Abstract
TiAl alloy offers advantages including low density, high specific strength and stiffness, and excellent high-temperature creep resistance. It is widely used in the aerospace, automotive, and chemical sectors, as well as in other fields. However, at temperatures of 800 °C and above, it forms a porous oxide film predominantly composed of TiO2, which fails to provide adequate protection. Applying high-temperature protective coatings is therefore essential. Oxides demonstrating protective efficacy at elevated temperatures include Al2O3, Cr2O3, and SiO2. The Pilling–Bedworth Ratio (PBR)—defined as the ratio of the volume of the oxide formed to the volume of the metal consumed—serves as a critical criterion for assessing oxide film integrity. A PBR value greater than 1 but less than 2 indicates superior film integrity and enhanced oxidation resistance. Among common oxides, Al2O3 exhibits a PBR value within this optimal range (1−2), rendering aluminum-based compound coatings the most extensively utilized. Aluminum coatings can be applied via methods such as pack cementation, thermal spraying, and hot-dip aluminizing. Pack cementation, being the simplest to operate, is widely employed. In this study, a powder mixture with the composition Al:Al2O3:NH4Cl:CeO2 = 30:66:3:1 was used to aluminize γ-TiAl intermetallic compound specimens via pack cementation at 600 °C for 5 h. Subsequent isothermal oxidation at 900 °C for 20 h yielded an oxidation kinetic curve adhering to the parabolic rate law. This treatment significantly enhanced the high-temperature oxidation resistance of the γ-TiAl intermetallic compound, thereby broadening its potential application scenarios.
1. Introduction
Three intermetallic compounds in the Ti–Al binary system have attracted significant research interest: Ti3Al, TiAl, and TiAl3 [1]. Among these, TiAl alloys have emerged as highly promising lightweight and high-temperature structural materials due to their advantageous properties, including high melting point, low density, high specific strength, excellent high-temperature creep resistance, and robust oxidation resistance at elevated temperatures [2]. These alloys are commonly employed in critical components such as gas turbine blades, engine exhaust valves, and compressor impellers. Rapid advancements in aviation, energy, and defense industries necessitate continuous improvements in energy utilization efficiency, driving progressive increases in turbine inlet gas temperatures. Combustion chamber temperatures in advanced aero-engines and large-scale ground-based gas turbines now reach 1700 °C [3]. However, Wu et al. [4] report that when TiAl alloys operate above 800 °C, they form predominantly non-protective rutile TiO2 scales or mixed TiO2/Al2O3 scales, which severely limit their extended applicability. Consequently, the application of high-temperature protective coatings is essential in practical implementations [5].
Typical high-temperature protective coatings include aluminum-based coatings, modified aluminum coatings, cladding coatings (MCrAlY coatings, where M = Fe, Ni, Co, or combinations thereof), and thermal barrier coatings [6]. Tang et al. [7] selected Ti-50Al-10Cr alloy as a coating material, depositing it onto γ-TiAl alloy via magnetron sputtering. After oxidizing the TiAlCr coating at 900 °C for 1000 h, cross-sectional analysis revealed a dense Al2O3 scale on the coating surface with minimal interdiffusion between the coating and substrate. Mengis et al. [8] aluminized Ti-48Al-2Cr-2Nb alloy through pack cementation at 600 °C for 4 h, obtaining a ∼10 μm thick coating. The coated sample exhibited a final mass gain at 700 °C approximately one-third that of the uncoated specimen. Following 1000 h of cyclic oxidation, a thin, adherent Al2O3 scale and an Al-rich interdiffusion zone formed on the aluminized surface, with internally precipitated TiO2 displaying α-Al2O3 and rutile structures. Swadzba et al. [9] synthesized silicon-modified aluminide coatings on Ti-45Al-5Nb-0.2B-0.2C using pack cementation (850 °C, 6 h) with a penetrant mixture of Si powder, Al powder, Al2O3 filler, and NH4Cl activator. The resulting ∼43 μm coating exhibited a bilayer structure: an external TiAl3 matrix and an intervening ∼400 nm equiaxed TiAl2 layer at the substrate interface, accompanied by dispersed Ti5Si3 phases. Isothermal oxidation at 950 °C for 3000 h produced a continuous, uniform ∼2.5 μm α-Al2O3 scale. Hao et al. [10] fabricated Al2O3–Y2O3 composite coatings on γ-TiAl alloy via electrophoretic deposition (EPD). These coatings significantly enhanced the alloy's oxidation and scale spallation resistance by suppressing outward Ti diffusion from the substrate while promoting selective Al oxidation within the γ-TiAl alloy.
