Review on Metal (-Oxide, -Nitride, -Oxy-Nitride) Thin Films: Fabrication Methods, Applications, and Future Characterization Methods
Abstract
1. Introduction
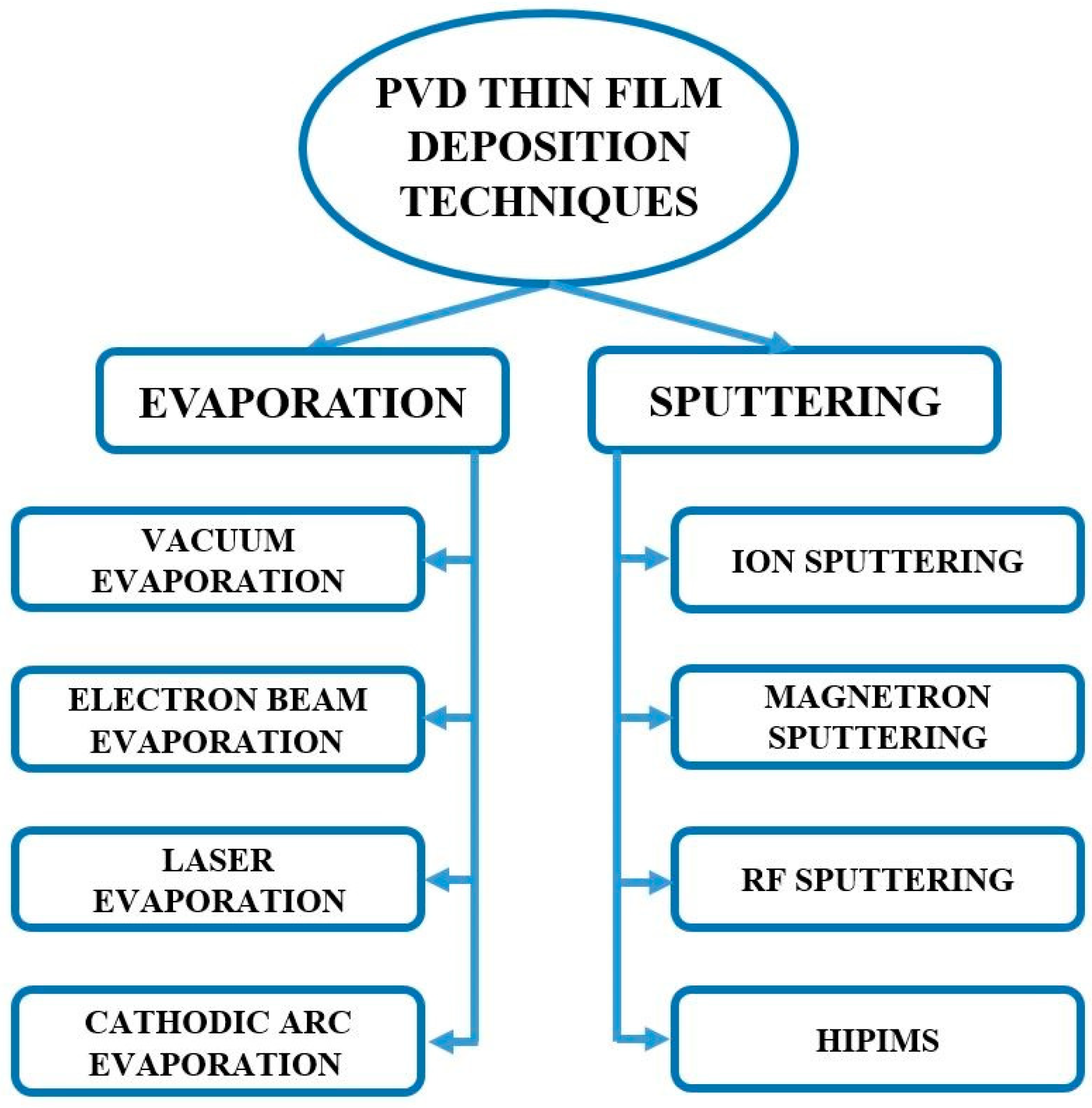
2. PVD Techniques for Thin-Film Deposition
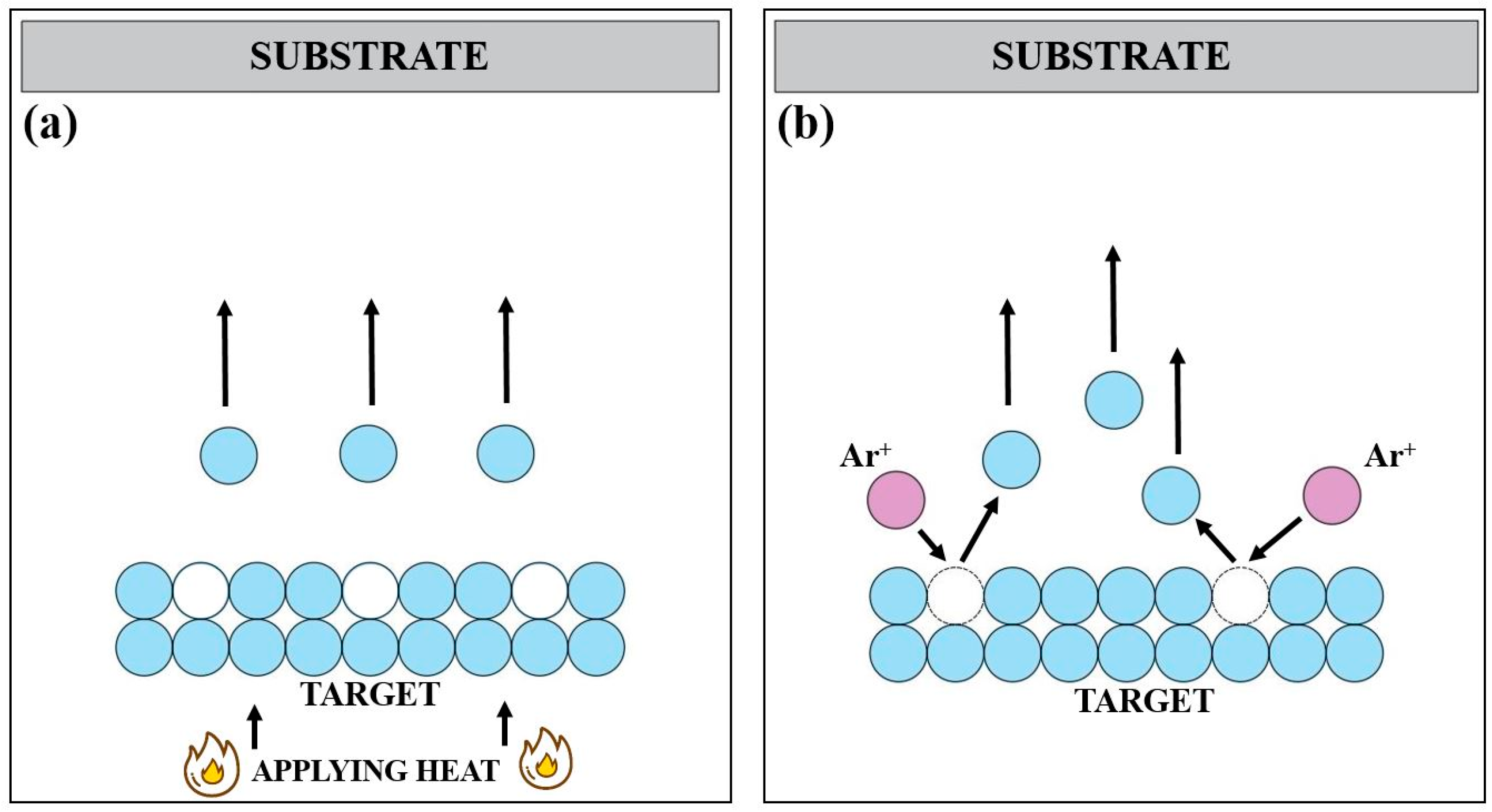
2.1. Fundamentals of Forming Thin Films Using PVD Techniques
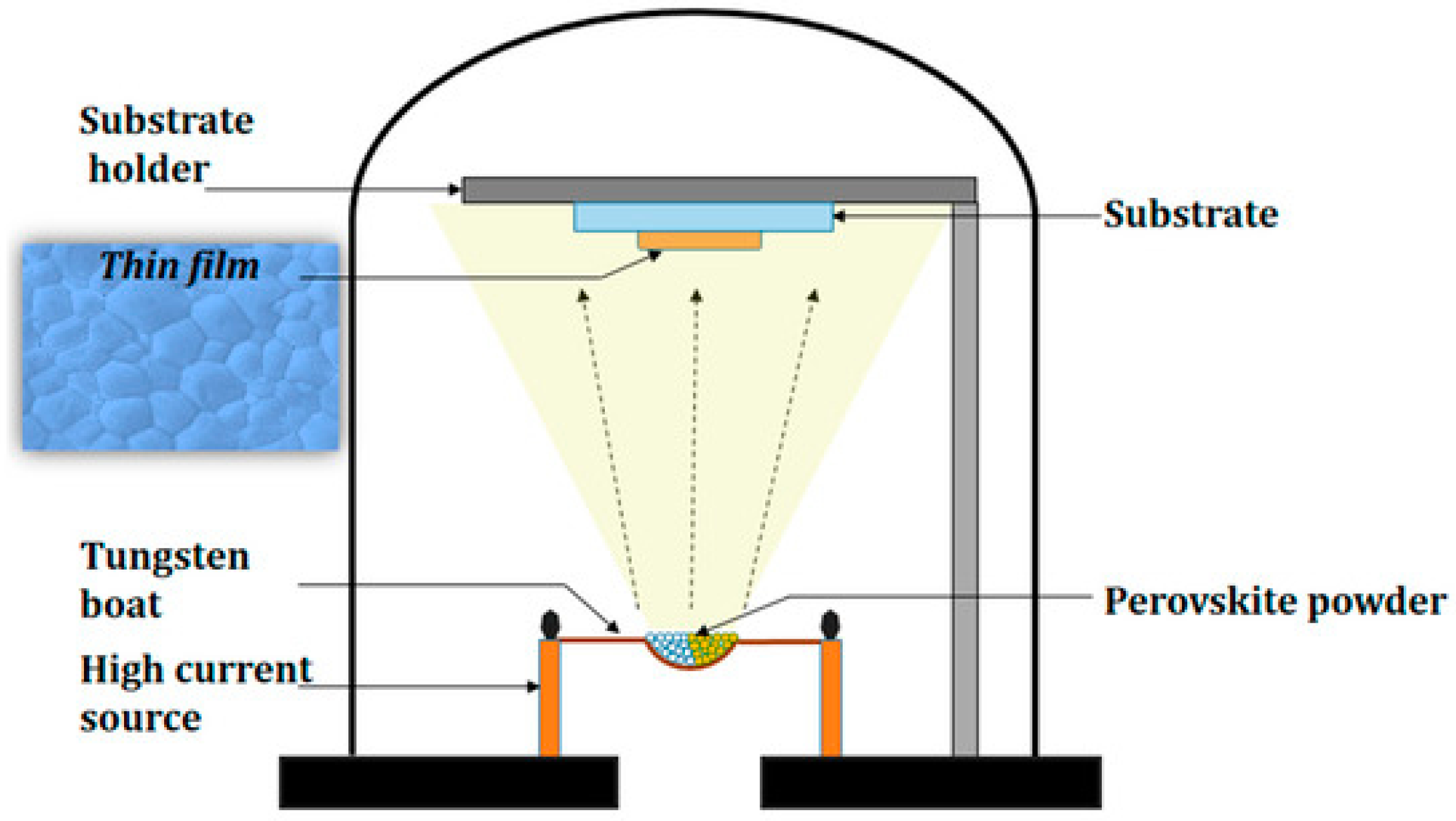
2.2. Vacuum Evaporation
2.3. Electron Beam-Physical Vapor Deposition (EBPVD)
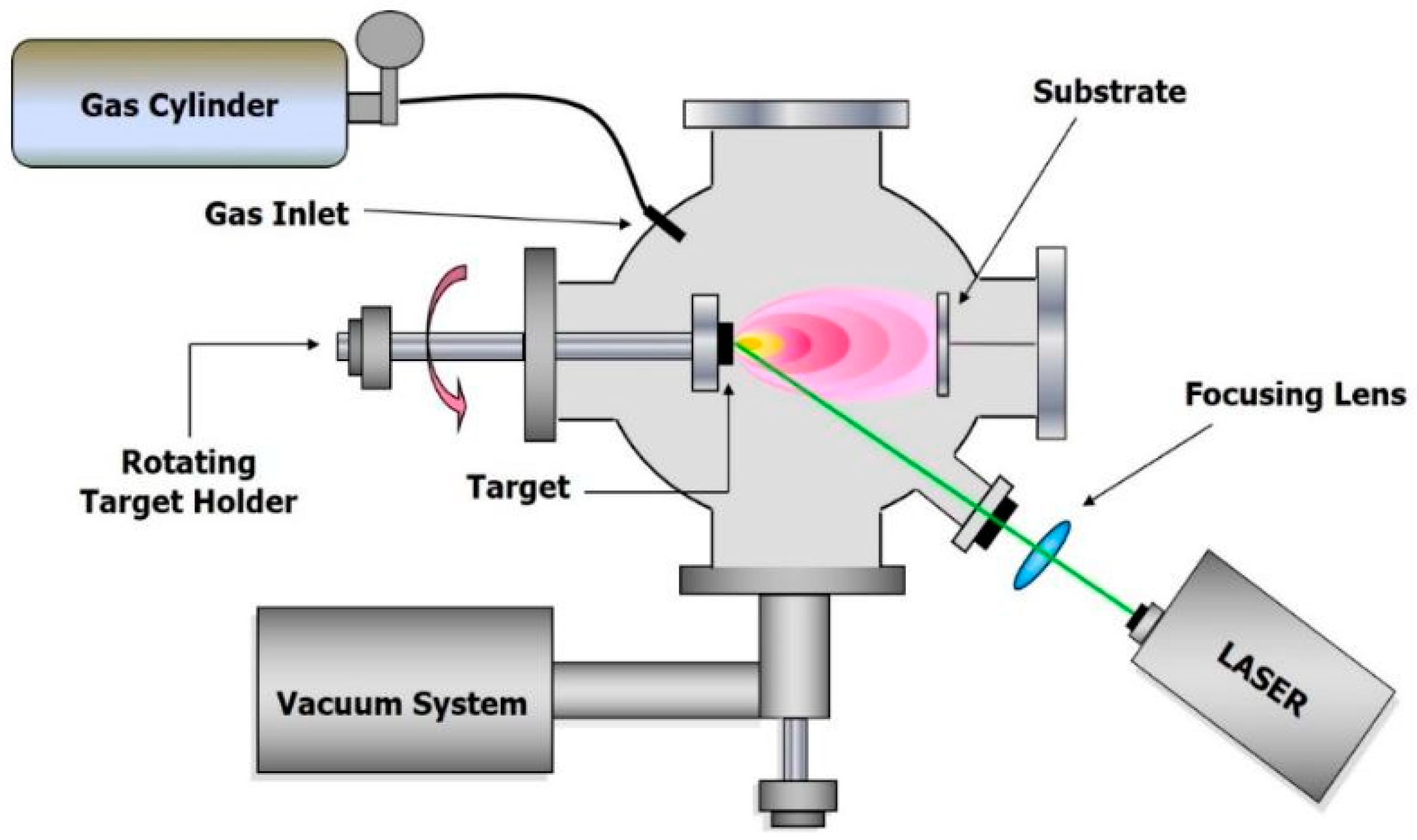
2.4. Pulsed Laser Deposition (PLD)
2.5. Cathodic Arc Evaporation
2.6. Ion Sputtering
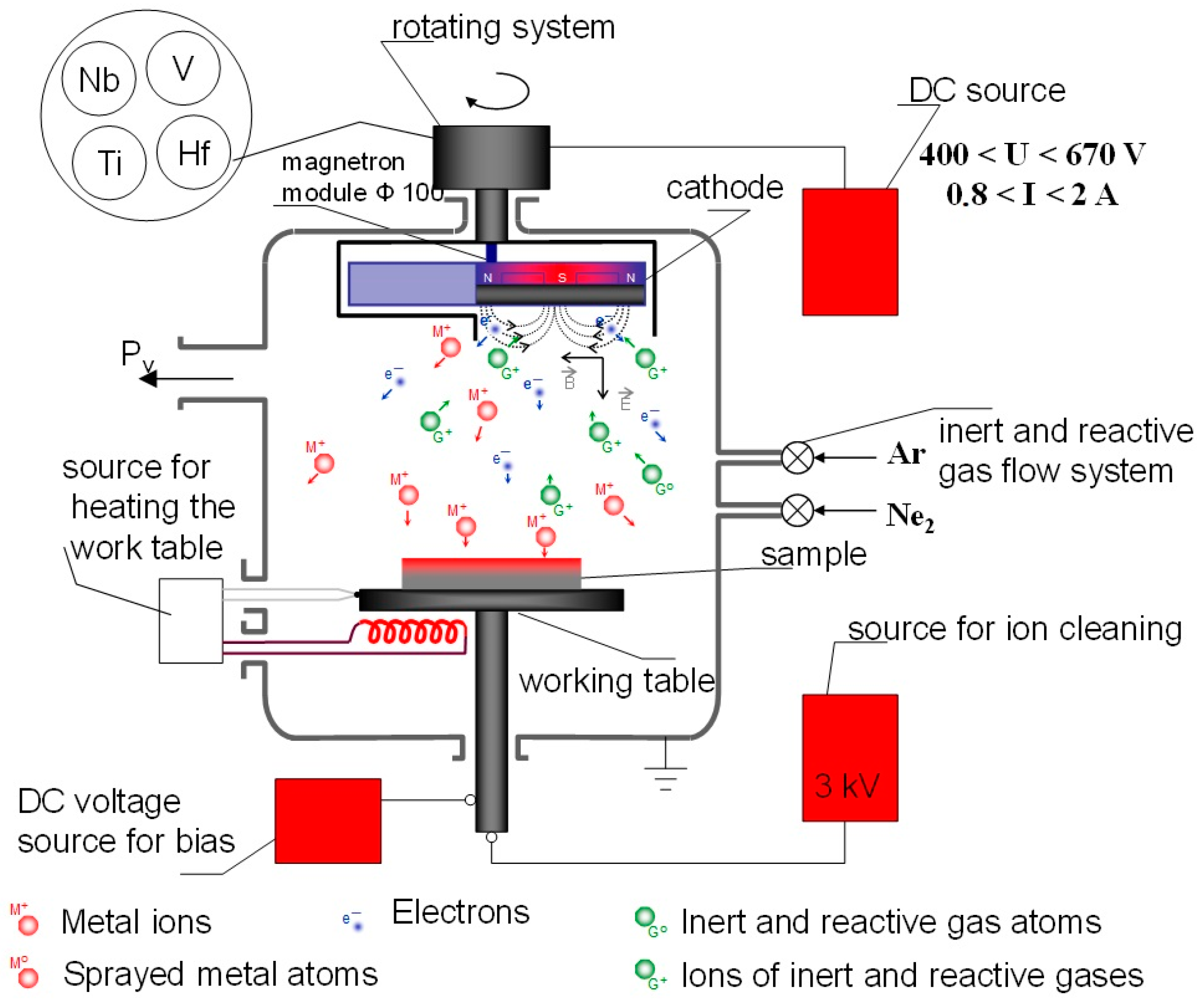
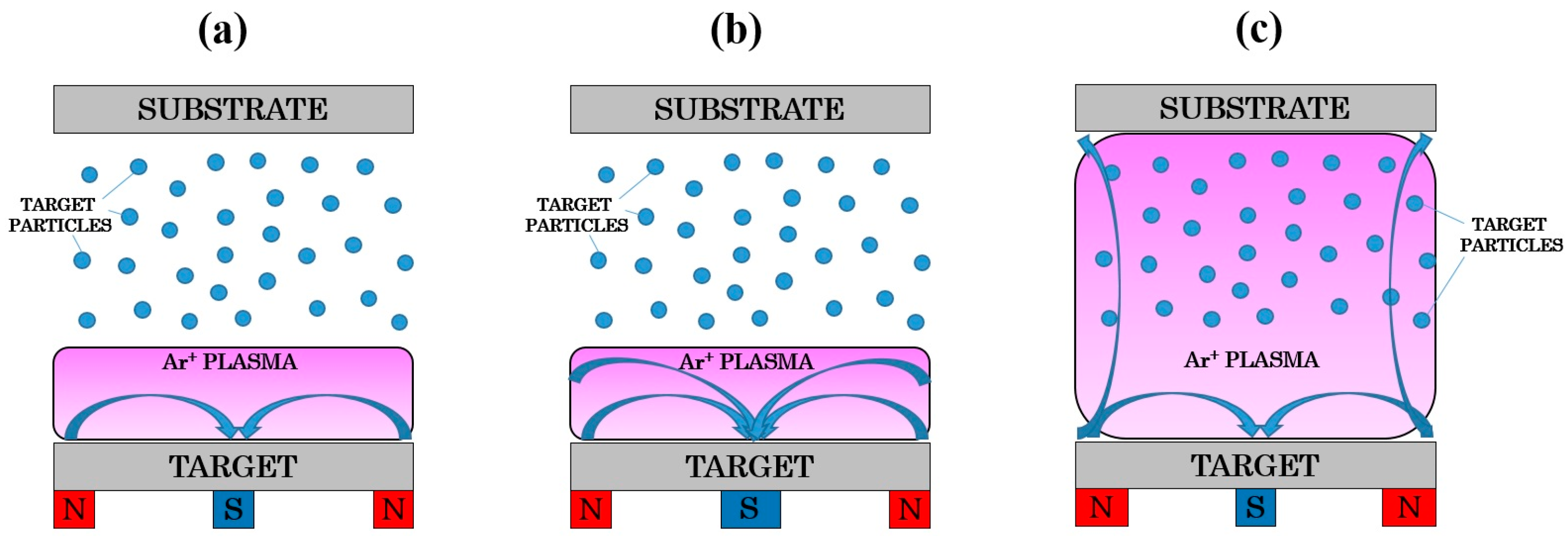
2.7. Magnetron Sputtering
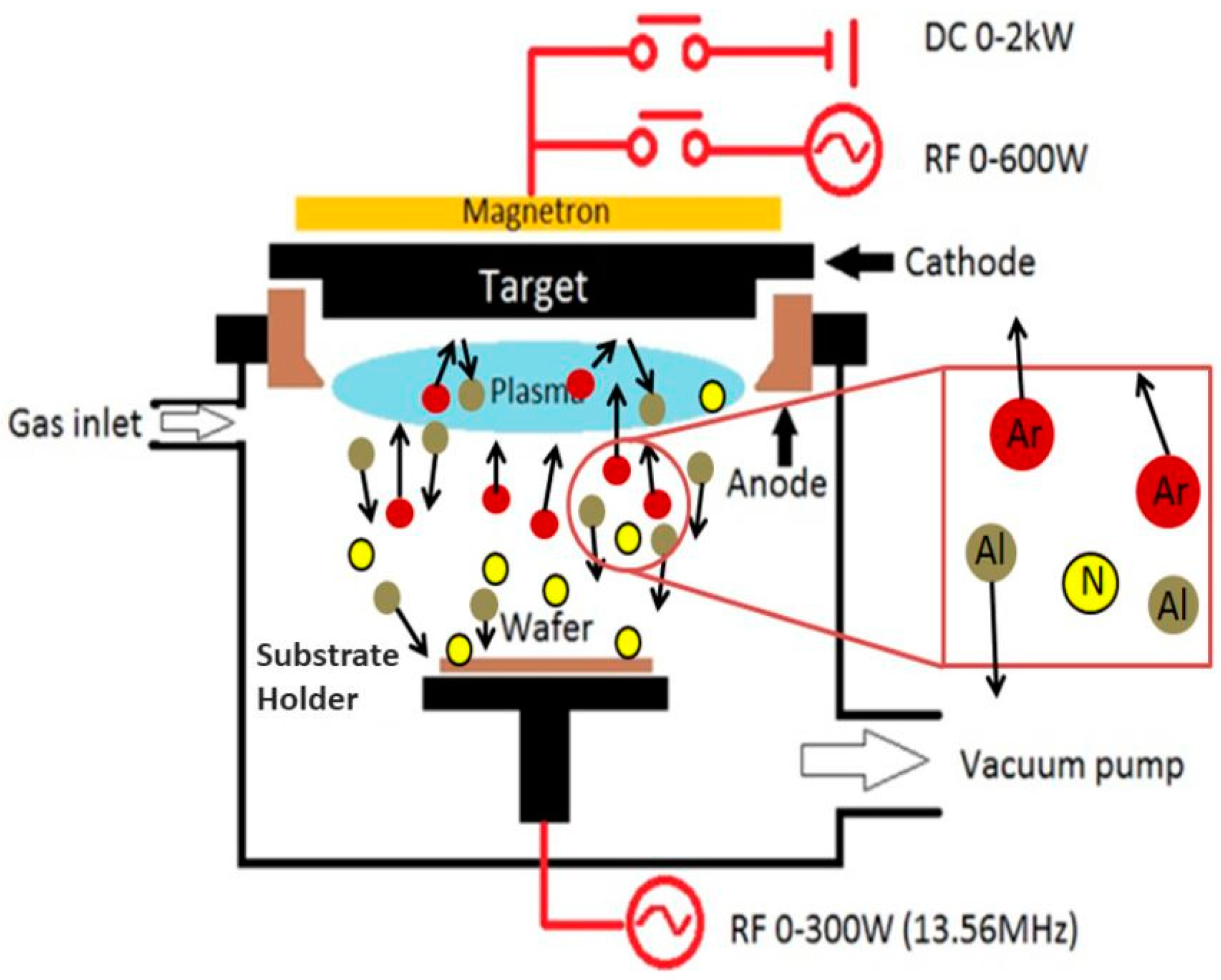
2.8. Radio-Frequency (RF) Magnetron Sputtering
2.9. High-Power Impulse Magnetron Sputtering (HiPIMS)
2.10. Summary of Sputtering Techniques
3. Formation of Metal-Oxide Thin Films and Their Applications
4. Formation of Nitride Thin Films and Their Applications
5. Comparison Between the Properties and Applications of TMO and TMN
6. Formation of Oxy-Nitride Thin Films and Their Applications
7. Electrical Impedance Spectroscopy and Its Application in Thin-Film Characterization
8. Summary
9. Future Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, Q.; Chua, M.; Ong, P.; Lee, J.; Chin, K.; Wang, S.; Kai, D.; Ji, R.; Kong, J.; Dong, Z.; et al. Recent advances in nanotechnology-based functional coatings for the build environment. Mater. Today Adv. 2022, 15, 100270. [Google Scholar] [CrossRef]
- Ramsden, J. Applied Nanotechnology: The Conversion of Research Results to Products; William Andrew: Oxford, UK, 2018. [Google Scholar]
- Saini, A.; Singh, G.; Mehta, S.; Singh, H.; Dixit, S. A review on mechanical behavior of electrodeposited Ni-composite coatings. Int. J. Interact. Des. Manuf. 2023, 17, 2247–2258. [Google Scholar] [CrossRef]
- Hamdiah, I.; Solehudin, A.; Hamdani, A.; Hasanah, L.; Khairurrijal, K.; Kurniawan, T.; Mamat, R.; Maryanti, R.; Nandiyanto, A.; Hammouti, B. Corrosion of copper alloys in KOH, NaOH, NaCl, and HCL electrolyte solutions and its impact to the mechanical properties. Alex. Eng. J. 2021, 60, 2235–2243. [Google Scholar] [CrossRef]
- Zhang, B.; Ghassemi, M.; Zhang, Y. Insulation materials and systems for power electronics modules: A review identifying challenges and future research needs. IEEE Trans. Dielectr. Electr. Insul. 2021, 28, 290–302. [Google Scholar] [CrossRef]
- Borghei, M.; Ghassemi, M. Insulation materials and systems for more- and all-electric aircraft: A review identifying challenges and future research needs. IEEE Trans. Transp. Electrif. 2021, 7, 1930–1953. [Google Scholar] [CrossRef]
- Habib, A.; Hasan, M.; Issa, G.; Singh, D.; Islam, S.; Ghazal, T. Lithium-ion battery management for electric vehicles: Constraints, challenges, and recommendations. Batteries 2023, 9, 152. [Google Scholar] [CrossRef]
- Islam, M.; Wolff, M.; Yassine, M.; Schonweger, G.; Christian, B.; Kohlstedt, H.; Ambacher, O.; Lofink, F.; Kienle, L.; Fichtner, S. On the exceptional temperature stability of ferroelectric Al1-xScxN thin films. App. Phys. Lett. 2021, 118, 232905. [Google Scholar] [CrossRef]
