Abstract
Halogenated flame retardants have been amongst the most widely used and effective solutions for enhancing fire resistance. However, their use is currently strictly regulated due to serious health and environmental concerns. In this context, phosphorus-based and mineral flame retardants have emerged as promising alternatives. Despite this, their combined use is neither straightforward nor guaranteed to be effective. This study scrutinizes the interactions between these two classes of flame retardants (FR) through a systematic analysis aimed at elucidating the antagonistic pathways that arise from their coexistence. Specifically, this study focuses on two inorganic fillers, mineral huntite and chemically precipitated magnesium hydroxide, both of which produce basic oxides upon thermal decomposition. These fillers were incorporated into a poly(butylene terephthalate) (PBT) matrix to be utilized as advanced-mattress FR coating fabric and were subjected to a series of flammability tests. The pyrolysis products of the prepared polymeric composite compounds were isolated and thoroughly characterized using a combination of analytical techniques. Thermogravimetric analysis (TGA) and differential thermogravimetric analysis (dTGA) were employed to monitor decomposition behavior, while the char residues collected at different pyrolysis stages were examined spectroscopically, using FTIR-ATR and Raman spectroscopy, to identify their structure and the chemical reactions that led to their formation. X-ray diffraction (XRD) experiments were also conducted to complement the spectroscopic findings in the chemical composition of the resulting char residues and to pinpoint the different species that constitute them. The morphological changes of the char’s structure were monitored by scanning electron microscopy with energy dispersive X-ray spectroscopy (SEM-EDS). Finally, the Limited Oxygen Index (LOI) and UL94 (vertical sample mode) methods were used to assess the relative flammability of the samples, revealing a significant drop in flame retardancy when both types of flame retardants are present. This reduction is attributed to the neutralization of acidic phosphorus species by the basic oxides generated during the decomposition of the basic inorganic fillers, as confirmed by the characterization techniques employed. These findings underscore the challenge of combining organophosphorus with popular flame-retardant classes such as mineral or basic metal flame retardants, offering insight into a key difficulty in formulating next-generation halogen-free flame-retardant composite coatings.
1. Introduction
Polybutylene Terephthalate (PBT) is a rigid, semicrystalline, thermoplastic polyester widely used in automotive, electronics, and electrical engineering industries, as well as in the production of masterbatches for filled PET yarns [1,2]. However, when PBT thermally decomposes, it releases a large amount of flammable gas, leaving minimal solid residue [3]. Its high flammability and dripping behavior during combustion points to the need to produce flame-retardant PBT composites, especially for domains where fire protection is required [4,5], like in mattress ticking fabric coatings.
To date, the most effective flame-retardants for PBT have been halogen-based. These materials have raised serious environmental and safety concerns as, when burned, halogenated flame-retardants generate toxic fumes and smoke [5,6,7], resulting in stricter regulations regarding their use. Therefore, the development of non-toxic and environmentally sustainable flame-retardant systems is crucial.
Phosphorus-based fillers, such as APP [8], DOPO [9], encapsulated red phosphorus [10] etc., are halogen-free flame-retardant additives widely employed to create intumescent flame-retardant (IFR) systems [11,12]. Organophosphorus compounds, such as phosphinates, phosphonates, or phosphate esters, are often combined with polyester-based polymer matrices, such as PBT [13,14]. Upon combustion, these compounds act as acid donors, producing H3PO4, which condense to pyrophosphoric structures by releasing water. Simultaneously, the decomposing polyester produces hydroxyl-containing species that act as a carbon source [15,16]. As the temperature increases, the phosphoric acidic species catalyze the dehydration of the newly formed hydroxyl groups, promoting the production of double bonds that contribute to the formation of carbonaceous solid residue (char). Furthermore, the pyrolysis of the ester groups generates carbon-rich fragments capable of undergoing crosslinking and polymerization. Specifically, according to Camino et al. [16], Diels–Alder reactions might occur, leading to the formation of cycloalkene, aromatic, and fused-ring char structures. The water released during the condensation of H3PO4 catalyzes ester hydrolysis reactions that lead to the formation of aldehyde groups or ether groups that can act as bridging moieties. In the gas phase, the flame-retardant mechanism of organophosphorus compounds includes the dilution of the oxygen available for combustion via the release of water vapor, along with the evolution of phosphorus-based radicals, such as HPO2−, HPO−, PO2−, and PO. These free radicals scavenge the volatile OH− and H2− free radicals produced by the polymer matrix, thereby suppressing flame propagation [3,17].
Another type of cost-effective and environmentally friendly flame-retardant filler are mineral entities, such as huntite, hydromagnesite [18], dolomite [19], etc., and inorganic fillers, like Mg(OH)2, Al(OH)3, etc. [20,21]. Achieving significant flame-retardant effects with these fillers typically requires high loadings of up to 60–70% [22].
This study focuses on Mg(OH)2 and huntite, both of which are thermally compatible with PBT due to their decomposition temperatures exceeding the PBT processing temperature range of 220–260 °C [23]. Mg(OH)2 can be either chemically precipitated or derived from hydromagnesite–huntite mixtures, as reported by Andrikopoulos et al. [18]. It decomposes at around 350 °C by releasing water vapor and forming a protective inorganic MgO layer [24] that insulates the polymer from oxygen and other combustible gases [25]. However, due to its alkaline nature, Mg(OH)2 is inherently incompatible with PBT, as it catalyzes ester hydrolysis [25,26,27]. To address this issue, Mg(OH)2 was surface-modified with stearic acid (SA) to render it more hydrophobic and thus compatible with polyester matrices, as described by Zhan et al. [27].
Huntite (Mg3Ca(CO3)4), between 400 °C and 700 °C, produces MgO and CaO inorganic solid residues, along with CO2 vapor [18,28]. Due to their coexistence in natural deposits, obtaining completely pure huntite is difficult [18,29]. While hydromagnesite (Mg3(CO3)4(OH)2·3H2O) contributes additional flame-retardant benefits by releasing water during its decomposition, its low decomposition temperature (~220 °C) makes it unsuitable for PBT processing [18,30,31]. Moreover, pure huntite has not shown significant flame-retardant efficacy for polyester matrices, due to its high decomposition onset temperature of 400 °C, relative to the flash point of PBT (~350 °C). Nevertheless, Hollingbery et al. [31] highlighted the synergistic effects observed in huntite–hydromagnesite blends.
The question was reasonably raised as to whether the combination of organophosphate flame retardants with mineral or inorganic fillers would yield improved flame-retardant properties. Liao et al. [32] have already reported that phosphorus-based and basic mineral flame-retardants exhibit antagonistic behavior when combined together. Specifically, in their study, a phosphorus-containing epoxy resin was doped with Mg(OH)2 resulting in a fire retardancy drop, attributed to the formation of magnesium phosphate salts.
In the present work, a PBT matrix containing a commercial undisclosed phosphorus-based flame retardant (PES FR 1304) was blended with either a huntite-rich mineral or surface-treated Mg(OH)2 to thoroughly investigate this apparent competitive behavior. To this end, an in-depth spectroscopic characterization of the char residues produced during the pyrolysis of relevant PBT composites has been conducted. ATR-FTIR, Raman, and XRD, supported by TGA and SEM combined with EDX, are employed to identify species that are regenerated and to clarify the mechanism of their formation. This study ultimately aims to provide a comprehensive chemical analysis of the species involved in this competitive interaction and the cause of this action, and to offer critical knowledge to advance the understanding of intumescent flame-retardant (IFR) systems.
2. Materials and Methods
2.1. Materials
The PBT polymer (CAS No. 30965-26-5) in pellet form was sourced from SASA A.S., Adana, Turkey, while the PBT-based PES FR 1304, a commercial flame retardant containing 59,000 ppm phosphorus, was a product of Setaş Colour Company. HyperCarb 2090, a rich in huntite (approx. 90% by weight) mineral product, was supplied by LKAB Minerals, Luleå, Sweden. Magnesium Dihydroxide (CAS No. 1309-42-8) and Stearic acid (CAS No. 57-11-4) were purchased from Carlo Erba (Cornaredo, Italy) and Sigma Aldrich (St. Louis, MO, USA), respectively.
2.2. Surface Modification of Mg(OH)2
Initially, 0.3 g of stearic acid (SA) was added to 100 mL of water at 75 °C, and the mixture was stirred until the SA fully melted. Subsequently, 4.7 g of magnesium hydroxide, Mg(OH)2, were introduced into the mixture, which was stirred for another 30 min to ensure uniform dispersion. The resulting dispersion was then subjected to ultrasonic treatment for 1.5 h [27]. Finally, the modified filler was collected and dried in an oven.
2.3. Compound Sample Preparation
A series of PES FR 1304 compounds was produced with varying concentrations of huntite (HUN) or Mg(OH)2 (MDH) modified with stearic acid (m-MDH). Two control samples based on a pristine PBT polymer matrix were also prepared. All the samples are listed in Table 1.

Table 1.
List of specimens prepared and their filler content.
The polymer compounds were prepared by combining the PES FR 1304 or PBT and the powdered fillers (MDH or HUN), using a homemade melt mixer under a nitrogen atmosphere at 250 °C, which is the typical processing temperature for PBT-based polymer matrices, for a duration of 10 min. Compounding of PBT-MDH is usually challenging due to the hydrolytic degradation of PBT in the presence of MDH [25,26], a difficulty also encountered in this study. In contrast, no such issues were encountered while blending PES FR 1304 with MDH filler that had been surface-modified with SA, referred to as m-MDH.
Specimens for Limited Oxygen Index (LOI) measurements, with dimensions conforming to ASTM D2863, were then prepared by melting the compounds in an aluminum mold using a hot press at 30 bar and 250 °C. The flame retardancy performance of selected FR compounds was determined according to UL94V, as well. For each examined compound, 5 specimens in the form of bars of ca. 125 × 13 mm2 and thickness varying from ca. 0.7 to 1.0 mm were prepared by compression molding at 250 °C and ca. 20 bar and measured according to the standard.
In this work the term “char” is descriptive of the solid residues of all the samples based on the PES FR 1304 polymeric matrix no matter the simultaneous presence of inorganic solid residue from the HUN and MDH filler content.
2.4. Characterization and Analysis Methods
The FTIR-ATR (Fourier Transform Infrared—Attenuated Total Reflectance) spectra were obtained using an Alpha-II Diamond ATR Spectrometer (Bruker Optics GmbH, Ettlingen, Germany). Raman spectra were recorded on a T-64000 spectrometer (Jobin Yvon, Horiba Group) equipped with a Cobolt Fandango™ ISO laser operating at 514.5 nm as the excitation source.
Thermogravimetric analysis (TGA) and differential thermogravimetric analysis (dTGA) were performed using a TGA 550 analyzer (TA Instruments, New Castle, DE, USA) under air atmosphere with a flow rate of 20 mL/min and a 10 °C/min heating rate.
Limited Oxygen Index (LOI) measurements were conducted using an in-house apparatus equipped with two high-accuracy Alicat mass flow meters, following ASTM D2863 specifications.
UL-94 vertical burning tests were performed according to the UL 94 standard developed by Underwriters Laboratories to assess vertical flame retardancy and the V-0, V-1, or V-2 classification of the tested samples.
Scanning electron microscopy with Energy Dispersive X-ray Spectroscopy (SEM-EDS) was used to display the morphological features of the solid residues derived by different samples with the elemental analysis provided by EDS. A Zeiss SUPRA 35VP (Carl Zeiss AG, Oberkochen, Germany) system operating at 5 kV voltage was used for this purpose. Every sample was platinum-coated prior to imaging.