Among high-temperature protective coatings, aluminide coatings are widely employed industrially due to mature processing technology and low production costs. However, traditional aluminizing temperatures typically range from 850 to 1050 °C. These elevated temperatures have a limitation: when applied at high temperatures, they can cause damage and degradation to the substrate material [11]. Combined with thermal stresses, this promotes internal cracking and deformation, further exacerbating mechanical property degradation [12]. Lower aluminizing temperatures also offer significant energy savings. Current research on low-temperature aluminizing primarily focuses on formula development, achieving reduced processing temperatures through addition of infiltration promoters, active elements, and compositional adjustments. Nevertheless, temperatures rarely fall below 650 °C. In this study, TiAl alloy served as the substrate. Through optimized formula design and incorporation of 0-1% rare earth oxide CeO2 [13,14], aluminizing was successfully performed at 600 °C. This process yielded an aluminum-based coating exhibiting excellent high-temperature oxidation resistance.
2. Materials and Methods
2.1. Coating Process
The substrate material was a γ-TiAl-based alloy supplied by the Titanium Alloy Department, Institute of Metal Research, Chinese Academy of Sciences. Table 1 presents the nominal composition and actual composition (determined by electron probe microanalysis, EPMA-1610, Shimadzu, Kyoto, Japan) of the γ-TiAl intermetallic compound (wt.%). Specimens were sectioned into 15 × 10 × 2 mm sheets via wire-electrode cutting, followed by sequential preparation: mechanical grinding (120−800# SiC abrasive paper), edge chamfering, ultrasonic cleaning in organic solvents (acetone and ethanol), drying, and storage. All substrates underwent surface sandblasting prior to processing.

Table 1.
The nominal chemical composition of γ-TiAl intermetallic compounds.
In the experiments of this article, the method of embedding with aluminizing was chosen, and the schematic diagram is shown in Figure 1. Prior to experimentation, the pack cementation powder (excluding the NH4Cl activator) was uniformly blended in a ball mill according to the designated weight percentages (wt.%). The mixed powder was then placed in a stainless steel crucible and dried at 400 °C for 2 h under an inert atmosphere. Subsequently, the NH4Cl activator was added in the prescribed proportion and thoroughly mixed. The dried powder mixture was transferred to a corundum crucible. Substrates were positioned within the powder bed at one-third to one-half of the crucible height, ensuring complete powder envelopment. The powder was compacted firmly to maximize substrate-powder contact. The crucible was heat-treated in a muffle furnace at 600 °C for specified durations, followed by air cooling. Samples were then extracted, rigorously cleaned, and prepared for testing. For cross-sectional analysis, a protective nickel layer was electrodeposited onto coated specimens using a plating solution containing 200 g/L NiSO4·6H2O, 40 g/L H3BO3, 40 g/L NiCl2, and 120 g/L Na3C6H5O7·2H2O (trisodium citrate dihydrate) to prevent alumina layer damage during metallographic preparation. The pack cementation composition is detailed in Table 2.

Figure 1.
Schematic cross-section view of an assembled pack box.

Table 2.
The formula components and the proportion of each component of the diffusion agent in the powder-embedding aluminizing process.
2.2. Isothermal Oxidation
To evaluate the oxidation resistance of coated specimens, isothermal oxidation tests were conducted in static air at 800 °C and 900 °C for 20 h, respectively. Sample mass changes were continuously monitored using a thermobalance. Post-oxidation mass measurements were recorded with a precision of 0.00001 g.
2.3. Analyzing Methods
Phase analysis of the coatings was performed using X-ray diffraction (XRD) (Panalytical, B.V., Almelo, The Netherlands). Microstructural morphology (surface and cross-section) of specimens before and after ablation testing was characterized by field-emission scanning electron microscopy (FE-SEM) (FEI Co., Hillsboro, OR, USA) (with coupled energy-dispersive spectroscopy (EDS) (Oxford instruments Co., Oxford, UK).
