1. Introduction
A sensor is a device that converts a specific change in a target into a measurable signal. Generally, it consists of a sensing element, a conversion element, a conversion circuit, and an auxiliary power supply [
1]. The measurement is directly sensed by the sensitive element, and a physical quantity signal related to the measurement is output. The role of the conversion element is to transform the physical quantity signal output from the sensitive element into an electrical signal; the conversion circuit is responsible for the amplification of the electrical signal output from the conversion element. Additionally, the conversion element and the conversion circuit typically require an auxiliary power supply to function effectively [
2]. Sensor detection technology, characterized by rapid response, high sensitivity, and accuracy, is widely used for detecting target analytes [
3,
4]. Glucose sensors are of significant importance across various fields, particularly in the medical field [
5]. In the food industry, sensors are widely used to determine glucose levels in food raw materials and finished products; for example, glucose sensors are used to detect the glucose concentration in sugary foods such as fruit juices and beverages [
6,
7,
8]. In the medical domain, glucose sensors are extensively employed in the diagnosis and treatment of diabetes by detecting the blood glucose levels of patients through the sensors, and the diet, exercise, and medication regimens of the patients can be guided accordingly [
9,
10].
Initially, glucose sensors were based on GOx [
11], which specifically catalyzes glucose to react with oxygen. Since the concept was first proposed in 1960 by Clark [
12], enzyme-based sensors have undergone three generations of innovation. The sensors have fast response and high sensitivity, but they are also limited by oxygen dependence, structural complexity, short-term stability, and enzyme activity [
13]. In recent years, the emergence of nanomaterials has significantly advanced the performance of non-enzymatic glucose sensors. With their large specific surface area, excellent electrical conductivity, and abundant surface functional sites, nanomaterials have not only enhanced catalytic activity and electron transfer efficiency but also markedly improved the stability and anti-interference capabilities of sensors [
14]. Researchers have extensively explored a variety of nanomaterials for constructing non-enzymatic sensing electrodes, including noble metal nanomaterials, non-noble transition metal-based materials, multi-metallic composites, and carbon-based nanomaterials such as graphene and carbon quantum dots [
15,
16,
17,
18,
19]. These materials have shown great potential in improving the sensitivity, selectivity, and response speed of non-enzymatic glucose sensors.
Electrochemical, fluorescence, and colorimetric sensing are effective non-enzymatic strategies for glucose detection, as they reduce or eliminate reliance on biological enzymes and achieve concentration measurements through different signal transduction mechanisms [
20]. With the continued development of nanomaterials, their advantageous properties, such as high surface area, superior conductivity, and abundant active sites, play a critical role in various aspects of sensor design, including electrode modification, fluorescence regulation, and colorimetric signal response, making them a central enabler in the performance enhancement of non-enzymatic glucose sensors [
21].
The information about the concentration and properties of the target analyte could be obtained by measuring the quantification of physical parameter variations, such as voltage and current, during the reaction process, which is the principle of electrochemical analysis. A typical three-electrode system consisting of WE, RE, and CE is often used to detect the target analyte. Among them, the oxidation of glucose occurs at the WE, which is usually made of materials with high point catalytic activity, such as noble metal nanomaterials, metal oxide nanomaterials, and carbon-based nanomaterials [
22,
23,
24,
25].
In addition to electrochemical techniques for glucose detection, optical sensing technologies such as fluorescence sensing and colorimetric sensing have also shown great potential [
26,
27]. Glucose concentration detection methods based on fluorescence principles use changes in fluorescence signals to reflect changes in glucose concentration. The intensity of the fluorescence signal is directly related to the concentration of the target analytes and is less susceptible to interference from other substances, thus providing better sensitivity and selectivity. In addition, fluorescent sensors have promising applications in the field of dynamic glucose monitoring because they can provide real-time, continuous monitoring, which is an important advantage for long-term glucose management [
28]. Colorimetric glucose detection methods [
29,
30], based on colorimetric mechanisms, achieve visual detection of glucose concentration through redox or enzyme-mimetic reactions catalyzed by nanomaterials that trigger color changes. Both detection strategies fall under the category of optical sensing, relying on changes in optical signals to perform analytical tasks, and share common advantages such as non-contact operation, fast response, and strong scalability [
31]. With the support of nanomaterials, these two optical methods continue to break through performance bottlenecks and have become indispensable components of non-enzymatic glucose sensor systems, providing strong technical support for achieving intelligent and versatile glucose monitoring [
32,
33,
34].
2. Enzymatic Glucose Sensor
The enzymatic glucose sensor operates by leveraging the catalytic activity of enzymes to measure glucose concentration [
35]. Its biologically active center is glucose oxidase. Owing to their exceptional sensitivity and selectivity, they are widely applied in monitoring the blood glucose of diabetic patients. The concept of enzyme-based electrodes was first introduced by Clark and Lyons in 1962 [
36], and since then, enzymatic glucose sensors have undergone three technological innovations that have significantly improved their performance. The development of the three generations of enzymatic glucose sensors is shown in
Figure 1.
The first generation of enzymatic glucose sensors can detect the consumption of glucose by detecting the oxygen consumption or hydrogen peroxide production during the electrode reaction [
37]. Its principle and manufacture are relatively simple, but the concentration of oxygen and other chemicals in the blood greatly interferes with the normal operation of the sensor, affecting the sensor’s response and detection linear range [
38]. At the same time, the first generation of sensors often used platinum as the anode material; the platinum electrode needs to apply a high potential, resulting in some reducing molecules (such as ascorbic acid, acetaminophen, uric acid, and lactic acid) being easily oxidized. In summary, the sensitivity, detection range, and accuracy of the first-generation glucose sensor are not satisfactory. The second generation of enzymatic glucose sensors uses an artificial electron acceptor that replaces oxygen to pass electrons between the FAD center of GOx and the electrode surface [
39]. This measure can address the oxygen dependence of first-generation sensors and allow the sensor to have shorter response times, higher sensitivity, and a wider range of applications [
40]. Without the presence of a medium such as oxygen and an artificial electron acceptor, the third generation of enzymatic glucose sensors transfers electrons directly from the enzyme to the electrode, with a faster response rate and higher sensitivity [
41]. The achievement of direct transfer of electrons is difficult because the FAD is coated with a thick layer of protein. The rapid development of nanoscale materials with porous structure has significantly improved the kinetic performance and provided electrodes with a larger surface. These materials are particularly important for third-generation glucose biosensors because they could be constructed as electrodes that are able to capture and immobilize the enzyme, thus facilitating direct electron transfer from the enzyme to the electrode [
42].
Over the past 60 years, significant advancements have been made in the performance of enzymatic glucose sensors. Innovations in materials, structural designs, and electrochemical analysis techniques have unlocked the full potential of these sensors. The introduction of novel materials, such as porous substances, nanomaterials, and electrocatalytic compounds, has significantly enhanced the effectiveness of enzyme immobilization, minimized environmental interference, and accelerated electron transfer rates [
43,
44]. These improvements have collectively boosted the stability, sensitivity, and response speed of the sensors. However, challenges remain, including high production costs, limited stability, and intricate electrode architectures. Despite these drawbacks, enzymatic glucose sensors continue to hold commercial value due to the exceptional selectivity of enzymes for glucose, making them a practical and meaningful solution in the field. For instance, Yang et al. [
45] developed a highly sensitive glucose sensor based on a tapered optical fiber functionalized with GOx/AuNPs/GO. The catalysis of glucose is carried out by GOx, which is immobilized on the functionalized surface of the tapered fiber. GOx specifically reacts with glucose, catalyzing its conversion into gluconic acid and H
2O
2. This reaction alters the local refractive index of the sensing region, thereby inducing a redshift in the LSPR signal and enabling highly sensitive detection of glucose concentration.
3. Non-Enzymatic Electrochemical Glucose Sensor
The invention of non-enzymatic electrodes has promoted the development of glucose sensors. Non-enzymatic glucose sensors have excellent performance in chemical stability and rapid response in electrode material detection, due to their electrodes can directly oxidize glucose, bypassing the need for sensitive and usually complex enzymes [
46]. The three-electrode system is often used for electrochemical research, usually composed of WE, RE, and CE. Herein, RE is connected to WE via a test loop, while CE is connected to WE through a current loop. The four fundamental elements of electrochemical techniques are potential, impedance, current, and time. From these elements, several electrochemical measurement methods have been developed, including CV, LSV, EIS, DPV, and amperometric methods [
47,
48,
49,
50]. The incorporation of nanomaterials has further significantly enhanced the performance of non-enzymatic electrochemical glucose sensors. Their high specific surface area provides more catalytically active sites, effectively improving the adsorption and oxidation efficiency of glucose. Their excellent electrical conductivity facilitates rapid electron transfer between the electrode and glucose molecules during oxidation, thereby increasing the response speed and signal intensity. Meanwhile, nanomaterials exhibit good anti-interference capability against common interfering substances such as UA and AA, thus enhancing the selectivity of the sensor. By forming composites with CPs, carbon-based materials, or MOFs, nanomaterials can also construct multifunctional and highly stable electrode systems [
51,
52,
53]. This section describes the progress of noble metal materials and non-noble transition metal compound materials in a non-enzymatic electrochemical glucose sensor.