X-ray diffraction (XRD) was used to identify the crystalline phase of all composites. XRD measurements were carried out by using a Bruker D8 Advance diffractometer (Bruker Optics GmbH, Karlsruhe, Germany) equipped with a Cu lamp (λCuKa = 1.54046 Å) at a scanning rate of 2°/min over a range of 5–80° (2θ). Temperature-dependent XRD measurements were also carried out using the same XRD instrument equipped with an XRK900 reactor chamber (Anton Paar GmbH, Graz, Austria).
3. Results
3.1. Characterization of the PES FR 1304 Matrix
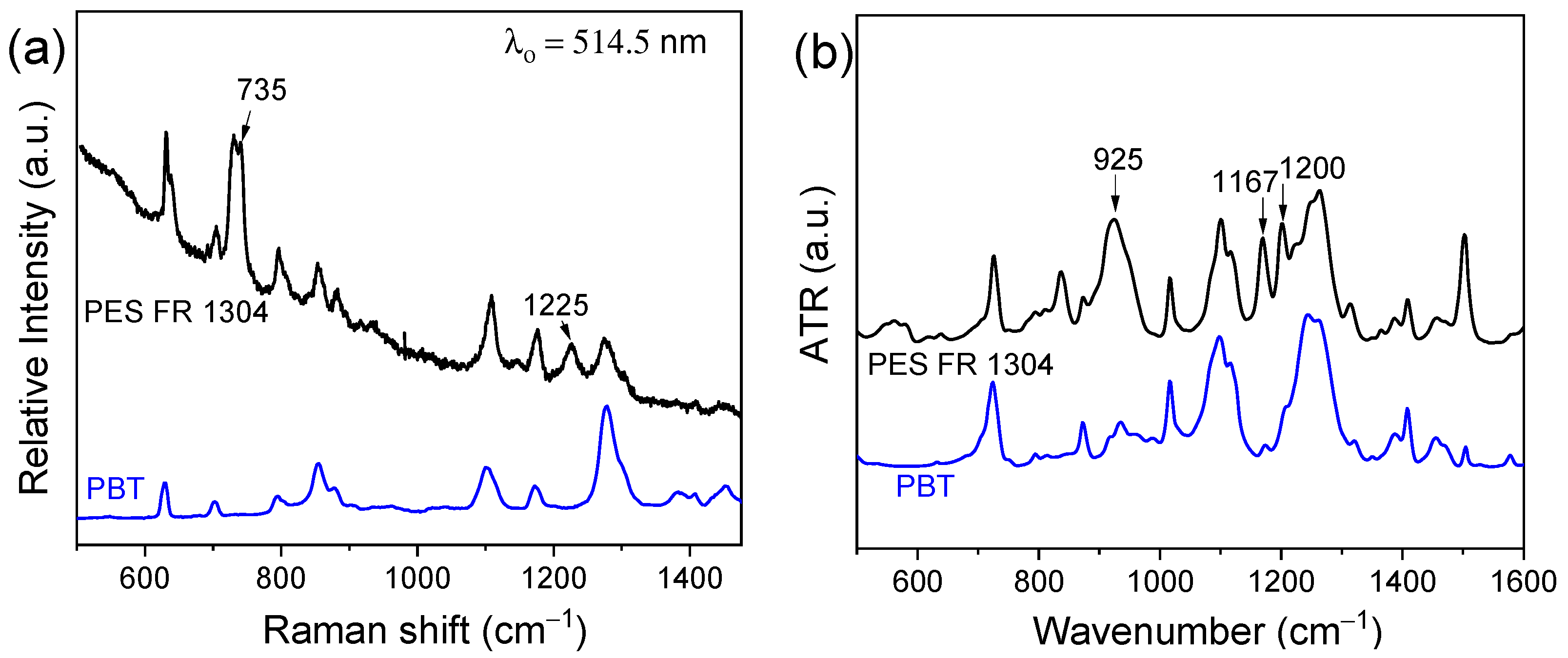
PES FR 1304 is a commercial polymer matrix containing an undisclosed type of phosphorus-based flame retardant. Figure 1a presents the Raman spectra of pure PBT (blue) and PES FR 1304 (black), both based on a PBT matrix. Two additional peaks appear in the PES FR 1304 spectrum at 735 cm−1 and 1225 cm−1, corresponding to the stretching vibrations of the P-C bond and the P-O-Ar group, respectively, indicating the presence of a phosphinate or phosphonate compound [33,34]. Figure 1b displays the FTIR-ATR spectra of pure PBT (blue) and PES FR 1304 (black). Peaks at 1167 cm−1 and 1200 cm−1 are attributed to the stretching vibration of organic phosphorus P=O bond [33], while the peak at 925 cm−1 is assigned to the symmetrical stretch of the P-O-Ar group, suggesting the presence of an aryl group. These functional groups are indicative of the presence of either a phosphinate or phosphonate flame-retardant filler [34].
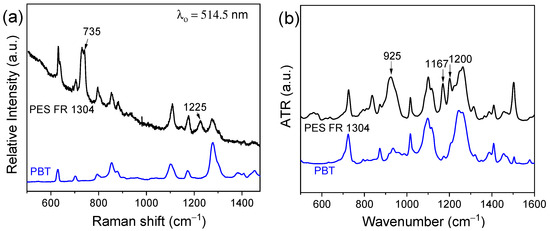
Figure 1.
Raman spectra (a) and FTIR-ATR spectra (b) of PES FR 1304 (black-top) and PBT (blue-bottom).
3.2. Characterization of the SA-Modified MDH Filler
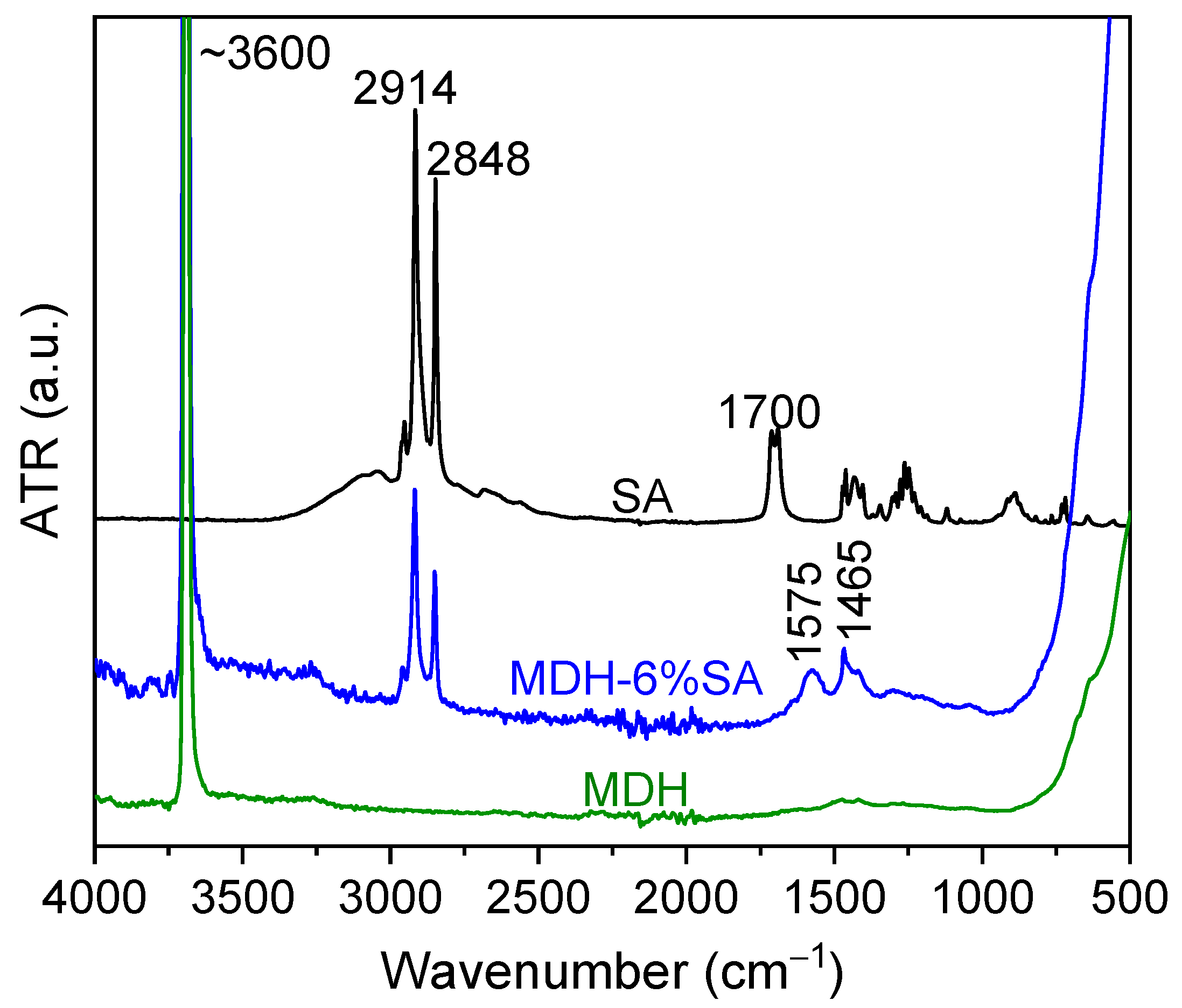
Figure 2 displays the FTIR-ATR spectra of the surface-modified magnesium hydroxide filler (m-MDH), alongside those of pure SA and unmodified MDH. The MDH spectrum shows the characteristic O-H bond near 3600 cm−1, while the SA additive displays C-H symmetric and asymmetric stretching peaks at 2848 and 2914 cm−1, respectively. The spectrum of pure SA presents the characteristic peak at 1700 cm−1 attributed to the carbonyl C=O bond vibration. The absence of this peak in the modified MDH (MDH-6% SA) indicates that no unreacted SA was detectable. Instead, peaks at 1576 and 1465 cm−1 appear, corresponding to the symmetric and asymmetric stretching of the carboxylate (COO−) group, suggesting chemical bonding between SA and Mg(OH)2 [27].
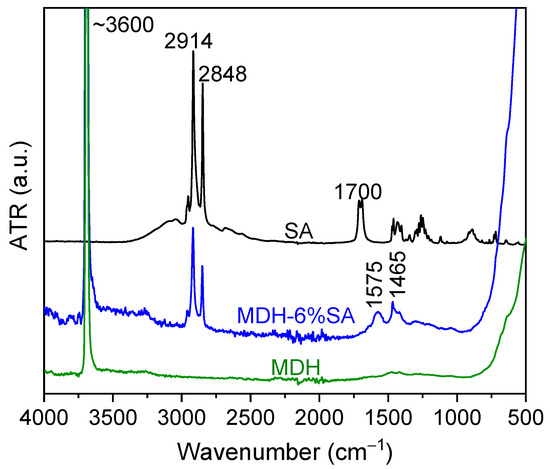
Figure 2.
FTIR-ATR spectra of pure MDH (green), MDH modified with 6% SA (m-MDH) (blue), and of pure SA (black).
3.3. Flame-Retardancy Assessment—Limited Oxygen Index (LOI) and UL94V Test Results
LOI measurements were conducted to evaluate the effect of m-MDH and HUN fillers on the flame retardancy of PES FR 1304. The LOI was calculated using the following Equation:
where [O2] and [N2] correspond to the concentrations of oxygen and nitrogen delivered in the testing compartment. The LOI was measured for each sample in triplicate. As shown in Table 2, the LOI of PES FR 1304 decreased with increasing content of either m-MDH or huntite. This decline suggests a potential antagonistic interaction between the phosphorus-based flame retardant and the chosen fillers.

Table 2.
LOI values of prepared samples.
To be able to quantify the antagonistic effect between the basic inorganic fillers and PES FR 1304, the Lewin concept of synergistic effectiveness, ES, was applied [35,36]. The ES value is calculated from the LOI values of the samples that contain 20% of HUN or MDH. In Equation (2), (FP)p is the flame-retardancy of pure PBT, (FP)fr is that of PES FR 1304, (FP)s is that of PBT containing either HUN or MDH, and (FP)[fr+s] is that of the formulation based on PES FR 1304 containing the same respective concentrations of HUN or MDH.