3. Results
3.1. The Influence of the Composition of the Diffusion Accelerator on the γ-TiAl-Intermetallic-Compound-Powder-Embedded Aluminized Coating
Figure 2 presents the microstructural morphology of aluminide coatings deposited on γ-TiAl intermetallic compounds via pack cementation with 0% and 1% nano-CeO2 additions. The results demonstrate nano-CeO2’s significant promotive effect. As shown in Figure 2A,D, CeO2-modified coatings exhibit greater thickness (~40 μm) than unmodified counterparts, with enhanced density, continuity, and absence of porosity or penetration features. This improvement stems from rare earth doping strengthening coating–substrate adhesion [15]. Conversely, conventional aluminide coatings display interfacial inhomogeneity and cracking (Figure 2A,B). The CeO2-modified coating surface appears uniformly smooth. Figure 3 describes the diffusion process of aluminizing on Ti6Al4V alloy. Similar to this paper, throughout the diffusion process, active Al atoms deposited on the surface diffuse into the matrix. Meanwhile, due to the high Ti content in the substrate, Ti atoms inevitably migrate from regions of high chemical potential (substrate) to low chemical potential (coating) to approach chemical equilibrium, consistent with Fick's diffusion law [16]. Given that Al diffusion rates in nickel-based systems exceed those of Ti [17], the coating thickness depends primarily on Al diffusion. It can be seen that the coating doped with CeO2 (~40 μm) is thicker than its undoped counterpart (~19 μm), indicating indirectly that Ce presence enhances Al interdiffusion into the substrate.

Figure 2.
Effect of the addition of CeO2 on aluminized coating of γ-TiAl intermetallic compound at 950 ℃ for 3 h. (A) Surface of addition of 0% CeO2, (B) surface of addition of 1% CeO2, (C) cross-section of addition of 0% CeO2, (D) cross-section of addition of 1% CeO2.
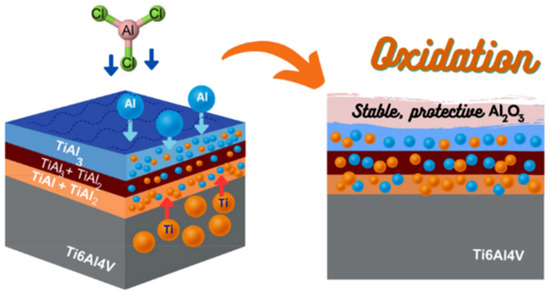
Figure 3.
Schematic representation of aluminizing process on Ti6Al4V alloy [16].
This is because rare earth elements have very strong chemical reactivity. NH4Cl decomposes into HCl and Cl2 at 450 ℃. HCl reacts with CeO2 as follows [18]:
2CeO2 + 2HCl = Ce2O3 + Cl2 + H2O
Ce2O3 + 6HCl = 2CeCl3 + 3H2O
Ce2O3 + 3Cl2 = 2CeCl3 + 3/2O2
CeCl3 diffuses onto the substrate surface and is reduced to active Ce atoms. The deposited rare earth Ce activates the coating surface. As Ce atoms possess a larger atomic radius than Ti atoms, their incorporation induces significant lattice distortion while reducing surface free energy [19]. This phenomenon enhances Al adsorption and diffusion, rapidly forming a high-Al-concentration surface layer. The resultant concentration gradient facilitates rapid inward Al diffusion.
3.2. The Influence of Different Aluminizing Times
Using Formula 1, coatings were heat-treated at 600 °C for 1, 3, and 5 h. Figure 4 presents the principal elemental composition and dominant phases on aluminized coating surfaces at varying durations. Following substrate sandblasting pretreatment, pack cementation at 600 °C yielded TiAl3 intermetallic compound coatings. EDS analysis (Figure 4E) confirmed a substantial increase in surface aluminum content from 32.2% to 78.78% post-treatment.

Figure 4.
Surface morphologies of aluminized coating of γ-TiAl intermetallic compound at 600 ℃ for different soaking times and XRD patterns. (A) 1 h, (B) 3 h, (C) 5 h, (D) surface element analysis by EDS, (E) XRD patterns.
