1. Introduction
Hydrogen has emerged as a highly promising alternative energy carrier owing to its exceptionally high gravimetric energy density and environmentally benign nature, as its utilization produces only water as a by-product, resulting in zero carbon emissions [
1,
2,
3]. Among the various hydrogen generation methods, electrochemical water splitting stands out as a clean and efficient route, particularly when powered by renewable energy sources such as solar, wind, or hydropower [
4,
5]. This process involves two half-reactions: the oxygen evolution reaction (OER) at the anode and the hydrogen evolution reaction (HER) at the cathode. The overall water-splitting reaction requires a minimum thermodynamic potential of 1.23 V, but due to kinetic barriers, practical operation often demands higher voltages, mainly attributed to the sluggish multi-electron transfer process of the OER [
1,
6,
7,
8]. The HER, in contrast, is a two-electron process and generally proceeds more rapidly. However, in alkaline media, where hydroxide ions are involved instead of protons, the HER is comparatively slower than in acidic environments due to additional water dissociation steps [
6,
9]. Therefore, the development of highly active, stable, and low-cost HER catalysts is essential to promote efficient hydrogen evolution in alkaline water electrolysis. Traditionally, noble metals such as platinum (Pt) for the HER and ruthenium/iridium oxides (Ru, Ir) for the OER have been recognized as the benchmark electrocatalysts due to their outstanding catalytic activity and low overpotentials. However, their high cost, scarcity, and poor long-term stability in alkaline environments hinder their large-scale deployment in commercial electrolyzers. This has stimulated considerable research interest in the development of earth-abundant, low-cost, and stable transition metal-based alternatives [
9,
10,
11].
Transition metals such as cobalt (Co), nickel (Ni), iron (Fe), and their respective compounds (oxides, sulfides, phosphides, and carbides) have emerged as promising non-precious metal catalysts for electrochemical water splitting. These materials exhibit multiple valence states, high intrinsic redox activity, and structural tunability, which are advantageous for tailoring their catalytic performance. In particular, the synergistic combination of different transition metal phases has been shown to enhance electronic conductivity, promote charge transfer, and increase the density of active sites [
11,
12,
13]. Cobalt oxide (Co
3O
4), a typical spinel-type oxide, has demonstrated promising activity for both the HER and the OER due to its favorable electronic structure, thermal stability, and corrosion resistance [
1,
14]. It contains both Co
2+ and Co
3+ ions within its crystal lattice, which facilitates redox reactions through reversible valence changes. The Co
2+ ions with three unpaired d-electrons and Co
3+ ions with all paired electrons create a mixed valence system that promotes fast electron transfer and intermediate adsorption/desorption during electrocatalysis [
11]. Recently, a Co
3O
4@CDs nanocomposite was synthesized via a hydrothermal method, yielding an overpotential of 165 mV at a current density of 10 mA cm
−2 in alkaline electrolyte [
15]. Similarly, a Co
3O
4/MoS
2 p–p type heterojunction electrocatalyst featuring a nanoparticle/nanoflower hierarchical architecture was developed to exploit the synergistic interactions between the two semiconductors. When evaluated in 1 M KOH solution, the heterostructure exhibited an overpotential of 156 mV to drive a current density of 10 mA cm
−2, reflecting its superior electrocatalytic efficiency and interfacial charge transfer characteristics [
16].
Nickel disulfide (NiS
2), a typical transition metal dichalcogenide (TMD), has recently attracted growing attention for HER applications due to its high electrical conductivity, favorable metal–sulfur bonding, and abundant edge-active sites [
6]. NiS
2 exhibits excellent electron transport properties, which help to alleviate the inherent limitations of sluggish HER kinetics in alkaline conditions. Nanostructured NiS
2 has garnered considerable attention due to its intrinsic catalytic activity and ability to be engineered into high-surface-area architectures that enhance electrolyte accessibility and charge transport [
3]. A noteworthy development in this field is the fabrication of nitrogen-doped NiS
2 nanosheets grown directly on carbon fiber cloth (denoted by N-NiS
2/CF), forming an integrated electrode. The resulting N-NiS
2/CF electrode demonstrates excellent HER activity, requiring an overpotential of only 96 mV to achieve a current density of 10 mA cm
−2 in 1.0 M KOH electrolyte [
17]. In another study, a hybrid nanowire architecture was constructed by integrating Cu-doped MoS
2 nanosheets onto NiS
2 nanowires through a multi-step strategy involving electrospinning, sulfuration, and hydrothermal treatment. The heterostructured nanowires exhibit significantly enhanced HER performance with a low overpotential of 105 mV at 10 mA cm
−2 in alkaline medium [
18]. Moreover, a unique heterostructure was engineered by introducing early transition metal vanadium (V) species into a NiS/NiS
2 hybrid matrix. The resultant V-NiS/NiS
2 electrocatalyst exhibited remarkable catalytic behavior with an overpotential of only 94 mV at 10 mA cm
−2 in 1 M KOH solution [
19].