According to the Lewin definition, when ES > 1 it can be concluded that the two fillers work in synergy and a formulation is antagonistic when the ES value falls below zero. The ES values for the samples containing 20% HUN and 20% MDH are ES = 0.23 and ES = 0.19, respectively, as both are positive but <1. This leads to the conclusion that the inorganic fillers greatly blunt the performance of PES FR 1304 but do not diminish the LOI below the level achieved by either component on its own.
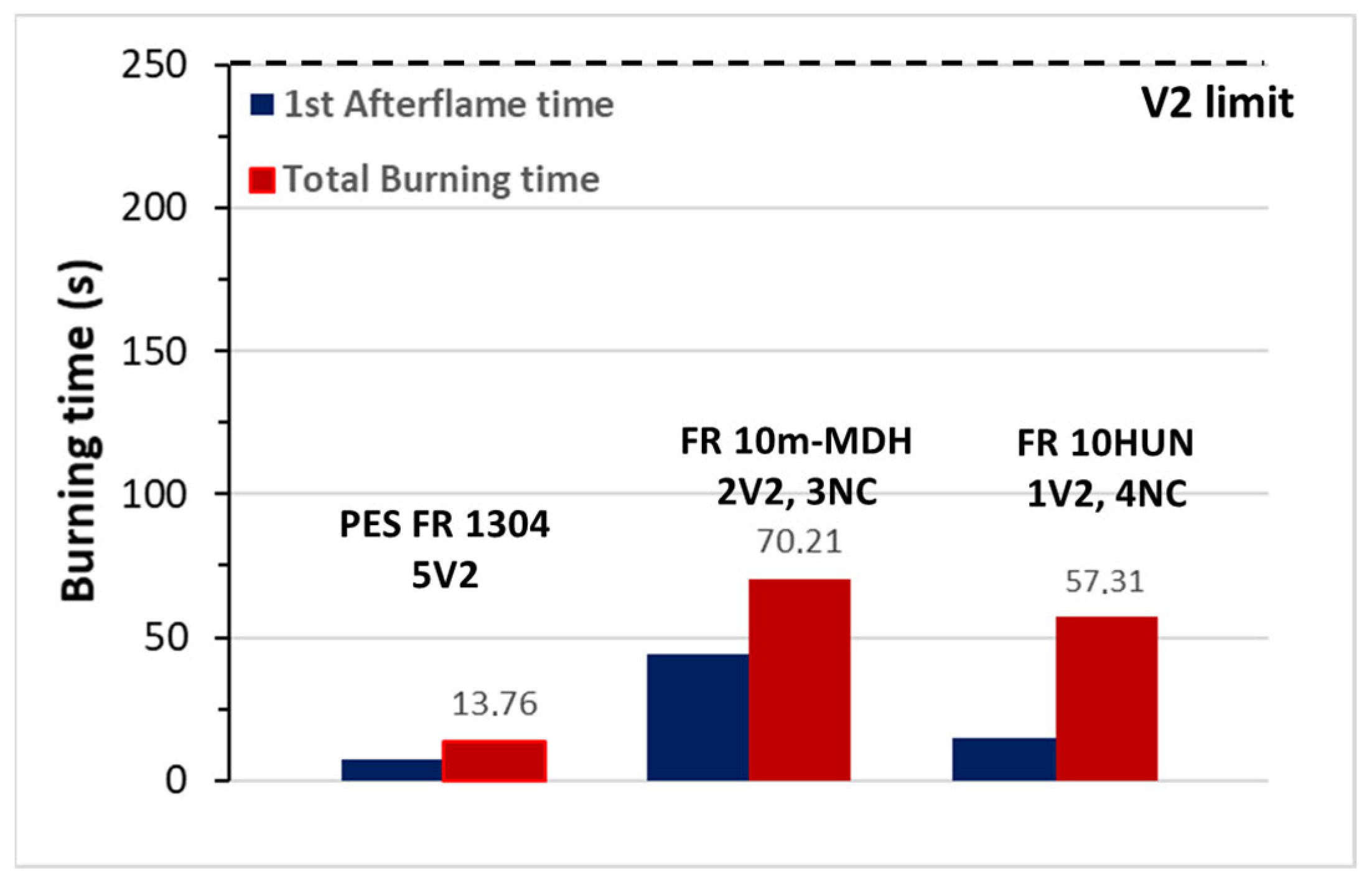
In order to further prove the antagonistic behavior between phosphorus-containing FRs and basic oxide-forming minerals, three compounds were selected, i.e., PES FR 1304, FR 10m-MDH, and FR 10HUN, and their FR performance was determined according the UL94V test. Accordingly, FR1304 samples clearly reached a V2 category, due to the observation of flame dripping (ignition of cotton indicator); although the recorded total burning time was <15 s for the five measured specimens (Figure 3). On the contrary, both FR 10m-DH and FR 10HUN exhibited inferior flame retardancy behavior, with almost all samples being burnt up to the holding clamp, and were thus categorized as non-classified (NC). Moreover, production of thick black smoke was observed upon the combustion of the pertinent specimens. The latter clearly reflects that the mineral FRs are indeed antagonistic to the respective phosphorous ones, which is completely in line with the reduced LOI values shown in Table 2.
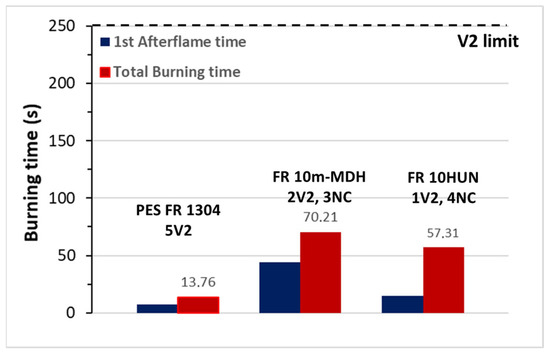
Figure 3.
UL94V test for PES FR 1304, FR 10m-MDH, and FR 10HUN samples. Total burning time per 5 specimens.
3.4. Sample Thermogravimetric Analysis Results
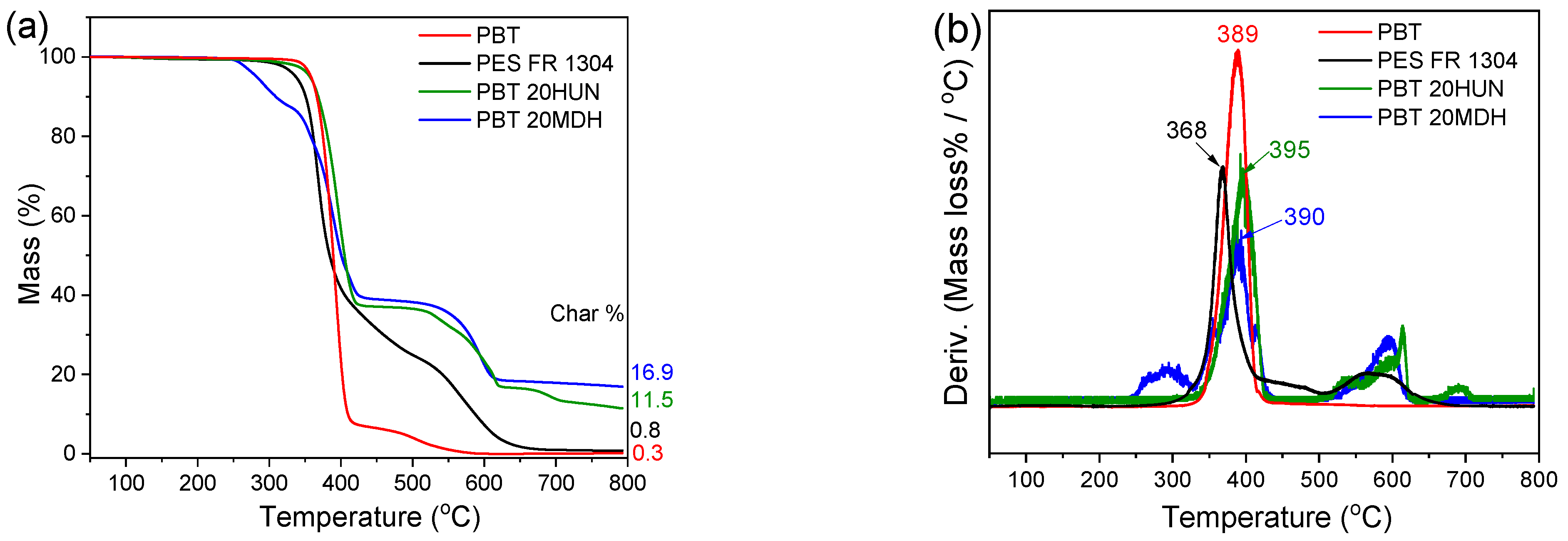
All the samples described in Table 1 were analyzed by TGA and dTGA up to 800 °C. The PES FR 1304 neat polymer decomposes similarly to PBT, with its primary degradation slightly before 400 °C (Figure 4), which is indicative of a typical PBT-based polymer. Its earlier degradation onset, relative to neat PBT, is confirmed by the shift of the dTGA main degradation peak towards lower temperatures. PES FR 1304 also presents a secondary mass loss region between 500 and 650 °C, associated with the degradation of the carbonaceous char typically forming in IFR systems.
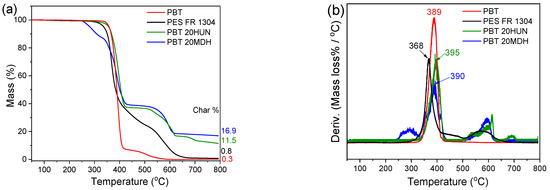
Figure 4.
TGA (a) and dTGA (b) of pure PBT (red), PES FR 1304 (black), PBT 20HUN (green), and PBT 20MDH (blue).
The carbonaceous char persists up to 650 °C before its full volatilization by 800 °C. The pure PBT sample does not follow this degradation pattern; however, this secondary mass loss region is also present in the PBT 20HUN and PBT 20MDH samples. These samples do not contain organophosphorus species, but only PBT and the corresponding filler additive, so the produced solid residue results from the formation of CaO and MgO decomposition products and their interaction with the PBT matrix.
