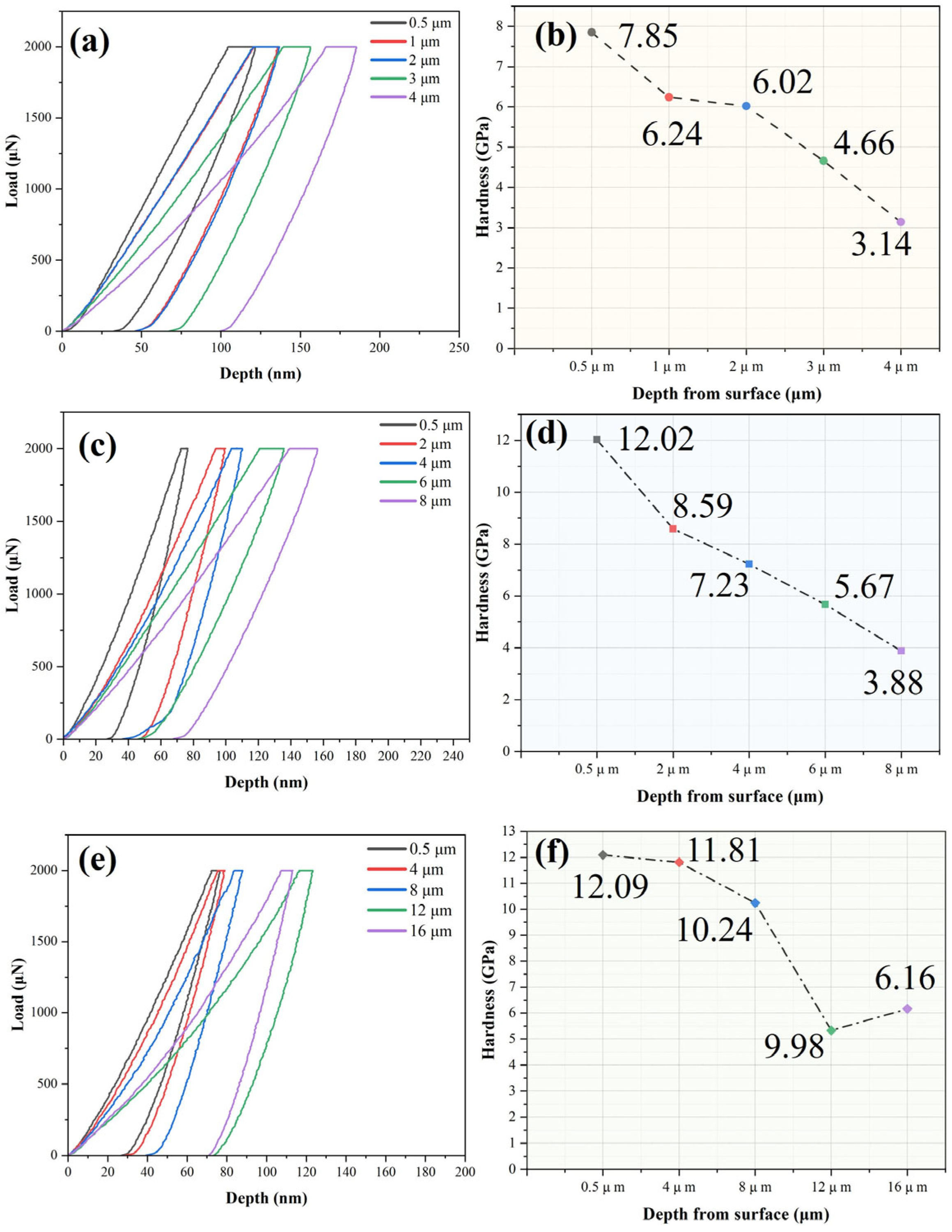
3.2. Mechanical Properties
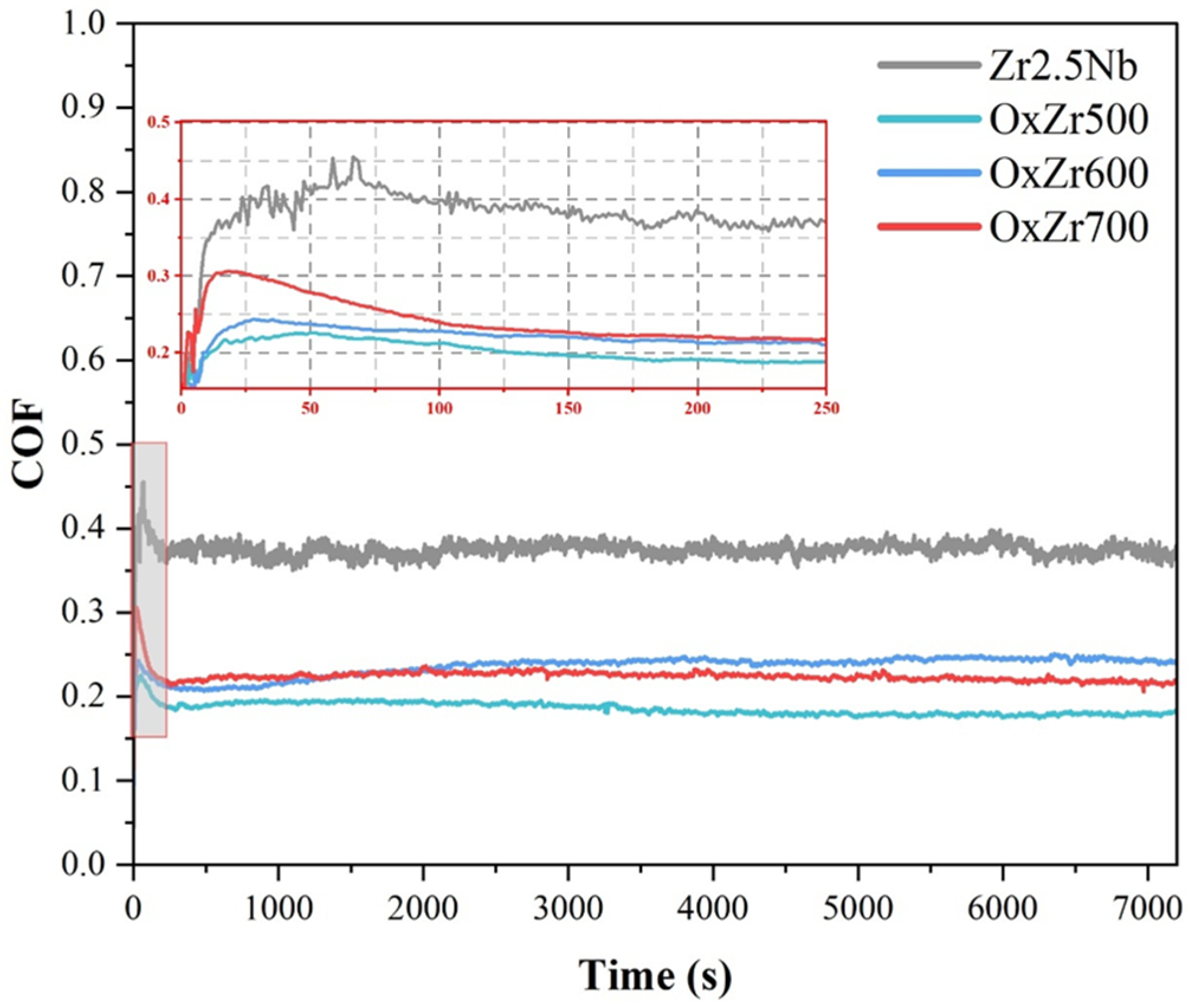
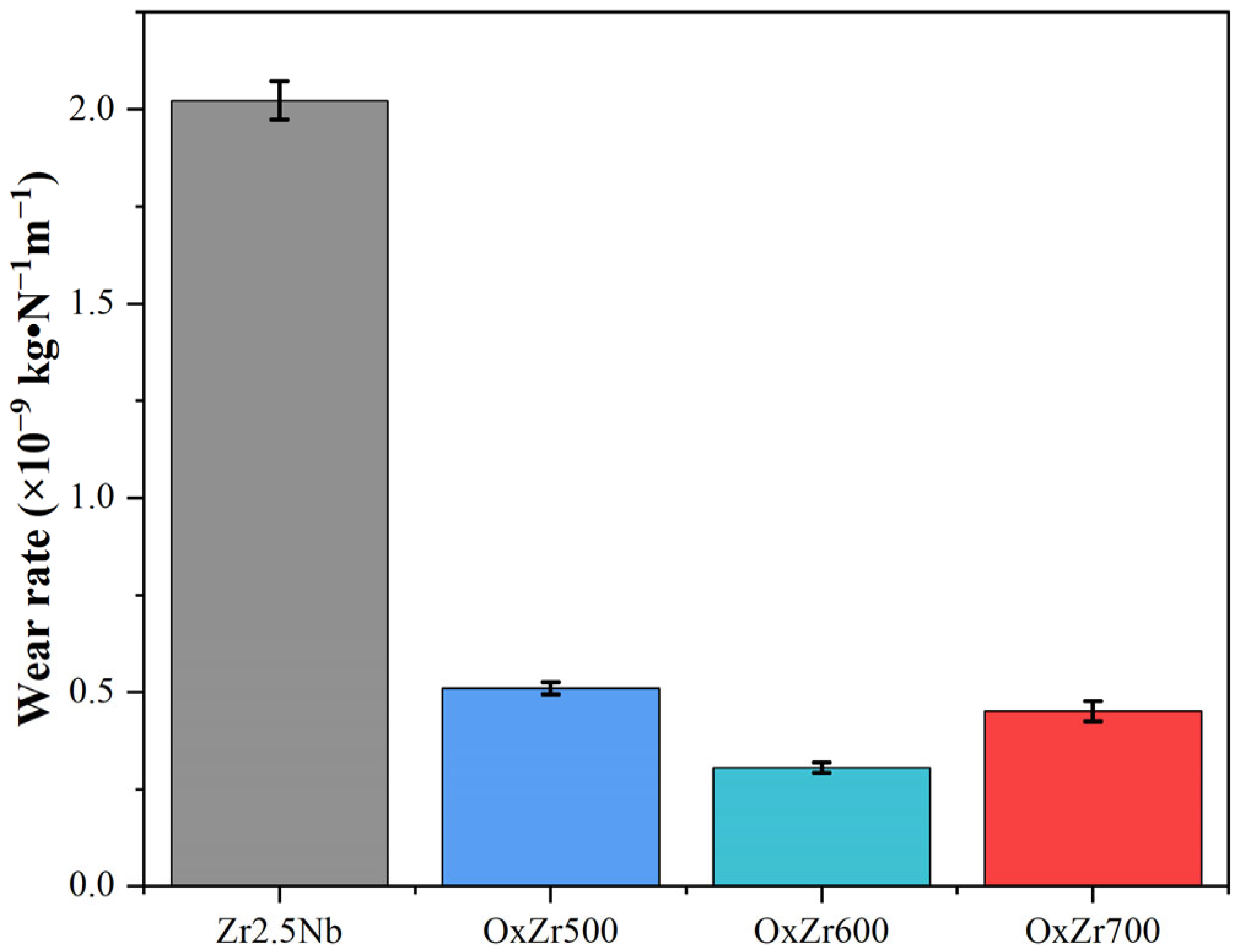
3.3. Tribological Behavior
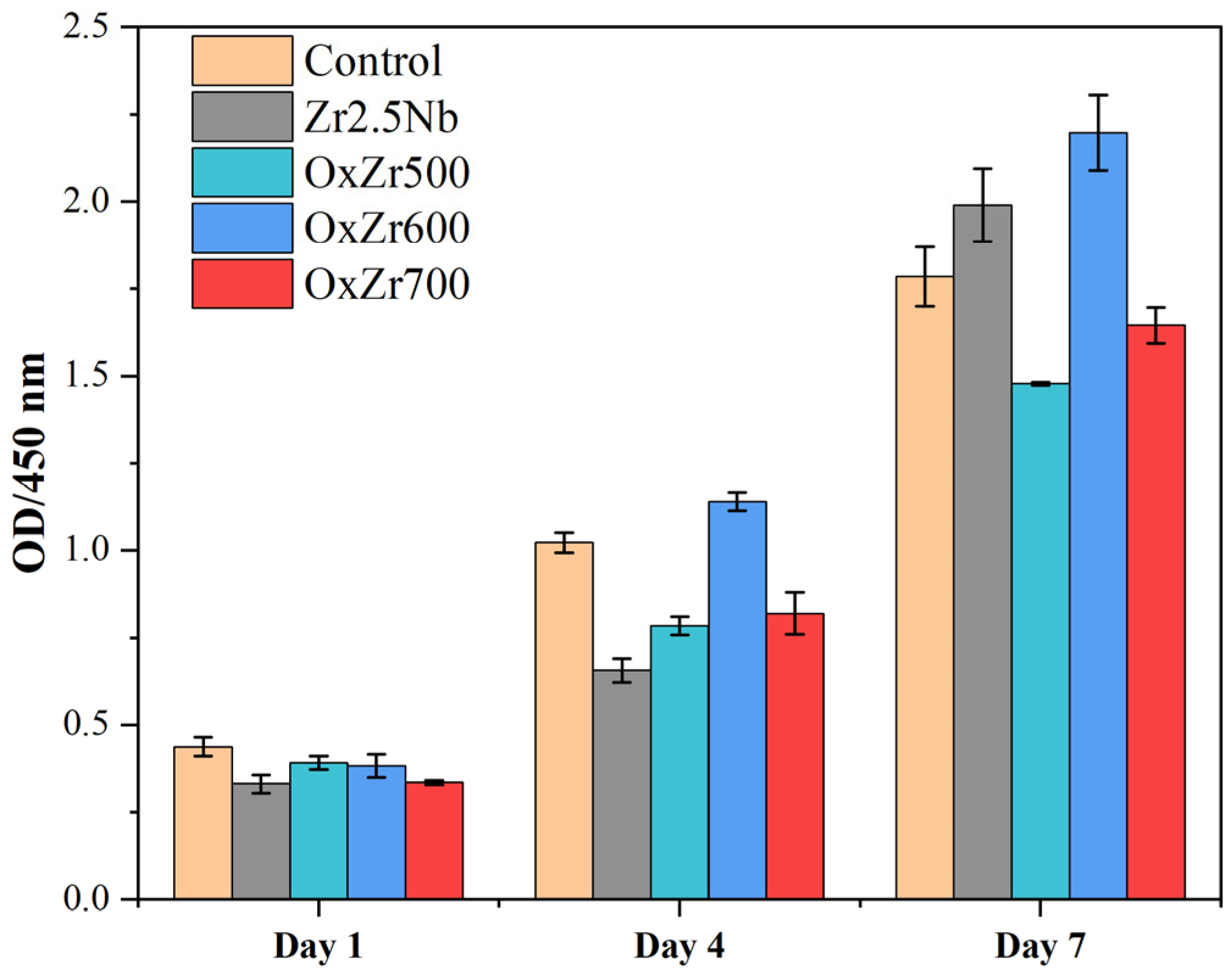
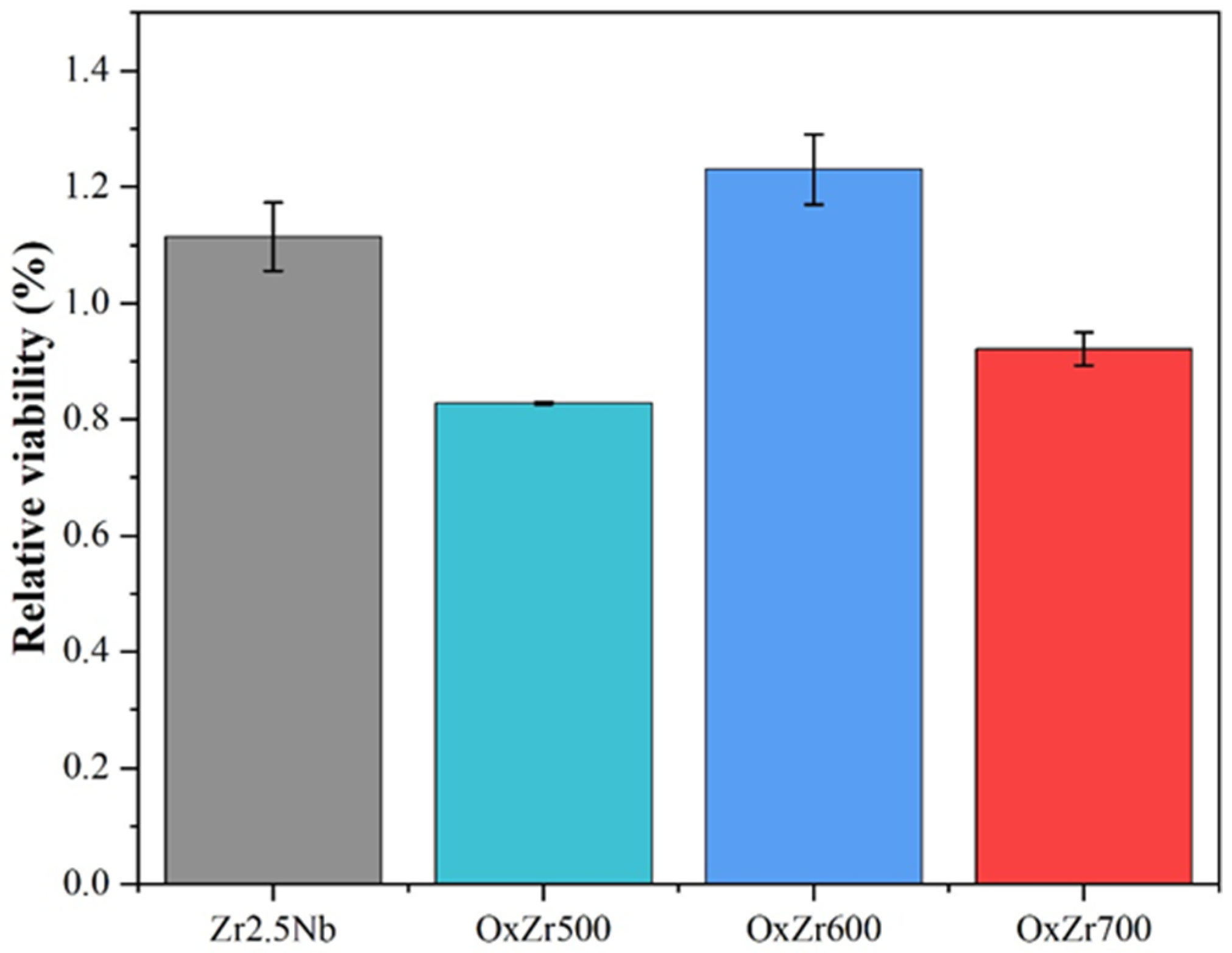
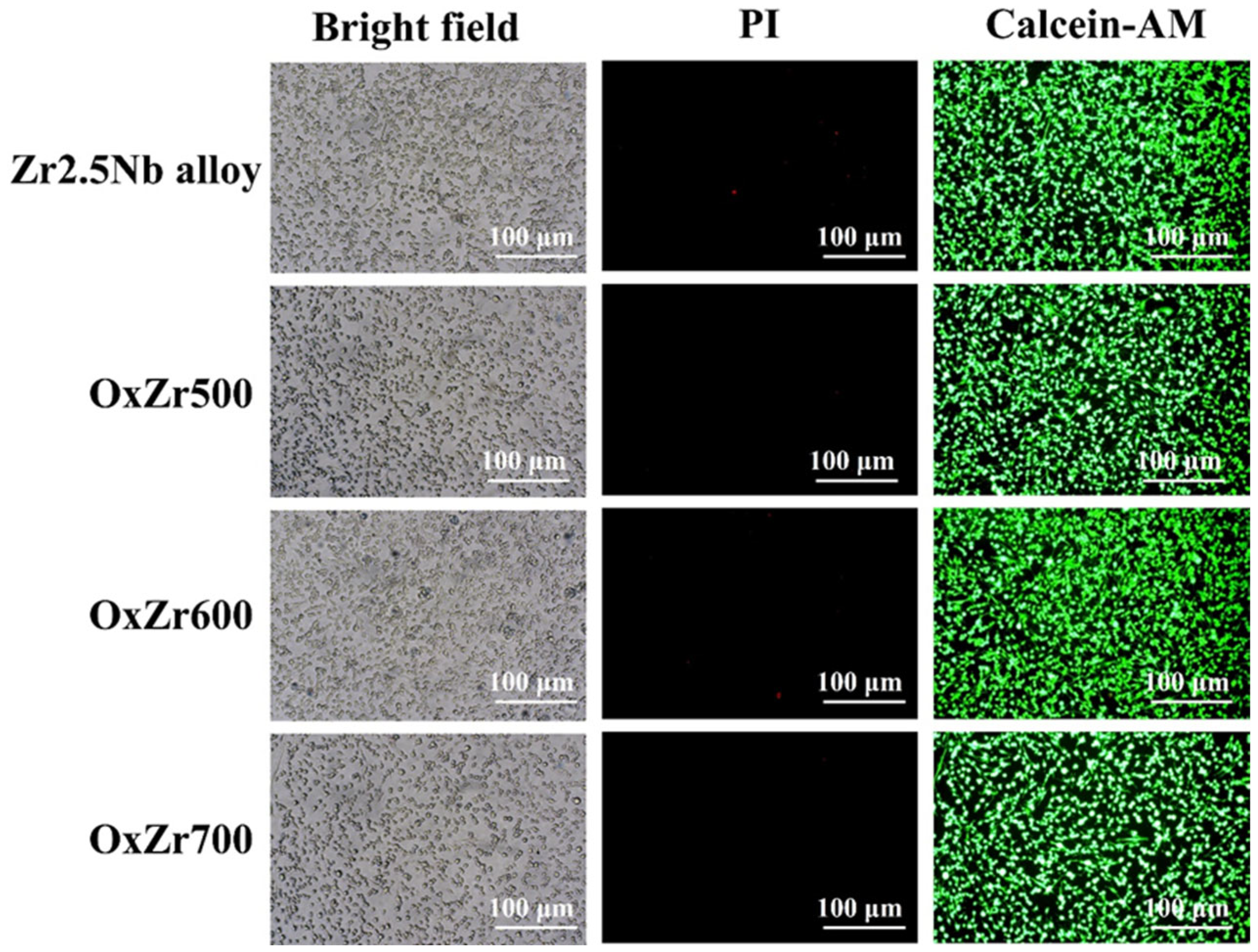
3.4. Biocompatibility
4. Conclusions
- (1)
- Oxide layers, primarily composed of m-ZrO2, formed on the surface of the Zr2.5Nb alloy. Increasing the temperature accelerated the growth of the oxide layer by enhancing oxygen diffusion, resulting in thicknesses that increased from 2.43 µm at 500 °C to 5.31 µm at 600 °C, and further to 13.59 µm at 700 °C. Nevertheless, this growth coincided with an increase in defect density;
- (2)
- Compared to the untreated Zr2.5Nb alloy, the thermally oxidized samples exhibited significantly enhanced mechanical properties. Specifically, the surface hardness increased from the original 244.24 ± 17.13 HV to 329.25 ± 23.69, 564.53 ± 42.01, and 778.28 ± 37.85 HV for samples oxidized at 500 °C, 600 °C, and 700 °C, respectively. Additionally, all oxidized samples displayed characteristic nanohardness gradients that decreased with depth;
- (3)