However, despite these advancements, many of the reported electrocatalysts still face inherent limitations such as relatively high overpotentials (typically >90 mV at 10 mA cm−2), insufficient long-term stability, or complex multi-step synthesis routes that hinder large-scale applicability. In this regard, there remains a critical need for rationally designed, compositionally tunable hybrid materials that offer both high catalytic activity and operational robustness in alkaline HER environments. To overcome these limitations, the current study reports the successful synthesis of a Co3O4–NiS2 (Co–Ni) nanocomposite through a straightforward coprecipitation strategy. The resulting hybrid material was systematically evaluated as a highly active electrocatalyst for the hydrogen evolution reaction (HER) in alkaline environments. To address these challenges, the present study introduces a Co3O4-NiS2 (Co-Ni) nanocomposite successfully synthesized via a facile coprecipitation method and investigated as an efficient electrocatalyst for the HER in alkaline media. Comprehensive spectroscopic and structural analyses confirmed the successful formation of the heterostructured composite, with nanosheet-like features presumably derived from sheet-structured NiS2 uniformly integrated with the Co3O4 matrix. Among the series of fabricated samples, the Co-Ni-2 electrocatalyst exhibited superior HER activity, delivering a low overpotential of 84 mV at a current density of 10 mA cm−2, along with a favorable Tafel slope of 67.5 mV dec−1, as evaluated in a three-electrode configuration. Furthermore, the electrochemical durability of the optimized electrocatalyst was rigorously assessed through extended cyclic voltammetry (CV) tests, demonstrating remarkable structural and catalytic stability under prolonged alkaline operation. These superior electrocatalytic properties are attributed to the synergistic interaction between Co3O4 and NiS2, which enhances charge transport, increases the electrochemically active surface area, and facilitates rapid hydrogen evolution kinetics. This work provides valuable insights into the development of high-performance electrocatalysts for sustainable hydrogen production.
3. Results and Discussion
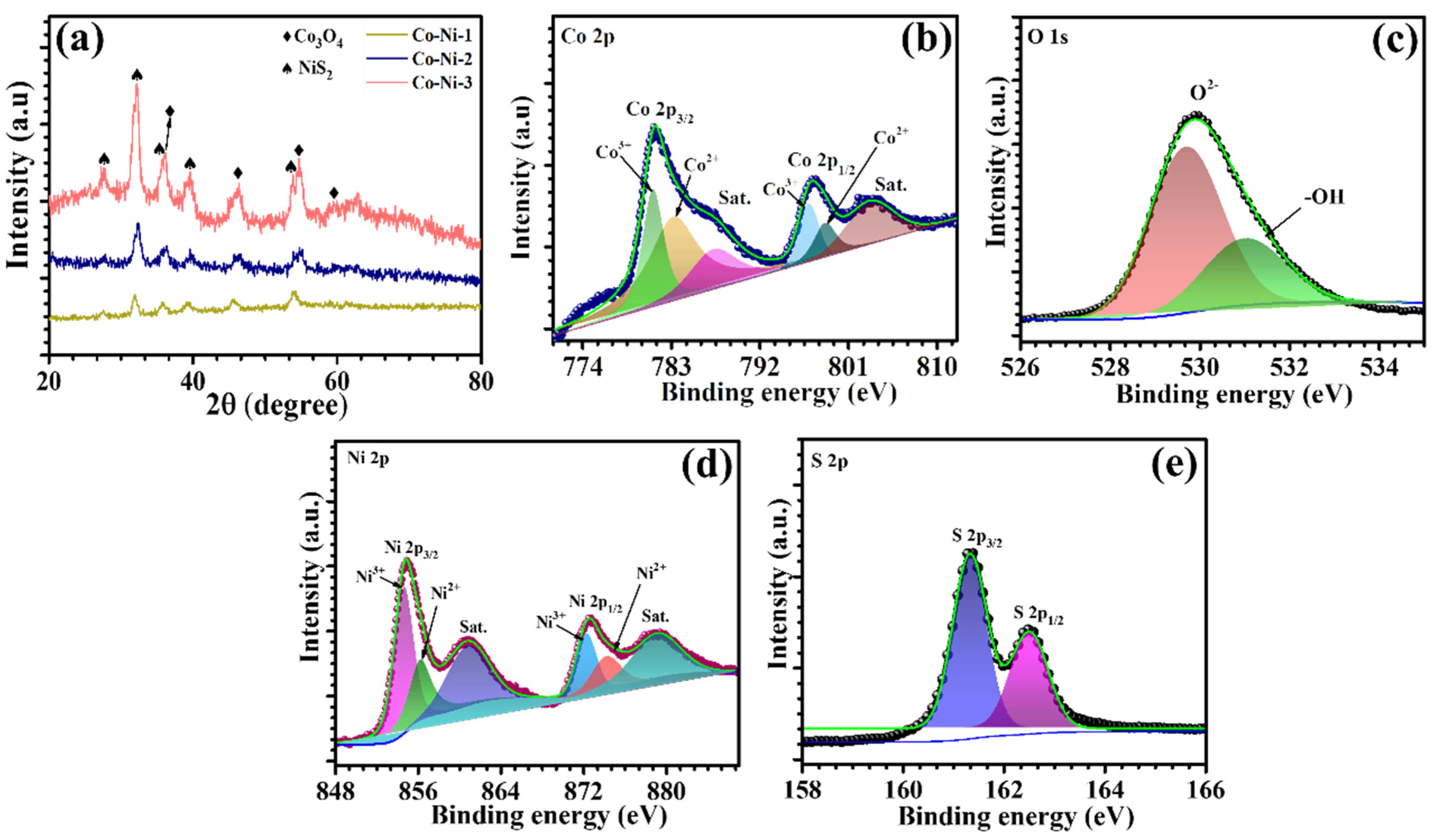
The crystalline phases and structural integrity of the synthesized Co-Ni composites were investigated using XRD, as shown in
Figure 1a. The diffraction patterns clearly confirm the coexistence of both Co
3O
4 and NiS
2 phases within the composite system. Distinct reflections of Co
3O
4 are observed at 2θ of 36.46°, 45.2°, 54.8°, and 59.02°, which are indexed to the (311), (400), (422), and (511) planes, respectively, of the cubic spinel phase (JCPDS No. 01-080-1540). The presence of these well-defined peaks indicates the successful formation of phase-pure Co
3O
4 with high crystallinity. Simultaneously, additional prominent peaks appearing at 2θ of 27.71°, 32.3°, 35.9°, 39.5°, and 54.1° correspond to the (111), (200), (210), (211), and (311) planes of cubic NiS
2 (JCPDS No. 01-080-0376), confirming the incorporation of the NiS
2 phase. The concurrent appearance of both sets of diffraction peaks without any extraneous signals affirms the formation of a clean binary composite with no secondary or impurity phases. Importantly, no observable peak shifting was detected, suggesting that both Co
3O
4 and NiS
2 maintain their individual crystal structures without significant lattice distortion or interdiffusion. With increasing NiS
2 content, a progressive enhancement in the intensity of NiS
2-related peaks is noted. This trend highlights the increasing contribution of NiS
2 in the composite and confirms its effective integration within the Co
3O
4 matrix.
