1. Introduction
Human joints are the connecting structures between bones, which contribute to the flexible movement of bones. The articular cartilage attached to the joint surface was known as the most efficient lubricating and load-bearing interface in nature, mainly due to its extremely low friction coefficient and efficient load-bearing capacity [
1,
2]. A healthy knee joint can bear 7–9 times human body weight and maintain efficient lubrication under physiological high pressure with a friction coefficient as low as 0.001 [
3]. Generally, a healthy synovial can withstand more than 70 years of movement without injury. However, symptoms such as joint pain, swelling and functional disorders caused by reasons such as accidental trauma, aging, genetic diseases and obesity can seriously affect the quality of life [
4,
5]. Damaged articular cartilage is difficult to self-repair due to the absence of blood and lymphatic vessels [
6]. At present, general physical therapy or drug treatment can only relieve symptoms and cannot prevent the further degeneration of articular cartilage or underlying bone. Eventually, artificial joint replacement surgery must be performed to effectively relieve joint pain and restore the normal function of the joint [
7,
8].
Although artificial joint replacement surgery has been regarded as one of the most successful surgical operations in the 21st century, there are still many drawbacks. After joint replacement surgery, the lubrication performance of artificial joint is usually poor, and the postoperative revision rate is as high as 20% [
9]. This is mainly due to the removal of natural articular cartilage and joint capsules, lacking the cushioning and shock-absorbing functions of natural cartilage as well as the lubricating effect of synovial fluid. In recent years, Polyvinyl alcohol (PVA) hydrogel materials have become a potential candidate for cartilage repair due to their excellent chemical stability, three-dimensional porous network structure similar to human articular cartilage, high water content and good biocompatibility [
10,
11,
12]. However, the poor mechanical strength and limited biological activity of pure PVA hydrogels limit their practical application in high-load cartilage replacement. Recent studies have incorporated various biopolymers (such as agar, chitosan, silk nanofiber, gellan gum) into PVA-based hydrogels to form dual-network hydrogels, effectively enhancing mechanical, thermal and biological properties [
13,
14,
15]. For instance, the synergistic interactions between silk nanofibers, chitosan, and PVA enable the composite hydrogels to exhibit improved tensile strength (up to 4 MPa) and high-water content (87%) [
16]. Under the synergistic effects of multiple networks, hydrogen bonds and nano-reinforcement, cellulose nanofiber-reinforced polyacrylamide/PVA composite hydrogel demonstrated outstanding tensile strength (1.41 MPa), elongation at break (1332%), and high transparency (89.8 %) with just one freeze-thaw cycle [
17]. As bionic articular cartilage, the above studies have overlooked the exploration of lubrication performance. Therefore, it is particularly important to develop self-lubricating PVA hydrogels (0.1–2 MPa) that can withstand the contact pressure of the knee or hip joint.
Here, a high-performance PVA-based bionic interpenetrating network hydrogel was designed and fabricated. Sodium alginate nanofibers were introduced into the hydrogel of the PVA network to improve the mechanical and tribological properties. The multiple hydrogen bonds formed between PVA chains and alginate fibers act as sacrificial bonds, achieving effective energy dissipation through reversible cleavage during deformation process [
18,
19]. In addition, the introduction of rigid alginate fibers endows the hydrogel with superior load-bearing performance. This alginate fiber-reinforced PVA hydrogel provides a new strategy for cartilage replacement.
2. Experimental Section
2.1. Materials
Sodium chloride (NaCl) was purchased form Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Sodium alginate (SA) and polyvinyl alcohol (PVA) (>99% hydrolyzed, average degree of polymerization is 1700) were obtained from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Phosphate buffer solution (PBS) was provided by Guangzhou Xiang Bo Biological Technology Co., Ltd. (Guangzhou, China). Calf serum was supplied by Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China). The deionized water was purified by the equipment from Shanghai Hetai Instrument Co., Ltd. (Shanghai, China). All reagents were of AR grade and used as received.
2.2. Preparation of High-Performance Hydrogel
To imitate the super-lubricated articular cartilage, the sodium alginate fiber-reinforced polyvinyl alcohol hydrogel was prepared through physical blending and freezing/thawing methods. First, different amounts of SA (0.2 g, 0.4 g, 0.6 g, 0.8 g, 1.0 g) were respectively added to 100 mL of 2 mg/mL NaCl solution, and the solution was magnetically stirred at room temperature until it became uniform. Then, the obtained SA/NaCl solution was placed for 12 h to obtain SA fibers. Subsequently, 12 g PVA powder was added to the above-mentioned 88 mL SA/NaCl solution. Then, it was continuously stirred in a water bath at 95 °C for 6 h to obtain a uniform PVA/SA/NaCl hydrogel precursor solution. To remove the excess bubbles, the above solution was raised to a higher temperature, and left to stand for 1 h. The hydrogel precursor solution was poured into the self-made mold. Finally, it was placed in the freezer for 21 h and thawed at room temperature for 3 h. The freezing/thawing process was repeated 5 times, and the as-obtained samples were denoted as PVA/SA/NaCl hydrogels. The samples with SA masses of 0.2 g, 0.4 g, 0.6 g, 0.8 g, 1.0 g were respectively marked as PVA/0.2SA, PVA/0.4SA, PVA/0.6SA, PVA/0.8SA, PVA/1.0SA. The schematic diagram of PVA/SA/NaCl hydrogel preparation process was shown in
Figure 1.