- The thermally oxidized samples all exhibited significantly improved wear resistance. In particular, the Zr2.5Nb alloy sample oxidized at 600 °C, owing to its optimal structural integrity and uniformity, showed a wear rate reduced by 85% compared to the untreated Zr2.5Nb alloy. Furthermore, this sample also possessed the best biocompatibility, as evidenced by the relative viability of MC3T3-E1 cells cultured on it after 7 days, reaching 123.07 ± 6.02%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, J.A.; Yu, S.; Chen, L.; Cleveland, J.D. Rates of Total Joint Replacement in the United States: Future Projections to 2020–2040 Using the National Inpatient Sample. J. Rheumatol. 2019, 46, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Clar, C.; Leitner, L.; Koutp, A.; Hauer, G.; Rasic, L.; Leithner, A.; Sadoghi, P. The worldwide survival rate of total hip arthroplasties is improving: A systematic comparative analysis using worldwide hip arthroplasty registers. EFORT Open Rev. 2024, 9, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Li, J.X.; Wen, J.P.; Ou, S.J.; Qu, Y.D.; Xia, C.L.; Li, W.J.; Yang, Y.; Liu, J.B.; Yang, R.; Zeng, W.; et al. Wear particles-induced periprosthetic osteolysis from a new perspective based on single-cell RNA-seq: Crosstalk of osteoblast PANoptosis and macrophage efferocytosis in osteoimmunology microenvironment. Chem. Eng. J. 2025, 515, 163509. [Google Scholar] [CrossRef]