XPS was carried out to investigate the surface chemical states and electronic interactions within the Co-Ni-2 composite. The high-resolution Co 2p spectrum (
Figure 1b) reveals two prominent peaks at 781.16 eV and 796.9 eV, corresponding to Co 2p
3/2 and Co 2p
1/2, respectively. These peaks exist along with the deconvoluted peaks at 783.41 eV and 798.7 eV. The deconvoluted peaks confirm the coexistence of Co
2+ and Co
3+ oxidation states, with satellite features near 787.6 eV and 803.3 eV characteristic of the spinel Co
3O
4 phase. This mixed-valence state is critical for promoting reversible redox reactions during electrocatalytic water splitting. The O 1s spectrum (
Figure 1c) further supports this framework, with a strong peak at 529.71 eV assigned to lattice oxygen (M–O) and additional peaks at 531.08 eV corresponding to surface hydroxyl groups. These surface oxygenated species are known to enhance catalytic activity by facilitating intermediate adsorption [
22]. The high-resolution Ni 2p spectrum (
Figure 1d) reveals a well-defined set of spin–orbit doublets accompanied by shake-up satellite features, indicative of the coexistence of Ni
2+ and Ni
3+ oxidation states. As illustrated in
Figure 1d, the deconvoluted peaks located at binding energies of 854.7 eV and 872.26 eV correspond to Ni
3+ (2p
3/2 and 2p
1/2), while those at 856.36 eV and 874.35 eV are attributed to Ni
2+ species. The observed satellite peaks further confirm the presence of divalent nickel, a common feature in sulfide-based materials. This dual-valence configuration reflects the redox flexibility of nickel within the NiS
2 matrix and is anticipated to play a crucial role in enhancing electrochemical kinetics. The high-resolution S 2p spectrum, presented in
Figure 1e, exhibits two well-defined peaks centered at binding energies of 161.34 eV and 162.56 eV, which are assigned to the S 2p
3/2 and S 2p
1/2 spin–orbit components, respectively. The observed spin–orbit splitting of approximately 1.22 eV is consistent with the typical signature of sulfide (S
2-) anions in nickel-sulfur frameworks such as NiS
2. The symmetrical and sharp nature of these peaks confirms the dominance of S
2- species without detectable contributions from oxidized sulfur states, indicating that the sulfur environment remains chemically intact during synthesis [
23]. These results strongly validate the formation of stoichiometric NiS
2.
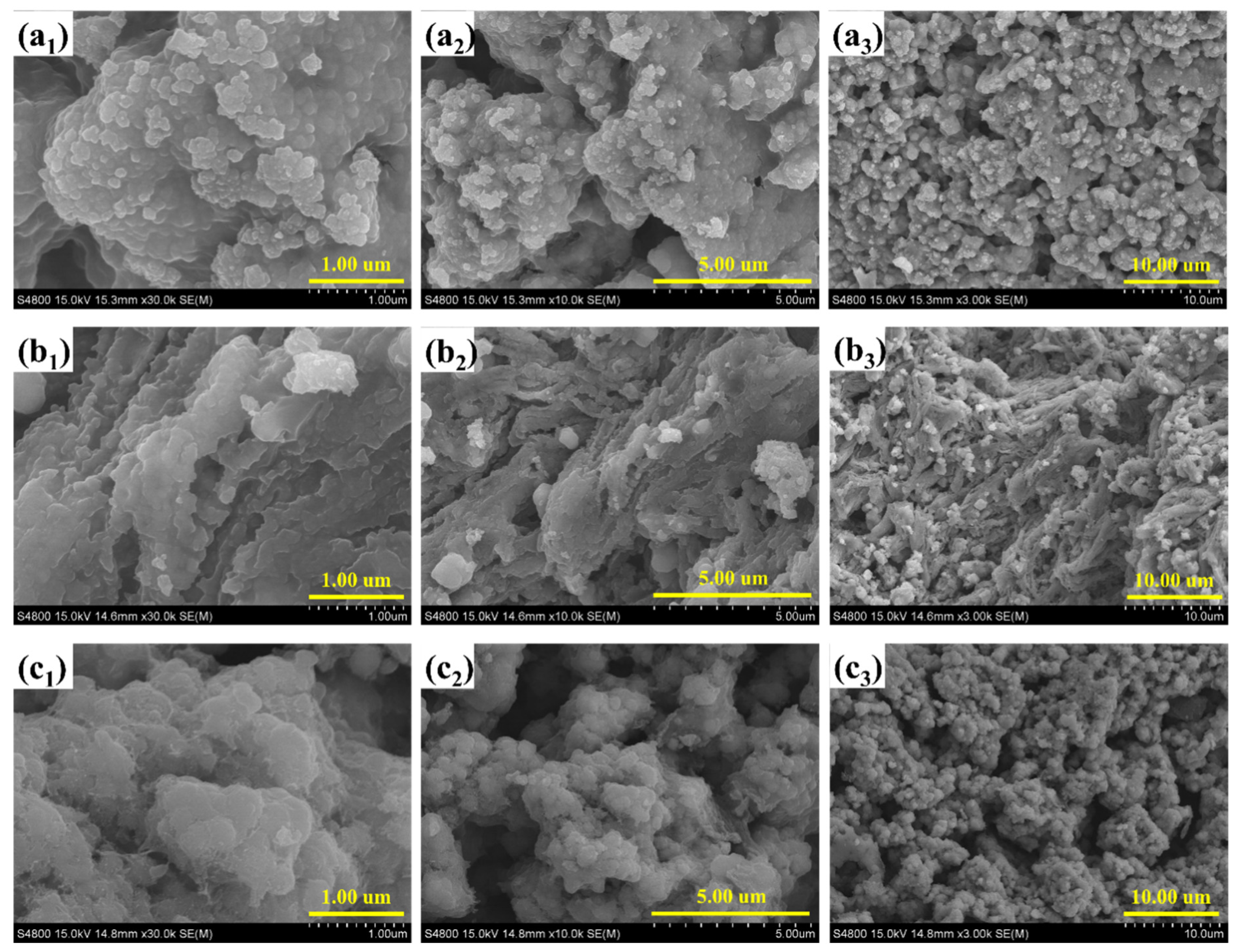
FESEM was employed to investigate the surface morphology and microstructural evolution of the Co-Ni composites with varying NiS