3.1. Noble Metal-Based Glucose Sensor
Noble metal nanomaterials can directly catalyze the oxidation of glucose, which makes glucose sensors based noble metal materials without the use of enzymes and avoids the problem of enzyme instability. Moreover, the unique structure of noble metal nanomaterials offers an increased surface area for loading and a higher density of active sites. These features enhance the absorption of glucose molecules and accelerate electron transfer, resulting in superior catalytic performance. As a result, these materials have driven the advancement of non-enzymatic electrochemical glucose sensors. By optimizing their structural properties and creating noble metal-based nanocomposites, these sensors achieve significantly enhanced sensitivity, specificity, and stability. In recent years, a variety of noble metal nanomaterials, including Au, Pt, and Pd, have been employed for electrocatalytic biosensing applications [
54,
55,
56].
3.1.1. Au-Based Glucose Sensor
Zhao et al. [
57] demonstrate a flexible non-enzymatic glucose sensor, composed of two gauzes and three electrodes. The schematic illustration of the flexible sensitive sensor is shown in
Figure 2a, and the actual situation of each part of the prepared sensor is demonstrated (from
Figure 2b–e). The AuNFs synthesized on the carbon cloth serve as WE for non-enzymatic glucose catalysis, and the researchers used CV and LSV to study the electrocatalytic principle of the AuNFs@CC electrode.
AuNWs possess numerous advantageous properties, including chemical inertness, excellent catalytic activity, numerous active sites, swift electron transfer, and outstanding biocompatibility; what is more, this nanomaterial with controllable morphology could be easily obtained and orderly positioned on conductive substrates [
58]. Due to the structure of AuNWs directly synthesized on SSWS having high performance in flexibility and conductivity, it could be applied for constructing wearable, flexible, and sensitive glucose sensors. As shown in
Figure 2f, the prepared integrated electrode was fabricated with v-AuNWs chemically grown on bendable SSWS. Zhou et al. [
59] presented an enzymeless glucose sensor with a v-AuNWs/SSWS electrode. Three types of v-AuNWs with different morphologies and lengths were synthesized by immersing SSWS electrodes, pre-seeded with gold nanoparticles, into the gold growth solution for durations of 5, 10, and 15 min. Specifically, the synthesized material with a reaction time of 5 minutes was named as v-AuNWs-5, with the corresponding electrode labeled as v-AuNWs-5/SSWS, and two other materials also followed the same nomenclature rule. The sensitivity of the glucose sensors constructed with these electrodes was separately estimated to be 50.5 µA mM
−1 cm
−2, 95.4 µA mM
−1 cm
−2, and 180.1 µA mM
−1 cm
−2. The detection limit for three glucose is calculated as 0.93 µM, 0.71 µM, and 0.65 µM. Overall, the v-AuNWs/SSWS glucose sensors exhibit high performance in sensitivity, low detection limit, and linear range.
Rahmania et al. [
60] have developed two types of non-enzymatic glucose sensors: one is composed of the gold AuNC and MeNTA, while the other is composed of AuNC and PPyNW, in which AuNC acts as a catalyst deposited on a metal substrate on a hole array template and a conductive polymer, respectively. The glucose sensors leverage the eco-friendly and easily accessible nature of AuNC, which can potentially enable faster sensing. The effective sensor-loading surface has a significant impact on the sensor performance. And the proposed AuNC/MeNTA and AuNC/PPyNW electrodes both adopt a 3D structure that enables a larger sensor-loading surface area and increases the quantity of deposited catalysts, thereby enhancing the performance of the sensors. The sensitivity of the AuNC/MeNTA glucose sensor is estimated to be 91.3 µA mM
−1 cm
−2, and the detection limit is calculated as 7.4 µM. It also demonstrates an excellent linear relationship in the range of 0.05 to 25 mM. In comparison, the AuNC/PPyNW glucose sensor has respective figures of 14.5 µA mM
−1 cm
−2, 48.2 µM, and a linear range from 0.05 to 10 mM.
Figure 2.
(
a) Schematic illustration of AuNFs@CC-based and flexible sensitive device [
57]. The front (
b) [
57] and back (
c) [
57] views of the fabricated flexible sensor. (
d) Configuration of the space layer-gauze, reference electrode-Ag/AgCl@CC, working electrode-Au NFs@CC, and counter electrode-CC [
57]. (
e) SEM image showing the working electrode with Au NFs grown on the surface of carbon cloth [
57]. (
f) Fabrication process of wearable v-AuNWs/SSWS glucose sensor [
59].
Figure 2.
(
a) Schematic illustration of AuNFs@CC-based and flexible sensitive device [
57]. The front (
b) [
57] and back (
c) [
57] views of the fabricated flexible sensor. (
d) Configuration of the space layer-gauze, reference electrode-Ag/AgCl@CC, working electrode-Au NFs@CC, and counter electrode-CC [
57]. (
e) SEM image showing the working electrode with Au NFs grown on the surface of carbon cloth [
57]. (
f) Fabrication process of wearable v-AuNWs/SSWS glucose sensor [
59].
3.1.2. Pd-Based Glucose Sensor
In recent years, Pd and its composite materials have garnered significant attention as highly effective electrocatalysts due to their exceptional electrocatalytic properties and cost-effectiveness. Tang et al. [
61] have proposed a non-enzymatic electrochemical glucose sensor with a PdNS-Cu/Cu
2O/FTO electrode. As illustrated in
Figure 3a, PdNS were synthesized on FTO modified with Cu/Cu
2O nanocomposites by the method of galvanic replacement reduction. The prepared electrodes show high electrochemical catalytic activity towards glucose oxidation. The researchers assess the performance of the glucose sensor by CV, LSV, and amperometry.
Eswaran et al. [
62] fabricate a flexible nanocomposite electrode of PI/Au-PAN/Pd for non-enzymatic glucose sensors. Due to the strong and synergistic metal–polymer interaction between the conducting PAN and Pd, the prepared electrode performs greatly in structural geometry, morphological views, and functional group. So it possesses excellent electrochemical properties, including high conductivity, rapid response, and high electron transfer rate. CPs are often applied in wearable, flexible electrodes. In this work, researchers synthesize a material compositing PANI and Pd NPs, which exhibits excellent electrochemical oxidizing properties and gives rise to strong and stable adherence with the electrode.
3.1.3. Pt-Based Glucose Sensor
Platinum is one of the most studied noble metals in the field of sensors and catalysts. Le et al. [
63] fabricated non-enzymatic glucose sensors through a one-step hydrothermal synthesis. The nanomaterials applied in this nanosensor are composed of 3D GOH and Pt NPs. Pt NPs functioned both as electrocatalysts for glucose oxidation and as spacers to prevent the agglomeration of graphene sheets, thereby increasing the surface area of the graphene oxide hydrogel. As illustrated in
Figure 3b, researchers investigated the impact of various platinum amounts in Pt/GOH on the sensitivity to glucose concentration. There was a positive correlation between the content of Pt NPs and sensitivity before 15 mg of HPt was used. However, the sensitivity of Pt/GOH decreased in the presence of 20 mg HPt for the agglomeration of Pt particles, which resulted in the loss of surface area and electrocatalytically active area. The synthesis of Pt/GOH is shown in
Figure 3c.
The application of platinum material as an electrocatalyst in glucose electrochemical sensors is limited by the active surface due to the active surface of Pt-based electrodes being easily poisoned by adsorbed intermediates, such as intermediates in the oxidation process of glucose, leading to reduced sensitivity and poor stability. To address these limitations, modifying Pt surfaces with ad-metals or exploring alternative electrode materials has emerged as a viable strategy. For instance, bimetallic nanomaterials containing Pt such as Pt-Pd have been shown to significantly enhance electrocatalytic performance for direct glucose oxidation.
Li et al. [
64] utilized platinum (Pt) to create Pt-Pd bimetallic nanostructures, comparing two distinct morphologies: Pt-Pd NWAs and Pt-Pd NTAs. Their findings demonstrated that Pt-Pd NTAs outperformed Pt-Pd NWAs in terms of catalytic activity, glucose oxidation, and non-enzymatic sensing capabilities. This superior performance was attributed to the larger active surface area and higher catalyst utilization efficiency of the nanotube structure. The ECSA of these materials was determined by analyzing the charge collected in the hydrogen adsorption/desorption region after correction. Pt-Pd NTAs possessed the largest ECSA (43.2 m
2g
−1Pt+Pd) and both of the proposed materials are larger than the flat Pt-Pd catalyst, highlighting the advantage of the nanotube morphology in providing more active sites.
Wang et al. [
65] employed Pt-containing bimetallic nanomaterials to develop catalysts and sensors for neutral GORs. By adjusting the concentration of Pt precursors, they synthesized various Pd@Pt NCs, and
Figure 3d shows the 3D illustrations of these morphologies. As the concentration of H
2PtCl
6 increased, the surface morphology evolved from Pd@Pt CNCC to Pd@Pt CNC, Pd@Pt CNCI, and finally to Pd@Pt SNC; what is more, all of these structures were effective in electrocatalysis for neutral GORs.
Figure 3.
(
a) The fabrication of PdNS-Cu/Cu
2O electrodes by the electrical replacement method [
61]. (
b) Sensitivity of GOH and Pt/GOH at various HPt contents [
63]. (
c) Schematic diagram of Pt/GOH synthesis [
63]. (
d) The 3D illustrations of Pd@Pt CNCC, Pd@Pt CNC, Pd@Pt CNCI, and Pd@Pt SNC [
65].
Figure 3.
(
a) The fabrication of PdNS-Cu/Cu
2O electrodes by the electrical replacement method [
61]. (
b) Sensitivity of GOH and Pt/GOH at various HPt contents [
63]. (
c) Schematic diagram of Pt/GOH synthesis [
63]. (
d) The 3D illustrations of Pd@Pt CNCC, Pd@Pt CNC, Pd@Pt CNCI, and Pd@Pt SNC [
65].