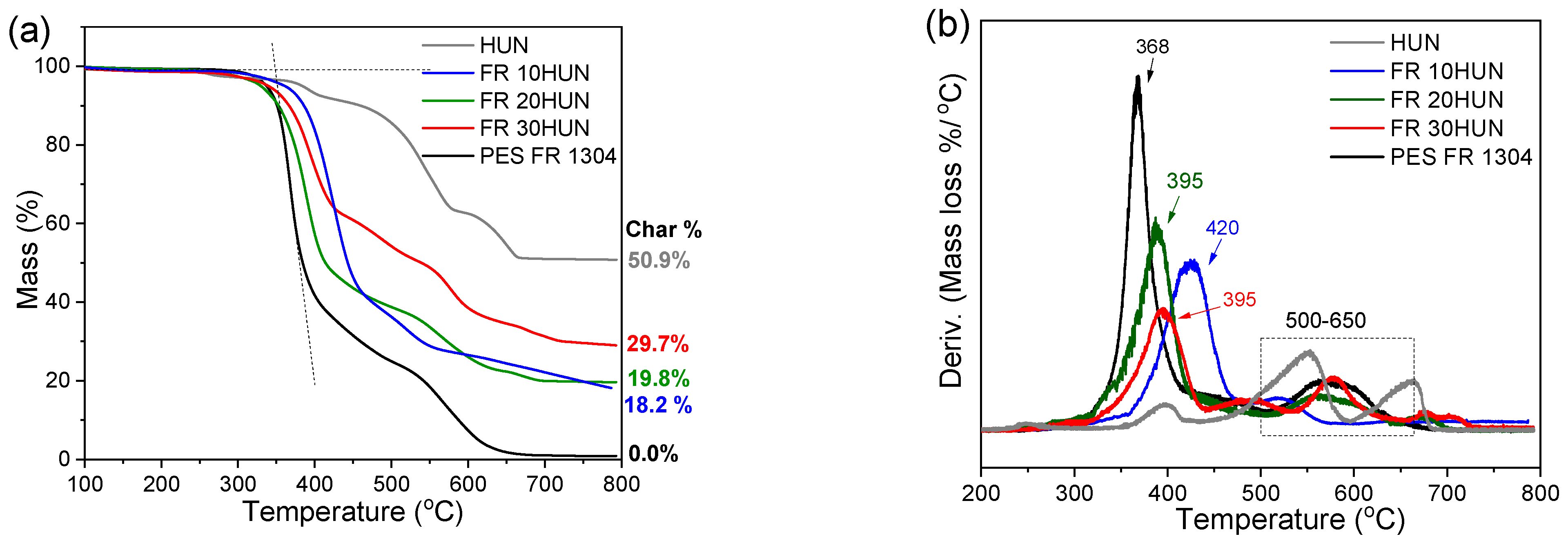
Figure 5 and Figure 6 present TGA/dTGA curves of the FR HUN and FR m-MDH samples, respectively. The mass loss region attributed to char degradation for the FR HUN samples is between 500 and 650 °C and for the FR m-MDH samples between 470 and 700 °C. As the PES FR 1304 matrix decomposes, the H3PO4 acid produced catalyzes the carbonaceous char formation. For PES FR 1304-based samples containing HUN and m-MDH, the basic oxides formed during these fillers’ decomposition neutralize H3PO4, suppressing its catalytic role in char formation. As a result, each sample forms a unique char structure depending on filler type and content. Despite structural differences, thermal stability remains comparable across the series, as presented in the thermograms of Figure 4 and Figure 5.
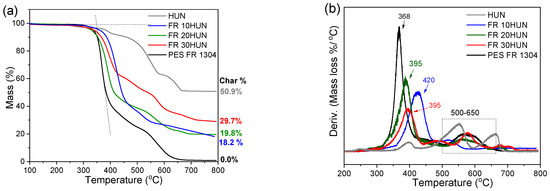
Figure 5.
TGA (a) and dTGA (b) of pure PES FR 1304 (black), FR 10HUN (blue), FR 20HUN (green), FR 30HUN (red), and pure HUN (grey).
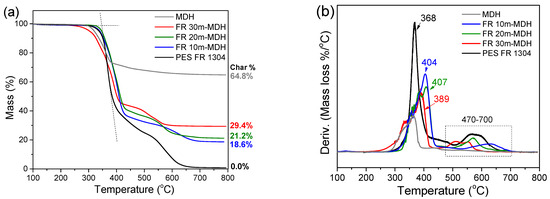
Figure 6.
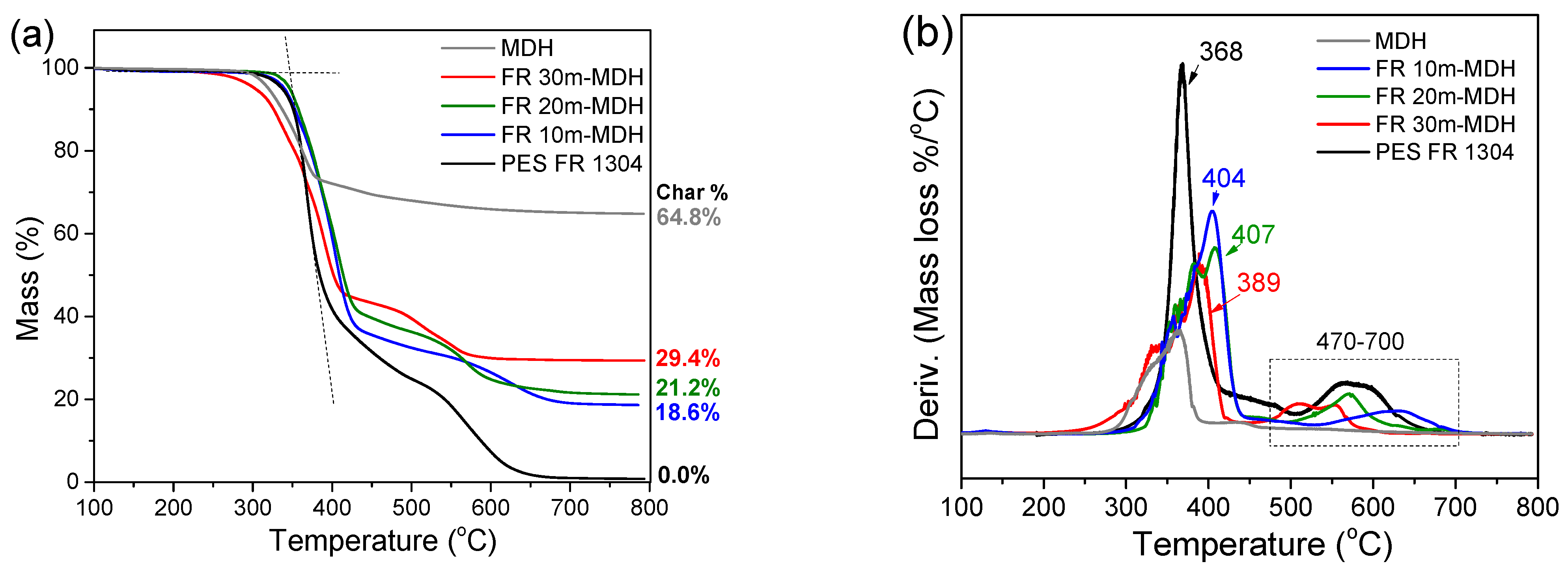
TGA (a) and dTGA (b) of pure PES FR 1304 (black), FR 10m-MDH (blue), FR 20m-MDH (green), FR 30m-MDH (red), and pure MDH (grey).
Figure 6 also shows the varying thermal stability of the char in the FR m-MDH samples. The FR 30m-MDH sample’s char decomposes first, between 465 and 600 °C, followed by the FR 20m-MDH sample’s char, between 500 and 650 °C, and lastly the FR 10m-MDH sample’s char between 540 and 700 °C. This trend likely arises from the early thermal decomposition of the SA coating, which leaves the m-MDH filler uncoated to interact with and hydrolyze the polyester, reducing the length of its chains [26]. Consequently, the hydrolyzed polyester of reduced molecular weight forms a char that is less thermally stable.
As can be seen in Table 3, a significant shift of the PES FR 1304 main decomposition peak from 368 °C to higher temperatures was noticed upon the inclusion of m-MDH and HUN fillers indicating a more thermally stable compound. On the other hand, the FR m-MDH samples begin their decomposition at notably lower temperatures, with FR 30m-MDH starting at 307 °C. This probably happens due to hydrolysis-induced early polymer decomposition. Premature polyester hydrolysis can also be noticed in Figure 4 for the PBT 20MDH sample’s thermogram that contains uncoated filler. The same effect was also noticed and reported by Viretto et al. [26]. The final solid residue at 800 °C for all the samples varies in mass according to filler percentage.

Table 3.
Data obtained from TGA analysis.
3.5. Spectroscopic Analysis of TGA-Derived Solid Residues
3.5.1. Analysis of the Solid Residue Collected After the Sample’s Thermal Decomposition at 800 °C
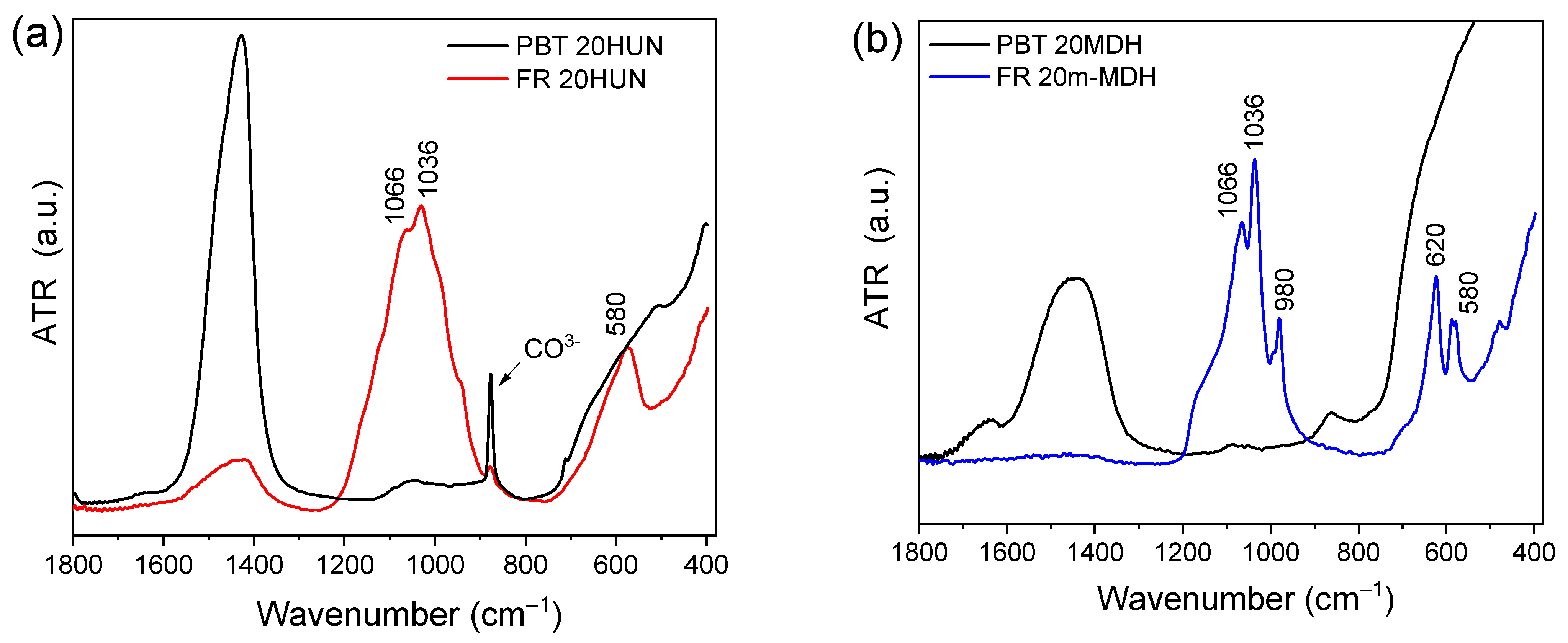
FTIR-ATR and Raman spectroscopy were used to characterize the residue collected from the TGA analysis at 800 °C. Figure 7 presents the FTIR-ATR spectra of FR 20m-MDH, FR 20HUN, PBT 20MDH (using unmodified MDH), and PBT 20HUN samples. For the PES FR 1304-based samples (FR 20HUN and FR 20m-MDH), it is suggested that the MgO and CaO formed from the decomposition of MDH and HUN reacted with the H3PO4 generated by the phosphorus-based flame retardant forming various magnesium, calcium phosphate, and pyrophosphate salts [32]. This occurred because the MgO and CaO are basic oxides, while H3PO4 is a strong acid. This process is highlighted in the reactions below:
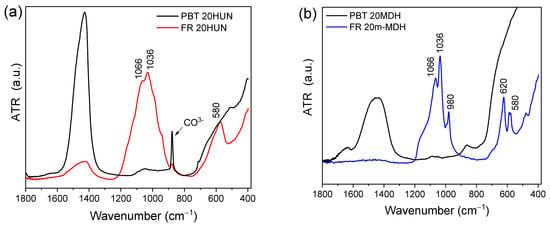
Figure 7.