2 content, as depicted in
Figure 2. The images provide insights into the impact of compositional tuning on the particle distribution, agglomeration behavior, and surface texturing, all of which are critical for optimizing electrochemical activity in water-splitting applications. In the case of Co-Ni-1 (
Figure 2(a
1–a
3)), which corresponds to the lowest NiS
2 loading, the surface is dominated by densely packed, cauliflower-like nanoclusters. These agglomerates are composed of interconnected nanoscale subunits, resulting in a moderately porous structure. The relatively uniform distribution and moderate texturing suggest limited NiS2 integration at this stage, with Co
3O
4 features largely preserved. With the intermediate NiS
2 content in Co-Ni-2 (
Figure 2(b
1–b
3)), the surface morphology undergoes a significant transformation. Nanosheet-like layers, presumably originating from exfoliated or sheet-structured NiS
2, become prominently integrated with the underlying Co
3O
4 matrix. These wrinkled and interlaced sheets impart a higher degree of surface roughness and hierarchical porosity. The partial embedding of small Co
3O
4 particles within the sheet matrix creates abundant interfacial contact, which is beneficial for facilitating electron transport and ion diffusion. This sample exhibits the most balanced morphology, combining structural integrity, surface area, and interconnectivity, suggesting it as the optimized configuration for catalytic applications. In contrast, Co-Ni-3 (
Figure 2(c
1–c
3)), which contains the highest NiS
2 content, shows a marked departure from the earlier architectures. The surface appears densely populated with aggregated spherical particles, leading to the formation of a granular and highly compact structure. While the increased presence of NiS
2 is evident, the excessive particle crowding results in reduced porosity and less-defined surface features. This morphology may hinder electrolyte accessibility and limit active site exposure, ultimately negatively affecting catalytic performance when compared to Co-Ni-2. Overall, the progressive incorporation of NiS
2 distinctly alters the microstructure of the Co
3O
4-based composites. Co-Ni-2 demonstrates the most favorable morphology, characterized by a well-integrated Co
3O
4–NiS
2 hybrid framework with high surface accessibility and interfacial connectivity. Such hierarchical texturing and synergistic nano-architectures are essential for enhancing electrochemical kinetics in redox-based catalytic systems.