Figure 5 presents cross-sectional morphologies of aluminide coatings obtained after pack cementation durations of 1, 3, and 5 h at 600 °C. Coating thickness increased progressively from ∼12 μm (1 h) to ∼28 μm (3 h), reaching ∼31 μm after 5 h. While thickness continuously grew, the growth rate decelerated with extended processing time. This behavior arises because temperature dominantly governs diffusion coefficients. When aluminizing temperature remains constant and duration exceeds a critical threshold, atomic diffusion approaches completion. At this stage, increased coating thickness reduces atomic diffusivity, thereby diminishing growth kinetics. Consequently, in this experiment, we chose 5 h as the embedding aluminizing duration when the aluminizing temperature is 600 ℃

Figure 5.
Cross- sectional morphologies of aluminized coating at 600 ℃ for different soaking times. (A) 1 h, (B) 3 h, (C) 5 h.
3.3. Surface Morphology Analysis
Figure 6 presents the surface morphology of the aluminide coating deposited on γ-TiAl intermetallic compound via 600 °C pack cementation for 5 h. As shown in Figure 6A, the coating surface exhibits uniform topography devoid of visible cracking. Higher-magnification imaging (Figure 6B) reveals a microstructure comprising irregularly sized blocky particles with adhered fine particulates. Interparticle voids are evident, while the blocky particles demonstrate non-uniform spatial distribution lacking discernible orientation.
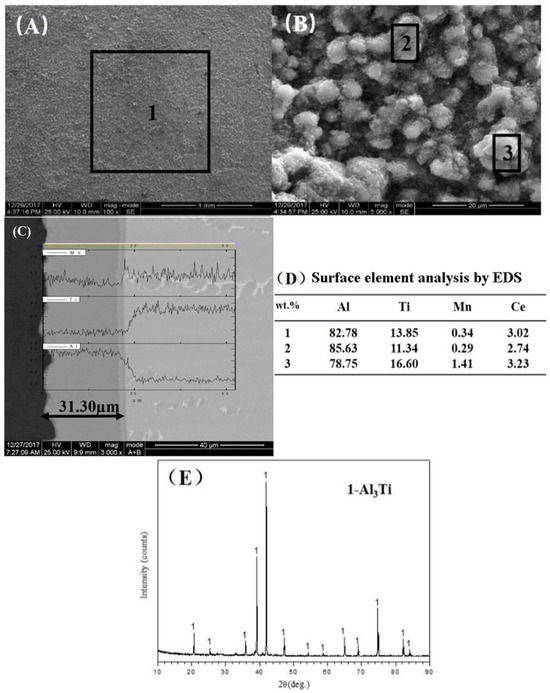
Figure 6.
Microstructure morphologies of aluminized coating of γ-TiAl intermetallic compound which is prepared at 600 ℃ for 5h and XRD patterns. (A) Surface, (B) amplification of A, (C) cross-section, (D) surface element analysis by EDS, (E) XRD patterns.
Figure 6D presents EDS elemental analysis of representative regions. Selected areas at 100× and 5000× magnification revealed a composition dominated by Al, Ti, and Mn. Comparative analysis of regions 2 and 3 (5000×) showed minimal variation in Al/Ti/Mn weight percentages, confirming compositional homogeneity throughout the coating.
Figure 6C presents the cross-sectional SEM morphology and elemental depth profile of the aluminide coating deposited on γ-TiAl intermetallic compound via 600 °C pack cementation for 5 h. Line scanning analysis (left-to-right: coating surface to substrate) reveals a uniform ∼30 μm thick coating with a sharp coating–substrate interface. The coating exhibits full density without through-thickness cracking. EDS line scan analysis indicates a significant decrease in Al concentration at 29 μm from the surface, coinciding with increased Ti intensity. Combined with XRD analysis, the coating was identified as primarily TiAl3 phase.
3.4. Research on High-Temperature Oxidation Resistance
Coatings prepared per Formula 1 (600 °C, 5 h) underwent isothermal oxidation at 800 °C and 900 °C for 20 h, respectively. Oxidation kinetics curves were derived from unit area mass gain measurements, with subsequent microstructural analysis performed via SEM/EDS.
