Degradation of HVOF-MCrAlY + APS-Nanostructured YSZ Thermal Barrier Coatings
Abstract
1. Introduction
2. Materials and Methods
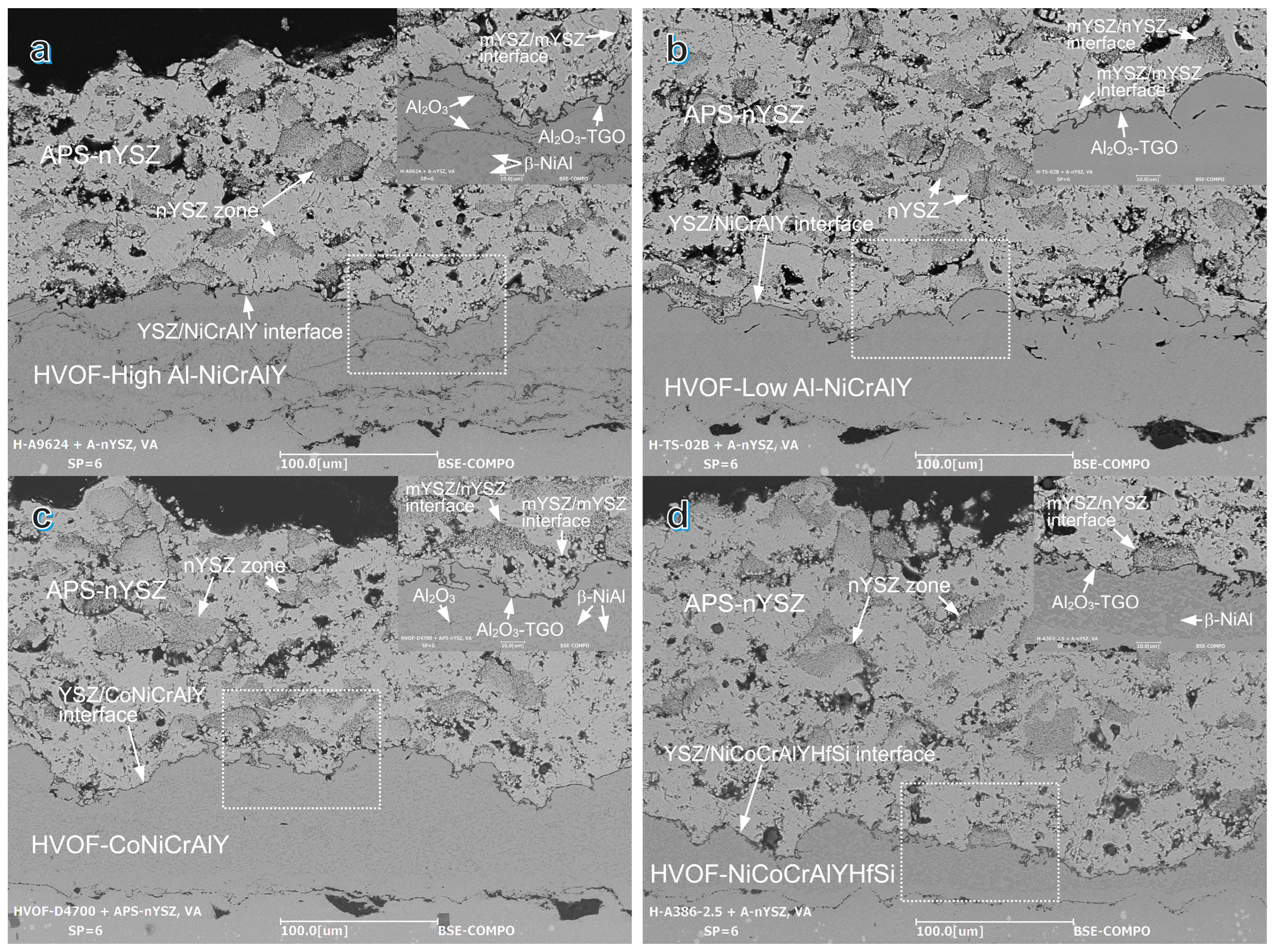
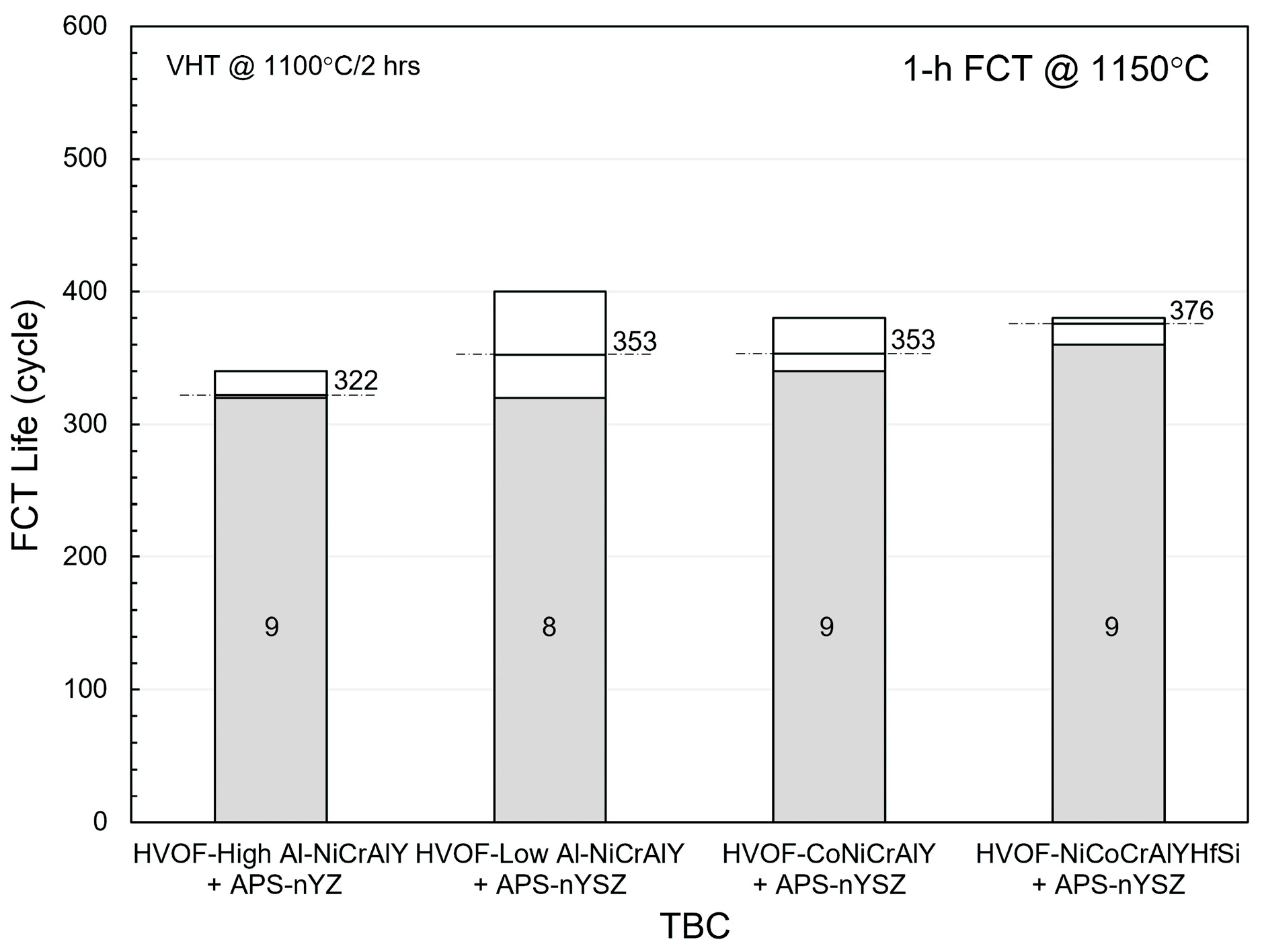
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicholls, J.R. Advances in Coating Design for High-Performance Gas Turbines. MRS Bull. 2003, 28, 659–670. [Google Scholar] [CrossRef]
- Walston, W.S. Coating and surface technologies for turbine airfoils. In Superalloys 2004; Green, K.A., Pollock, T.M., Harada, H., Howson, T.E., Reed, R.C., Schirra, J.J., Walston, S., Eds.; The Minerals, Metals & Materials Society: Champion, PA, USA, 2004; pp. 579–588. [Google Scholar]
- Feuerstein, A.; Knapp, J.; Taylor, T.; Ashary, A.; Bolcavage, A.; Hitchman, N. Technical and Economical Aspects of Current Thermal Barrier Coating Systems for Gas Turbine Engines by Thermal Spray and EBPVD: A Review. J. Therm. Spray Technol. 2008, 17, 199–213. [Google Scholar] [CrossRef]
- Hardwicke, C.U.; Lau, Y.-C. Advances in Thermal Spray Coatings for Gas Turbines and Energy Generation: A Review. J. Therm. Spray Technol. 2013, 22, 564–576. [Google Scholar] [CrossRef]
- Darolia, R. Thermal barrier coatings technology: Critical review, progress update, remaining challenges and prospects. Int. Mater. Rev. 2013, 58, 315–348. [Google Scholar] [CrossRef]
- Bose, S. High Temperature Coatings, 2nd ed.; Elsevier: Cambridge, MA, USA, 2017; ISBN 9780128046227. [Google Scholar]
- Dorfman, M.R.; Dwivedi, G.; Dambra, C.; Wilson, S. Perspective: Challenges in the Aerospace Marketplace and Growth Opportunities for Thermal Spray. J. Therm. Spray Technol. 2022, 31, 672–684. [Google Scholar] [CrossRef] [PubMed]
- Sulzer. Low Pressure Plasma Spray LPPS. Available online: www.sulzer.com/en/shared/services/low-pressure-plasma-spray-lpps (accessed on 2 July 2025).
- Höganäs. Vacuum Plasma Spray/Low Pressure Plasma Spray (VPS/LPPS). Available online: www.hoganas.com/en/powder-technologies/surface-coating/vps-lpps/ (accessed on 2 July 2025).
- Dorfman, M.R.; Sporer, D.; Meyer, P. Thermal Spray Technology Growth in Gas Turbine Applications. In Thermal Spray Technology, ASM Handbook; Tucker, R.C., Jr., Ed.; ASM International: Almere, The Netherlands, 2013; Volume 5A, pp. 280–286. [Google Scholar]