3.2. Non-Noble Transition Metal-Based Glucose Sensor
Non-noble transition metals and their compounds are widely employed in creating highly effective non-enzymatic glucose sensors. Positioned in the third row of the periodic table, these metals are prized for their cost-effectiveness and swift interaction with glucose molecules, which ensures exceptional sensitivity. In recent years, a multitude of studies have explored the use of non-noble transition metals in glucose detection. This review will highlight the latest progress in non-enzymatic glucose sensors that leverage electrochemical methods, with a particular emphasis on Ni, copper Cu, iron Fe, and cobalt Co [
66,
67,
68].
3.2.1. Ni and Its Compounds
Imanzadeh et al. [
69] have synthesized a novel nanocomposite material combining NiO with rGO: NiO/C@rGO. The synthesis process is illustrated in
Figure 4a. Herein, rGO could be a matrix for incorporating Ni-based compounds to enhance their performance in glucose sensing. The outstanding electrochemical performance of the prepared glucose sensors is attributed to the catalytic properties of NiO and the high electrical conductivity and large surface area of rGO. Moreover, they determined glucose concentrations in human serum samples by the biosensor based on NiO/C@rGO/GCE. Similarly, Cui et al. [
70] used a 3D graphene skeleton as an effective carrier for loading redox probes, including Ni and Cu micro-/nanostructured redox probes. The porous structure of the 3D graphene provided a large surface area, enhancing electrolyte–electrode contact and facilitating glucose adsorption, thereby achieving low detection limits and high sensitivity. The fabricated Cu/Ni/graphene/Ta electrode exhibited high electrocatalytic activity for glucose oxidation.
3.2.2. Cu and Its Compounds
Copper-based electrodes and nickel-based electrodes have similar functions in the electrochemical oxidation of glucose. CuO nanomaterials, an efficient material for enzymeless glucose detection, have been utilized in sensors. The CuO-based sensors show outstanding abilities in sensitivity, chemical stability, and electrochemical properties towards glucose.
Taşaltın et al. [
71] synthesized Cu/CuO/SiO
2 nanospheres using an ultrasonic-assisted method. SEM analysis of the prepared Cu core–shell nanosphere revealed a homogeneous distribution with minimal agglomeration, as shown in
Figure 4b. The large specific surface area and numerous active sites of the Cu core–shell nanospheres facilitated glucose molecule transport by providing multiple channels for adsorption. Cu core–shell nanospheres were applied in a non-enzymatic glucose electrochemical biosensor, which demonstrated high sensitivity (8 µA mM
−1 cm
−2) for detecting glucose in the concentration range of 3–12 mM. Mesoporous materials have several advantages, such as a higher adsorption capacity and a faster diffusion of the analyte molecules through the pores.
Solhi et al. [
72] have produced mesoporous copper oxide powders with various sizes by controlling the reaction time of 5, 7, and 10 s, respectively. By reducing the reaction time, they produced smaller particle sizes. And the minimal particle had an average size of about 63 nm, with a reaction time of 5 s, and their TEM images are shown in
Figure 4c. Furthermore, the electrode modified with prepared nanomaterials demonstrated wonderful glucose sensing capability in the alkaline pH range with a sensitivity of 733.3 µA mM
−1 cm
−2, which is better than that of a non-porous copper oxide electrode (371 µA mM
−1 cm
−2); it also exhibited a reasonable detection limit of 0.05 µM.
The core–shell structure not only protects copper nanoparticles from oxidation but also facilitates efficient electron transfer from the copper core to the carbon layer. Leveraging this advantage, Ye et al. [
73] developed a sensor using Cu@C core–shell nanocubes as the active electrocatalyst. These nanocubes, characterized by a large specific surface area and improved electrical conductivity, were synthesized from cubic Cu
2O nanoparticles and water-soluble PVP. The assembly process for the well-aligned Cu@C core–shell nanocubes is illustrated in
Figure 4d, while the proposed electrocatalytic oxidation mechanism for the Cu@C/Nafion/GCE system is depicted in
Figure 4e.
Wang et al. [
74] proposed a non-enzymatic glucose sensor based on Pt
1/Cu
2O@CF. For the Cu
2O@CF, Cu
2O nanowires were dispersed on a copper foam substrate to provide a large surface area and enhance the number of active sites, and a SAC of Pt was embedded on Cu
2O@CF through a simple electrochemical deposition process, which is beneficial to the adsorption of glucose molecules. The fabrication process of Pt
1/Cu
2O@CF is shown in
Figure 4f. The prepared possessed a high sensitivity of the biosensor (31.55 mA mM
−1 cm
−2) and a much lower LOD (1 µM).
3.2.3. Fe and Its Compounds
Iron stands out as a unique electron facilitator due to its reversible Fe3+/Fe2+ redox pair. Various iron oxides, such as Fe2O3 and Fe3O4, are commonly synthesized and widely used in analytical research because metallic iron is unstable in air.
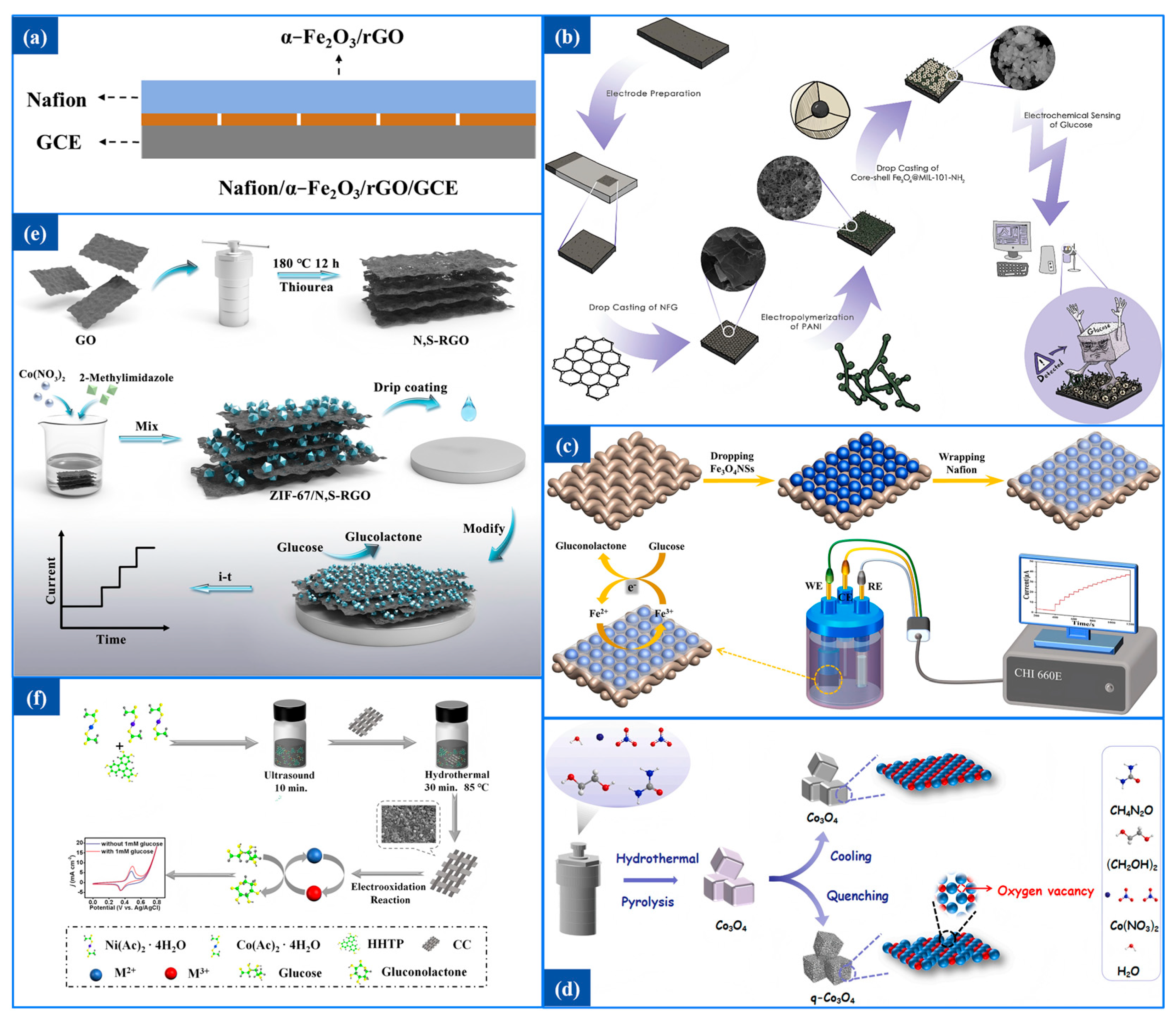
α-Fe
2O
3, with its variable valency, is capable of participating in redox reactions and offers multiple benefits such as environmental friendliness, low cost, high chemical and electrochemical stability, and excellent redox reversibility. Yadav et al. [
75] utilized the biosynthesis method to fabricate the α-Fe
2O
3/rGO nanocomposite using Syzygium aromaticum or clove extract for the first time. This biosynthetic approach offers numerous benefits, including being cost-effective, rapid, non-toxic, feasible, and utilizing abundant natural resources. Moreover, rGO enhances the conductivity of the prepared nanomaterials. This α-Fe
2O
3/rGO nanocomposite with a high surface-to-volume ratio was used to manufacture Nafion/α-Fe
2O
3/rGO/GCE, and the schematic diagram of Nafion/α-Fe
2O
3/rGO/GCE is given in
Figure 5a.