3.4.1. Oxidation Kinetics Curve
Figure 7 presents oxidation kinetic curves for γ-TiAl intermetallic compound substrates and corresponding pack-cementation aluminide coatings after 20 h isothermal oxidation at 800 °C and 900 °C. As shown in Figure 7A, both materials exhibit mass gain during initial 800 °C exposure (<10 h). However, the uncoated substrate experienced oxide scale spallation after 10 h, accompanied by mass loss, indicating complete loss of oxidation resistance. In contrast, aluminized specimens demonstrated rapid initial mass gain followed by parabolic kinetics stabilization. This behavior signifies prompt formation of a continuous, dense Al2O3 scale that impedes oxygen permeation and suppresses further oxidation. Consequently, aluminized specimens exhibited minimal net mass gain with oxidation rates substantially lower than the substrate. Figure 7B reveals distinct oxidation mechanisms at 900 °C. While the substrate followed linear oxidation kinetics (indicating no protective scale formation), aluminized specimens maintained parabolic kinetics. This confirms sustained formation of protective Al2O3 scales that effectively isolate the substrate from oxygen ingress. Thus, aluminized coatings again demonstrated significantly reduced oxidation rates compared to the unprotected substrate. From Figure 7C, it can be seen that during the mid-stage oxidation, the oxidation kinetics curve shows a slow increase/exhibits a gradual upward trend.

Figure 7.
Thermostatic oxidation kinetics curves of substrate of γ-TiAl intermetallic compound and aluminized coating of γ-TiAl intermetallic compound. (A) 800 ℃ for 20h, (B) 900 ℃ for 20 h, (C) 800 ℃ for 20 h,900 ℃ for 20 h.
3.4.2. Analysis of the Oxidation Resistance of Aluminized Coating After Constant Temperature Oxidation at 800 ℃ for 20 H
Following 20 h isothermal oxidation at 800 °C in static air, pack-cementation aluminide samples exhibited a color transition from light gray to dark gray. Figure 8 presents post-oxidation microstructural characteristics. As shown in Figure 8A, the oxide scale remains intact without cracking or spallation. Surface morphology at 5000× magnification (Figure 8B) reveals coarsened blocky particles with increased surface particulates compared to pre-oxidation states (Figure 7). EDS analysis (Figure 8D) confirms regions 1−4 consist primarily of Al2O3 with minor TiO2. Cross-sectional examination (Figure 8C) shows no distinct oxide layer, attributed to the thin (<1 μm) scale intertwining with the electrodeposited nickel layer during metallographic preparation, rendering it SEM-indistinguishable. As indicated by the EDS line scan in Figure 8C, aluminum content peaks at ~70% on the coating surface and progressively decreases toward the substrate. XRD analysis (Figure 8E) identifies TiAl3 and δ-Al2O3 as dominant surface phases.

Figure 8.
Microstructure morphologies of aluminized coating of γ-TiAl intermetallic compound which is oxidized at 800 ℃ for 20 h and XRD patterns. (A) Surface, (B) amplification of A, (C) cross-section, (D) surface element analysis by EDS, (E) parallel light detection.
3.4.3. Analysis of the Oxidation Resistance of Aluminized Coating After Constant Temperature Oxidation at 900 ℃ for 20 H
Following 20 h of isothermal oxidation at 900 °C in static air, pack-aluminized samples transitioned from light gray to dark gray, with Figure 9 characterizing the post-oxidation microstructure as exhibiting: (i) an intact oxide scale without cracking/spallation (Figure 9A), (ii) agglomerated block-like oxides under 10,000× magnification, (iii) predominant Al2O3-TiO2 composition via EDS (Figure 9D,E), (iv) a sharp coating–substrate interface with minimal oxide thickness (Figure 9C,D), (v) Kirkendall voids at the oxide–coating boundary (magnified inset in Figure 9D) formed by vacancy accumulation due to divergent Ti/Al diffusion kinetics during oxide consumption—where outward Al diffusion depletes coating aluminum faster than inward Ti migration, and (vi) surface-phase dominance of TiAl and α-Al2O3 confirmed by parallel-light spectroscopy (Figure 9F), all corroborated by EDS line-scan data showing an elevated Al concentration at the coating surface, progressive Al depletion toward the substrate, and corresponding Ti enrichment (Figure 9D, the inset profiles).