- Hao, X.; Jiang, B.; Wu, J.; Xiang, D.; Xiong, Z.; Li, C.; Li, Z.; He, S.; Tu, C.; Li, Z. Nanomaterials for bone metastasis. J. Control. Release 2024, 373, 640–651. [Google Scholar] [CrossRef]
- Li, Z. Advancements of biomaterial in hip replacement technology incorporating ceramic materials. J. Orthop. 2025, 62, 27–35. [Google Scholar] [CrossRef]
- Morano, C.; Garofalo, S.; Bertuccio, P.; Sposato, A.; Zappone, I.; Pagnotta, L. A Comprehensive Literature Review of Total Hip Arthroplasty (THA): Part 1-Biomaterials. J. Funct. Biomater. 2025, 16, 179. [Google Scholar] [CrossRef]
- Knecht, C.; Polakof, L.; Behrens, J.; Goodman, S.B. Wear debris in metal-on-metal bearings and modular junctions What have we learned from the last decades? Orthopadie 2023, 52, 206–213. [Google Scholar]
- Alvarez-Vera, M.; Ortega, J.A.; Ortega-Ramos, I.A.; Hdz-Garcia, H.M.; Munoz-Arroyo, R.; Diaz-Guillen, J.C.; Acevedo-Davila, J.L.; Hernandez-Rodriguez, M.A.L. Tribological and microstructural characterization of laser microtextured CoCr alloy tested against UHMWPE for biomedical applications. Wear 2021, 477, 203819. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, H.J.; Lee, S.J.; Yoo, J.J. Ceramic-on-Ceramic or Metal-on-Polyethylene: The Bearing of Choice after Ceramic Component Fracture in Total Hip Arthroplasty along with Concise Follow-Up of the Previous Cohort. Orthop. Surg. 2023, 15, 2864–2871. [Google Scholar] [CrossRef]
- Abdelghafour, K.M.; Jaques, A.; Shah, N. Ceramic-on-ceramic bearings: Can we avoid the complications? Long-term outcomes of big head CoC bearings in primary total hip arthroplasty. Eur. J. Orthop. Surg. Traumatol. 2025, 35, 80. [Google Scholar] [CrossRef] [PubMed]
- Merola, M.; Affatato, S. Materials for Hip Prostheses: A Review of Wear and Loading Considerations. Materials 2019, 12, 495. [Google Scholar] [CrossRef]
- Nasiri-Tabrizi, B.; Basirun, W.J.; Walvekar, R.; Yeong, C.H.; Phang, S.W. Exploring the potential of intermetallic alloys as implantable biomaterials: A comprehensive review. Biomater. Adv. 2024, 161, 213854. [Google Scholar] [CrossRef]
- Good, V.; Widding, K.; Hunter, G.; Heuer, D. Oxidized zirconium—A potentially longer lasting hip implant. Mater. Des. 2005, 26, 618–622. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Kocagoez, S.; Arnholt, C.; Huet, R.; Ueno, M.; Walter, W.L. Advances in zirconia toughened alumina biomaterials for total joint replacement. J. Mech. Behav. Biomed. Mater. 2014, 31, 107–116. [Google Scholar] [CrossRef]