2.3. Characterization
The chemical state of SA monomer, PVA and PVA/SA/NaCl samples were measured through Fourier Transform Infrared Spectrometers (ATR-FTIR, Nicolet IS20, Waltham, MA, USA). The microscopic morphologies of the hydrogels were characterized using scanning electron microscopy (SEM, JSM-IT500HR, Tokyo, Japan). Before observation, Hydrogels were freeze-dried for 24 h and sprayed with a layer of gold. The three-dimensional contour morphology and surface roughness values of the hydrogels were obtained by the laser scanning confocal microscope (VK-X150, Keyence, Shanghai, China).
To evaluate the hydrophilicity of the hydrogel surfaces, the static water contact angle on the hydrogel surface was measured using an optical contact goniometer (JC2000D, Shenzhen, China). The water content and swelling behavior of the hydrogel in deionized water were determined by the weighing method. Water content and Swelling rate at time points (
) of the hydrogel were calculated respectively through the following Formulas (1) and (2):
where,
represented the mass of the hydrogel after complete swelling in deionized water,
referred the mass of the hydrogel after complete drying in a freeze dryer, and
represented the mass of a completely dried hydrogel swelling in deionized water at different times (1 h, 2 h, 3 h, 6 h, 12 h, 24 h, 48 h, 60 h). Each sample was tested three times and the average value was taken.
The mechanical properties of the hydrogel were characterized by a universal mechanical testing machine (UTM6104, Shenzhen, Shenzhen, China). Hydrogels were cut into dumbbell-shaped standard specimens with a length of 20 mm, a width of 5 mm and a thickness of 3 mm as tensile specimens. The stretching rate was set to 4 mm/min. The tensile strength and elongation at break of the hydrogel specimens were obtained according to the tensile stress-strain curve. Moreover, the fracture energy was calculated through the area integral of the tensile stress-strain curve to quantitatively evaluate the toughness.
Hydrogels were prepared into cylinders with a diameter of 8 mm and a height of 3 mm as compression specimens. During the test, the compression rate was set at 0.5 mm/min, and 60% of the compression strain was set as the termination line. For comparative analysis, the stress values under this strain were all selected as the compressive strength.
The tribological properties of the composite hydrogels were evaluated by using a multifunctional reciprocating friction testing machine (UMT-2, Bruker, Karlsruhe, Germany). A stainless steel ball with a diameter of 6 mm was used as the upper friction pair, and the prepared hydrogel sample was used as the lower friction pair. The reciprocating stroke was set at 2 mm. Before the test, the hydrogel was cut into a rectangular prism with a size of 20 mm × 20 mm × 3 mm and fixed in a custom acrylic mold with screws. During the test, the applied load, sliding rate and friction time were set as 2–8 N, 2–10 mm/s and 10 min respectively. To further evaluate its durability, the preferred hydrogel was subjected to a 60-min friction test in different biomimetic physiological solutions (PBS buffer, Calf serum).
3. Results
The FTIR spectra of SA monomer, PVA and PVA/SA/NaCl samples are shown in
Figure 2. In the spectra of pure PVA hydrogel, the absorption peaks at 3265 cm
−1 and 2910 cm
−1 are respectively attributed to the stretching vibration peaks of -OH groups and C-H. The deformation vibration peak at 1404 cm
−1 corresponds to CH
2, and the absorption peak at 1083 cm
−1 corresponds to the tensile vibration peak of C-O. For the SA monomer, the absorption peak at 2952 cm
−1 is the stretching vibration peak of C-H, and the absorption peaks at 1596 cm
−1 and 1412 cm
−1 correspond respectively to the asymmetric and symmetric stretching vibration peaks of -COO-. The introduction of NaCl did not result in a new peak. The PVA/1.0SA spectrum shows the characteristic peaks related to PVA and SA. The absorption peaks at 1646 cm
−1 and 1416 cm
−1 are the asymmetric and symmetric stretching vibrations of the carboxylate groups in SA, which indicates the successful preparation of the PVA/SA/NaCl composite hydrogel. This is consistent with the data reported in previous literature [
20,
21].