FTIR-ATR spectra of the solid residue collected at 800 °C from TGA measurements corresponding to the following: (a) PBT 20 HUN (black) and FR 20HUN (red) and (b) corresponding to PBT 20MDH (black) and FR 20m-MDH (blue) on the right.
Magnesium Dihydroxide (MDH) decomposition [26]:
350–400 °C: Mg(OH)2 → MgO + H2O
H3PO4 + MgO → Mg3(PO4)2
Huntite (HUN) decomposition [18]:
400–550 °C: Mg3Ca(CO3)4 → 3MgO + CaCO3 + 3CO2
H3PO4 + MgO → Mg3(PO4)2
550–700 °C: CaCO3 → CaO + CO2
H3PO4 + CaO → Ca3(PO4)2
The portion of H3PO4 that reacts with the basic oxides is neutralized, and is thus prevented from catalyzing the char formation. The main peaks corresponding to the magnesium and calcium phosphate salts are observed in Figure 7. Specifically, for the samples FR 20m-MDH and FR 20HUN, the characteristic peak of O-P-O is noticed in the 500–700 cm−1 region while peaks corresponding to P-O appear in the 900–1200 cm−1 region [37,38]. On the other hand, the PBT 20MDH and PBT 20HUN samples present a broad band in the 1400 cm−1 region that corresponds to the formation of MgO and CaO oxides. The peak at 878 cm−1, present on the spectra of the PBT 20HUN sample, is attributed to the presence of .
3.5.2. Analysis of the Solid Residue Collected During Sample’s Degradation
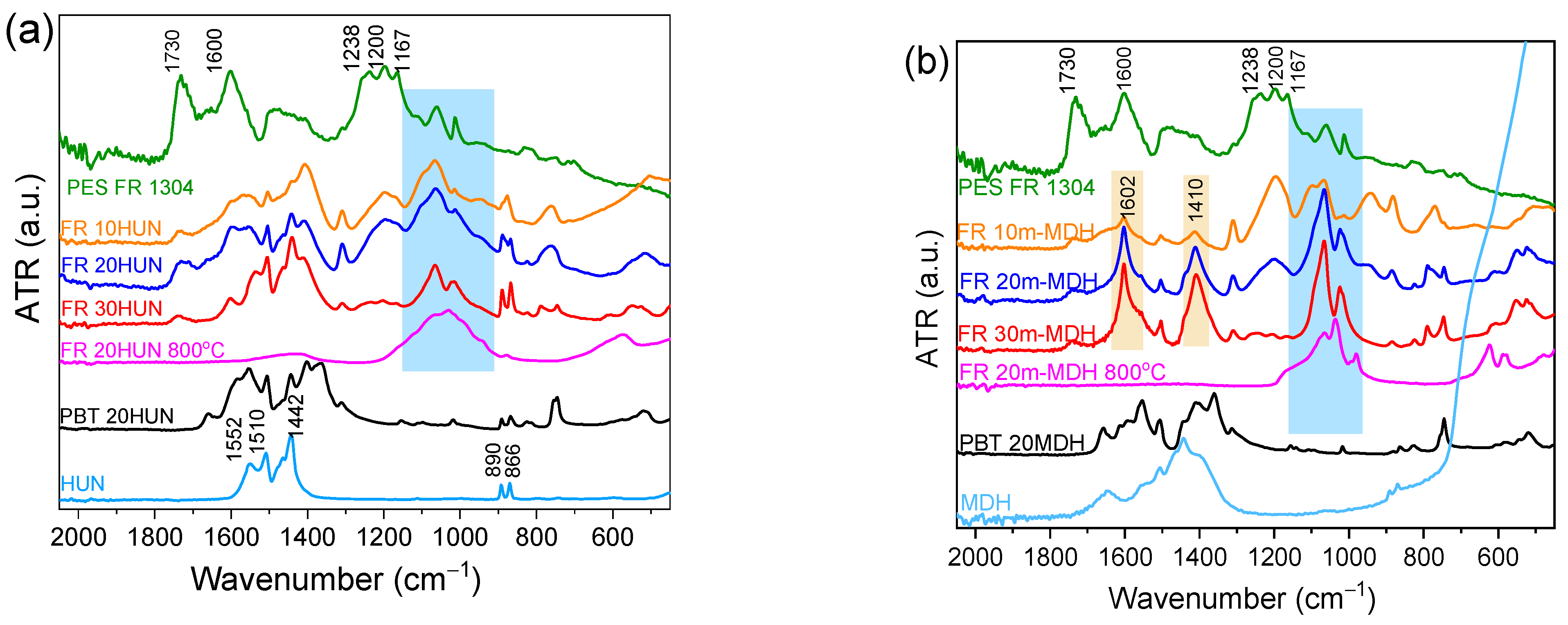
In an effort to understand the nature of the species created during sample degradation, the TGA measurements corresponding to each sample were interrupted exactly at the point before the flame-retardant char begins to decompose. This is considered the point at which the sample contains the maximum amount of insulating char before this starts to volatilize. Figure 8a shows the FTIR-ATR spectra of the solid residues collected at approximately 500 °C, from the FR 10HUN, FR 20HUN, FR 30HUN, PES FR 1304, PBT 20HUN, and pristine HUN samples, along with the spectra of the char derived by the FR 20HUN sample upon heating to 800 °C. Similarly, Figure 8b shows the FTIR-ATR spectra of the solid residues collected at approximately 500 °C of the FR 10m-MDH, FR 20m-MDH, FR 30m-MDH, PES FR 1304, PBT 20MDH, and pristine MDH samples, along with the spectra of the char derived by the FR 20m-MDH sample upon heating to 800 °C.
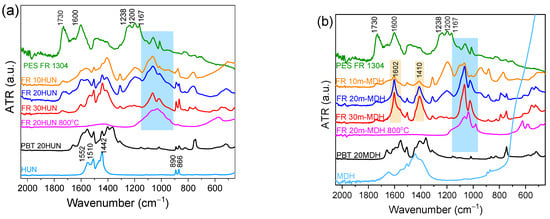
Figure 8.
(a) FTIR-ATR spectra of the residue derived around 500 °C from PES FR 1304 (green), FR 10HUN (orange), FR 20HUN (blue), FR 30HUN (red), PBT 20HUN (black), HUN (cyan), and from PBT 20HUN at 800 °C (magenta). (b) FTIR-ATR spectra of the residue derived around 500 °C from PES FR 1304 (green), FR 10m-MDH (orange), FR 20m-MDH (blue), FR 30m-MDH (red), PBT 20MDH (black), MDH (cyan), and from FR 20m-MDH at 800 °C (magenta).
It is worth noting that the FTIR-ATR spectra of the inorganic solid residue from samples PBT 20HUN and PBT 20MDH do not exhibit any peaks in the 900–1200 cm−1 range (Figure 8). This confirms that the peaks observed in this spectral region for the other samples shown in Figure 8 are attributed to the phosphorus species present in PES FR 1304 and its decomposition products. These peaks correspond to the inorganic magnesium and calcium phosphate salts present in the char collected from the FR 20HUN (both magnesium and calcium salts) and FR 20m-MDH (magnesium salts only) samples at 800 °C [37,38]. These peaks are also noticed in the spectra of the rest of the samples that contain PES FR 1304 with HUN or MDH, indicating the occurrence of neutralization reactions between H3PO4 and the MgO/CaO oxides that lead to the formation of phosphate salts. These peaks are attributed to species and specifically to the P-O bonds. It could be argued that the same peaks appear also on the spectra of the pure PES FR 1304 where the MDH and HUN fillers are absent; so, these peaks could not be related to the salts produced during the composite decomposition. In this case, these peaks are attributed to phosphonate or phosphinate residues and polyphosphoric or pyrophosphoric fragments, all of which are active in the 900–1200 cm−1 and attributed to P-O bonds. We could conclude that the char obtained from PES FR 1304 samples containing either HUN or m-MDH, probably contains both organic and inorganic phosphorus compounds simultaneously. This issue will be elucidated in the next sections. The peaks at 1167 and 1200 cm−1, which were present also in the FTIR-ATR spectra of pure PES FR 1304 depicted on Figure 1, are attributed to organic phosphorus P=O bonds, while the 1238 cm−1 peak is attributed to the asymmetrical stretching of the P-O-Ar bond [34]. These peaks are still strong in the spectra of the char collected from the FR 10HUN and FR 10m-MDH samples even though slightly diminished. They diminish even further in the FR 20HUN, FR 20m-MDH, FR 30HUN, and FR 30m-MDH samples, indicating the conversion of the organic phosphorus species present in the char into inorganic phosphorus species. The 1600 cm−1 peak observed in the pure PES FR 1304 spectrum indicates the formation of carbonaceous char, probably containing graphite-like and fused ring structures; this peak also diminishes greatly with the presence of HUN and m-MDH indicating the absence of char of this type. Moreover, the 1730 cm−1 peak, which is tentatively attributed to the presence of oxidated entities integrated on the char structure, diminishes as the percentage of HUN and m-MDH increases, indicating the prohibition of char formation. In contrast, the spectral characteristics attributed to organic and inorganic phosphorus compounds and the formation of carbonaceous char are not presented in the solid residues corresponding to PBT 20HUN and PBT 20MDH presented on Figure 8.
The characteristic peaks of the huntite mineral at 866, 890, 1442, 1510, and 1552 cm−1 appear in all the samples containing HUN filler suggesting huntite’s presence in the collected solid residues at 500 °C. On the other hand, for samples containing MDH, we observe two peaks at 1410 cm−1 and 1602 cm−1, which are attributed to the formation of MgO, a product of the MDH decomposition that happens at 350 °C [39].
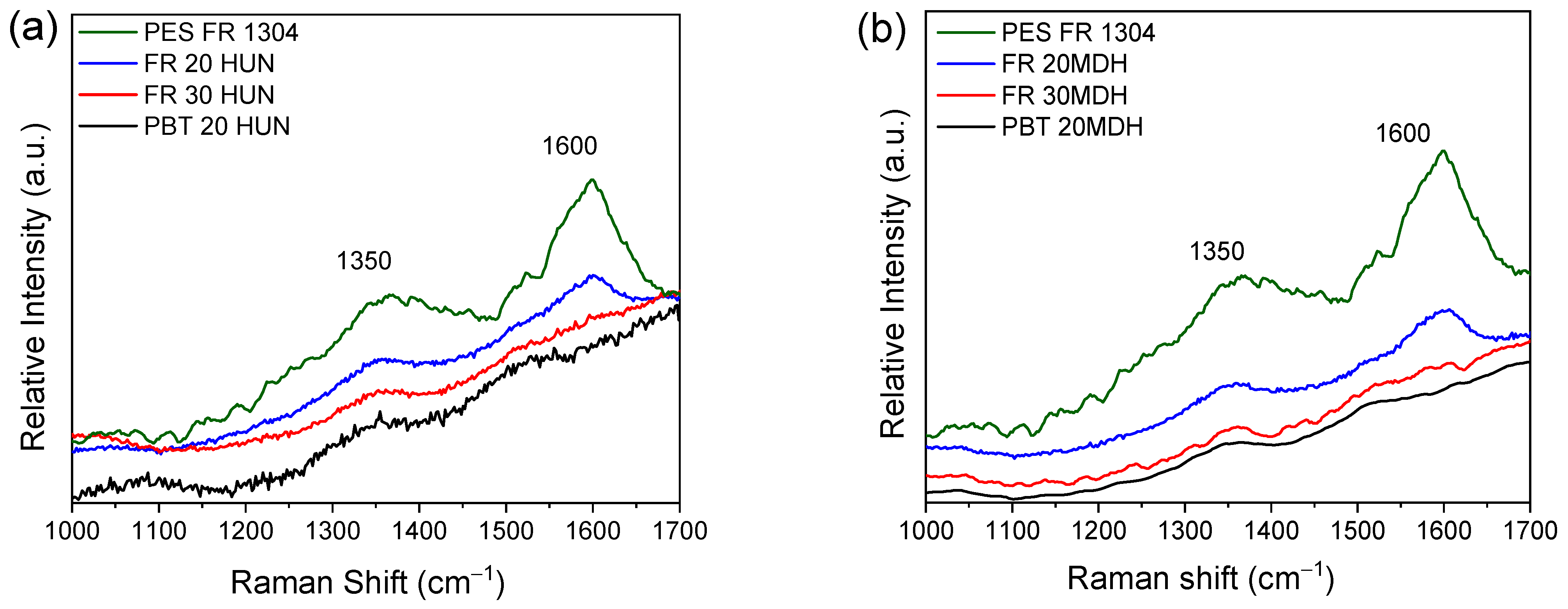
The Raman spectra depicted in Figure 9 further support the findings from the FTIR-ATR analysis. Specifically, the ~1600 cm−1 and ~1350 cm−1 peaks attributed to graphite-like species are present clearly in the PES FR 1304 sample’s char. Upon the addition of the basic oxide-forming fillers, the intensity of these two peaks is greatly reduced in the spectra of the char of the samples containing 20% of HUN or m-MDH and is non-detectable in the samples containing 30%. Likewise, they are non-detectable on the spectra of the inorganic solid residue of the PBT 20HUN and PBT 20MDH.