- Leto, A.; Zhu, W.; Matsubara, M.; Pezzotti, G. Bioinertness and fracture toughness evaluation of the monoclinic zirconia surface film of Oxinium femoral head by Raman and cathodoluminescence spectroscopy. J. Mech. Behav. Biomed. Mater. 2014, 31, 135–144. [Google Scholar] [CrossRef]
- DesJardins, J.D.; Burnikel, B.; LaBerge, M. UHMWPE wear against roughened oxidized zirconium and CoCr femoral knee components during force-controlled simulation. Wear 2008, 264, 245–256. [Google Scholar] [CrossRef]
- Arima, T.; Miyata, K.; Idemitsu, K.; Inagaki, Y. Oxidation properties of Zr-Nb alloys at 973–1273 K in air. Prog. Nucl. Energy 2009, 51, 307–312. [Google Scholar] [CrossRef]
- Duriez, C.; Drouan, D.; Pouzadoux, G. Reaction in air and in nitrogen of pre-oxidised Zircaloy-4 and M5™ claddings. J. Nucl. Mater. 2013, 441, 84–95. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, S.; Xia, C.; Zou, X.; Liu, D.; Wang, Y.; Yang, T.; Li, Q. Thermal oxidation of novel Zr-Ti-Al-V alloy with high strength and toughness and its influence on the corrosion behavior. Surf. Coat. Technol. 2021, 423, 127576. [Google Scholar] [CrossRef]