- Guller, O.; Varol, T.; Alver, U.; Biyik, S. The wear and arc erosion behavior of novel copper based functionally graded electrical contact materials fabricated by hot pressing assisted electroless plating. Adv. Powder Technol. 2021, 32, 2873–2890. [Google Scholar] [CrossRef]
- Dongquoc, V.; Seo, D.; Anh, C.; Lee, J.; Park, J.; Kim, E. Controlled surface morphology and electrical properties of sputtered titanium nitride thin film for metal-insulator-metal structures. Appl. Sci. 2022, 12, 10415. [Google Scholar] [CrossRef]
- Grigoriev, S.; Dosko, S.; Vereschaka, A.; Zelenkov, V.; Sotova, C. Diagnostic techniques for electrical discharge plasma used in PVD coating processes. Coatings 2023, 13, 147. [Google Scholar] [CrossRef]
- Grechanyuk, I.; Konoval, P.; Grechanyuk, G.; Badliuk, G.; Myroniuk, D. Properties of Cu-Mo materials produced by physical vapor deposition for electrical contacts. Powder Metall. Metal Ceram. 2021, 60, 183–190. [Google Scholar] [CrossRef]
- Kelly, P.; Arnell, R. Magnetron sputtering: A review of recent developments and applications. Vacuum 2000, 56, 159–172. [Google Scholar] [CrossRef]
- Efeoglu, I.; Arnell, R.; Teer, D. The mechanical and tribological properties of titanium aluminium nitride coatings formed in a four magnetron closed-field sputtering system. Surf. Coat. Technol. 1993, 57, 117–121. [Google Scholar] [CrossRef]
- Peron, M.; Cogo, S.; Bjelland, M.; Afif, A.; Dadlani, A.; Greggio, E.; Berto, F.; Torgersen, J. On the evaluation of ALD TiO2, ZrO2 and HfO2 coatings on corrosion and cytotoxicity performances. J. Magnes. Alloys 2021, 9, 1806–1819. [Google Scholar] [CrossRef]
- Randhawa, S. A state-of-the-art review on advanced ceramic materials: Fabrication, characteristics, applications, and wettability. Pigment Resin Technol. 2024, 53, 768–785. [Google Scholar] [CrossRef]
- Manohar, A.; Bretschger, O.; Nealson, K.; Mansfeld, F. The use of electrochemical impedance spectroscopy (EIS) in the evaluation of the electrochemical properties of a microbial fuel cell. Bioelectrochemistry 2008, 72, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Pejcic, B.; Marco, R. Impedance spectroscopy: Over 35 years of electrochemical sensor optimization. Electrochim. Acta 2006, 51, 6217–6229. [Google Scholar] [CrossRef]
- Chang, B.; Park, S. Electrochemical Impedance Spectroscopy. Annu. Rev. Anal. Chem. 2010, 3, 207–229. [Google Scholar] [CrossRef] [PubMed]
- Arnell, R.; Kelly, P. Recent advances in magnetron sputtering. Surf. Coat. Technol. 1999, 112, 170–176. [Google Scholar] [CrossRef]
- Awan, T.; Afsheen, S.; Kausar, S. Physical vapor deposition techniques. In Thin Film Deposition Techniques; Springer: Singapore, 2025. [Google Scholar]
- Rossnagel, S. Thin filim deposition with physical vapor deposition and related technologies. J. Vac. Sci. Technol. A 2003, 21, S74–S87. [Google Scholar] [CrossRef]
- Gates, S. Surface chemistry in the chemical vapor deposition of electronic materials. Chem. Rev. 1996, 4, 1519–1532. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; King, D.; Weimer, A. Ceramic ultra-thin coatings using atomic layer deposition. In Ceramic Nanocomposites; Woodhead Publishing: Sawston, UK, 2013. [Google Scholar]
- Vorobyova, M.; Biffoli, F.; Giurlani, W.; Martinuzzi, M.; Linser, M.; Caneschi, A.; Innocenti, M. PVD for Decorative Applications: A Review. Materials 2023, 16, 4919. [Google Scholar] [CrossRef] [PubMed]