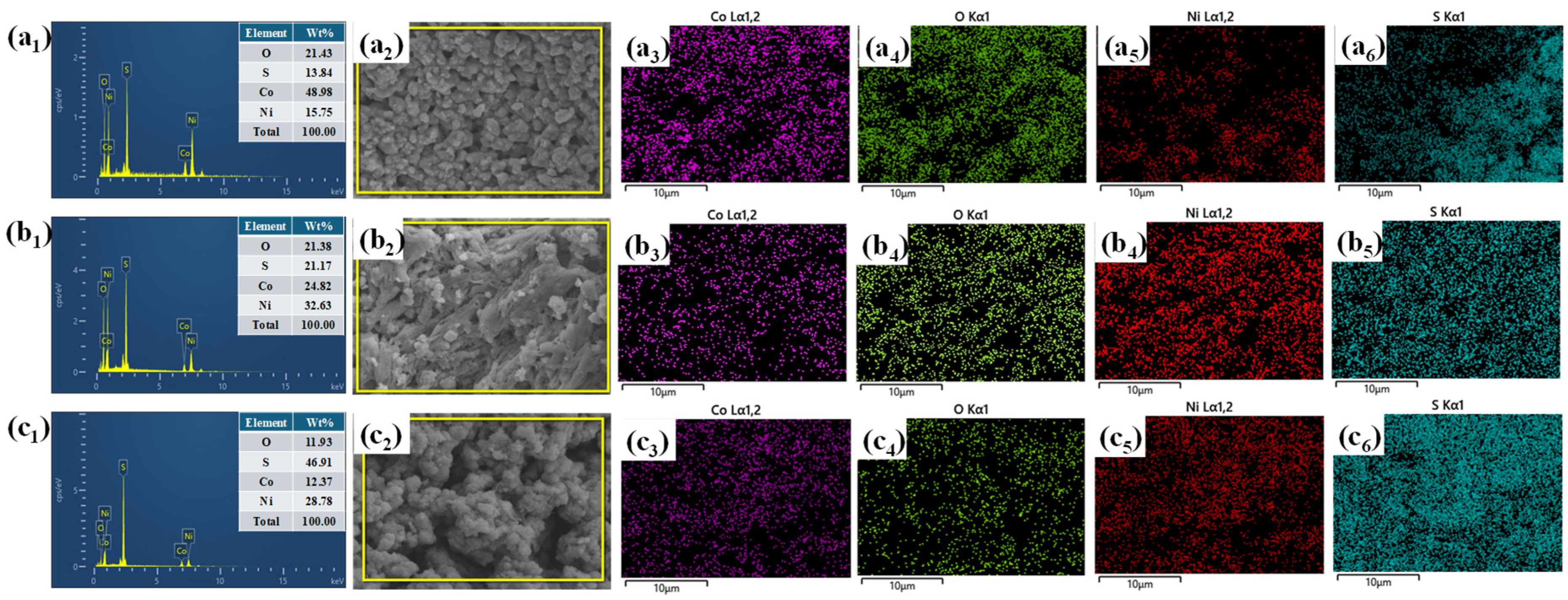
EDS and elemental mapping analyses were carried out to examine the elemental composition and spatial distribution of Co, Ni, S, and O in the Co
3O
4–NiS
2 composites. All samples exhibit the expected elements without any impurity signals, confirming the successful formation of the intended heterostructure. In Co-Ni-1 (
Figure 3(a
1–a
6)), Co dominates the composition, with relatively lower amounts of Ni and S, indicating a Co
3O
4-rich phase. In contrast, Co-Ni-3 (
Figure 3(c
1–c
6)) shows a significant increase in Ni and S contents with a considerable reduction in Co, suggesting excessive NiS
2 loading that may suppress the redox-active oxide phase. Co-Ni-2 (
Figure 3(b
1–b
6)) exhibits a well-balanced elemental ratio with uniform and dense distributions of all elements across the surface, as evident from the mapping images. This compositional synchronization and homogeneous dispersion are expected to enhance interfacial charge transfer, structural stability, and electrochemical activity.
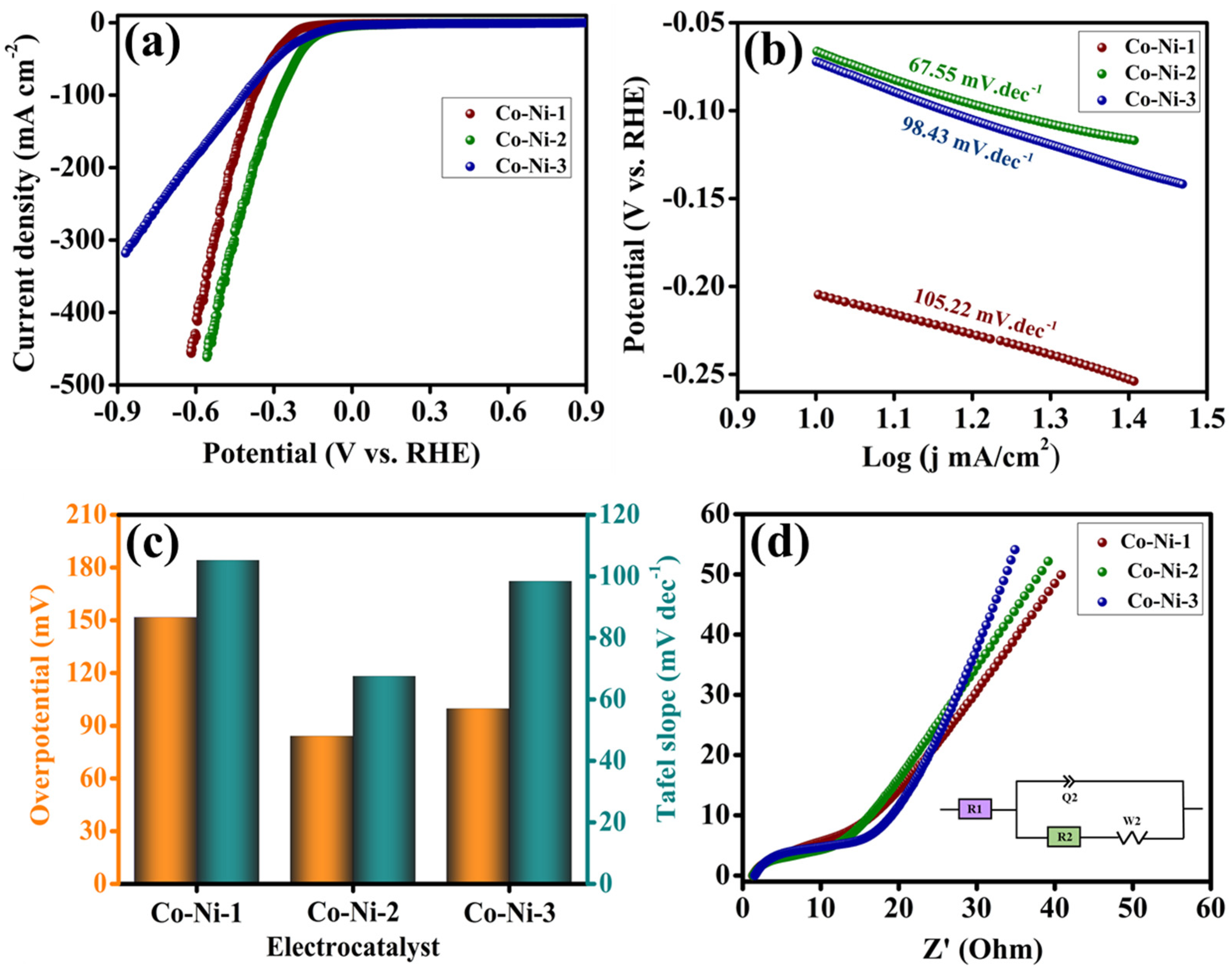
The electrocatalytic activity of the synthesized Co-Ni-based materials toward the HER was systematically evaluated using a standard three-electrode configuration in 1 M KOH aqueous electrolyte. LSV spectra were obtained to compare the HER performance of the Co-Ni-1, Co-Ni-2, and Co-Ni-3 electrocatalysts, and the corresponding polarization curves are presented in
Figure 4a. Among the tested samples, Co-Ni-2 exhibited the most promising HER activity, requiring a minimal overpotential of 84 mV to achieve a benchmark current density of 10 mA cm
−2. In comparison, Co-Ni-1 and Co-Ni-3 required overpotentials of 151 mV and 99 mV, respectively, to reach the same current density, as shown in
Figure 4a,c. This marked reduction in overpotential for Co-Ni-2 indicates its superior intrinsic catalytic activity, which can be attributed to its optimized composition, enhanced active surface exposure, and favorable electronic configuration that promotes efficient proton adsorption and subsequent hydrogen evolution. To gain deeper insights into the HER kinetics, Tafel plots were constructed from the LSV data, as shown in
Figure 4b,c. The Tafel slope of Co-Ni-2 was determined to be 67.5 mV dec
−1, which is substantially lower than those of Co-Ni-1 (105.2 mV dec
−1) and Co-Ni-3 (98.4 mV dec