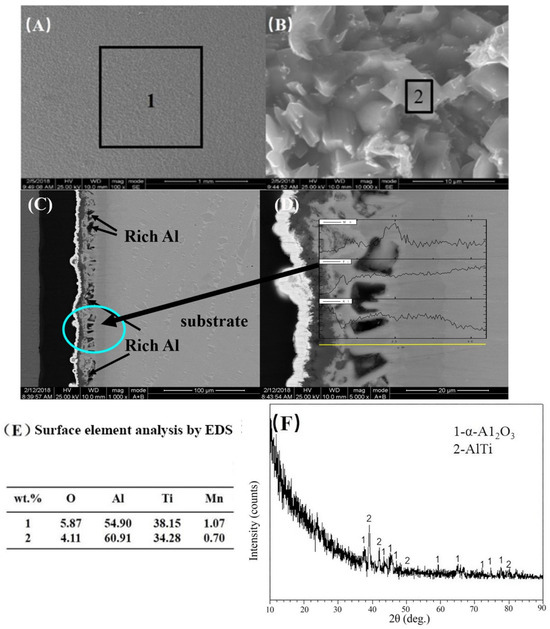
Figure 9.
Microstructure morphologies of aluminized coating of γ-TiAl intermetallic compound which is oxidized at 900 ℃ for 20 h and XRD patterns. (A) Surface, (B) amplification of A, (C) cross-section, (D) amplification of C, (E) surface element analysis by EDS, (F) parallel light detection.
4. Discussion
Thermodynamically, the Gibbs free energy for Al2O3 formation is marginally lower than for TiO2 formation (ΔG difference: 218 kJ/mol at standard pressure [20]). This proximity enables competitive oxidation. Consequently, TiAl intermetallic compounds exposed to air form mixed Al2O3/TiO2 scales rather than exclusive Al2O3 layers. Kinetically, Ti diffusion through TiO2 substantially exceeds Al diffusion through Al2O3, accelerating TiO2 growth. These factors collectively drive dual-oxide formation on TiAl alloys during air oxidation.
Numerous studies [21,22,23] have examined the high-temperature oxidation mechanism of TiAl intermetallic compounds. Research indicates that during initial oxidation, Al2O3 and TiO2 form discontinuously as island structures. TiO2 exhibits bidirectional growth (lateral and vertical), consuming surrounding Ti while locally enriching Al, thereby promoting Al2O3 nucleation. Consequently, the initial oxide scale comprises mixed TixOy (various valence states) and Al2O3 phases. A thin Ti-rich oxide overlayer forms due to Al's significantly lower oxidation kinetic tendency compared to Ti. As oxidation progresses, outermost TixOy phases transform into stable TiO2 upon prolonged air exposure. Kirkendall porosity develops at the TiO2/(Al2O3 + TiO2) interface due to differential cation diffusion. Furthermore, rapid growth of the non-protective porous TiO2 scale creates weak adhesion between the mixed oxide layer and TiAl substrate, ultimately causing scale spallation and loss of protective function. However, as aluminizing produces an aluminum-rich TiAl3 phase on γ-TiAl intermetallic compounds, the coating surface achieves 85.63 wt.% aluminum content. During oxidation, this enables formation of a continuous, dense, and adherent protective Al2O3 scale. This barrier effectively inhibits inward oxygen diffusion and outward metal ion transport, significantly reducing oxidation kinetics. A TiAl2 interlayer persists between the aluminide coating's TiAl3 phase and the γ-TiAl substrate during high-temperature oxidation.
The literature [23,24] demonstrates that above 800 °C, TiAl2 exhibits greater stability than TiAl3 and Ti. The diffusion rate of Al follows TiAl > TiAl3 > TiAl2. Consequently, TiAl3 persists throughout intermediate oxidation stages. After sustained exposure > 800 °C, the scale primarily consists of Al2O3 and residual TiAl3, consistent with XRD analysis. After oxidation at 900 °C for 20 h, a small amount of TiO2 phase formed in the oxide layer. This occurred because the rapid oxidation rate during the initial stage at 900 ℃ led to partial TiO2 formation. As oxidation proceeded, a continuous and dense α-Al2O3 film developed, effectively inhibiting oxygen ingress and trapping residual TiO2 on the alumina surface. Concurrently, elevated temperature accelerated the decomposition of TiAl3 and enhanced Al diffusion, promoting TiAl phase formation.
During prolonged oxidation, inherent brittleness of the aluminide coating and continuous Al depletion for Al2O3 formation generate internal stresses. These induce microcracking, wrinkling, and eventual spallation in the oxide scale, leading to progressive thinning. Consequently, mid-stage oxidation exhibits diminished mass gain, reflected in the kinetics curve's attenuated slope. As oxidation persists, spalled regions expose the substrate. However, the coating's self-healing capacity facilitates continuous Al2O3 reformation, maintaining substrate protection.