- Padture, N.P.; Gell, M.; Jordan, E.H. Thermal Barrier Coatings for Gas-Turbine Engine Applications. Science 2002, 296, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.W.; Glynn, M.L.; Ott, R.T.; Hufnagel, T.C.; Hemker, K.J. Characterization and modeling of a martensitic transformation in a platinum modified diffusion aluminide bond coat for thermal barrier coatings. Acta Mater. 2003, 51, 4279–4294. [Google Scholar] [CrossRef]
- Liang, B.; Ding, C. Thermal shock resistances of nanostructured and conventional zirconia coatings deposited by atmospheric plasma spraying. Surf. Coat. Technol. 2005, 197, 185–192. [Google Scholar] [CrossRef]
- Wang, W.Q.; Sha, C.K.; Sun, D.Q.; Gu, X.Y. Microstructural feature, thermal shock resistance and isothermal oxidation resistance of nanostructured zirconia coating. Mater. Sci. Eng. A 2006, 424, 1–5. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, C.; Peng, H.; Gong, S.; Xu, H. Influence of thermal shock on insulation effect of nano-multilayer thermal barrier coatings. Surf. Coat. Technol. 2007, 201, 6340–6344. [Google Scholar] [CrossRef]
- Lima, R.S.; Marple, B.R. Thermal Spray Coatings Engineered from Nanostructured Ceramic Agglomerated Powders for Structural, Thermal Barrier and Biomedical Applications: A Review. J. Therm. Spray Technol. 2007, 16, 40–63. [Google Scholar] [CrossRef]
- Wu, J.; Guo, H.; Zhou, L.; Wang, L.; Gong, S. Microstructure and Thermal Properties of Plasma Sprayed Thermal Barrier Coatings from Nanostructured YSZ. J. Therm. Spray Technol. 2010, 19, 1186–1194. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, Y.; Yuan, T.; Chen, H.Y.; Kang, Y.X.; Shi, W.J.; Song, X.L.; Li, B.Q. Failure analysis of fine-lamellar structured YSZ based thermal barrier coatings with submicro/nano-grains. Surf. Coat. Technol. 2017, 319, 95–103. [Google Scholar] [CrossRef]
- Bai, Y.; Han, Z.H.; Li, H.Q.; Xu, C.; Xu, Y.L.; Wang, Z.; Ding, C.H.; Yang, J.F. High performance nanostructured ZrO2 based thermal barrier coatings deposited by high efficiency supersonic plasma spraying. Appl. Surf. Sci. 2011, 257, 7210–7216. [Google Scholar] [CrossRef]