The surface morphologies of the as-obtained samples prepared with different SA contents are presented in
Figure 3. It can be clearly seen that a three-dimensional honeycomb-like porous network structure is formed on the hydrogel surface. With the increase of SA content, the size and number of holes decrease accordingly, and the surface presents a relatively dense structure accompanied by some uneven small holes. This is because that the PVA molecular chain cross-links with SA, generating a stronger hydrogen bond effect and thus forming a denser network structure.
The surface morphology of the hydrogel has an important influence on tribological properties. Generally, hydrogels with relatively smooth and flat surfaces tend to achieve lower friction. The relevant parameters of the three-dimensional surface topography and surface roughness of the prepared samples are shown in
Figure 4. It can be clearly seen that the hydrogel surface is actually not completely smooth and there are certain undulations on the surface. When the SA is introduced, the roughness of the hydrogel surface shows a trend of increasing first and then decreasing. When the content of SA exceeds 0.6 g, its surface roughness decreases instead. However, the surface roughness Ra values of all samples are below 10 μm, which indicates that the surfaces of the prepared hydrogels were relatively smooth.
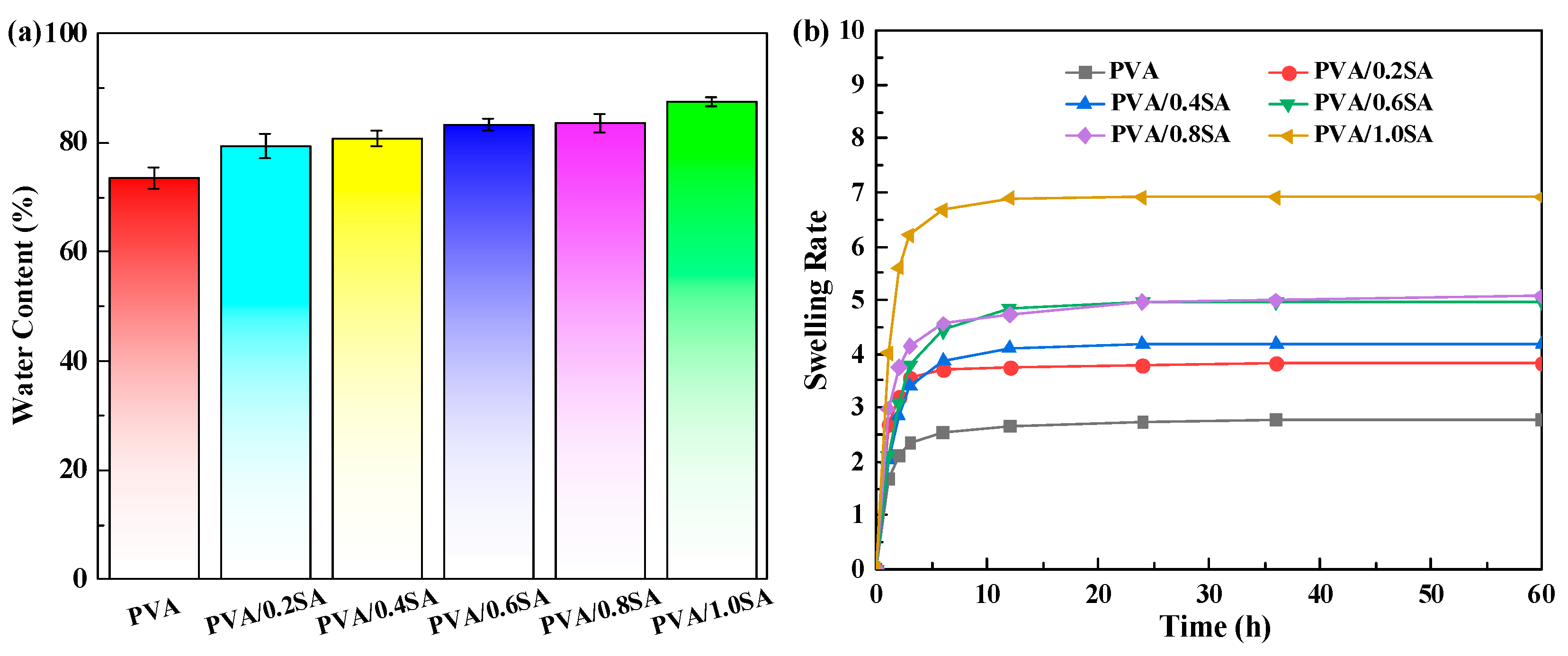
As a substitute for articular cartilage, the tests of water content and swelling performance of hydrogels are also indispensable. The influence of SA content on the water content and swelling behavior of PVA/SA/NaCl hydrogel is shown in
Figure 5. It can be seen from
Figure 5a that the prepared PVA/SA/NaCl hydrogel samples all have a relatively higher water content, and the water content of all hydrogels is above 75%. Furthermore, the water content of the PVA/SA/NaCl hydrogel increases with the increase of SA content. When the SA content increased to 1 g, the water content of the hydrogel reached 87%. This is mainly due to the increase in SA content, which raises the number of hydroxyl and carboxyl groups in the hydrogel network, thus enabling more water to be adsorbed. The swelling rate of the hydrogel samples was also determined by the mass method, as shown in
Figure 5b. With the increase of SA content, its swelling rate also gradually increases, which is consistent with the trend of water content. When the dried PVA/SA/NaCl hydrogel is soaked in the stripping water again, the sample will rapidly absorb water within the first three hours, and then the rate of water absorption gradually slows down. After 12 h, the weight of the sample basically reached a stable state, which indicates that the prepared PVA/SA/NaCl hydrogel exhibits good swelling performance.