−1). A smaller Tafel slope indicates more favorable reaction kinetics, implying that the hydrogen evolution reaction (HER) proceeds through a more efficient electron transfer pathway. A lower Tafel slope signifies faster kinetics and suggests that the HER proceeds. The improved reaction kinetics further support the superior catalytic behavior of the Co-Ni-2 catalyst. A detailed analysis of the Tafel slope offers valuable insights into the underlying HER mechanism. Generally, a Tafel slope in the range of 30–40 mV dec
−1 is indicative of a Volmer–Tafel pathway, wherein the recombination of surface-adsorbed hydrogen atoms (H*) constitutes the rate-limiting step. In contrast, Tafel slopes between 40 and 120 mV dec
−1 are typically associated with the Volmer–Heyrovsky mechanism, characterized by the electrochemical desorption of H* as the rate-determining step [
24]. For the Co-Ni-2 electrocatalyst, a measured Tafel slope of 67.5 mV dec
−1 suggests that the HER proceeds predominantly via the Volmer–Heyrovsky route. This mechanism involves an initial discharge step where water molecules are reduced to generate adsorbed hydrogen species (H*) [
25,
26]:
This is followed by the electrochemical desorption of H* to produce molecular hydrogen:
Consequently, targeted optimization aimed at enhancing H* desorption efficiency is critical for achieving substantial improvements in HER kinetics for this electrocatalytic system. Electrochemical impedance spectroscopy (EIS) was conducted to evaluate the interfacial charge transfer resistance (R
ct) and probe the conductivity characteristics of the electrode–electrolyte interface. The Nyquist plots displayed in
Figure 4d, along with the equivalent circuit model shown in the inset, allowed for measurable extraction of R
ct values. The Co-Ni-2 electrocatalyst exhibited the smallest semicircle diameter, reflecting the lowest R
ct value of 7.7 Ω compared to the other tested samples, Co-Ni-1 (12.5 Ω) and Co-Ni-3 (10.6 Ω). This lower R
ct is indicative of rapid electron transport and efficient interfacial charge transfer, which plays a critical role in enhancing HER kinetics. The improved conductivity and reduced resistance observed for Co-Ni-2 are in line with its superior HER performance, strengthening the synergetic effect between cobalt and nickel in optimizing the electronic structure and accelerating reaction dynamics.