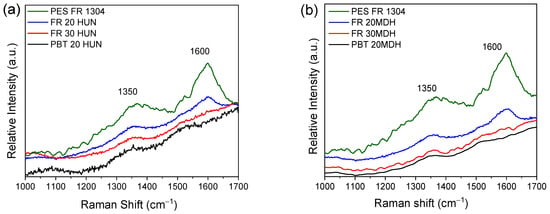
Figure 9.
(a) Raman spectra of the residue derived around 500 °C from PES FR 1304 (green), FR 20HUN (blue), FR 30HUN (red), and PBT 20HUN (black). (b) Raman spectra of the residue derived around 500 °C from PES FR 1304 (green), FR 20m-MDH (blue), FR 30m-MDH (red), and PBT 20MDH (black).
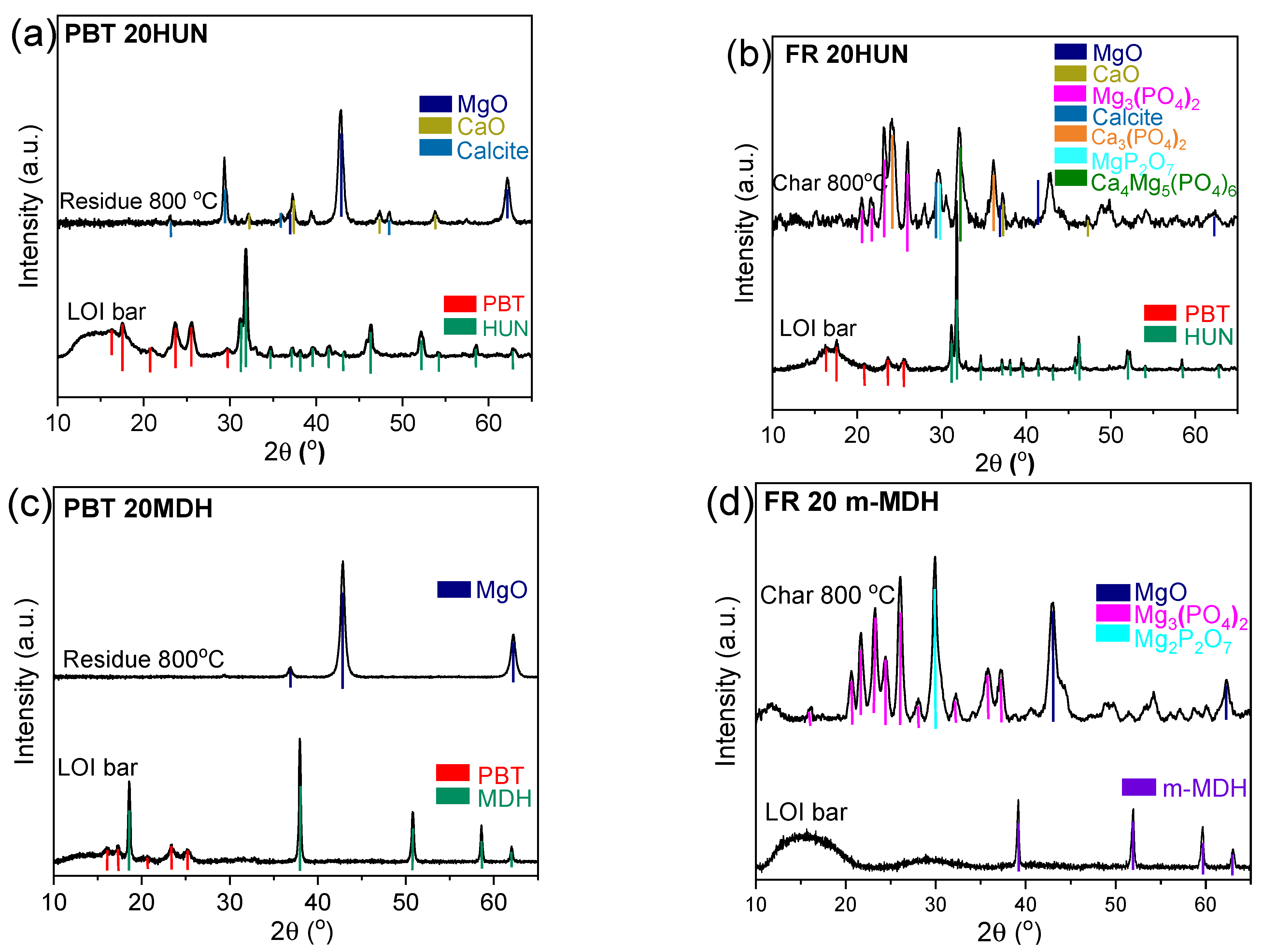
3.6. XRD Diffractograms of the Solid Residues at 800 °C
XRD was performed on the solid residue from samples treated up to 800 °C (Figure 10). The inorganic residue of the PBT 20HUN sample (Figure 10a), presents the peaks of MgO, CaO, and calcite [18,40], is typical for its huntite content. On the other hand, the char of the FR 20HUN sample (Figure 10b) shows additional peaks that belong to the Mg3(PO4)2, Ca3(PO4)2, MgP2O7, and Ca4Mg5(PO4)6 species [41,42,43]. Similarly, the inorganic residue of the PBT 20MDH sample (Figure 10c) mostly consists of MgO [18], due to the decomposition of MDH, while the char of the FR 20m-MDH sample (Figure 10d) also contains the peaks corresponding to the presence of different phosphate salts, mainly Mg3(PO4)2 and MgP2O7. These results further reinforce the experimental results of the FTIR-ATR and Raman analysis, as they document the formation of the phosphate salts previously discussed in the char of the PES FR 1304-based samples.
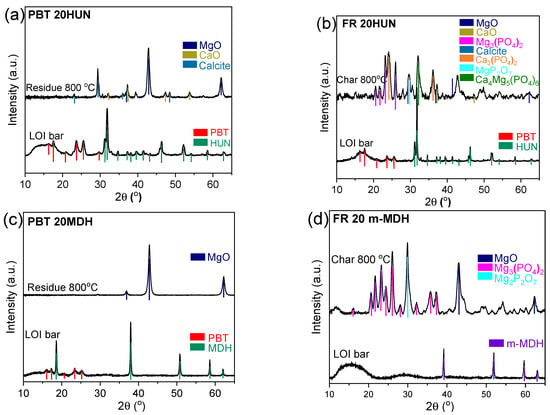
Figure 10.
Diffractograms of the solid residues collected at 800 °C from the PBT 20HUN and FR 20HUN (a,b) and PBT 20MDH and FR 20m-MDH (c,d), compared with the corresponding LOI bars.
3.7. Scanning Electron Microscopy (SEM) Images
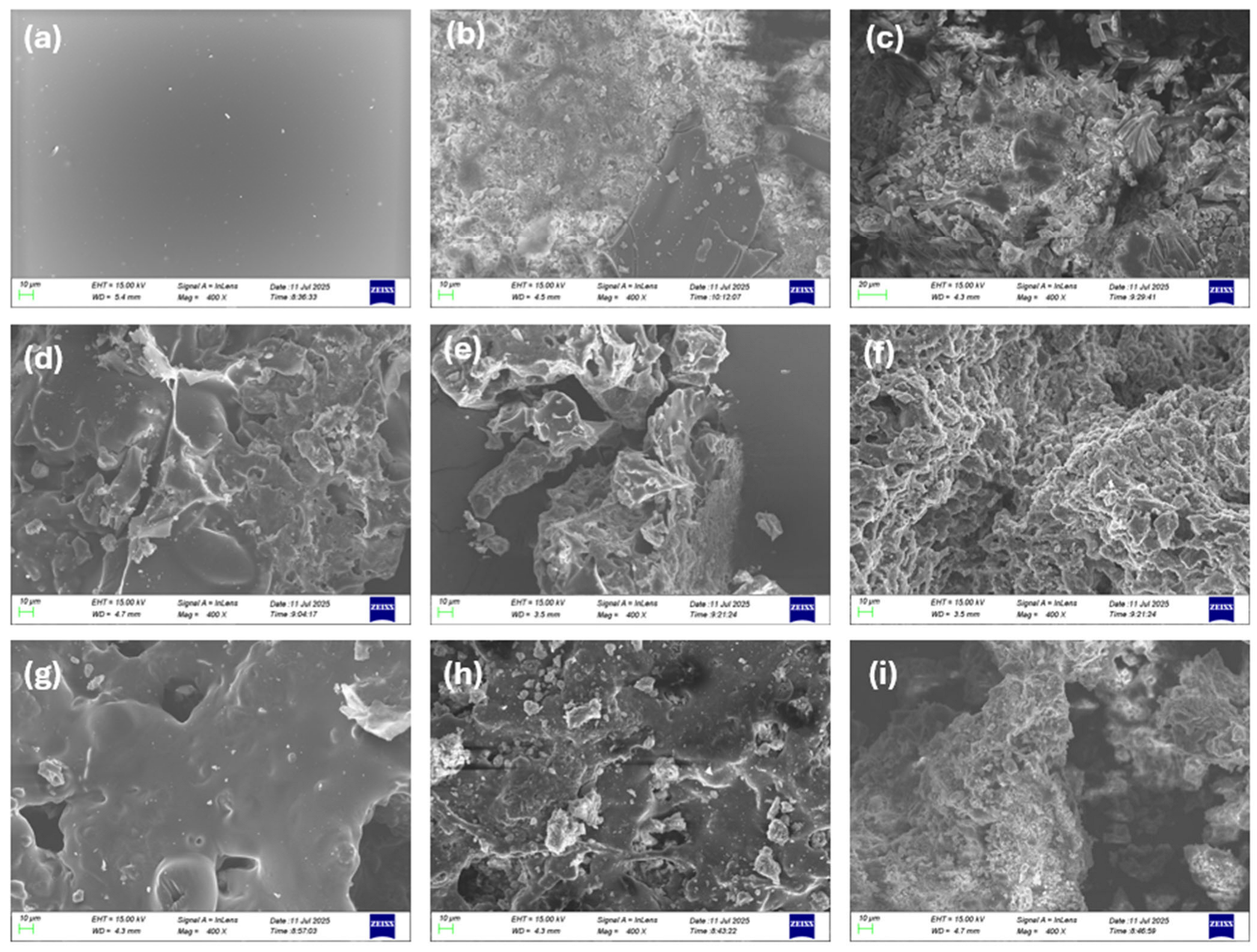
SEM-EDS images were obtained from the TGA solid residues formed up to 500 °C for each composite. These images reveal a pattern of deterioration of the intumescent char’s integrity upon the incorporation of the HUN or MDH filler. Specifically, in Figure 11, the char of PES FR 1304 presented in Figure 11a appears to be smooth and uniform. Upon the addition of 10% m-MDH (Figure 11d) and 10% HUN (Figure 11g), the morphology of the char changes as it loses its smooth appearance. Its appearance changes more drastically upon the addition of 20% filler either of m-MDH (Figure 11e) or HUN (Figure 11h), and filler agglomerations begin to appear visibly. Also, its smooth appearance is fractured, and its texture is coarser, indicating a disruption in the normal formation of the carbonaceous char that appears in Figure 11a of the pure PES FR 1304 sample. For 30% filler addition, the initial morphology of the char is completely destroyed as the FR 30m-MDH sample’s char appears like a fine powder, probably because of the MgO’s presence (Figure 11f), and similarly the FR 30HUN (Figure 11i) sample’s char also appears porous with huntite residue particles being discernible. The reason that the SEM images of PBT 20MDH (Figure 11b) and PBT 20HUN (Figure 11c) were also collected was for comparison, and neither of these samples presented a smooth solid residue with regular morphology. These observations lead to the conclusion that upon the introduction of HUN and MDH in the PES FR 1304 matrix, its formerly smooth intumescent char is destroyed along with its flame-retardant properties.