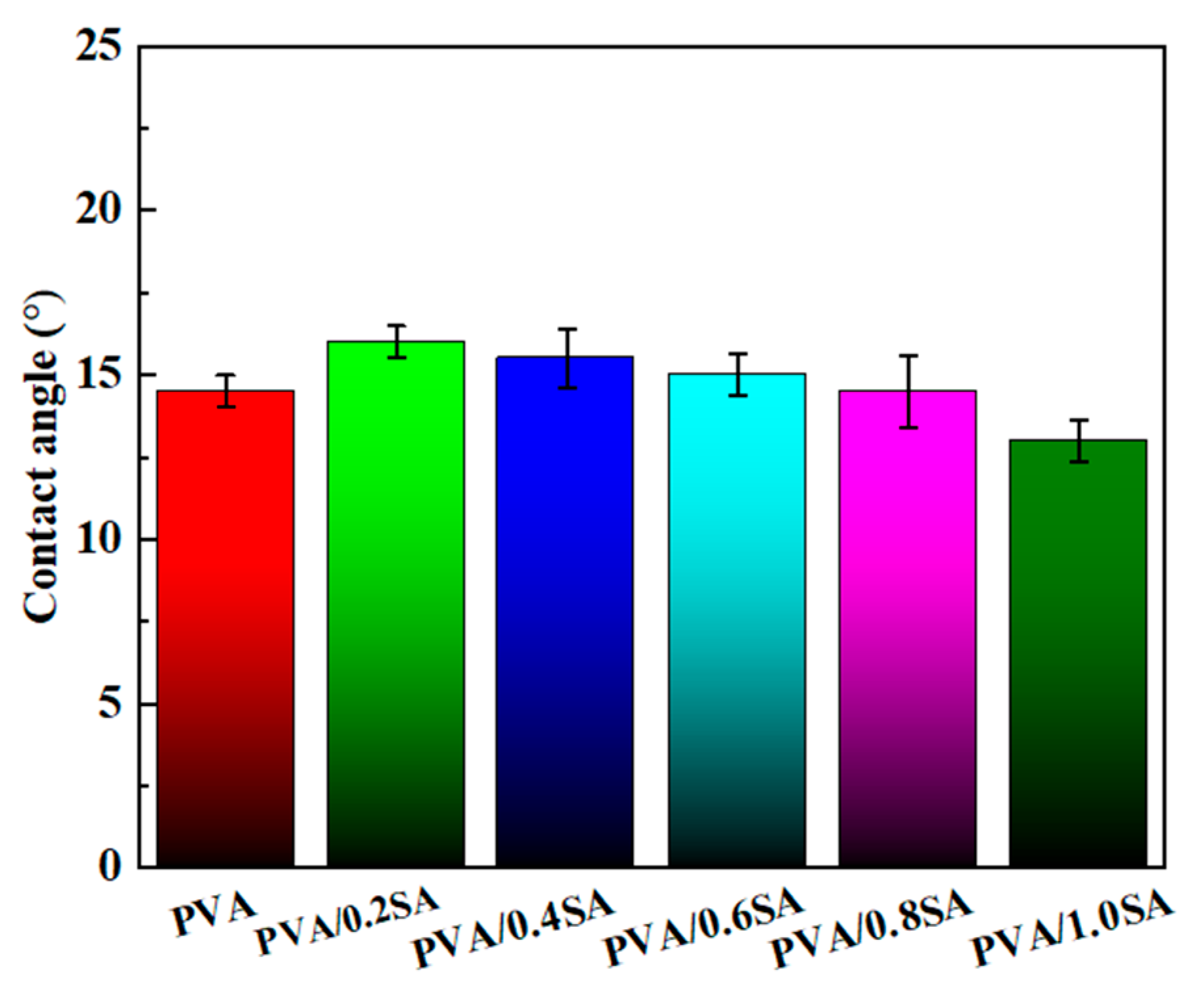
The excellent hydrophilicity of natural articular cartilage is conducive to promoting protein adsorption and cell adhesion [
22,
23]. The static water contact angles on the surface of the composite hydrogel samples prepared with different SA contents are shown in
Figure 6. With the increase of SA content, the contact angle of the hydrogel samples shows a gradually decreasing trend, which indicates that the addition of SA improves the hydrophilicity of the surface of the composite samples. Most importantly, after several seconds, the surfaces of all hydrogels were in a completely wetted state, with a static water contact angle of 0°. This further demonstrates that the prepared PVA/SA/NaCl composite hydrogels have excellent surface wettability.