- Qaid, S.; Ghaithan, H.; Al-Asbahi, B.; Aldwayyan, A. Single-source thermal evaporation growth and the tuning surface passivation layer thickness effect in enhanced amplified spontaneous emission properties of CsPb(Br0.5Cl0.5)3 Perovskite Films. Polymers 2020, 12, 2953. [Google Scholar] [CrossRef] [PubMed]
- Elanjeitsenni, V.; Vadivu, K.; Prasanth, B. A review on thin films, conducting polymers as sensor devices. Mater. Res. Express 2022, 9, 022001. [Google Scholar] [CrossRef]
- Silva, N.; Goncalves, L.; Carvalho, H. Deposition of conductive materials on textile and polymeric flexible substrates. J. Mater. Sci. Mater. Electron. 2013, 24, 635–643. [Google Scholar]
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. On coating techniques for surface protection: A review. J. Manuf. Mater. Process. 2019, 3, 28. [Google Scholar] [CrossRef]
- Kotlarski, G.; Valkov, S.; Andreeva, A.; Mateev, V.; Marinova, I.; Petrov, P. Electrical contact resistance of tungsten coatings deposited on Cu and Al conductors. J. Phys. Conf. Ser. 2021, 1859, 012063. [Google Scholar] [CrossRef]
- Kuanr, S.; Vinothkumar, G.; Babu, K. Substrate temperature dependent structural orientation of EBPVD deposited NiO films and its influence on optical, electrical property. Mater. Sci. Semicond. Process. 2018, 75, 26–30. [Google Scholar] [CrossRef]
- Ogugua, S.; Ntwaeaborwa, O.; Swart, H. Latest development on pulsed laser deposited thin films for advanced luminescence applications. Coatings 2020, 10, 1078. [Google Scholar] [CrossRef]
- Karnati, P.; Haque, A.; Taufique, N.; Ghosh, K. A Systematic study on the structural and optical properties of vertically aligned zinc oxide nanorods grown by high pressure assisted pulsed laser deposition technique. Nanomaterials 2018, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Osiac, M.; Cioatera, N.; Jigau, M. Structural, morphological, and optical properties of iron doped WO3 thin film prepared by pulsed laser deposition. Coatings 2020, 10, 412. [Google Scholar] [CrossRef]
- Zhang, Y.; Russo, E.; Mao, S. Femtosecond laser assisted growth of ZnO nanowires. Appl. Phys. Lett. 2005, 87, 133115. [Google Scholar] [CrossRef]
- Duta, L.; Ristoscu, C.; Stan, C.; Husanu, M.; Besleaga, C.; Chifiriuc, M.; Lazar, V.; Bleotu, C.; Miculescu, F.; Mihailescu, N.; et al. New bio-active, antimicrobial and adherent coatings of nanostructured carbon double-reinforced with silver and silicon by Matrix-Assited Pulsed Laser Evaporation for medical applications. Appl. Surf. Sci. 2018, 441, 871–883. [Google Scholar] [CrossRef]
- Bonis, A.; Teghil, R. Ultra-short pulsed laser deposition of oxides, borides and carbides of transition elements. Coatings 2020, 10, 501. [Google Scholar] [CrossRef]
- Lux, H.; Edling, M.; Lucci, M.; Kitzmann, J.; Villringer, C.; Siemroth, P.; De Matteis, F.; Schrader, S. The role of substrate temperature and magnetic filtering for DLC by cathodic arc evaporation. Coatings 2019, 9, 345. [Google Scholar] [CrossRef]
- Vyskocil, J.; Musil, J. Cathodic arc evaporation in thin film technology. J. Vac. Sci. Technol. A 1992, 10, 1740–1748. [Google Scholar] [CrossRef]