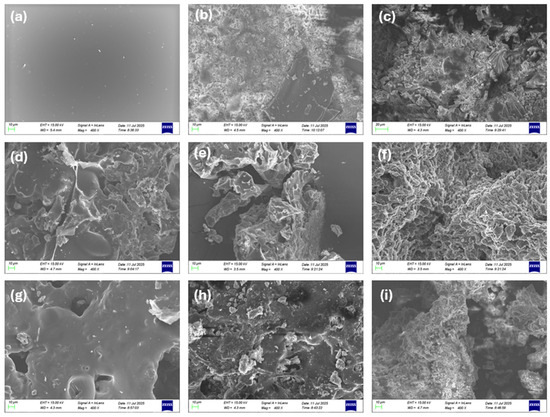
Figure 11.
SEM images at 400× magnification of the solid residues at 500 °C of (a) PES FR 1304, (b) PBT 20HUN, (c) PBT 20MDH, (d) FR 10m-MDH, (e) FR 20m-MDH, (f) FR 30m-MDH, (g) FR 10HUN, (h) FR 20HUN, and (i) FR 30HUN.
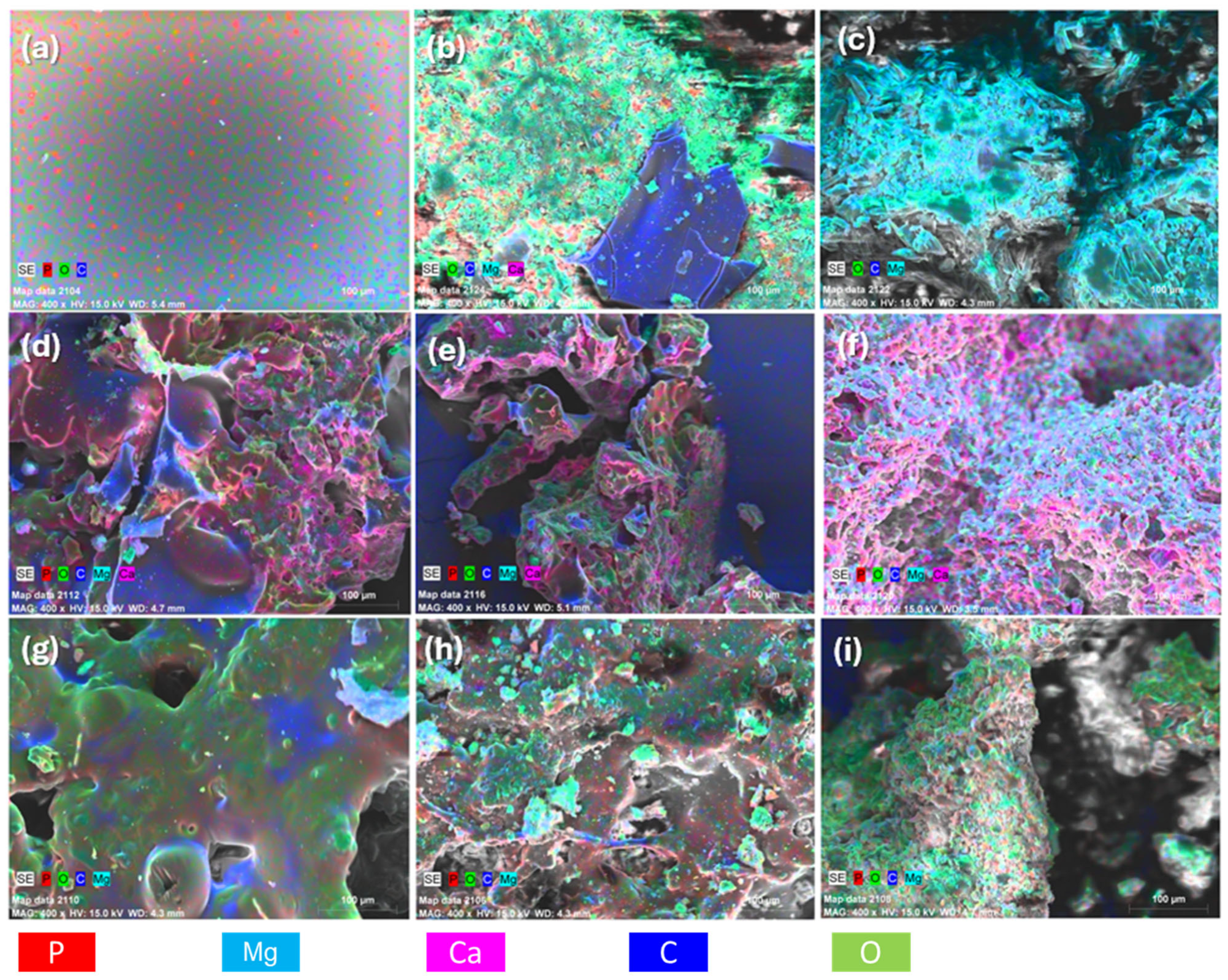
Elemental compositions of the solid residues for the FR-PP formed up to 500 °C for each composite were assessed by EDS measurements as shown in Figure 12 and Table 4. It is evident that the char residues of the PES FR 1304-based samples contain phosphorus, whereas those based on PBT did not. Additionally, in the char of the samples containing huntite, a relatively constant magnesium to calcium ratio is observed (mean value: 2.54), indicating a consistent presence of these elements in the resulting phosphate salts; this ratio loosely reflects the 3:1 Mg:Ca ratio found in the crystal structure of huntite. Regarding phosphorus content, the char derived from pure PES FR 1304 contains 1.4% phosphorus, while the char from samples containing HUN and m-MDH show higher phosphorus levels. These findings align with other evidence supporting the formation of phosphate salts and the transformation of the char structure from carbonaceous and smooth to irregular and rich in inorganic residues.
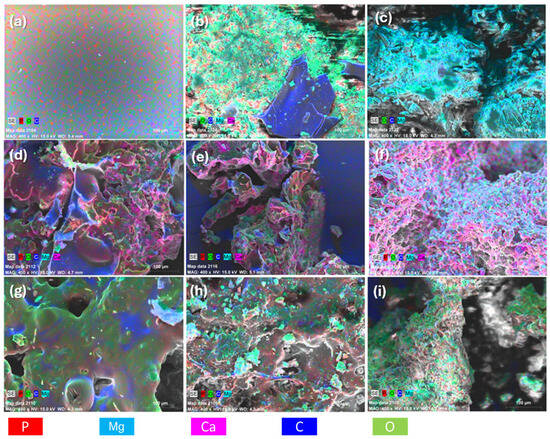
Figure 12.
EDS mapping of (a) PES FR 1304, (b) PBT 20HUN, (c) PBT 20MDH, (d) FR 10m-MDH, (e) FR 20m-MDH, (f) FR 30m-MDH, (g) FR 10HUN, (h) FR 20HUN, and (i) FR 30HUN.

Table 4.
Elemental compositions of solid residues at 500 °C.
4. Conclusions
The present study demonstrates in detail why combining basic oxide-forming fillers with phosphorus-based flame retardants results in a clear antagonistic effect. Basic MgO and CaO oxides, produced during the decomposition of HUN and m-MDH, react with the acidic phosphorus species evolved in generating inert phosphate salts. This leads to the suppression of the intumescent flame-retardant mechanism, as revealed by both the LOI and the UL94V tests. Thermogravimetric and spectroscopic analyses confirmed that the higher the HUN and m-MDH filler content, the fewer graphite-like and fused-ring structures remain in the char, ultimately diminishing its integrity and insulating capacity. XRD is used to confirm the emergence of the magnesium and calcium phosphate salts and identify their distinct structure. SEM images show that the once cohesive intumescent char of neat PES FR 1304 becomes porous and powder-like as basic inorganic filler loading rises. These findings underscore in detail that, when organophosphorus additives are paired with basic oxide-forming fillers in polymer flame-retardant coatings, the fillers must be carefully selected and modified to ensure that flame-retardant performance does not diminish. The EDS analysis of samples containing huntite indicates a consistent presence of magnesium and calcium in the resulting phosphate salts.
All these findings highlight and detail the reason why, when organophosphate additives are combined with basic oxide-forming fillers in flame-retardant polymer coatings, the fillers must be carefully selected and modified to ensure that the flame-retardant performance is not reduced. Beyond that, the present study constitutes a protocol for using a combination of specific techniques capable of revealing competitive or, hopefully, synergistic mechanisms of FR mixed compounds; the latter will be the subject of a future work.
Author Contributions
E.M.: Data curation, Formal Analysis, Validation, Writing—original draft; G.N.M.: Data curation, Formal Analysis, Investigation, Validation, Visualization, Writing—review & editing; A.S.B.: Conceptualization, Data Curation, Formal analysis, Investigation, Validation, Visualization, Writing—review & editing; A.P.: Data curation, Formal analysis, Investigation, Validation; V.D.: Data curation, Formal analysis, Investigation, Validation; K.K.: Data Curation, Formal Analysis, Investigation, Validation; T.C.: Conceptualization, Methodology; D.S.: Conceptualization, Methodology; C.G.: Conceptualization, Methodology, Project administration; J.K.: Conceptualization, Methodology, G.A.V.: Conceptualization, Methodology, Project administration, Writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
The results presented have received funding from the Eurostars 3: Call 3 joint program with co-funding from the European Union Horizon 2020 research and innovation program (ID Number 2314, Flame Retardant polymers composites for advanced mattress development -FR.poly.com).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data will be available from the corresponding author upon reasonable request.
Conflicts of Interest
Kerim Kılınç and Deniz Savci are currently employed by Polyteks Tekstil Company and were employed by Polyteks Tekstil while contributing to this manuscript. Otherwise Theodosios Chatzinikolaou is currently employed by LKAB Minerals GmbH and was employed by LKAB Minerals GmbH while contributing to this manuscript. Their contributions to this work and manuscript were made independently without any requirement, guidance or input by their employer. They received no financial compensation from any source for the contributions they made to this scientific work and manuscript.