Choudhary et al. [
76] created a γ-Fe
2O
3-modified exfoliated graphite-based electrode. This prepared flexible paper electrode, utilized in a non-enzymatic glucose sensor, shows a large surface-to-volume ratio, a high degree of porosity, and lower charge transfer resistance. Researchers also prepared an ExGCP electrode that was compared with the γ-Fe
2O
3@ExGCP electrode. The glucose sensor characteristics of two electrodes were analyzed and compared through CV, EIS, and chronoamperometry. As a result, the electrode with ExGCP exhibited a higher sensitivity of 7.1 µA mM
−1 cm
−2, which is twice that of ExGCP. This improvement is attributed to the larger surface area provided by γ-Fe
2O
3 NPs. In addition, the LOD of the γ-Fe
2O
3@ExGCP electrode was 520 µM.
In the realm of glucose sensors, Fe
3O
4 has garnered significant attention due to its unique physicochemical properties, including excellent biocompatibility, nontoxicity, and ease of synthesis. Fe
3O
4 nanoparticles can catalyze the decomposition of hydrogen peroxide and directly electrocatalyze glucose oxidation through the redox reaction of Fe
3+/Fe
2+. Ghaffarirad et al. [
77] prepared a novel conductive nanocomposite including NFG, conductive PANI, and core–shell nanoparticles (Fe
3O
4@-MIL-101-NH
2). The presence of Fe
3O
4@MIL-101-NH
2 could improve the sensitivity of the electrode toward the oxidation of glucose. Then the prepared nanocomposite was coated on a graphite sheet to fabricate the electrode. The process of non-enzymatic sensor preparation and nanocomposite is presented in
Figure 5b.
Zhou et al. [
78] adjusted the morphology of hollow Fe
3O
4 NSs to improve glucose sensing performance due to the significant impact of nanostructure morphology on electrocatalytic capacity.
Figure 5c displays the construction process of the Nafion/Fe
3O
4NSs/SSWS glucose sensors. Fe
3O
4 NSs were synthesized via a solvothermal method, and the controllable morphologies were achieved by regulating the Fe
3+ concentration in the growth solution. These nanospheres were then deposited onto flexible SSWS fibers for use in non-enzymatic electrochemical glucose analysis. The as-synthesized Fe3O4NSs were marked as Fe
3O
4 NSs-1, Fe
3O
4 NSs-2, Fe
3O
4 NSs-3, Fe
3O
4 NSs-4 and Fe
3O
4 NSs-5. As the Fe
3+ concentration increased, the morphology of the Fe3O4NSs became rounder and rougher, exhibiting a surface close to a Gaussian random rough surface, which is beneficial for the performance of electrochemical sensors. So the Nafion/Fe
3O
4NSs-5/SSWS glucose sensor showed optimal performance in glucose detection.
3.2.4. Co and Its Compounds
Cobalt-based electrode materials have been widely applied in non-enzymatic glucose sensors for their low cost and higher effectiveness. Wang et al. [
79] prepared a novel cobalt-based nanomaterial named Hollow-Co
3O
4/GO through a simple chemical composition and annealing process. GO with excellent adsorption capability could improve the overall conductivity of Co
3O
4/GO materials. During the annealing process, substantial loads were generated on the surface of Co
3O
4, creating active sites that facilitated the oxidation reaction and enhanced catalytic activity towards glucose.
Yang et al. [
80] regulated the electrocatalytic activity of Co
3O
4 by generating oxygen vacancies on the material surface through quenching. Two different types of Co
3O
4 nanomaterials were synthesized, which were marked as Co
3O
4 and q-Co
3O
4, respectively.
Figure 5d showed the synthesis of these materials. The quenching treatment significantly influenced the surface structure of q-Co
3O
4 nanomaterials, leading to the accumulation of oxygen vacancy defects on their surface. As a result, q-Co
3O
4 exhibited stronger electrocatalytic ability and higher sensitivity for glucose detection compared to q-Co
3O
4.
The design of modified electrode materials plays a crucial role in improving the performance of glucose sensors. MOFs, known for their controllable structures, large surface areas, and tunable pore sizes, represent a highly promising category of porous materials. However, their relatively poor charge transfer efficiency often hinders their electrochemical sensing capabilities. This challenge can be effectively overcome by integrating MOFs with conductive materials, thereby enhancing their overall performance.
By an in situ synthesis method, Zhou et al. [
81] prepared co-based MOF functionalized N,S-RGO heterostructures (ZIF-67/N,S-RGO). The schematic illustration of the preparation of N,S-RGO, ZIF-67/N,S-RGO/GCE, and electrochemical response to glucose is given in
Figure 5e. The synergistic action of ZIF-67 and N,S-RGO not only provided more effective active sites and a larger surface area, but also improved the efficiency of electron transfer and electrocatalysis.
Xu et al. [
82] proposed a Ni/Co(HHTP)MOF/CC glucose sensor by direct growth of conductive Ni/Co bimetal MOF on carbon cloth via a facile hydrothermal method.
Figure 5f demonstrated the synthetic process of the Ni/Co(HHTP)MOF/CC. The nanocomposite could provide a larger surface area, more effective active sites, faster charge transfer, and electrocatalytic performance because synergic catalytic effect of Ni and Co elements and excellent conductivity between Ni/Co(HHTP)MOF and CC. Similarly, ZnCo
2O
4@MOF, a novel MOF template-derived ZnCo
2O
4 composite, was fabricated by Divyarani et al. [
83]. These nanocomposites were utilized to construct a glucose sensor with high sensitivity, long-term stability, and excellent anti-interference ability.
Figure 5.
(
a) Schematic diagram of modified Nafion/α-Fe
2O
3/rGO/GCE [
75]. (
b) Fabrication process scheme of the non-enzymatic glucose sensor [
77]. (
c) Construction and electrochemical test of Nafion/Fe
3O
4NSs/SSWS glucose sensor [
78]. (
d) Synthesis of Co
3O
4 and q-Co
3O
4 nanomaterials [
80]. (
e) Schematic illustration of the preparation of N, S-RGO,ZIF-67/N, S-RGO/GCE, and electrochemical response to glucose [
81]. (
f) Synthetic process of Ni/Co(HHTP)MOF/CC [
82].
Figure 5.
(
a) Schematic diagram of modified Nafion/α-Fe
2O
3/rGO/GCE [
75]. (
b) Fabrication process scheme of the non-enzymatic glucose sensor [
77]. (
c) Construction and electrochemical test of Nafion/Fe
3O
4NSs/SSWS glucose sensor [
78]. (
d) Synthesis of Co
3O
4 and q-Co
3O
4 nanomaterials [
80]. (
e) Schematic illustration of the preparation of N, S-RGO,ZIF-67/N, S-RGO/GCE, and electrochemical response to glucose [
81]. (
f) Synthetic process of Ni/Co(HHTP)MOF/CC [
82].
3.3. Multimetallic Material-Based Glucose Sensor
The application of multimetallic materials in non-enzymatic electrochemical sensors has attracted increasing attention. Compared to single-metal materials, these materials exhibit superior electrocatalytic performance, electron transfer efficiency, and structural stability due to their unique synergistic effects [
84,
85,
86].