- Kuprin, A.; Gilewicz, A.; Kuznetsova, T.; Lapitskaya, V.; Tolmachova, G.; Warcholinski, B.; Aizikovich, S.; Sadyrin, E. Structure and properties of ZrON coatings synthesized by cathodic arc evaporation. Materials 2021, 14, 1483. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.; Doyle, E.; Wong, Y.; Munroe, P.; Cairney, J.; Long, J. Reducing the macroparticle content of cathode arc evaporation TiN coatings. Surf. Coat. Technol. 2004, 183, 283–294. [Google Scholar] [CrossRef]
- Gilewicz, T.; Warcholinski, B.; Murzynski, D. The properties of molybdenum nitride coatings obtained by cathodic arc evaporation. Surf. Coat. Technol. 2013, 236, 149–158. [Google Scholar] [CrossRef]
- Olbrich, W.; Fessmann, J.; Kampschulte, G.; Ebberink, J. Improved control of TiN coating properties using cathodic arc evaporation with a pulsed bias. Surf. Coat. Technol. 1991, 49, 258–262. [Google Scholar] [CrossRef]
- Mwema, F.; Akinlabi, E.; Oladijo, O.; Majumdar, J. Effect of varying low substrate temperature on sputtered aluminum films. Mater. Res. Express 2019, 6, 056404. [Google Scholar] [CrossRef]
- Rabadzhiyska, S.; Dechev, D.; Ivanov, N.; Ivanova, T.; Strijkova, V.; Katrova, V.; Rupetsov, V.; Dimcheva, N.; Valkov, S. Wear and Corrosion Resistance of ZrN Coatings Deposited on Ti6Al4V Alloy for Biomedical Applications. Coatings 2024, 14, 1434. [Google Scholar] [CrossRef]
- Qin, X.; Sui, C.; Di, L. Influence of substrate temperature on the morphology and structure of bismuth thin films deposited by magnetron sputtering. Vacuum 2019, 166, 316–322. [Google Scholar] [CrossRef]
- Dorri, M.; Thornberg, J.; Hellgren, N.; Palisaitis, J.; Petruhins, A.; Klimashin, F.; Hultman, L.; Petrov, I.; Persson, P.; Rosen, J. Synthesis and characterization of CrB2 thin films grown by DC magnetron sputtering. Scr. Mater. 2021, 200, 113915. [Google Scholar] [CrossRef]
- Koshy, A.; Sudha, A.; Yadav, S.; Swaminathan, P. Effect of substrate temperature on the optical properties of DC magnetron sputtered copper oxide thin films. Phys. B Condens. Matter 2023, 650, 414452. [Google Scholar] [CrossRef]
- Valkov, S.; Parshorov, S.; Andreeva, A.; Bezdushnyi, R.; Nikolova, M.; Dechev, D.; Ivanov, N.; Petrov, P. Influence of electron beam treatment of Co-Cr alloy on the growing mechanism, surface topography, and mechanical properties of deposited TiN/TiO2 coatings. Coatings 2019, 9, 513. [Google Scholar] [CrossRef]
- Vega-Moron, R.; Castro, G.; Melo-Maximo, D.; Mendez-Mendez, J.; Melo-Maximo, L.; Oseguera-Pena, J.; Meneses-Amador, A. Adhesion and mechanical properties of Ti films deposited by DC magnetron sputtering. Surf. Coat. Technol. 2018, 349, 1137–1147. [Google Scholar] [CrossRef]
- Iqbal, A.; Mohd-Yasin, F. Reactive sputtering of Aluminium nitride (002) thin films for piezoelectric applications: A review. Sensors 2018, 18, 1797. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Yan, S.; Zhao, R.; Liu, Z. Structure and properties of (AlCrMoNiTi)Nx and (AlCrMoZrTi)Nx films by reactive RF sputtering. Surf. Coat. Technol. 2013, 235, 764–772. [Google Scholar] [CrossRef]
- Baba, S.; Kinbara, A.; Kajiwara, T.; Watanabe, K. Internal stress and adhesion of r.f. sputtered MgO films on glass substrates. Thin Solid Films 1988, 164, 169–174. [Google Scholar] [CrossRef]
- Yizhe, W.; Zha-ma, T.; Zhenming, Y.; Hui, S.; Jianhong, G.; Jun, G. Research on adhesion strength and optical properties of SiC films obtained via RF magnetron sputtering. Chin. J. Phys. 2020, 64, 79–86. [Google Scholar] [CrossRef]