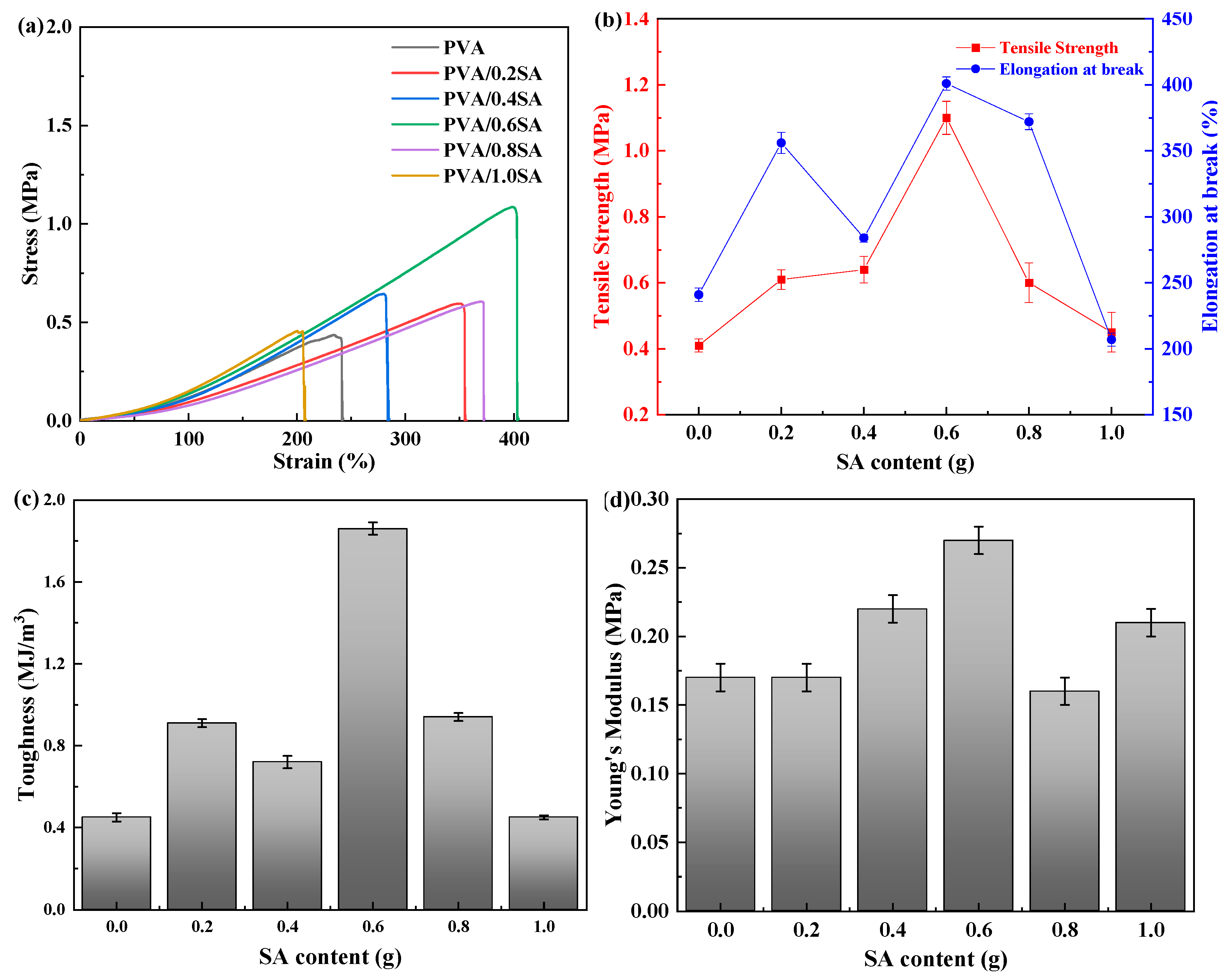
The tensile properties of PVA/SA/NaCl hydrogels with different SA contents are presented in
Figure 7. The corresponding toughness and Young’s modulus values of the samples were also calculated based on the stress-strain curve. The tensile strength of the pure PVA hydrogel is relatively low (0.4 MPa), and the maximum elongation at break is 240%. The toughness and Young’s modulus of pure PVA hydrogel were 0.42 MJ/m
3 and 0.17 MPa, respectively. When a small amount of SA is introduced, the crosslinking density within the hydrogel network increases, forming a more stable structure. Therefore, the maximum elongation at break and tensile strength of the PVA/SA/NaCl hydrogel also increase accordingly. When the content of SA increased to 0.6 g, the maximum elongation at break and tensile strength of the PVA/0.6SA hydrogel reached the maximum, which were 404% and 1.08 MPa respectively. At this point, the toughness and Young’s modulus of the PVA/0.6SA hydrogel also reached the maximum, which were 1.86 MJ/m
3 and 0.27 MPa respectively. Compared with the pure PVA hydrogel, the maximum elongation at break increased by 68% and the tensile strength increased by 170%. However, when the content of SA is further increased, the tensile strength and elongation at break of the composite hydrogel decrease. This is mainly because the SA molecular chain contains abundant carboxyl groups (-COOH) and hydroxyl groups (-OH), which form additional hydrogen bonds with the hydroxyl groups of PVA, significantly increasing the density of crosslinking points. When the SA content is moderate (0.6 g), SA molecules can be uniformly dispersed in the PVA network, forming a uniform interpenetrating network. When the amount of SA continues to increase, the growth of hydrogen bond crosslinking points slows down. Due to the significant increase in crosslinking density, excessive SA instead leads to a harder texture and poorer uniformity of the hydrogel. Therefore, the PVA/0.6SA stretch is significantly more than the other hydrogels.