- Wu, Z.; Ni, L.; Yu, Q.; Zhou, C. Effect of Thermal Exposure on Mechanical Properties of a Plasma-Sprayed Nanostructured Thermal Barrier Coating. J. Therm. Spray Technol. 2012, 21, 169–175. [Google Scholar] [CrossRef]
- Jamalin, H.; Mozafarinia, R.; Razavi, R.S.; Ahmadi-Pidani, R. Comparison of thermal shock resistances of plasma-sprayed nanostructured and conventional yttria stabilized zirconia thermal barrier coatings. Ceram. Int. 2012, 38, 6705–6712. [Google Scholar] [CrossRef]
- Pourbafrani, M.; Razavi, R.S.; Bakhshi, S.R.; Loghman-Estarki, M.R.; Jamali, H. Effect of microstructure and phase of nanostructured YSZ thermal barrier coatings on its thermal shock behaviour. Surf. Eng. 2015, 31, 64–73. [Google Scholar] [CrossRef]
- Masoule, S.T.; Valefi, Z.; Ehsani, N.; Lavasani, H.Q. Thermal Insulation and Thermal Shock Behavior of Conventional and Nanostructured Plasma-Sprayed TBCs. J. Therm. Spray Technol. 2016, 25, 1684–1691. [Google Scholar] [CrossRef]
- Ashofteh, A.; Seifollahpour, S. Role of nano-zones in enhancing the performance of YSZ coatings under thermal shock conditions. J. Am. Ceram. Soc. 2024, 107, 1201–1218. [Google Scholar] [CrossRef]
- Brumm, M.W.; Grabke, H.J. The oxidation behaviour of NiAl-I. Phase transformations in the alumina scale during oxidation of NiAl and NiAl-Cr alloys. Corros. Sci. 1992, 33, 1677–1690. [Google Scholar] [CrossRef]
- Evans, H.E.; Donaldson, A.T.; Gilmour, T.C. Mechanisms of breakaway oxidation and application to a chromia-forming steel. Oxid. Met. 1999, 52, 379–402. [Google Scholar] [CrossRef]
- Gurrappa, I.; Weinbruch, S.; Naumenko, D.; Quadakkers, W.J. Factors governing breakaway oxidation of FeCrAl-based alloys. Mater. Corros. 2000, 51, 224–235. [Google Scholar] [CrossRef]
- Niranatlumpong, P.; Ponton, C.B.; Evans, H.E. The Failure of Protective Oxides on Plasma-Sprayed NiCrAlY Overlay Coatings. Oxid. Met. 2000, 53, 241–258. [Google Scholar] [CrossRef]
- Pindera, M.; Aboudi, J.; Arnold, S.M. The effect of interface roughness and oxide film thickness on the inelastic response of thermal barrier coatings to thermal cycling. Mater. Sci. Eng. 2000, A284, 158–175. [Google Scholar] [CrossRef]