- Vossen, J. Control of film properties by rf-sputtering techniques. J. Vac. Sci. Technol. 1971, 8, S12–S30. [Google Scholar] [CrossRef]
- Maurya, D.; Sardarinejad, A.; Alameh, K. Recent developments in R.F. magnetron sputtered thin films for pH sensing applications—An overview. Coatings 2014, 4, 756–771. [Google Scholar] [CrossRef]
- Gudmundsson, J. Physics and technology of magnetron sputtering discharges. Plasma Sources Sci. Technol. 2020, 29, 113001. [Google Scholar] [CrossRef]
- Kouznetsov, V.; Macak, K.; Schneider, J.; Helmersson, U.; Petrov, I. A novel pulsed magnetron sputter technique utilizing very high target power densities. Surf. Coat. Technol. 1999, 122, 290. [Google Scholar] [CrossRef]
- Gudmundsson, J.; Brenning, N.; Lundin, D.; Helmersson, U. High power impulse magnetron sputtering discharge. J. Vac. Sci. Technol. A 2012, 30, 030801. [Google Scholar] [CrossRef]
- Lundin, D.; Sarakinos, K. An introduction to thin film processing using high-power impulse magnetron sputtering. J. Mater. Res. 2012, 27, 780–792. [Google Scholar] [CrossRef]
- Anders, A. Tutorial: Reactive high power impulse magnetron sputtering (R-HiPIMS). J. Appl. Phys. 2017, 121, 171101. [Google Scholar] [CrossRef]
- Breilmann, W.; Maxzl, C.; Benedikt, J.; Keudell, A. Dynamic of the growth flux at the substrate during high-power pulsed magnetron sputtering (HiPIMS) of titanium. J. Phys. D Appl. Phys. 2013, 46, 485204. [Google Scholar] [CrossRef]
- Greczynski, G.; Lu, J.; Jensen, J.; Bolz, S.; Kolker, W.; Schiffers, C.; Lemmer, O.; Greene, J.; Hultman, L. A review of metal-ion-flux-controlled growth of metastable TiAlN by HIPIMS/DCMS co-sputtering. Surf. Coat. Technol. 2014, 257, 15–25. [Google Scholar] [CrossRef]
- Sittinger, V.; Lenck, O.; Vergohl, M.; Szyszka, B.; Brauer, G. Applications of HIPIMS metal oxides. Thin Solid Films 2013, 548, 18–26. [Google Scholar] [CrossRef]
- Losquai, S.; Baloukas, B.; Zabeida, O.; Klemberg-Sapieha, J.; Martinu, L. HiPIMS-deposited thermochromic VO2 filims on polymeric substrates. Sol. Energy Mat. Sol. Cells 2016, 155, 60–69. [Google Scholar] [CrossRef]
- Devan, R.; Patil, R.; Lin, J.; Ma, Y. One-dimensional metal-oxide nanostructures: Recent developments in synthesis, characterization, and applications. Adv. Funct. Mater. 2012, 22, 3326–3370. [Google Scholar] [CrossRef]
- Ashik, U.P.M.; Kudo, S.; Hayashi, J. An overview of metal oxide nanostructures. In Micro and Nano Technologies, Synthesis of Inorganic Nanomaterials, 1st ed.; Bhagyaraj, S., Olumafemi, O., Kalarikkal, N., Thomas, S., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 19–57. [Google Scholar]
- Baptista, A.; Silva, F.; Porteiro, J.; Miguesz, J.; Pinto, G.; Fernandes, L. One the physical vapour deposition (PVD): Evolution of magnetron sputtering processes for industrial applications. Procedia Manuf. 2018, 17, 746–757. [Google Scholar] [CrossRef]
- Ramezani, M.; Mohd Ripin, Z.; Pasang, T.; Jiang, C.-P. Surface engineering of metals: Techniques, characterizations and applications. Metals 2023, 13, 1299. [Google Scholar] [CrossRef]
- Petti, L.; Munzenrieder, N.; Vogt, C.; Faber, H.; Buthe, L.; Cantarella, G.; Bottacchi, F.; Anthopoulos, T.; Troster, G. Metal oxide semiconductor thin-film transistors for flexible electronics. Appl. Phys. Rev. 2016, 3, 021303. [Google Scholar] [CrossRef]