5. Conclusions
This formulation, namely the use of the 30%Al-66%Al2O3-3%NH4Cl-1%CeO2 composition, successfully deposited aluminide coatings on γ-TiAl intermetallic compounds at 600 °C for 5 h. The coating thickness increased progressively with aluminizing duration, though growth decelerated over time. Beyond a critical period, thickness asymptotically approaches a maximum value. The optimal coating thickness was achieved after 5 h at 600 °C.
The addition of the rare earth oxide CeO2 promoted the diffusion of aluminum atoms, achieving an oxide film approximately 31.30 µm thick at a temperature of 600 °C for 5 h.
The oxidation kinetics of both γ-TiAl intermetallic substrates and low-temperature aluminized specimens conformed to parabolic laws after 20 h at 800 °C. At 900 °C, however, the substrate exhibited linear oxidation kinetics, demonstrating no high-temperature oxidation resistance. In contrast, aluminized specimens maintained parabolic kinetics. These results confirm the coating's significant enhancement of substrate oxidation resistance.
Author Contributions
Conceptualization, J.S., J.W. and M.C.; methodology, J.S., Y.L. (Yunmei Long), J.W., Y.H. and M.C.; investigation, J.S., Y.L. (Yunmei Long) Y.L.(Yichen Li), D.H. and X.W.; data curation, J.S., Y.L. (Yunmei Long), Y.L. (Yichen Li), D.H. X.W. and Y.G.; writing—original draft preparation, J.S. and J.W.; writing—review and editing, J.S., J.W., Y.L. (Yunmei Long), Y.G. and M.C.; visualization, J.W., Y.G. and M.C. All authors have read and agreed to the published version of the manuscript.
Funding
The authors declare that this study received funding from the National Natural Science Foundation of China under Grant (51671053 and 51801021), the Fundamental Research Funds for the Central Universities (No. N25DCG001), and the Ministry of Industry and Information Technology Project (No. MJ-2017-J-99). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contain within the article.
Conflicts of Interest
Author Yan Gu was employed by the company AECC Shenyang Liming Aeroengine Corporation Ltd Technology Center. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Yamaguchi, M.; Inui, H.; Ito, K. High-temperatures structural intermetallics. Acta Mater. 2000, 48, 307–322. [Google Scholar] [CrossRef]
- Clemens, H.; Mayer, S. Design, processing, microstructure, properties, and applications of advanced intermetallic TiAl alloys. Adv. Eng. Mater. 2013, 15, 191. [Google Scholar] [CrossRef]
- Vaben, R.; Jarligo, M.O.; Steinke, T.; Mack, D.E.; Stöver, D. Overview on advanced thermal barrier coatings. Surf. Coat. Technol. 2010, 205, 938–942. [Google Scholar] [CrossRef]
- Wu, L.; Wu, J.; Wu, W.; Hou, G.-Y.; Cao, H.-Z.; Tang, Y.-P.; Zhang, H.-B.; Zheng, G.-Q. High temperature oxidation resistance of γ-TiAl alloy with pack aluminizing and electrodeposited SiO2 composite coating. Corros. Sci. 2018, 146, 18–27. [Google Scholar] [CrossRef]
- Brady, M.P.; Brindley, W.J.; Smialek, J.L.; Locci, I.E. The oxidation and protection of gamma titanium aluminides. JOM 1996, 48, 46–50. [Google Scholar] [CrossRef]
- Gurrappa, I.; Rao, S.A. Thermal barrier coatings for enhanced efficiency of gas turbine engines. Surf. Coat. Technol. 2006, 201, 3016–3029. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, F.; Wu, W. Effect of a sputtered TiAlCr coating on the oxidation resistance of TiAl intermetallic compound. Oxid. Met. 1997, 48, 511–525. [Google Scholar] [CrossRef]