- Pawar, V.; Weaver, C.; Jani, S.C. Physical characterization of a new composition of oxidized zirconium—2.5 wt% niobium produced using a two step process for biomedical applications. Appl. Surf. Sci. 2011, 257, 6118–6124. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, W.; Zhang, S.; Lu, Y.; Wang, J.; Ma, G.; Lin, J.; Hosseinkhani, S.; Ma, J.; Wang, Q. Corrosion fatigue behavior of porous Cu-bearing Ti alloy fabricated by selective laser melting. J. Mater. Res. Technol. 2023, 23, 1630–1643. [Google Scholar] [CrossRef]
- Rao, Q.; Zhang, J.; Chen, Y.; Yang, Y.; Chen, X.; Liu, D.; Zhu, R.; Li, A.; Lv, Y.; Zheng, S. Research Progress of the Coatings Fabricated onto Titanium and/or Titanium Alloy Surfaces in Biomaterials for Medical Applications for Anticorrosive Applications. Coatings 2025, 15, 599. [Google Scholar] [CrossRef]
- Abriata, J.P.; Garcés, J.; Versaci, R. The O−Zr (Oxygen-Zirconium) system. Bull. Alloy Phase Diagr. 1986, 7, 116–124. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, X.; Xiao, P. In Situ Measurement of Stresses and Phase Compositions of the Zirconia Scale During Oxidation of Zirconium by Raman Spectroscopy. Oxid. Met. 2013, 81, 331–343. [Google Scholar] [CrossRef]