- Al-harbi, L.; Alsulami, Q.; Farea, M.; Rajesh, A. Tuning optical, dielectric, and electrical properties of Polyethylene oxide/Carboxymethyl cellulose doped with mixed metal oxide nanoparticles for flexible electronic devices. J. Mol. Struct. 2023, 1272, 134244. [Google Scholar] [CrossRef]
- Cao, Y.; He, Y.; Gang, H.; Wu, B.; Yan, L.; Wei, D.; Wang, H. Stability study of transition metal oxide electrode materials. J. Power Sources 2023, 560, 232710. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Fazio, E.; Spadaro, S.; Corsaro, C.; Neri, G.; Leonardi, S.; Neri, F.; Lavanya, N.; Sekar, C.; Donato, N.; Neri, G. Metal-oxide based nanomaterials: Synthesis, characterization and their applications in electrical and electrochemical sensors. Sensors 2021, 21, 2494. [Google Scholar] [CrossRef] [PubMed]
- Saruhan, B.; Fomekong, R.; Nahirniak, S. Review: Influences of semiconductor metal oxide properties on gas sensing characteristics. Frontiers 2021, 2, 657931. [Google Scholar] [CrossRef]
- Kumar, K.; Atchuta, S.; Prasad, M.; Barshilia, H. Review on selective absorber coatings: A catalyst for enhanced solar energy conversion efficiency. Sol. Energy Mater. Sol. Cells 2024, 277, 113080. [Google Scholar] [CrossRef]
- Agravat, D.; Patel, S.; Alsalman, O. Nanostructured metal-oxide materials for solar energy absorption and conversion for industrial heater applications. Int. J. Therm. Sci. 2024, 200, 108951. [Google Scholar] [CrossRef]
- Jain, N.; Kumar, D.; Bhardwaj, K.; Sharma, R.; Holovsky, J.; Mishra, M.; Mishra, Y.; Sharma, S. Heterostructured core-shell metal oxide-based nanobrushes for ultrafast UV photodetectors. Mater. Sci. Eng. R Rep. 2024, 160, 100826. [Google Scholar] [CrossRef]
- Nunes, D.; Pimentel, A.; Goncalves, A.; Pereira, S.; Branquinho, R.; Barquinha, P.; Fortunato, E.; Martins, R. Metal oxide nanostructures for sensor applications. Semicond. Sci. Technol. 2019, 34, 043001. [Google Scholar] [CrossRef]
- Zhao, C.; Yao, S.; Li, C.; Zhao, S.; Sun, X.; Wang, K.; Zhang, X.; Ma, Y. Recent advances in transition metal oxides as anode materials for high-performance lithium-ion capacitors. Chem. Eng. J. 2024, 497, 154535. [Google Scholar] [CrossRef]
- Park, B.; Nam, S.; Kang, Y.; Jeon, S.; Jo, J.; Park, S.; Kim, Y. Cation doping strategy for improved carrier mobility and stability in metal-oxide Heterojunction thin-film transistors. Mater. Today Electron. 2024, 8, 100090. [Google Scholar] [CrossRef]
- Wang, Z.; Su, J.; Qi, H.; Pan, P.; Jiang, M. Porous nanocrystalline WO3 thin films: Fabrication, electrical and optical properties. Surf. Inn. 2020, 9, 214–221. [Google Scholar] [CrossRef]
- Banyamin, Z.; Kelly, P.; West, G.; Boardman, J. Electrical and Optical Properties of Fluorine Dopes Tin Oxide Thin Films Prepared by Magnetron Sputtering. Coatings 2014, 4, 732–746. [Google Scholar] [CrossRef]
- Yu, X.; Marks, T.; Facchetti, A. Metal oxides for optoelectronic applications. Nat. Mater. 2016, 15, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Kumbhakar, P.; Gowda, C.; Mahapatra, P.; Mukherjee, M.; Malviya, K.; Chaker, M.; Chandra, A.; Lahiri, B.; Ajayan, P.; Jariwala, D.; et al. Emerging 2D metal oxides and their applications. Mater. Today 2021, 45, 142–168. [Google Scholar] [CrossRef]