- Busso, E.P.; Lin, J.; Sakurai, S.; Nakayama, M. A mechanistic study of oxidation-induced degradation in a plasma-sprayed thermal barrier coating system. Part I: Model formulation. Acta Mater. 2001, 49, 1515–1528. [Google Scholar] [CrossRef]
- Limarga, A.M.; Widjaja, S.; Yip, T.H.; Teh, L.K. Modeling of the effect of Al2O3 interlayer on residual stress due to oxide scale in thermal barrier coatings. Surf. Coat. Technol. 2002, 153, 16–24. [Google Scholar] [CrossRef]
- Kumar, A.; Moledina, J.; Liu, Y.; Chen, K.; Patnaik, P.C. Nano-micro-structured 6%–8% YSZ thermal barrier coatings: A comprehensive review of comparative performance analysis. Coatings 2021, 11, 1474. [Google Scholar] [CrossRef]
- Chen, W.R.; Wu, X.; Marple, B.R.; Nagy, D.R.; Patnaik, P.C. TGO growth behaviour in TBCs with APS and HVOF bond coats. Surf. Coat. Technol. 2008, 202, 2677–2683. [Google Scholar] [CrossRef]
- Karaoglanli, A.C.; Grund, T.; Turk, A.; Lampk, T. A comparative study of oxidation kinetics and thermal cyclic performance of thermal barrier coatings (TBCs). Surf. Coat. Technol. 2019, 371, 47–67. [Google Scholar] [CrossRef]
- Trunova, O.; Beck, T.; Herzog, R.; Steinbrech, R.W.; Singheiser, L. Damage mechanisms and lifetime behavior of plasma sprayed thermal barrier coating systems for gas turbines—Part I: Experiments. Surf. Coat. Technol. 2008, 202, 5027–5032. [Google Scholar] [CrossRef]
- Bargraser, C.; Mohan, P.; Lee, K.; Yang, B.; Suk, J.; Choe, S.; Sohn, Y.H. Life approximation of thermal barrier coatings via quantitative microstructural analysis. Mater. Sci. Eng. A 2012, 549, 76–81. [Google Scholar] [CrossRef]
- Cui, Q.; Seo, S.M.; Yoo, Y.S.; Lu, Z.; Myoung, S.W.; Jung, Y.G.; Paik, U. Thermal durability of thermal barrier coatings with bond coat composition in cyclic thermal exposure. Surf. Coat. Technol. 2015, 284, 69–74. [Google Scholar] [CrossRef]
- Pint, B.A.; Haynes, J.A.; More, K.L.; Schneibel, J.H.; Zhang, Y.; Wright, I.G. The Performance of Pt-Modified Alumina-Forming Coatings and Model Alloys. In Superalloys 2008; Reed, R.C., Green, K.A., Caron, P., Gabb, T.P., Fahrmann, M.G., Huron, E.S., Woodard, S.A., Eds.; The Minerals, Metals & Materials Society: Champion, PA, USA, 2008; pp. 641–650. [Google Scholar]