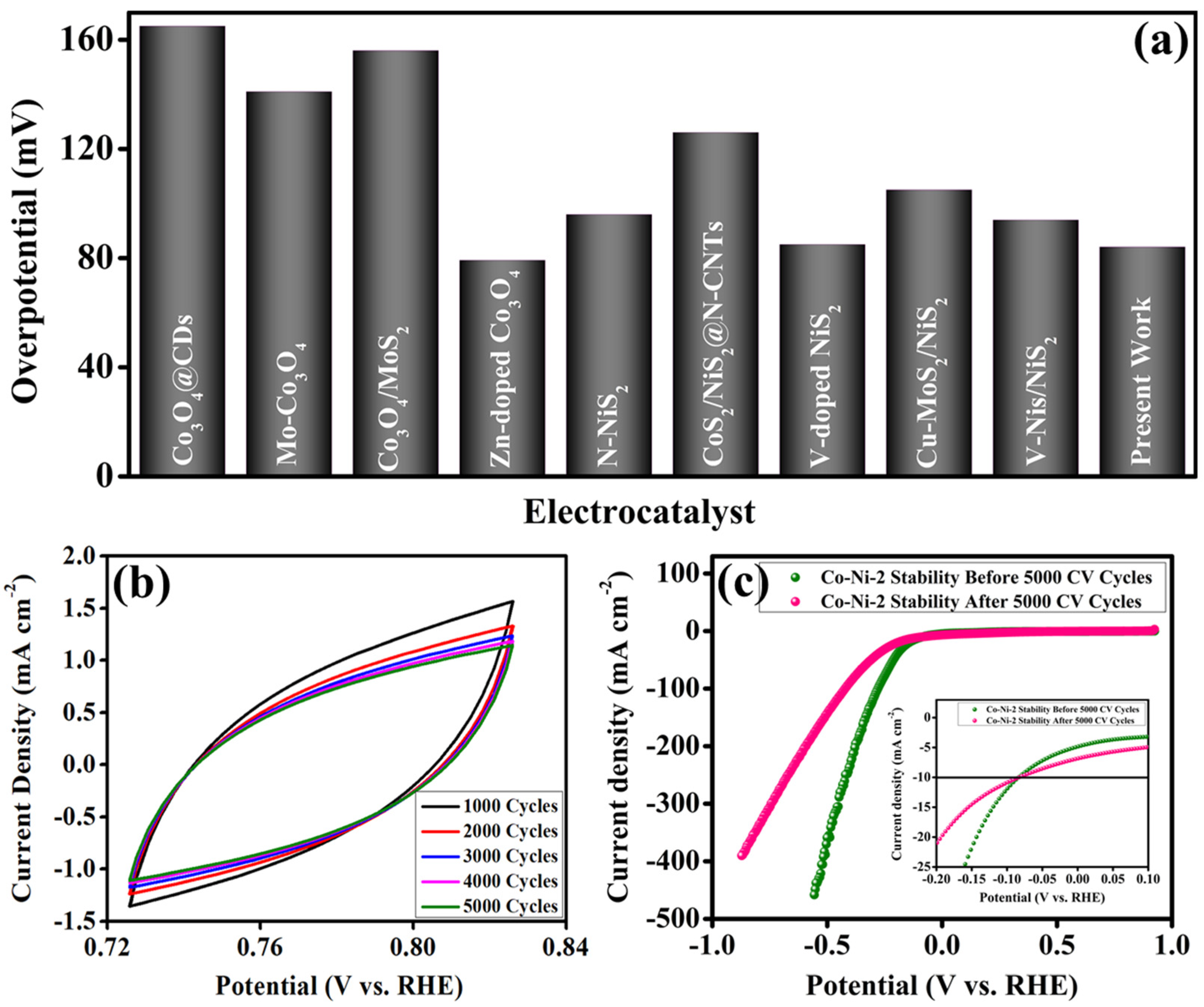
A comparative analysis of the overpotential values for Co-Ni-2 and previously reported electrocatalysts is presented in
Figure 5a and
Table 1. The data clearly indicate that the electrocatalytic performance of Co-Ni-2 is highly competitive, demonstrating similar or superior HER activity relative to other benchmark materials. This enhanced performance can be attributed to the synergistic integration of Co
3O
4 and NiS
2 phases within the composite, which collectively contribute to optimized electronic conductivity, increased active site density, and improved reaction kinetics. Beyond activity, long-term electrochemical stability is a critical principle in evaluating the practical applicability of electrocatalysts. To assess the stability of Co-Ni-2, continuous CV measurements were performed over 5000 cycles in alkaline media. The CV profiles recorded throughout the cycling test are illustrated in
Figure 5b. Additionally, the corresponding polarization curves before and after the 5000 CV cycles are shown in
Figure 5c. Remarkably, the Co-Ni-2 electrode maintained an overpotential of 84.11 mV at 10 mA cm
−2 even after prolonged cycling, showing negligible deviation from its initial performance. This negligible change in HER overpotential after extensive electrochemical cycling indicates excellent structural integrity, chemical robustness, and long-term operational stability of the Co-Ni-2 electrocatalyst under harsh alkaline conditions. These results establish Co-Ni-2 as a durable and high-performance candidate for sustained hydrogen evolution applications.
Table 1.
Comparison of present electrocatalyst’s HER performance with that of other reported electrocatalysts in 1.0 M KOH electrolyte.
Table 1.