References
- Deshmukh, G.S.; Peshwe, D.R.; Pathak, S.U.; Ekhe, J.D. A study on effect of mineral additions on the mechanical, thermal, and structural properties of poly(butylene terephthalate) (PBT) composites. J. Polym. Res. 2010, 18, 1081–1090. [Google Scholar] [CrossRef]
- Karsli, N.G.; Ozkan, C.; Aytac, A.; Deniz, V. Characterization of poly(butylene terephthalate) composites prepared by using various types of sized carbon fibers. Mater. Des. 2015, 87, 318–323. [Google Scholar] [CrossRef]
- Hastie, J.W.; Bonnell, D.W. Molecular Chemistry of Inhibited Combustion Systems; Report NBSIR 80-2169; National Bureau of Standards: Gaithersburg, MD, USA, 1980.
- Gallo, E.; Braun, U.; Schartel, B.; Russo, P.; Acierno, D. Halogen-free flame retarded poly(butylene terephthalate) (PBT) using metal oxides/PBT nanocomposites in combination with aluminium phosphinate. Polym. Degrad. Stab. 2009, 94, 1245–1253. [Google Scholar] [CrossRef]
- Keshavarzian, A.; Haghighi, M.N.; Taromi, F.A.; Abedini, H. Phosphorus-based flame retardant poly (butylene terephthalate): Synthesis, flame retardancy and thermal behavior. Polym. Degrad. Stab. 2020, 180, 109310. [Google Scholar] [CrossRef]
- Niu, L.; Xu, J.; Yang, W.; Ma, J.; Zhao, J.; Kang, C.; Su, J. Study on the Synergetic Fire-Retardant Effect of Nano-Sb2O3 in PBT Matrix. Materials 2018, 11, 1060. [Google Scholar] [CrossRef] [PubMed]
- Suzanne, M.; Ramani, A.; Ukleja, S.; McKee, M.; Zhang, J.; Delichatsios, M.; Patel, P.; Clarke, P.; Cusack, P. Fire performance of brominated and halogen-free flame retardants in glass-fiber reinforced poly(butylene terephthalate). Fire Mater. 2017, 42, 18–27. [Google Scholar] [CrossRef]
- Lim, K.-S.; Bee, S.-T.; Sin, L.T.; Tee, T.-T.; Ratnam, C.T.; Hui, D.; Rahmat, A.R. A review of application of ammonium polyphosphate as intumescent flame retardant in thermoplastic composites. Compos. Part B Eng. 2016, 84, 155–174. [Google Scholar] [CrossRef]
- Varganici, C.-D.; Rosu, L.; Bifulco, A.; Rosu, D.; Mustata, F.; Gaan, S. Recent advances in flame retardant epoxy systems from reactive DOPO–based phosphorus additives. Polym. Degrad. Stab. 2022, 202, 110020. [Google Scholar] [CrossRef]
- Gibertini, E.; Carosio, F.; Aykanat, K.; Accogli, A.; Panzeri, G.; Magagnin, L. Silica-encapsulated red phosphorus for flame retardant treatment on textile. Surf. Interfaces 2021, 25, 101252. [Google Scholar] [CrossRef]
- Bereska, A.; Kafarski, P.; Bereska, B.; Tkacz, B.; Iłowska, J.; Lenża, J. The application of organophosphorus flame-retardants in epoxy resin. J. Vinyl Addit. Technol. 2015, 23, 142–151. [Google Scholar] [CrossRef]
- Bai, Z.; Tian, Y.; Wang, Z.; Wu, D.; Wang, X.; Yu, J.; Yuan, R.; Li, F. A macromolecular intumescent flame retardant with thermal cross-linking groups for poly(ethylene terephthalate) with enhanced fire safety. Polym. Degrad. Stab. 2024, 225. [Google Scholar] [CrossRef]
- Li, N.; Jiang, G.; Zhou, G. Fire retardant and charring effect of cyclic phenolphthalein (phenylene phosphonate) oligomer on polybutylene terephthalate. RSC Adv. 2015, 6, 2512–2519. [Google Scholar] [CrossRef]
- Goedderz, D.; Weber, L.; Markert, D.; Schießer, A.; Fasel, C.; Riedel, R.; Altstädt, V.; Bethke, C.; Fuhr, O.; Puchtler, F.; et al. Flame retardant polyester by combination of organophosphorus compounds and an NOR radical forming agent. J. Appl. Polym. Sci. 2019, 137, 47876. [Google Scholar] [CrossRef]
- Lv, P.; Wang, Z.; Hu, K.; Fan, W. Flammability and thermal degradation of flame retarded polypropylene composites containing melamine phosphate and pentaerythritol derivatives. Polym. Degrad. Stab. 2005, 90, 523–534. [Google Scholar] [CrossRef]
- Camino, G.; Costa, L.; di Cortemiglia, M.L. Overview of fire retardant mechanisms. Polym. Degrad. Stab. 1991, 33, 131–154. [Google Scholar] [CrossRef]
- Laoutid, F.; Ferry, L.; Lopez-Cuesta, J.M.; Crespy, A. Red phosphorus/aluminium oxide compositions as flame retardants in recycled poly(ethylene terephthalate). Polym. Degrad. Stab. 2003, 82, 357–363. [Google Scholar] [CrossRef]
- Andrikopoulos, K.S.; Bounos, G.; Lainioti, G.C.; Ioannides, T.; Kallitsis, J.K.; Voyiatzis, G.A. Flame Retardant Nano-Structured Fillers from Huntite/Hydromagnesite Minerals. Nanomaterials 2022, 12, 2433. [Google Scholar] [CrossRef]
- Malaysia, M.U.T.; Saidi, M.A.A.; Mazlan, F.S.; Hassan, A.; Rashid, R.A.; Rahmat, A.R. Flammability, Thermal and Mechanical Properties of Polybutylene Terephthalate/Dolomite Composites. J. Phys. Sci. 2019, 30, 175–189. [Google Scholar] [CrossRef]
- Piperopoulos, E.; Scionti, G.; Atria, M.; Calabrese, L.; Proverbio, E. Flame-Retardant Performance Evaluation of Functional Coatings Filled with Mg(OH)2 and Al(OH)3. Polymers 2022, 14, 372. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Feng, J.; Tsui, C.; Tang, C.; Liu, D.; Zhang, S.; Huang, W. Mechanical properties and flame-retardant of PP/MRP/Mg(OH)2/Al(OH)3 composites. Compos. Part B Eng. 2015, 71, 74–81. [Google Scholar] [CrossRef]
- Huang, H.; Tian, M.; Liu, L.; Liang, W.; Zhang, L. Effect of particle size on flame retardancy of Mg(OH)2-filled ethylene vinyl acetate copolymer composites. J. Appl. Polym. Sci. 2006, 100, 4461–4469. [Google Scholar] [CrossRef]
- Hale, W.; Pessan, L.; Keskkula, H.; Paul, D. Effect of compatibilization and ABS type on properties of PBT/ABS blends. Polymer 1999, 40, 4237–4250. [Google Scholar] [CrossRef]
- Youn, Y.; Kim, T.W.; Cho, K.; Hong, S.K. Investigation on the Initial Stage of the Dehydration Process in Mg(OH)2 by Density Functional Theory Calculations. ACS Appl. Mater. Interfaces 2024, 16, 23122–23129. [Google Scholar] [CrossRef]
- Ok, J.; Ahn, M.-C. Novel PBT Blends with Magnesium Hydroxide Flame Retardant. 2004. Available online: https://www.analyticalsynthesis.net/about/papers/L3425.pdf (accessed on 24 June 2025).
- Viretto, A.; Sonnier, R.; Taguet, A.; Otazaghine, B.; Ferry, L.; Lopez-Cuesta, J.; Lagrève, C. Thermal degradation of polyesters filled with magnesium dihydroxide and magnesium oxide. Fire Mater. 2015, 40, 445–463. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, H.; Su, Z. Surface treatment of magnesium hydroxide to improve its dispersion in organic phase by the ultrasonic technique. Appl. Surf. Sci. 2007, 253, 7393–7397. [Google Scholar] [CrossRef]
- Hollingbery, L.; Hull, T. The thermal decomposition of huntite and hydromagnesite—A review. Thermochim. Acta 2010, 509, 1–11. [Google Scholar] [CrossRef]
- Hull, T.R.; Witkowski, A.; Hollingbery, L. Fire retardant action of mineral fillers. Polym. Degrad. Stab. 2011, 96, 1462–1469. [Google Scholar] [CrossRef]
- Hollingbery, L.A. Decomposition and Fire Retardancy of Naturally Occurring Mixtures of Huntite and Hydromagnesite. Ph.D. Thesis, University of Central Lancashire, Preston, UK, 2011. [Google Scholar]
- Hollingbery, L.; Hull, T. The fire retardant behaviour of huntite and hydromagnesite—A review. Polym. Degrad. Stab. 2010, 95, 2213–2225. [Google Scholar] [CrossRef]
- Liao, H.; Liu, Y.; Jiang, J.; Li, J.; Liu, Y. Synergism and antagonism of phosphorus-containing epoxy resin combined with different metal hydroxides. J. Fire Sci. 2016, 34, 3–12. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Barańska, H.; Łabudzińska, A.; Terpiński, J. Laser Raman Spectrometry: Analytical Applications; Office of Scientific and Technical Information (OSTI): Oak Ridge, TN, USA, 1987.
- Horrocks, A.R.; Smart, G.; Nazaré, S.; Kandola, B.; Price, D. Quantification of Zinc Hydroxystannate** and Stannate** Synergies in Halogen-Containing Flame-Retardant Polymeric Formulations. J. Fire Sci. 2009, 28, 217–248. [Google Scholar] [CrossRef]
- Lewin, M. Synergism and catalysis in flame retardancy of polymers. Polym. Adv. Technol. 2001, 12, 215–222. [Google Scholar] [CrossRef]
- Mahajan, R.; Prakash, R.; Kumar, S.; Kumar, V.; Choudhary, R.; Phase, D. Surface and luminescent properties of Mg3(PO4)2:Dy3+ phosphors. Optik 2021, 225, 165717. [Google Scholar] [CrossRef]
- Massit, A.; El Yacoubi, A.; Kholtei, A.; El Idrissi, B.C. XRD and FTIR analysis of magnesium substituted tricalcium calcium phosphate using a wet precipitation method. Biointerface Res. Appl. Chem. 2021, 11, 8034–8042. [Google Scholar]
- Sharma, L.; Kakkar, R. Hierarchical Porous Magnesium Oxide (Hr-MgO) Microspheres for Adsorption of an Organophosphate Pesticide: Kinetics, Isotherm, Thermodynamics, and DFT Studies. ACS Appl. Mater. Interfaces 2017, 9, 38629–38642. [Google Scholar] [CrossRef] [PubMed]
- Sompech, S.; Dasri, T.; Thaomola, S. Preparation and Characterization of Amorphous Silica and Calcium Oxide from Agricultural Wastes. Orient. J. Chem. 2016, 32, 1923–1928. [Google Scholar] [CrossRef]
- Ramteke, S.K.; Yerpude, A.N.; Kokode, N.S.; Dhoble, S.J. Optical properties of rare earth-activated Ca3(PO4)2:RE (RE = Eu3+ and Dy3+) phosphors prepared by wet chemical synthesis. Bull. Mater. Sci. 2021, 44, 1–5. [Google Scholar] [CrossRef]
- Nagpure, I.; Shinde, K.; Dhoble, S.; Kumar, A. Photoluminescence characterization of Dy3+ and Eu2+ ion in M5(PO4)3F (M=Ba, Sr, Ca) phosphors. J. Alloy. Compd. 2009, 481, 632–638. [Google Scholar] [CrossRef]
- Zhang, S.; Li, L.; Lv, X. Synthesis and characterization of a novel Mg3(PO4)2 ceramic with low dielectric constant. J. Mater. Sci. Mater. Electron. 2016, 28, 1620–1623. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).