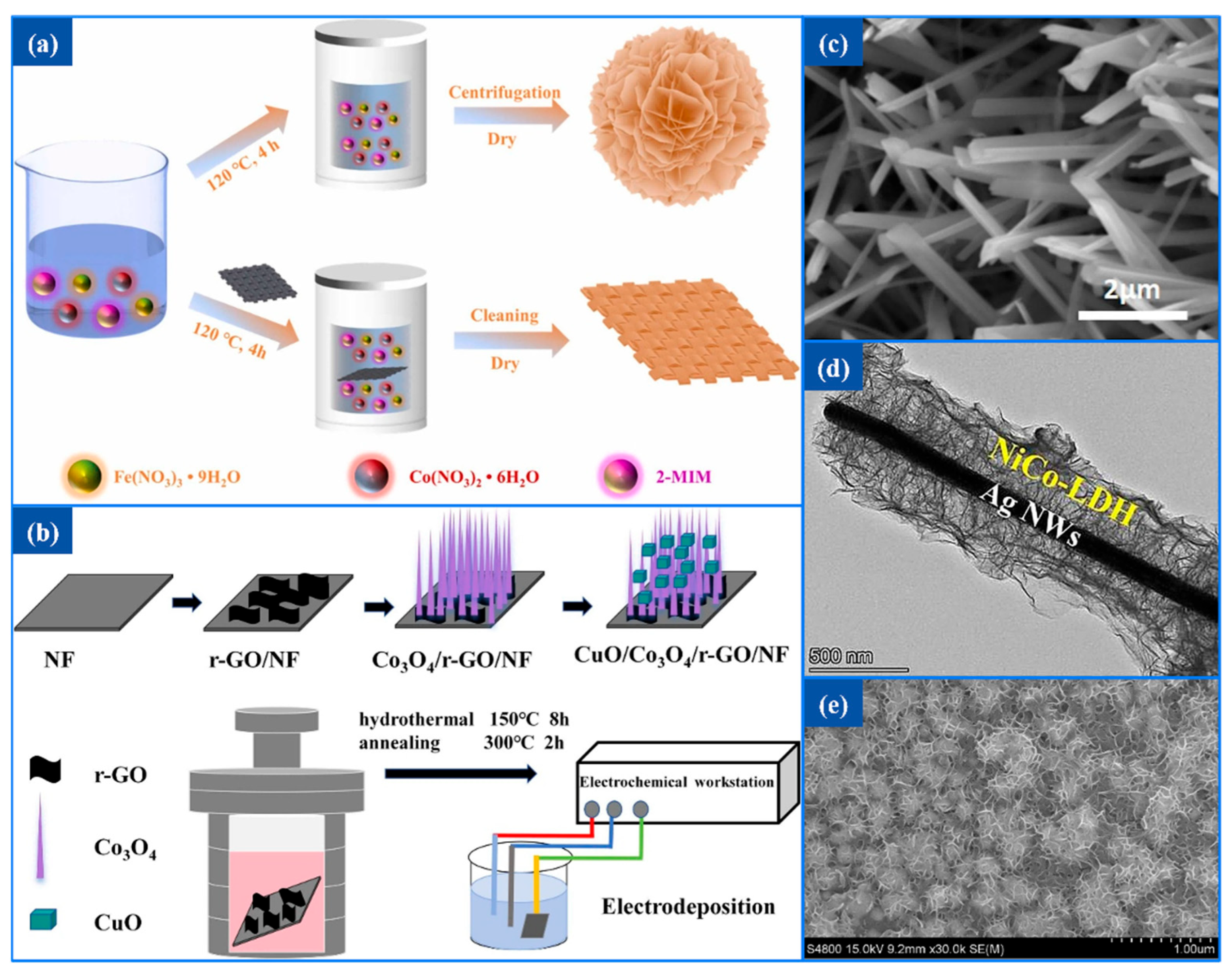
Liu et al. [
87] synthesized Co
0.95Fe
0.05-ZIF/CC with an open flower-like nanosheet structure by introducing a small amount of Fe
3+ ions into Co-ZIF, as illustrated in
Figure 6a. The incorporation of Fe
3+ ions disrupted the original rhombohedral dodecahedral structure, significantly increasing the specific surface area and the exposure of metal active sites. This structural modification improved electron conductivity and ion diffusion, thereby enhancing the overall sensing performance of the material.
Tao et al. [
88] fabricated a bimetallic oxide electrode, CuO/Co
3O
4/r-GO/NF, with the synthesis process illustrated in
Figure 6b. CuO, a photosensitive semiconductor material, can catalyze the oxidation of glucose under alkaline conditions. Its photoelectrical activity helps enhance the photocurrent response, thereby improving the catalytic efficiency. In the composite electrode, Co
3O
4 serves as an intermediate structural layer, growing in the form of nanorods or nanowires on the nickel foam substrate modified with rGO. Through hydrothermal synthesis followed by high-temperature annealing, a three-dimensional porous network structure with high specific surface area is formed. This structure provides a rough and highly adherent surface, enhancing the mechanical stability and spatial support capability of the composite material.
By partially substituting Ni
2+ in Ni LDH with Cu
2+, Ni-Cu LDH can be synthesized. Due to the similar lattice constants of Cu and Ni, the chemical composition is easily tunable, which facilitates the optimization of electrochemical performance. Zhao et al. [
89] fabricated a Ni-Cu LDH@Cu(OH)
2 NWs/CuF electrode material using copper foam as a conductive substrate. Vertically aligned Cu(OH)
2 nanowires were grown in situ on the surface to construct an ordered one-dimensional nanostructured scaffold. Subsequently, a Ni-Cu LDH shell layer was deposited on the nanowires, forming a morchella-like folded morphology with a highly porous three-dimensional structure. This significantly increased the reactive interface, exposed more catalytically active sites, and enhanced glucose molecule adsorption and diffusion, thereby improving catalytic performance. As shown in
Figure 6c, Cu(OH)
2 nanowires uniformly cover the surface of CuF. Building on previous work, Huang et al. [
90] further optimized the support framework and proposed a novel NiCo-LDH@Ag NWs composite material.
Figure 6d illustrates the resulting core–shell structure: Ag NWs serve as a highly conductive one-dimensional backbone, effectively enhancing electron transport efficiency. NiCo-LDH nanosheets uniformly coat the Ag NWs, forming a three-dimensional core–shell structure characterized by continuous coverage and interconnection of ultrathin layers. This architecture substantially increases the specific surface area and the exposure of electrocatalytically active sites.
Similarly, Shen et al. [
91] prepared a NiCo-LDH@Au/Cu material with a honeycomb-like surface, as shown in
Figure 6e. In this system, the introduction of Au NPs and the CuF substrate together constructed an efficient three-dimensional conductive structure. The porous 3D architecture of the copper foam provides a large specific surface area and a stable structural framework, which is beneficial for material loading and electron transport. The Au nanoparticles significantly enhance the electrode’s conductivity and electrocatalytic activity, promoting the oxidation of glucose. The synergistic effect of the two components effectively improves the sensor’s sensitivity, stability, and selectivity.
Liu et al. [
92] used a Cu-Ag-Zn disk electrode as a precursor material to construct a nanoporous Cu-Ag thin film with high electrocatalytic activity through a spontaneous dealloying method. During the immersion of the electrode in an alkaline KOH solution, Zn was completely dissolved and Cu was partially dissolved, leading to the gradual evolution of a highly porous structure on the electrode surface. Zn only participates in structural reconstruction and does not contribute to the oxidation of glucose. Due to its higher electronegativity compared to Cu, Ag can attract electrons from adjacent Cu atoms, resulting in a decreased electron density on the surface of Cu species and thereby enhancing their electrocatalytic activity toward glucose oxidation. Additionally, Ag interacts electronically with Cu through the formation of interfaces such as Ag-Cu, Ag-CuO, and Ag-CuOOH, which further improves the catalytic efficiency of the electrode in glucose oxidation reactions.
4. Fluorescent Glucose Sensor
Electrochemical sensors frequently face issues such as interference from competing electroactive substances, high overpotentials, and the necessity for controlled laboratory environments, including highly alkaline solutions. Moreover, certain electrochemical biosensors depend on additional reducing or oxidizing agents, such as dyes or enzymes, which adds complexity to their design and functionality. By comparison, fluorescence detection analysis is a widely used and promising sensing method with strong specificity, high sensitivity, and high efficiency. Fluorescence-based glucose detection methods not only avoid the use of enzymes but also effectively circumvent the aforementioned limitations of electrochemical sensors [
93,
94].
4.1. Fluorescence Mechanism
Fluorescence-based detection has been extensively applied for analyzing diverse analytes, with the “turn-off” and “turn-on” modes being the predominant strategies. In the turn-off approach, glucose commonly acts as a quencher, initiating the fluorescence quenching process of the donor fluorophore and leading to a decline in fluorescence emission. FRET is a phenomenon of energy transfer between fluorescent molecules [
95]. Under specific conditions, the donor fluorescent molecule absorbs a photon and is excited to a higher electronic energy state, producing an oscillating dipole that resonates with the acceptor fluorescent molecule. After the acceptor fluorescent molecule absorbs the energy released by the former, it emits fluorescence, thus achieving the transfer of energy to a neighboring molecule [
96].
In the literature [
97], CQDs function as the donor fluorophore, with glucose being the acceptor. As shown in
Figure 7a, upon exposure to UV light, photoexcited electrons from the VB of CQDs may transition to the HOMO of glucose. Higher glucose concentrations enhance the probability of electron acceptance. Furthermore, the intensified FRET effect results in more electrons being transferred from CQDs to glucose, diminishing the number of electrons available for fluorescence emission and causing a steady decrease in peak intensity. Thus, a change in fluorescence intensity can respond to a change in glucose concentration, and the change could be measured by using customized smartphone applications. In the literature [
98], a non-enzymatic “switch-OFF” fluorescence sensing mechanism was achieved via using a CQD/Dex/PVA nanocomposite. Upon the addition of glucose to the CQD/Dex/PVA composite, the fluorescence intensity diminishes. This reduction is attributed to the robust interaction between the hydroxyl groups of glucose and those within the CQD/Dex/PVA composite.
In the “turn-on” mode, a commonly used fluorescence “switch-on” strategy is the turn on–off–on method. This technique starts by utilizing quenchers to suppress the fluorescence of the donor fluorophore. Afterward, the introduction of glucose to the system activates the removal of the quenching effect, thereby restoring the fluorescence of the donor fluorophore. The fluorescent nanosensor described in the literature [
99] introduced a novel and straightforward fluorescence “turn-on” mechanism, which directly enhances the fluorescence of CDs in response to H
2O
2. This approach eliminates the need for the complex process of fluorescence quenching and subsequent recovery typical in conventional “switch-on” mode.
Figure 7b illustrates the fluorescence turn-on detection of H
2O
2 and glucose using green fluorescent CDs. Additionally, a new nanomaterial, S and N co-doped graphene quantum dots modified by boric acid, was proposed in the literature [
100] for glucose detection. The functional mechanism of the (B)/S,N-GQDs nanosensor is demonstrated in
Figure 7c. The PL intensity of the prepared materials is enhanced by the restriction of the intramolecular motions by the (B)/S,N-GQDs-glucose interactions.
4.2. Fluorescence Sensing in Glucose Detection
With their high sensitivity, rapid response, biocompatibility, and non-invasive nature, fluorescent sensors are well-suited for portable and real-time glucose monitoring, particularly in diabetes management.