- Meier, S.M.; Nissley, D.M.; Sheffler, K.D. Thermal barrier coating life prediction model development. In Proceedings of the International Gas Turbine and Aeroengine Congress and Exposition, American Society of Mechanical Engineers, Orlando, FL, USA, 3–6 June 1991. Paper # ASME 91-GT-40. [Google Scholar]
- Beck, T.; Herzog, R.; Trunova, O.; Offermann, M. Damage mechanisms and lifetime behavior of plasma-sprayed thermal barrier coating systems for gas turbines—Part II: Modeling. Surf. Coat. Technol. 2008, 202, 5901–5908. [Google Scholar] [CrossRef]
- Chen, W.R. Degradation of a TBC with HVOF-CoNiCrAlY Bond Coat. J. Therm. Spray Technol. 2014, 23, 876–884. [Google Scholar] [CrossRef]
- Smialek, J.L. Compiled furnace cyclic lives of EB-PVD thermal barrier coatings. Surf. Coat. Technol. 2015, 276, 31–38. [Google Scholar] [CrossRef]
- Chen, W.R. Unpublished Research; Oerlikon Metco US, Inc.: Westbury, NY, USA, 2019. [Google Scholar]
- Li, C.; Wu, J.; Li, H.; Huang, T.; Wang, L.; Chen, W.R. Impact of nanostructure on thermal cycling behaviour of a HVOF-NiCrAlY + APS-YSZ thermal barrier coating. Mater. High Temp. 2025, 42, 129–136. [Google Scholar] [CrossRef]







| MCrAlY | Product and Manufacture | Chemistry (wt.%) | Powder Size (µm) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ni | Co | Cr | Al | Y | Hf | Si | |||
| High Al-NiCrAlY | OM Amdry 9624 | Bal | 21–23 | 9–11 | 0.8–1.2 | −37 + 11 | |||
| Low Al-NiCrAlY | IMR TS-02B | Bal | 24–26 | 4–6 | 0.3–0.7 | −45 + 20 | |||
| CoNiCrAlY | OM Diamalloy 4700 | 29–35 | Bal | 18–24 | 5–11 | 0.1–0.8 | −45 + 15 | ||
| NiCoCrAlYHfSi | OM Amdry 386-2.5 | Bal | 19–26 | 14–21 | 11–14 | 0.2–0.8 | 0.1–0.5 | 0.1–0.7 | −63 + 22 |
| Bond Coat | Chemistry (wt.%) | β-NiAl (%) | Ra (µm) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ni | Co | Cr | Al | Y | Hf | Si | |||
| HVOF-high Al-NiCrAlY | Bal | 20.88 ± 0.47 | 9.30 ± 0.52 | 0.41 ± 0.19 | 30.80 ± 6.23 | 8.63 ± 1.79 | |||
| HVOF-low Al-NiCrAlY | Bal | 25.37 ± 0.27 | 5.65 ± 0.19 | 0.40 ± 0.15 | 0 | 9.42 ± 1.34 | |||
| HVOF-CoNiCrAlY | 31.73 ± 0.44 | Bal | 21.59 ± 0.20 | 7.59 ± 0.15 | 0.35 ± 0.15 | 39.41 ± 6.46 | 9.03 ± 1.94 | ||