- Wong, Y.H.; Cheong, K.Y. Thermal oxidation and nitridation of sputtered Zr thin film on Si via N2O gas. J. Alloys Compd. 2011, 509, 8728–8737. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, W.; Tian, M.; Teng, S. Thermal oxidation of Ti6Al4V alloy and its biotribological properties under serum lubrication. Tribol. Int. 2015, 89, 67–71. [Google Scholar] [CrossRef]
- Brachet, J.C.; Rouesne, E.; Ribis, J.; Guilbert, T.; Urvoy, S.; Nony, G.; Toffolon-Masclet, C.; Le Saux, M.; Chaabane, N.; Palancher, H.; et al. High temperature steam oxidation of chromium-coated zirconium-based alloys: Kinetics and process. Corros. Sci. 2020, 167, 108537. [Google Scholar] [CrossRef]
- Qi, J.; Guan, D.; Nutter, J.; Wang, B.; Rainforth, W.M. Insights into tribofilm formation on Ti-6Al-4V in a bioactive environment: Correlation between surface modification and micro-mechanical properties. Acta Biomater. 2022, 141, 466–480. [Google Scholar] [CrossRef]
- Guan, J.; Jiang, X.; Xiang, Q.; Yang, F.; Liu, J. Corrosion and tribocorrosion behavior of titanium surfaces designed by electromagnetic induction nitriding for biomedical applications. Surf. Coat. Technol. 2021, 409, 126844. [Google Scholar] [CrossRef]

| Nb | Hf | Fe | O | C | H | Zr |
|---|---|---|---|---|---|---|