In recent years, CDs have gained recognition as highly promising zero-dimensional fluorescent nanomaterials for sensing applications. They are widely appreciated for their straightforward synthesis, exceptional photostability, biocompatibility, minimal toxicity, excellent water dispersibility, and the abundance of raw materials required for their production. Hu et al. [
99] have introduced an innovative “turn-on” fluorescence sensor designed to detect glucose as well as H
2O
2. This sensor utilizes green fluorescent CDs and exhibits remarkable selectivity and sensitivity for glucose detection, achieving a detection limit of 0.12 µM. Their research makes a significant contribution to the field by broadening the potential uses of carbon nanomaterials, particularly those with enhanced fluorescence emission characteristics. Kansay et al. [
97] presented a novel fluorescent nanosensor probe based on boronic acid-functionalized and heteroatom-doped carbon CQDs. This fluorescence probe, along with paper-based analytical devices and a hydrophilic cotton thread-based microfluidic channel, collectively form a wearable, disposable, and non-enzymatic fluorescent nanosensor that is shown in
Figure 7d.
Masteri-Farahani et al. [
100] designed a fluorescent nanosensor for glucose detection using boric acid-modified S,N-GQDs. Upon the addition of glucose, the fluorescence of the boric acid-functionalized S,N-GQDs was enhanced at 455 nm. This fluorescence response offers a simpler, more cost-effective, and efficient alternative to prior boric acid-based fluorescent sensing methods. Similarly, Patra et al. [
98] developed a novel method to produce highly functional, dispersible, and stable CQDs from aloe vera through carbonization. They used a simple solution-mixing technique to prepare CQD/Dex/PVA nanocomposites. These nanocomposites demonstrated high selectivity for glucose detection in human serum, effectively differentiating glucose from other similar sugars like fructose, galactose, mannose, arabinose, and xylose. By combining dextran, PVA and CQD, they designed a highly sensitive and selective biosensor with improved accuracy and stability. Overall, fluorescent nanosensors exhibit several notable advantages in glucose detection. They operate without enzymes, offering excellent chemical stability and prolonged operational life. High specificity arises from well-designed fluorophore-glucose interactions, while their sensitivity benefits from strong signal amplification. Additionally, the compatibility of fluorescent probes with smartphone-based platforms facilitates real-time, portable, and non-invasive glucose monitoring. These merits make fluorescence-based sensing a promising strategy for next-generation glucose diagnostics [
101,
102,
103].
5. Colorimetric Glucose Sensor
Similarly to fluorescent glucose sensors, colorimetric sensors also belong to the category of optical sensors. Due to their ease of operation and intuitive response, colorimetric sensors have received widespread attention in the field of biological analysis, such as glucose detection. In recent years, with the development of nanomaterials, the introduction of nanostructures into colorimetric sensors has greatly enhanced their sensitivity, selectivity, and integrability, becoming one of the key approaches to improving sensor performance [
104,
105].
5.1. Colorimetric Detection Mechanism
The principle of colorimetric detection is to indirectly reflect the concentration of the target analyte by observing the color change after a specific reaction with the analyte. It is a common and effective method in the field of optical sensing. Typically, the detection is based on a classic two-step reaction mechanism: first, H
2O
2 is generated through an enzyme-catalyzed reaction; then, H
2O
2 participates in a subsequent colorimetric reaction. The extent of the color change in the chromogenic substrate indirectly reflects the glucose concentration. After the color reaction occurs, glucose concentration can be determined using techniques such as UV-Vis absorption spectroscopy and DIC [
106,
107]. The former usually monitors the absorbance change in the colored product at a specific wavelength, with the absorbance intensity showing a linear or quasi-linear relationship with glucose concentration; the latter uses a smartphone or camera to capture an image of the reaction system, and the glucose concentration is correlated to the RGB color component changes extracted from the image, reflecting the depth of color and establishing a corresponding relationship between concentration and color parameters [
108,
109].
5.2. Colorimetric Sensing in Glucose Detection
Nanomaterials, such as metal oxide nanoparticles, metal nanoparticles, and carbon-based nanomaterials, are employed in colorimetric sensors to significantly enhance the catalytic activity, response sensitivity, and adaptability to complex detection environments [
110]. In most designs, nanomaterials are involved in the second step of the reaction and are used in the subsequent colorimetric reaction after H
2O
2 is generated from glucose oxidation, thereby producing a color change [
111].
Saranchina et al. [
112] embedded Au
0 NPs into a PMM matrix to form a composite sensor material, PMM-Au
0, which significantly enhanced the stability of the gold nanoparticles in air and solution. Upon reacting with a glucose solution, H
2O
2 generated from the enzymatic oxidation of glucose oxidizes I
− to I
2. The resulting I
2 reacts with Au NPs, leading to surface etching and a shift in the SPR absorption peak. Consequently, the color of the PMM-AuI
2 sensor changes to red-purple. The color change is captured using a smartphone camera, and RGB values are extracted using the ColourGrab app to enable rapid quantitative analysis of glucose concentration.
Basiria et al. [
113] developed a sensor based on AgNPs-Fe
2+-H
2O
2 for glucose detection. Glucose is first oxidized to produce H
2O
2. The H
2O
2 then undergoes a Fenton reaction to generate highly oxidative hydroxyl radicals, which react with AgNPs, oxidizing Ag(0) to Ag+. This leads to a decrease in the SPR intensity of the AgNPs, causing the solution color to change from yellow to colorless. The glucose concentration is quantitatively analyzed using UV-Vis spectroscopy.
Figure 8a shows that the change in absorbance of the sensor exhibits a good linear relationship with glucose concentration in the range of 0–25 μM.
Ly et al. [
114] developed a Fe
3O
4@PDA-based colorimetric sensor. Upon the addition of exogenous H
2O
2, Fe
3O
4 catalyzes the decomposition of H
2O
2 to generate hydroxyl radical, which oxidizes TMB into TMB+. After the introduction of glucose, the glucose molecules bind to the recognition cavities on the MIP surface within the PDA layer, hindering the contact between H
2O
2 and the catalytic sites of Fe
3O
4. This suppresses the generation of hydroxyl radicals and the subsequent oxidation of TMB, causing the solution color to gradually fade from blue to nearly colorless, as shown in
Figure 8b.
Ngo et al. [
115] synthesized a nanocomposite material composed of MnFe
2O
4 and g-C
3N
4, where g-C
3N
4 provided a high specific surface area and excellent electron transport properties, thereby enhancing the overall catalytic efficiency. MnFe
2O
4 played a key role in the generation of hydroxyl radicals. As shown in
Figure 8c, with increasing glucose concentration, the solution gradually turned blue in color. Furthermore, the study developed a detection platform that integrates smartphone-based image analysis.
Figure 8d illustrates that the platform utilizes an ordinary smartphone camera to capture images of the reaction solution and processes the images using a built-in artificial neural network-based application to analyze RGB data. This enables visual identification of the color change and quantitative detection of glucose concentration.
Currently, most studies focus on chromogenic reactions, in which the color change is achieved by either promoting or inhibiting the subsequent reactions of H
2O
2. Clearly, H
2O
2 plays a central role in colorimetric glucose sensing; however, it must be either generated enzymatically via GOx or supplied externally. In contrast, Pathak et al. [
116] proposed a non-enzymatic dual-mode colorimetric sensing strategy using a NiO nanofilm as the working electrode. Under an applied positive bias (+0.7 V), the NiO film is electrochemically oxidized from Ni(OH)
2 to NiOOH, accompanied by a distinct color change from light to dark (black/brown). Upon glucose injection, NiOOH is reduced back to Ni(OH)
2, resulting in a reversible color transition from dark to light. This process is accompanied by a significant change in optical transmittance at 500 nm, increasing from approximately 25% to 75%, corresponding to a maximum optical contrast (ΔT%) of about 66%. By measuring the transmittance and referencing a standard calibration curve, glucose concentration can be quantitatively determined, enabling non-enzymatic and rapid colorimetric detection.
6. Results and Discussion
Table 1,
Table 2 and
Table 3, respectively, demonstrate the key performance parameters of non-enzymatic electrochemical glucose sensors based on noble metal, non-noble transition metal, and multimetallic materials, including sensitivity, LOD, and linear detection range. The results indicate that the non-enzymatic electrochemical sensors mentioned in this review generally exhibit excellent performance in glucose detection, characterized by high sensitivity, a wide linear range, and a low detection limit. Moreover, their compatibility with various sample types and practical application environments has also attracted considerable research interest. The sensors presented in tables, along with the fluorescence and colorimetric sensors mentioned, primarily focus on non-enzymatic detection for monitoring glucose levels in human body fluids, with blood glucose monitoring being the predominant application. They also encompass detection in other fluids such as sweat, saliva, and tears. Most sensors were validated using human serum, plasma, or whole blood samples through spiking and recovery experiments, and their results were compared with commercial glucose meters, demonstrating good accuracy and clinical application potential. Some sensors have been extended to the detection of glucose in sweat, saliva, or even tears, with a portion developed into wearable platforms (such as flexible electrodes or paper-based colorimetric or fluorescent sensors) that can be attached to the skin and enable real-time monitoring via smartphones or other devices. Although the intended use of these sensors is detection in real biological fluids, the majority of experimental validations were conducted in standard buffer solutions (e.g., NaOH, PBS) or artificial body fluids, which are relatively idealized environments and lack a comprehensive evaluation of anti-interference performance in complex matrices. Some studies have carried out validation in real serum or sweat samples, achieving high recovery rates and consistent results. In addition, a limited number of studies ([
78,
82]) explored the application in beverage glucose detection, indicating their potential for use in non-biological glucose sensing applications.