- Mengis, L.; Oskay, C.; Donchev, A.; Galetz, M. Critical assessment of the cyclic oxidation resistance of the aluminized Ti-48Al-2Cr-2Nb TiAl alloy at 700 °C and its impact on mechanical properties. Surf. Coat. Technol. 2021, 406, 126646. [Google Scholar] [CrossRef]
- Swadźba, R.; Swadźba, L.; Mendala, B.; Witala, B.; Tracz, J.; Marugi, K.; Pyclik, Ł. Characterization of Si-aluminide coating and oxide scale microstructure formed on γ-TiAl alloy during long-term oxidation at 950 °C. Intermetallics 2017, 87, 81–89. [Google Scholar] [CrossRef]
- Gao, J.; He, Y.; Gao, W. Oxidation behavior of γ-TiAl based alloy with Al2O3–Y2O3 composite coatings prepared by electrophoretic deposition. Surf. Coat. Technol. 2011, 205, 4453–4458. [Google Scholar] [CrossRef]
- Xiang, Z.D.; Datta, P.K. Relationship between pack chemistry and aluminide coating formation for low-temperature aluminisation of alloy steels. Acta Mater. 2006, 54, 4453–4463. [Google Scholar] [CrossRef]
- Alam, M.Z.; Durgarao, K.Y.; Kumawat, M.; Banumathy, S. Microstructure, oxidation and mechanical properties of a diffusion aluminide (Al3Ti) coated lamellar γ-TiAl alloy. Surf. Coat. Technol. 2019, 380, 125071. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Hu, H.; Meng, J.; Zhao, X. Effect of Y2O3 content in the pack mixtures on the cyclic-oxidation of Y2O3-modified low temperature aluminide coatings on 309 stainless steel. Vacuum 2018, 158, 101–112. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, Y.G.; Li, W.; Tian, B.; Hu, S.; Qin, Q. Oxidation behavior of the Y2O3-modified aluminide coating on Ti-6Al-4V alloy. Mater. Sci. Eng. A 2007, 458, 34–38. [Google Scholar] [CrossRef]
- Cho, D.W.; Kim, I. Formation of pegs during high temperature oxidation of Fe3Al containing yttrium. Metall. Mater. Trans. A 2000, 31, 1685–1687. [Google Scholar] [CrossRef]
- Doleker, K.M.; Yener, T.; Erdogan, A.; Yılmaz, F.; Efe, G.C. Effect of Si and Cr on formation of aluminide coatings on Ti6Al4V alloy by low temperature aluminizing: Wear and oxidation behavior. Surf. Coat. Technol. 2025, 509, 132207. [Google Scholar] [CrossRef]
- Mehrer, H. Diffusion in Intermetallics. Mater. Trans. JIM 1996, 37, 1259–1280. [Google Scholar] [CrossRef]
- Gaviría, J.P.; Navarro, L.G.; Bohé, A.E. Chlorination of lanthanum oxide. J. Phys. Chem. A 2012, 116, 2062–2070. [Google Scholar] [CrossRef]
- West, G.; Perkins, J.; Lewis, M. The effect of rare earth dopants on grain boundary cohesion in alumina. J. Eur. Ceram. Soc. 2006, 27, 1913–1918. [Google Scholar] [CrossRef]
- Varlese, F.A.; Tului, M.; Sabbadini, S.; Pellissero, F.; Sebastiani, M.; Bemporad, E. Optimized coating procedure for the protection of TiAl intermetallic alloy against high temperature oxidation. Intermetallics 2013, 37, 76–82. [Google Scholar] [CrossRef]
- Yoshihara, M.; Kim, Y. Oxidation behavior of gamma alloys designed for high temperature applications. Intermetallics 2004, 13, 952–958. [Google Scholar] [CrossRef]
- Das, D.K.; Alam, Z. Cyclic oxidation behaviour of aluminide coatings on Ti-base alloy IMI-834 at 750 °C. Surf. Coat. Technol. 2006, 201, 3406–3414. [Google Scholar] [CrossRef]
- Lutfullin, R.Y.; Imayev, R.M.; Kaibyshev, O.; Hismatullin, F.; Imayev, V. Superplasticity and solid-state bonding of the tial intermetallic compound with microcrystalline and submicrocrystalline structure. Scr. Metall. Et Mater. 1995, 33, 1445–1449. [Google Scholar] [CrossRef]
- Goral, M.; Swadzba, L.; Moskal, G.; Jarczyk, G.; Aguilar, J. Diffusion aluminide coatings for TiAl intermetallic turbine blades. Intermetallics 2011, 19, 744–747. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).