Comparison of present electrocatalyst’s HER performance with that of other reported electrocatalysts in 1.0 M KOH electrolyte.
| Electrocatalyst | Overpotential @10 mA cm−2 (mV) | Ref. |
|---|
| Co3O4@CDs | 165 | [15] |
| Mo-Co3O4 | 141 | [11] |
| Co3O4/MoS2 | 156 | [16] |
| Zn-doped Co3O4 | 79.2 | [27] |
| N-NiS2 | 96 | [17] |
| CoS2/NiS2@N-CNTs | 126 | [28] |
| V-doped NiS2 | 85 | [29] |
| Cu-MoS2/NiS2 | 105 | [18] |
| V-NiS/NiS2 | 94 | [19] |
| Co3O4-NiS2 | 84 | [Present work] |
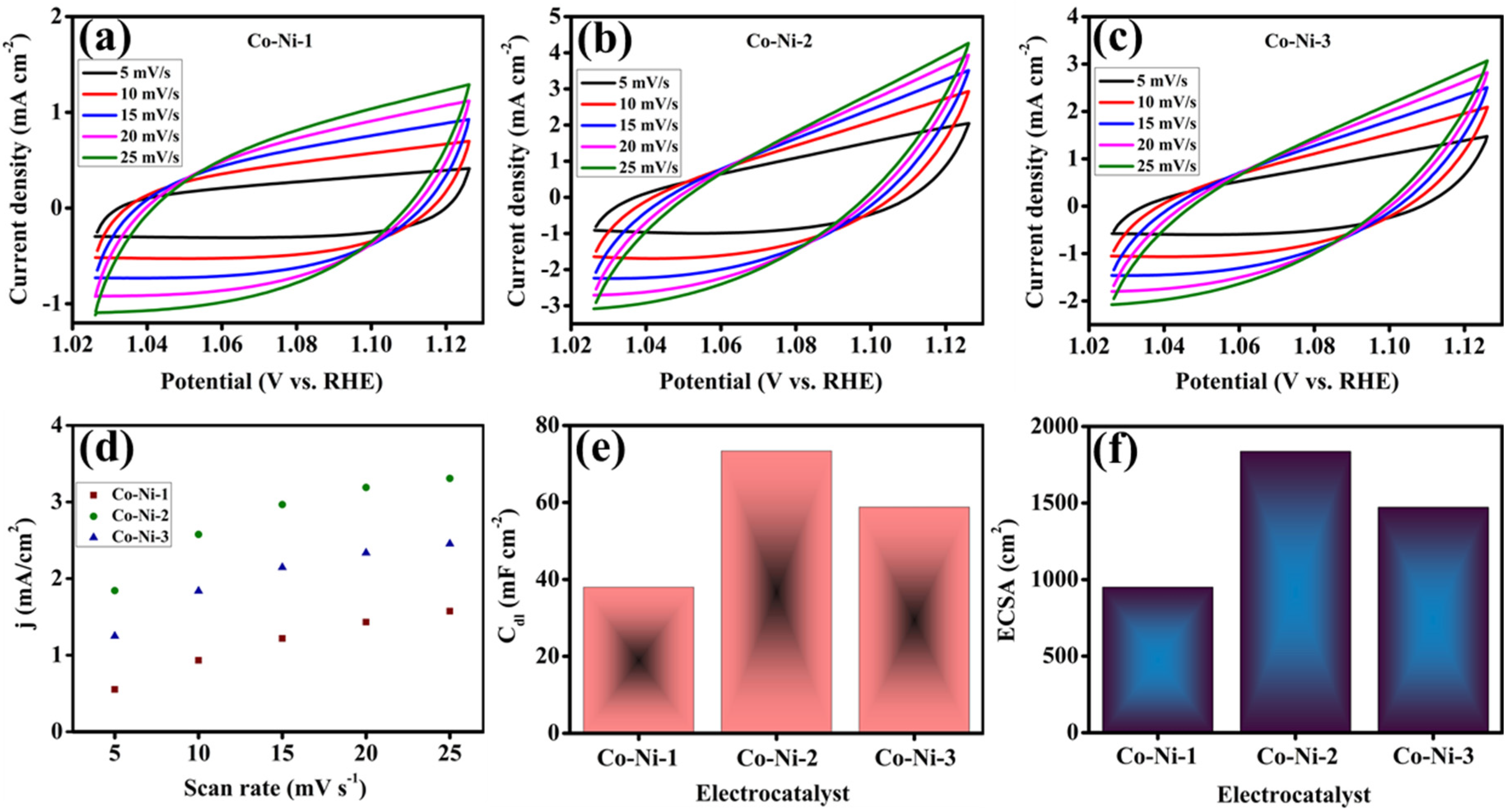
Figure 6 comprehensively illustrates the CV profiles of Co-Ni electrocatalysts with varying metal ratios recorded at different scan rates alongside the corresponding plots of current density versus potential. Specifically, the CV curves of Co-Ni-1 (
Figure 6a), Co-Ni-2 (
Figure 6b), and Co-Ni-3 (
Figure 6c) reveal a progressive increase in current density with increasing scan rate. This behavior highlights a strong scan rate dependence, suggesting rapid redox kinetics within the electrical double-layer region. To elucidate the relationship between electrochemical activity and the availability of catalytically active sites, the ECSA was estimated by evaluating 2C
dl and C
dl, which were derived from the non-Faradaic current response at different scan rates. The linear fitting of current density differences against the scan rate yielded the respective C
dl values for each sample, as shown in
Figure 6d,e. The C
dl values obtained for Co-Ni-1, Co-Ni-2, and Co-Ni-3 were 38.0, 73.4, and 58.8 mF cm
−2, respectively. The estimated ECSA values for Co-Ni-1, Co-Ni-2, and Co-Ni-3 were calculated to be 950.0, 1836.2, and 1471.2 cm
2, respectively, as presented in
Figure 6f. Among the studied compositions, Co-Ni-2 exhibits the highest ECSA, suggesting a substantial increase in electrochemically available active sites as a result of optimized nickel incorporation. The enhanced surface area not only promotes improved charge carrier accessibility but also facilitates a greater number of active centers for catalytic interaction during the HER.