Non-enzymatic electrodes are capable of sensitive responses at the micromolar level. Composite materials composed of metals and other functional components maintain high sensitivity while achieving broader detection concentration ranges, thereby enhancing the practicality and applicability of the sensors. In addition, multi-metallic composite materials through synergistic catalytic effects between different metals (such as Ni-Co, Ni-Cu, and Ni-Co-Au) demonstrate superior performance in electron transfer efficiency and active site exposure, as well as in structural stability and anti-interference capability. In the absence of enzymes, the electrode becomes the core factor determining the performance of a non-enzymatic electrochemical sensor. The reasons are that in non-enzymatic systems, glucose recognition and catalytic oxidation depend on the electrode material surface properties, electrocatalytic activity, and electron transfer efficiency. Therefore, designing electrode interfaces with high reactivity, good conductivity, and strong selectivity is essential for achieving high-performance glucose detection. The use of optimized electrodes can significantly improve sensor performance, and the rapid development of nanotechnology has provided new approaches and strategies for tailoring electrode materials.
To further enhance the performance of nanostructured electrodes, researchers have developed and applied a variety of materials, including expensive transition metals, non-noble metal oxides, multi-metallic composites, MOF materials, CPs, and carbon-based materials. These materials possess advantages such as strong catalytic activity, tunable structures, and large specific surface areas, making them widely used in the construction of high-performance non-enzymatic glucose sensors. The rational selection and structural design of electrode materials are key to achieving highly sensitive and stable glucose detection.
By changing the nanostructure of the material to have a larger sensor load surface area to provide more active sites, more active sites increase the contact between the sensor electrodes and glucose molecules, resulting in higher sensitivity and faster response speed. For example, the catalytic performance of the sensor is improved by adjusting the topology of the noble metal nanomaterials to have a larger specific surface area. In addition, the use of nanocomposite materials is also a common strategy to enhance sensor performance. The structural and interfacial complementarity between different types of materials helps provide more attachment sites, improves electron transfer rates and overall conductivity, thereby enabling the development of glucose sensors with superior performance.
Non-precious metal electrocatalysts based on Ni, Cu, Fe, and Co are very active in glucose sensing in an alkaline environment, have fast electrochemical technology, and produce high sensitivity that is sufficient to measure glucose in most samples. And, they have a poor response to common distractors, such as DA, AA, UA, AP, and other sugars. It is worth mentioning that non-precious transition metals are much cheaper and widely available than precious metals such as platinum. Multi-metallic composite materials exhibit superior performance due to the synergistic catalytic effects among their constituent elements. By rationally combining different metals (Ni-Co, Ni-Cu, or Ni-Co-Au), these composites can effectively modulate electronic structures, optimize reaction pathways, and increase the density of active sites and electron transfer efficiency. Such materials demonstrate significant advantages in enhancing catalytic activity, anti-interference capability, and electrode stability, and have become a key focus in the design of non-enzymatic electrode materials in recent years.
Optical sensing, particularly fluorescence and colorimetric sensors, has also shown significant development potential in non-enzymatic glucose detection. Carbon-based nanomaterials, such as CDs and GQDs, serve as important building blocks for fluorescence sensing platforms due to their excellent biocompatibility, tunable photoluminescence properties, and surface functionalization capability. Through modification strategies such as doping, surface functionalization, or composite formation with polymers, the structure of these materials can be tailored, their interactions with glucose molecules enhanced, and their stability and biocompatibility improved. These enhancements promote more efficient glucose adsorption and electron transfer, thereby increasing the sensitivity and selectivity of the sensors. Because of its fluorescence characteristics, the fluorescent sensor can provide a high sensitivity of detection, while the characteristic emission spectrum of the specific fluorophore guarantees the high specificity of the sensor.
Colorimetric sensors achieve visual glucose detection through chromogenic reactions and offer advantages such as intuitive signal output, simple operation, and low cost. In a typical two-step colorimetric detection system, the first step involves the catalytic oxidation of glucose to produce H2O2. Then, nanomaterials with peroxidase-like activity catalyze the oxidation of chromogenic substrates, resulting in a visible color change that can be analyzed by the naked eye or quantified using devices like spectrophotometers or smartphone cameras. In this process, nanomaterials such as metal oxides (e.g., Fe3O4, CuO, MnO2), metal nanoparticles (e.g., Au, Pt, Pd), and multi-metallic composites are widely used in the second chromogenic step. These materials not only offer good stability and catalytic activity but also exhibit enzyme-mimicking functions, effectively facilitating the colorimetric reaction. The catalytic efficiency, and consequently the sensitivity and selectivity of the colorimetric response, is greatly influenced by the nanomaterials’ particle size, morphology, specific surface area, and electronic structure. With the continuous emergence of novel nanomaterials and the advancement of signal amplification and anti-interference strategies, fluorescence and colorimetric glucose sensors are becoming important complements to electrochemical methods, providing strong support for the development of high-sensitivity, non-enzymatic glucose monitoring platforms.
The non-enzymatic glucose sensing strategies: electrochemical, fluorescent, and colorimetric, possess distinct advantages in terms of material selection, application scenarios, detection mechanisms, and system integration. However, they still face limitations in practical applications.
Table 4 summarizes three detection modalities in terms of their suitability for wearable/portable applications, cost and scalability, and practical challenges.
The practical application of non-enzymatic electrochemical sensors still has difficulties; for example, it is difficult to continuously produce these nanostructures on a large scale. Most of the measurement results are obtained in the laboratory environment, and the test environment is only similar to the biological environment, but there are some differences in practical applications. The high cost and scarcity of noble metals significantly limit application in non-enzymatic electrochemical glucose detection. Moreover, these materials are susceptible to poisoning by interfering species such as chloride ions in complex physiological environments, resulting in reduced sensor stability and compromised detection accuracy over time Also, an important limitation is the poor conductivity of transition metal oxides as well as other derivatives used for electrochemical biosensor applications. Therefore, it is necessary to find new and suitable conductive support, such as graphene, graphene derivatives, MOFs, and so on, to promote electronic transmission. Despite the significant advantages of multi-metallic materials in enhancing catalytic performance, several non-negligible challenges remain in their practical applications. On one hand, the synthesis of multi-metallic composites is relatively complex, as differences in the reactivity and physical properties of various metals can lead to issues such as uneven composition distribution and difficulties in controlling morphology. On the other hand, the performance of these materials is highly sensitive to the metal ratios and structural parameters, making their optimization and standardized preparation technically demanding. Therefore, while advancing the application of multi-metallic materials in non-enzymatic glucose sensors, it is essential to address key issues related to controllable synthesis and long-term stability.
The fluorescent glucose sensor also faces several limitations. Some fluorophores, such as quantum dots and organic dyes, require complex synthesis and are costly, hindering large-scale production. Though generally more stable than enzymatic systems, fluorescence signals are also subject to pH and temperature interference, and are prone to photobleaching, which compromises long-term signal reliability and accuracy. Additionally, the low and variable glucose concentrations in biofluids like sweat or tears show weak correlation with blood glucose, limiting their use in continuous glucose monitoring. As a result, fluorescence sensing remains more suitable for research and prototype development, with further advances needed in material robustness, system integration, and environmental tolerance before widespread commercialization becomes feasible.
For colorimetric sensing, interference from environmental variables remains one of the obstacles limiting the widespread application of colorimetric sensors in complex biological samples and wearable scenarios. Currently, colorimetric sensors face significant challenges in environmental adaptability. Their signal generation mechanisms are primarily based on redox reactions or the aggregation/corrosion of nanomaterials, both of which are subject to external factors such as pH, temperature, and humidity. In real physiological fluids, pH levels fluctuate widely, often leading to reduced reaction efficiency or unstable signal output. Temperature variations also affect reaction kinetics and system stability; elevated temperatures may cause uncontrolled reactions or enzyme deactivation, while lower temperatures can suppress color development. In wearable applications, fluctuations in skin surface temperature further compromise signal consistency. High humidity can cause swelling in paper-based materials, resulting in blurred color boundaries and signal diffusion, which interfere with color recognition and image analysis, ultimately undermining stability and repeatability. Additionally, some systems require external hydrogen peroxide or rely on enzymatic reactions, which reduces overall system robustness and user-friendliness. High-performance colorimetric sensors often depend on noble metal nanoparticles or complex optical structures, increasing material costs and complicating fabrication, thereby hindering large-scale production and deployment. At present, most research remains at the laboratory stage, lacking standardized readout methods and process control, which significantly limits commercialization and clinical translation.
7. Challenges
While the methods discussed exhibit considerable potential for non-enzymatic glucose detection, the following challenges still exist in both research and practical implementations.
- (1)
Lack of a universal standard evaluation system
Currently, many non-enzymatic glucose sensors demonstrate excellent performance in terms of sensitivity, selectivity, and detection limit. However, significant discrepancies in testing conditions, such as the type of electrolyte, working potential, detection method, and sample matrix, exist across different studies, leading to a lack of comparability among reported performance metrics. In addition, some studies fail to standardize electrode area or use inconsistent units for sensitivity, which further complicates direct comparisons. As a result, horizontal comparisons of analytical performance across studies are often limited and may be misleading. At present, the field lacks a unified evaluation standard system, and this issue urgently needs to be addressed in future research to enable standardized performance assessment and cross-study reproducibility.