- Rabadzhiyska, S.; Ormanova, M.; Valkov, S.; Dechev, D.; Terziyska, P.; Petrov, P. Study of the structure, roughness and optical properties of HfO2 coatings deposited on microscopic glass substrates. J. Phys. Conf. Ser. 2022, 2240, 012011. [Google Scholar] [CrossRef]
- Heard, C.; Cejka, J.; Opanasenko, M.; Nachtigall, P.; Centi, G.; Perathoner, S. 2D oxide nanomaterials to address the energy transition and catalysis. Adv. Mater. 2019, 31, 1801712. [Google Scholar] [CrossRef] [PubMed]
- Alhabradi, M.; Yang, X.; Alruwaili, M.; Chang, H.; Tahir, A.A. Enhanced photoelectrochemical performance using cobalt-catalyst-loaded PVD/RF-Engineered WO3 photoelectrodes. Nanomaterials 2024, 14, 259. [Google Scholar] [CrossRef] [PubMed]
- Ratova, M.; Sawtell, D.; Kelly, P. Micro-Patterning of Magnetron Sputtered Titanium Dioxide Coatings and Their Efficiency for Photocatalytic Applications. Coatings 2020, 10, 68. [Google Scholar] [CrossRef]
- Pourhashem, S.; Seif, A.; Saba, F.; Nezhad, E.; Ji, X.; Zhou, Z.; Zhai, X.; Mirzaee, M.; Duan, J.; Rashidi, A.; et al. Antifouling nanocomposite polymer coatings for marine applications: A review on experiments, mechanisms, and theoretical studies. J. Mater. Sci. Technol. 2022, 118, 73–113. [Google Scholar] [CrossRef]
- Desireddy, S.; Chacko, S. A review on metal oxide (FeOx/MnOx) mediated nitrogen removal processes and its application in wastewater treatment. Rev. Environ. Sci. Bio Technol. 2021, 20, 697–728. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Tzvetkov, I.; Dimitrova, T.V.; Ivanova, V.L.; Handzhiyski, Y.; Andreeva, A.; Valkov, S.; Ormanova, M.; Apostolova, M.D. Effect of Co-sputtered copper and titanium oxide coatings on bacterial resistance and cytocompatibility of osteoblast cells. Nanomaterials 2024, 14, 1148. [Google Scholar] [CrossRef] [PubMed]
- Kannan, P.; Maduraiveeran, G. Metal oxides nanomaterials and nanocomposite-based electrochemical sensors for healthcare applications. Biosensors 2023, 13, 542. [Google Scholar] [CrossRef] [PubMed]
- Thirugnanasambandam, A.; Gupta, M.; Murugapandian, R. Biocompatibility and corrosion resistance of Si/ZrO2 bioceramic coating on AZ91D using electron beam physical vapor deposition (EB-PVD) for advanced biomedical applications. Metals 2024, 14, 607. [Google Scholar] [CrossRef]
- Ilievska, I.; Ivanova, V.; Dechev, D.; Ivanov, N.; Ormanova, M.; Nikolova, M.P.; Handzhiyski, Y.; Andreeva, A.; Valkov, S.; Apostolova, M.D. Influence of Thickness on the Structure and Biological Response of Cu-O Coatings Deposited on cpTi. Coatings 2024, 14, 455. [Google Scholar] [CrossRef]
- Danish, M.; Bhattacharya, A.; Stepanova, D.; Mikhaylov, A.; Grilli, M.; Khosravy, M.; Senjyu, T. A Ssystematic review of metal oxide applications for energy and environmental sustainability. Metals 2020, 10, 1604. [Google Scholar] [CrossRef]
- Don, C.; Shalvey, T.; Sindi, D.; Lewis, B.; Swallow, J.; Bowen, L.; Fernandes, D.; Kubart, T.; Biswas, D.; Thakur, P.; et al. Reactive DC sputtered TiO2 electron transport layers for cadmium-free Sb2Se3 solar cells. Adv. Energy Mater. 2024, 14, 2401077. [Google Scholar] [CrossRef]