The compressive stress-strain curves of PVA/SA/NaCl hydrogel specimens with different SA contents are presented in
Figure 8. During the compression process, the water inside the hydrogel network is squeezed onto the surface. Generally, the greater the compressive strength of a hydrogel, the more difficult it is for the water inside the hydrogel network to be squeezed out. The compressive strength of the composite hydrogel shows a trend of increasing first and then decreasing with the increase of SA content. When the SA content increases from 0 g to 0.6 g, the compressive strength of the samples increases from 0.32 MPa to 0.44 MPa, increasing by approximately 1.5 times. This is due to the fact that the addition of SA increases the cross-linking density of hydrogen bonds between PVA molecules and SA, thereby enhancing its compressive strength. When the SA content increases to 1.0 g, the compressive strength of the PVA/1.0SA hydrogel is approximately the same as that of PVA/0.8SA.
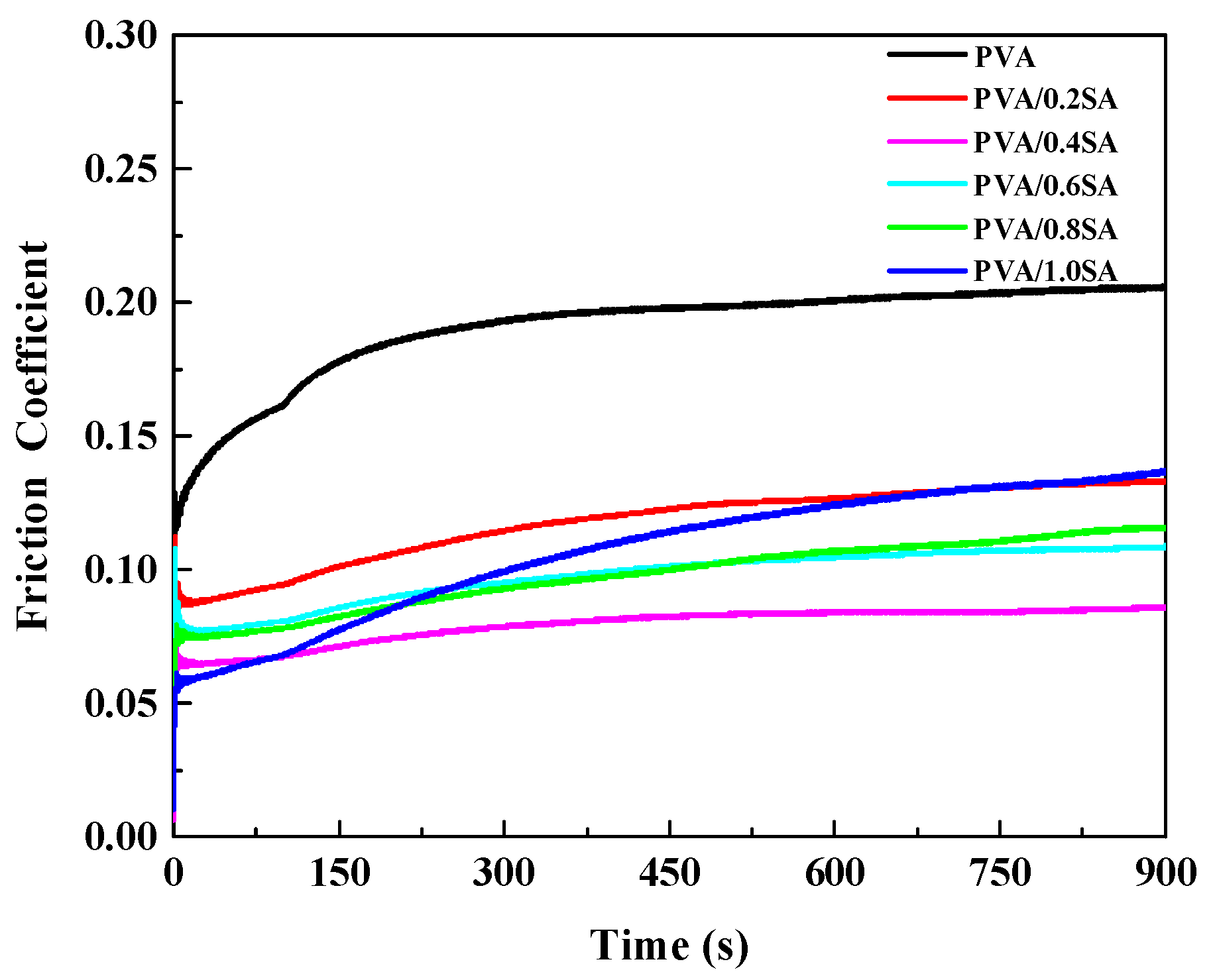
Excellent lubricating performance can reduce the shear force on the cartilage surface and delay degenerative diseases such as osteoarthritis. Here, the tribological properties of the composite hydrogels were evaluated through a reciprocating friction testing machine.
Figure 9 shows the curves of the friction coefficient of the composite hydrogel samples varying with time. The average friction coefficient of pure PVA hydrogel without SA addition is relatively large, around 0.19. With the increase of SA content, the friction coefficient decreases to a certain extent, and the overall trend shows a decrease first and then an increase. When the content of SA increased to 0.4 g, the PVA/0.4SA hydrogel sample obtained the lowest friction coefficient, approximately 0.079. When the SA content continued to increase to 0.6 g, 0.8 g and 1.0 g, the friction coefficients of the composite hydrogel samples increased to 0.097, 0.098 and 0.113 respectively.