| HVOF-NiCoCrAlYHfSi | Bal | 17.75 ± 0.72 | 15.25 ± 0.55 | 10.44 ± 0.27 | 0.57 ± 0.27 | 0.14 ± 0.25 | 0.39 ± 0.07 | 68.29 ± 4.19 | 9.46 ± 1.47 |
| Bond Coat | Chemistry (wt.%) | k | n | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ni | Co | Cr | Al | Y | Hf | Si | |||
| HVOF-high Al-NiCrAlY | Bal | 20.88 ± 0.47 | 9.30 ± 0.52 | 0.41 ± 0.19 | 0.828 | 0.392 | |||
| HVOF-low Al-NiCrAlY | Bal | 25.37 ± 0.27 | 5.65 ± 0.19 | 0.40 ± 0.15 | 1.051 | 0.284 | |||
| HVOF-CoNiCrAlY | 31.73 ± 0.44 | Bal | 21.59 ± 0.20 | 7.59 ± 0.15 | 0.35 ± 0.15 | 0.891 | 0.367 | ||
| HVOF-NiCoCrAlYHfSi | Bal | 17.75 ± 0.72 | 15.25 ± 0.55 | 10.44 ± 0.27 | 0.57 ± 0.27 | 0.14 ± 0.25 | 0.39 ± 0.07 | 0.895 | 0.348 |
| Bond Coat | Chemistry (wt.%) | Δa/ΔδTGO (μm/μm) | δstd-acc (μm) | δcrit (μm) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ni | Co | Cr | Al | Y | Hf | Si | ||||
| HVOF-high Al-NiCrAlY | Bal | 20.88 ± 0.47 | 9.30 ± 0.52 | 0.41 ± 0.19 | 33.668 | 5~5.5 | 7.51 ± 0.94 | |||
| HVOF-low Al-NiCrAlY | Bal | 25.37 ± 0.27 | 5.65 ± 0.19 | 0.40 ± 0.15 | 83.501 | 4~4.5 | 5.63 ± 0.84 | |||
| HVOF-CoNiCrAlY | 31.73 ± 0.44 | Bal | 21.59 ± 0.20 | 7.59 ± 0.15 | 0.35 ± 0.15 | 72.628 | 5~5.5 | 7.45 ± 0.75 | ||
| HVOF-NiCoCrAlYHfSi | Bal | 17.75 ± 0.72 | 15.25 ± 0.55 | 10.44 ± 0.27 | 0.57 ± 0.27 | 0.14 ± 0.25 | 0.39 ± 0.07 | 51.562 | 5~5.5 | 6.57 ± 0.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.R.; Li, C.; Cheng, Y.; Li, H.; Zhang, X.; Wang, L. Degradation of HVOF-MCrAlY + APS-Nanostructured YSZ Thermal Barrier Coatings. Coatings 2025, 15, 871. https://doi.org/10.3390/coatings15080871
Chen WR, Li C, Cheng Y, Li H, Zhang X, Wang L. Degradation of HVOF-MCrAlY + APS-Nanostructured YSZ Thermal Barrier Coatings. Coatings. 2025; 15(8):871. https://doi.org/10.3390/coatings15080871
Chicago/Turabian StyleChen, Weijie R., Chao Li, Yuxian Cheng, Hongying Li, Xiao Zhang, and Lu Wang. 2025. "Degradation of HVOF-MCrAlY + APS-Nanostructured YSZ Thermal Barrier Coatings" Coatings 15, no. 8: 871. https://doi.org/10.3390/coatings15080871
APA StyleChen, W. R., Li, C., Cheng, Y., Li, H., Zhang, X., & Wang, L. (2025). Degradation of HVOF-MCrAlY + APS-Nanostructured YSZ Thermal Barrier Coatings. Coatings, 15(8), 871. https://doi.org/10.3390/coatings15080871