- (2)
Interference and limitation of external environmental factors
Non-enzymatic glucose sensors exhibit superior structural stability compared to enzyme-based systems. However, the performance is still affected by environmental factors such as temperature, pH, and humidity. Electrochemical non-enzymatic sensors are generally more tolerant to temperature fluctuations, but their catalytic activity often relies on alkaline conditions and tends to decline under neutral or acidic environments. Fluorescent detection generally exhibits high thermal stability and maintains stable emission over a wide temperature range. However, certain organic probes, such as MOFs, are less thermally robust; elevated temperatures may cause degradation or desorption of surface groups or quenchers/ligands, indirectly affecting the fluorescence signal. In addition, these functional groups can undergo protonation or deprotonation with pH changes, altering the photoluminescence behavior and thereby lowering sensitivity. Colorimetric glucose sensing offers good environmental adaptability and could maintain stable chromogenic performance under mild conditions. However, most current systems still rely on exogenous H2O2 or GOx to generate the precursors required for the chromogenic reaction, which limits the potential advantages of non-enzymatic systems in terms of environmental interference resistance and stability.
- (3)
Stability and reproducibility of nanomaterial-based sensors
A wide range of nanomaterials are prone to aggregation, surface passivation, oxidation, or structural degradation during use, leading to gradual deterioration in sensing performance. Moreover, during device fabrication, the final morphology and composition of the materials are highly sensitive to synthesis conditions such as temperature, reaction time, and precursor concentration. This results in poor batch-to-batch reproducibility and inconsistent device performance. These issues not only compromise the reliability of long-term operation but also pose significant challenges for scalable industrial production.
- (4)
Limitations in cost-effectiveness
Compared with enzyme-based systems, non-enzymatic glucose detection possesses certain cost advantages in terms of material accessibility, synthesis conditions, and storage requirements. However, achieving high performance often requires the construction of complex nanostructures or the incorporation of noble metal modifications, which significantly increase material and processing costs. In addition, the complete sensor system involves components such as electrodes, packaging, and signal acquisition modules, which contribute substantially to the overall cost, particularly in wearable or continuous monitoring applications. As a result, the cost benefits of non-enzymatic sensors remain limited in practical, large-scale implementation.
- (5)
Regulatory hurdles for clinical deployment
Despite demonstrating favorable sensitivity and selectivity under laboratory conditions, non-enzymatic glucose sensors face significant regulatory challenges in clinical translation. Most studies remain at the proof-of-concept stage, lacking systematic preclinical evaluation and validation using human biological samples, which are essential to meet the stringent requirements of medical device regulations for safety, efficacy, and reproducibility. Standardized evaluation frameworks for key performance metrics have yet to be established, such as batch-to-batch consistency, long-term stability, and biocompatibility, which pose substantial barriers to formal regulatory approval. Therefore, advancing the clinical deployment of non-enzymatic glucose sensors requires continued efforts in regulatory compliance, product reliability, and standardized validation.
- (6)
Integration with digital technologies
Non-enzymatic glucose sensing is gradually integrating with digital technologies such as smartphones, leading to the development of intelligent monitoring systems characterized by wearability and portability. By incorporating wireless communication modules such as Bluetooth and NFC, or utilizing image recognition of colorimetric and fluorescent signals via smart terminals, these sensors enable real-time acquisition and remote transmission of glucose data, providing a technological foundation for personalized health management and telemedicine. However, several limitations remain in the deep integration with digital technologies, including high power consumption of communication modules, a lack of stable and universal data interfaces, susceptibility of image recognition to environmental interference, and the absence of standardized connectivity with health information platforms. Furthermore, insufficient consideration of data security and privacy protection constrains the broader application of these systems within clinical healthcare frameworks.
- (7)
Integration with existing healthcare systems
The application of non-enzymatic glucose sensors within medical systems remains limited, and their clinical value has yet to be widely established. Most sensors are still at the stage of laboratory validation, with signal output formats, measurement units, and data processing methods lacking standardization. As a result, there is no unified framework for translating sensor outputs into medically interpretable results. Although some studies have extended detection to alternative biofluids such as sweat and saliva, the inherently low glucose concentrations, high inter-individual variability, and susceptibility to external interference in these matrices continue to compromise detection accuracy and clinical consistency. Moreover, the vast majority of non-enzymatic sensors have not undergone systematic clinical trials or obtained regulatory approval, lacking the compliance foundation required for integration into healthcare systems. In addition, data security and privacy protection mechanisms are often insufficient, further limiting their large-scale adoption in chronic disease management, remote monitoring, and clinical decision support.
Considering the challenges outlined above, non-enzymatic glucose sensors, despite significant advancements in laboratory performance, have yet to be widely adopted in clinical or commercial settings. This is not due to inadequate analytical capability, as many sensors are already capable of meeting the requirements for glucose detection. This is not due to inadequate analytical capability, as many sensors are already capable of meeting the requirements for glucose detection, but rather results from a combination of limiting factors. Clinical translation remains hindered by a lack of systematic safety, biocompatibility, and long-term stability evaluations, which are essential for meeting regulatory requirements for medical approval. Additionally, most devices are still in the validation phase and lack compatibility with the existing medical information systems, restricting their effective integration into clinical workflows. Although some studies have extended detection to alternative biofluids such as sweat and saliva, the inherently low glucose concentrations, high inter-individual variability, and susceptibility to external interference compromise the clinical consistency and reliability of these results. Collectively, these multifaceted barriers have constrained the transition of non-enzymatic glucose sensors from laboratory research to practical clinical application.
8. Conclusions and Prospects
Glucose concentration detection plays a crucial role in the food industry, medical health, and disease diagnosis. With the development of nanotechnology, sensors based on nanomaterials have shown great potential in glucose detection due to their high sensitivity, strong anti-interference ability, and small volume. Although traditional enzyme-based glucose sensors have high selectivity and sensitivity, their instability and susceptibility to pH, temperature, and humidity make them insufficient in anti-interference ability. The progress of nanotechnology promoted the development of non-enzymatic glucose sensors, and it brought valuable nanostructures and methods; these nanostructures and methods have played a key role in the modernization of glucose sensors. Electrochemical detection strategy based on the direct oxidation of glucose by nanozymes, exhibiting excellent chemical stability and rapid response. Fluorescent nanomaterials are characterized by high quantum yield and good stability, which are important for long continuous detection. Therefore, the glucose fluorescence sensor has great potential in realizing real-time monitoring and dynamic detection of glucose. Meanwhile, the colorimetric glucose detection method, with its low cost, visually distinct color change, simple equipment, and ease of integration, holds a dominant position in rapid testing and portable application scenarios.
Nanosensors show broad application prospects in the field of glucose detection. With the development of new materials and optimization of sensor design, more breakthroughs are expected in the future to further improve the accuracy, sensitivity, and utility of glucose detection. However, in order to further meet the future needs of rapid, efficient, portable detection and real-time dynamic detection, the development of glucose sensors should focus on the following key directions:
Non-invasive Detection Technology: At present, glucose concentration monitoring in the medical and healthcare field is predominantly invasive, often causing pain and discomfort for patients. To improve patient comfort and convenience, glucose sensors should continue to advance toward non-invasive approaches. By detecting glucose levels in body fluids such as sweat or saliva, it is possible to achieve non-invasive evaluation of blood glucose levels, offering a gentler and more sustainable method for continuous monitoring.
Dynamic Real-time Monitoring Technology: Fluorescence and colorimetric methods, with their rapid response, ease of miniaturization, and visually intuitive outputs, are particularly well-suited for integration into wearable devices, making them promising tools for dynamic, real-time glucose monitoring. Nevertheless, the stability of these sensors over extended periods remains a critical challenge. Further improvements are needed to ensure the consistent accuracy and reliability of the data during continuous monitoring.
Development of Non-enzymatic Colorimetric Sensors: In colorimetric sensing, hydrogen peroxide is typically introduced externally or produced via glucose oxidation catalyzed by GOx. Future colorimetric detection strategies should aim to develop non-enzymatic systems that eliminate the reliance on enzymatic catalysis, thereby enhancing the sensors’ stability, shelf life, and applicability in complex environments.
Multi-modal Sensing Technology: Integrating electrochemical detection with fluorescence or colorimetric techniques allows for the complementary strengths of each method to be harnessed. For example, combining the high sensitivity of electrochemical methods with the visual intuitiveness of fluorescence or colorimetric readouts enables simultaneous rapid qualitative assessment and accurate quantitative analysis, offering a promising approach for more comprehensive and reliable glucose monitoring.
Integration with Digital Technology: Digital health technologies such as artificial intelligence, wireless communication, smartphone-based platforms, and cloud data systems present new opportunities for smart glucose monitoring. Combining non-enzymatic sensors with data processing algorithms, Bluetooth-enabled devices, or wearable IoT systems can enable continuous monitoring, personalized analysis, remote diagnostics, and cloud-based health management.
Application-Driven Development of Materials and Devices: To meet the practical requirements of glucose monitoring in biological fluids, future research should focus on developing electrode materials that remain stable and effective under neutral or mildly alkaline pH conditions, while offering strong anti-interference ability and long-term durability. Strategies such as heteroatom doping, core–shell nanostructure design, and surface functionalization can help improve selectivity and electrocatalytic activity. Meanwhile, integrating these high-performance materials into flexible, wearable platforms—combined with smartphones or wireless modules—will enable non-invasive, real-time, and user-friendly glucose detection suitable for daily health management and preclinical diagnostics.