The movement patterns of articular cartilage are significantly diverse, and it will withstand dynamically changing pressure loads and frequency characteristics under different biomechanical conditions. Therefore, the influence of the applied load and sliding speed on the tribological properties of the composite hydrogel was also evaluated, and the results are shown in
Figure 10. When the applied load increases from 2 N to 6 N, the average friction coefficient increases from 0.079 to 0.149. When the applied load continued to increase to 8 N, the friction coefficient of the PVA/0.4SA hydrogel did not continue to rise. Conversely, after being lubricated in deionized water for 150 s, its friction coefficient was slightly lower than that measured under a load of 6 N. As shown in
Figure 10b, the friction coefficient of PVA/0.4SA hydrogel shows a trend of first decreasing and then increasing with the increase of the sliding speed. When the sliding speed is 4 mm/s, its friction coefficient is the lowest, about 0.074.
To simulate the physiological environment, PBS buffer solution and calf serum were also used as lubricating media. In addition, to evaluate its stability, a 1 h friction test was conducted on the hydrogel samples, as shown in
Figure 11. All the friction curves are relatively flat without significant undulations. Importantly, when lubricated in PBS buffer solution, the friction coefficient of the PVA/SA/NaCl hydrogel is the lowest, around 0.02, while when lubricated in calf serum, its friction coefficient is the highest, approximately 0.22.
4. Discussion
At present, the most widely used hard artificial joints (such as metal/polyethylene, metal/ceramic) have certain durability, but they still have some limitations, such as high friction and wear. To solve this problem, by simulating the structure and lubrication mechanism of natural cartilage, the mechanical and tribological properties of PVA hydrogel were enhanced through introducing sodium alginate fibers. Moreover, the chemical composition, surface morphology, swelling performance, wettability, mechanical properties and tribological properties of hydrogels were studied systematically.