| 2.26% | 1.90% | 0.015% | 0.032% | <0.010% | <0.010% | Blance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Y.; Song, H.; Hu, T.; Luo, Y.; Xu, H.; Sun, X. Temperature-Dependent Performance of Thermally Oxidized Zr2.5Nb Alloy for Orthopedic Implants: Mechanical Properties, Wear Resistance, and Biocompatibility. Coatings 2025, 15, 940. https://doi.org/10.3390/coatings15080940
Xiao Y, Song H, Hu T, Luo Y, Xu H, Sun X. Temperature-Dependent Performance of Thermally Oxidized Zr2.5Nb Alloy for Orthopedic Implants: Mechanical Properties, Wear Resistance, and Biocompatibility. Coatings. 2025; 15(8):940. https://doi.org/10.3390/coatings15080940
Chicago/Turabian StyleXiao, Yunpeng, Hanke Song, Tangqing Hu, Yong Luo, Hao Xu, and Xiaolei Sun. 2025. "Temperature-Dependent Performance of Thermally Oxidized Zr2.5Nb Alloy for Orthopedic Implants: Mechanical Properties, Wear Resistance, and Biocompatibility" Coatings 15, no. 8: 940. https://doi.org/10.3390/coatings15080940
APA StyleXiao, Y., Song, H., Hu, T., Luo, Y., Xu, H., & Sun, X. (2025). Temperature-Dependent Performance of Thermally Oxidized Zr2.5Nb Alloy for Orthopedic Implants: Mechanical Properties, Wear Resistance, and Biocompatibility. Coatings, 15(8), 940. https://doi.org/10.3390/coatings15080940