Natural cartilage is a dense and highly hydrated connective tissue. When compressed, the synovial fluid stored in the joint moves from the inside to the surface, playing a role in load-bearing and lubrication. The FTIR spectra confirmed that SA fibers were introduced into the PVA hydrogel network (
Figure 2). Moreover, the increase in water content and swelling rate also proved the successfully introduction of SA fibers (
Figure 5). The obtained PVA/SA/NaCl composite hydrogel all exhibited good swelling performance, surface wettability and a three-dimensional porous network structure similar to articular cartilage [
24]. The tensile stress of the composite hydrogels showed a nonlinear variation trend with strain (
Figure 7). The tensile strength and elongation at break of PVA/SA/NaCl hydrogel exhibited a trend of increasing first and then decreasing with the increase of SA content. Moreover, the variation trend of the compressive strength of hydrogels with the content of SA was similar. Excessive SA instead led to the deterioration of the uniformity of the hydrogel, and the hardening of its texture, thus resulting in a decrease in mechanical properties.
In natural joints, the frictional force on the cartilage surface is proportional to the load borne by the solid phase. In other words, as long as the liquid-phase bearing remains at a high level for a long time, the friction coefficient will remain at a low level for a long time [
25,
26]. Since bionic cartilage hydrogels have similar structural characteristics to natural cartilage, the lubrication mechanism of the prepared PVA/SA/NaCl composite hydrogels can be explained by the above-mentioned biphasic lubrication mechanism. The friction coefficient of PVA/SA/NaCl hydrogel with different SA contents was compared in
Figure 9. The friction coefficient showed a trend of increasing first and then decreasing with the increase of SA content. When the SA content increased to 0.4 g, the friction coefficient of the PVA/0.4SA hydrogel was the lowest (0.079), similar to that of fresh porcine cartilage [
27]. This was because that the introduction of an appropriate amount of SA enhanced the hydrogen bonding within the hydrogel, making the network structure denser. When subjected to a load, the load-bearing capacity of the hydrogel increased and the deformation on hydrogel surface decreased. When the SA content was further increased, it caused the surface texture of the hydrogel to become harder and denser. When subjected to a load, the amount of deionized water seeping out from the pores of the hydrogel decreased, resulting in a reduced proportion of the liquid phase bearing.
Human daily physiological activities (such as walking, running, and jumping) involve diverse movement patterns, resulting in significant differences in the loads, frequencies, and sliding speeds borne by articular cartilage [
28,
29]. Therefore, to evaluate the potential application performance of hydrogels in simulating different motion scenarios, the effects of different applied loads and sliding speeds on the tribological properties of composite hydrogels were also characterized (
Figure 10). With the increase of the applied load (from 2 N to 8 N), the friction coefficient of the hydrogel showed a trend of first decreasing and then similar. This is mainly because that as the applied load increased, the deformation degree of the hydrogel would increase, which increased the contact area between stainless steel ball and the hydrogel surface, resulting in an increase in the friction coefficient. The variation trend of the sliding speed on the friction coefficient of the hydrogel was consistent with the applied load.
The strain hysteresis characteristic of hydrogels would lead to a decrease in the friction coefficient. When the sliding speed was further increased, the water inside the hydrogel network cannot be replenished in time to restore the deformation of the hydrogel, resulting in an increase in friction coefficient.
To fully simulate the service environment of human synovial fluid, the tribological properties of the preferred PVA/0.4SA hydrogel were tested in PBS buffer and calf serum. During the whole friction process, the friction coefficient curves of the composite hydrogel in deionized water, PBS buffer and calf serum are all relatively flat, without too much fluctuation (
Figure 11). When PVA/0.4SA hydrogel was lubricated in PBS buffer solution, its friction coefficient was the lowest (0.02). This was mainly attributed to the higher osmotic pressure of the PBS buffer solution. When PVA/0.4SA hydrogel was lubricated in calf serum, its friction coefficient was the highest, approximately 0.22. Because calf serum contains various biological macromolecules (plasma proteins, polypeptides, fats), they will aggregate at the friction interface, thereby resulting in higher friction. This was mainly attributed to the relatively high viscosity of calf serum. Moreover, the biological macromolecules (such as plasma proteins, polypeptides, fats) in calf serum would aggregate at the friction interface, resulting in higher friction. On the whole, PVA/0.4SA hydrogel all demonstrated excellent lubricating performance and durability in bionic physiological solutions, indicating that it had great application potential in physiological environments. In addition, the results obtained in this study (mechanical strength, toughness, friction) were compared with the properties of PVA hydrogel reinforced by other methods. The results show that they each have their advantages and disadvantages, and it is still difficult for them to achieve the unification of high mechanical strength and low friction [
13,
30,
31,
32,
33]. In conclusion, the PVA/SA/NaCl hydrogels prepared in this study exhibit superior tribological performance in biomimetic physiological solutions.