- Wojcieszak, D.; Mazur, M.; Indyka, J.; Jurkowska, A.; Kalisz, M.; Domanawski, P.; Kaczmarek, D.; Domaraszki, J. Mechanical and structural properties of titanium dioxide deposited by innovative magnetron sputtering process. Mater. Sci. 2015, 33, 660–668. [Google Scholar] [CrossRef]
- Aissani, L.; Alhussein, A.; Zia, A.W.; Mamba, G.; Rtimi, S. Magnetron sputtering of transition metal nitride thin films for environmental remediation. Coatings 2022, 12, 1746. [Google Scholar] [CrossRef]
- Bobzin, K.; Brogelmann, T.; Kruppe, N.; Janowitz, J. Smart PVD hard coatings with temperature sensor function. Surf. Coat. Technol. 2021, 423, 127631. [Google Scholar] [CrossRef]
- Popov, A.A.; Tikhonowski, G.V.; Shakhov, P.V.; Popova-Kuznetsova, E.A.; Tselikov, G.I.; Romanov, R.I.; Markeev, A.M.; Klimentov, S.M.; Kabashin, A.V. Synthesis of Titanium Nitride Nanoparticles by Pulsed Laser Ablation in Different Aqueous and Organic Solutions. Nanomaterials 2022, 12, 1672. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, C.; Wang, C.; Cao, X.; Ma, R.; Sui, X.; Hao, J.; Liu, W. Investigation of (CrAlTiNbV)Nx high-entropy nitride coatings via tailoring nitrogen flow rate for anti-wear applications in aviation lubricant. Appl. Surf. Sci. 2021, 557, 149813. [Google Scholar] [CrossRef]
- Yang, H.; Weng, C.; Wang, H.; Yuan, Z. Transition metal nitride-based materials as efficient electrocatalysts: Design strategies and prospective applications. Coord. Chem. Rev. 2023, 496, 215410. [Google Scholar] [CrossRef]
- Kadam, S.; Jose, L.; George, N.; Sreehari, S.; Nayana, D.; Pham, D.; Kadam, K.; Aravind, A.; Ma, Y. Recent progress in transition metal nitride electrodes for supercapacitor, water splitting, and battery applications. J. Alloys Compd. 2024, 976, 173083. [Google Scholar] [CrossRef]
- Dabees, S.; Mirzaei, S.; Kaspar, P.; Holcman, V.; Sobola, D. Characterization and evaluation of engineered coating techniques for different Ccutting tools—Review. Materials 2022, 15, 5633. [Google Scholar] [CrossRef] [PubMed]
- Qin, R.; Shan, G.; Hu, M.; Huang, W. Two-dimensional transition metal carbides and/or nitrides (MXenes) and their applications in sensors. Mater. Today Phys. 2021, 21, 100527. [Google Scholar] [CrossRef]
- Shirguppikar, S.; Patil, M. Experimental investigation on micro-electro discharge machining process using tungsten carbide and titanium nitride-coated micro-tool electrode for machining of Ti-6Al-4V. Adv. Mater. Process. Technol. 2020, 8, 187–204. [Google Scholar] [CrossRef]
- Yousefi, M.; Baghshahi, S.; Kerahroodi, M. Effect of titanium nitride, diamond-like carbon and chromium carbonitride coatings on the life time of an AISI M2 steel punch forming tool. J. Bio- Tribo-Corr. 2021, 7, 50. [Google Scholar] [CrossRef]
- Bakdemir, S.; Ozkan, D.; Turkuz, C.; Salman, S. Wear performance under dry and lubricated conditions of duplex treatment TiN/TiCrN coatings deposited with different numbers of CrN interlayers on steel substrates. Wear 2023, 526–527, 204931. [Google Scholar] [CrossRef]
- Indupuri, S.; Kumar, R.; Prasad, S.; Kumar, K.; Islam, A.; Masoom, S.; Pandey, S.; Keshri, A. Plasma sprayed aluminium nitride (AlN) coating: Microstructural, mechanical, tribological, and corrosion resistance performance. J. Eur. Ceram. Soc. 2024, 44, 1458–1469. [Google Scholar] [CrossRef]
- Krekeler, T.; Rout, S.; Krishnamurthy, G.; Stormer, M.; Arya, M.; Ganguly, A.; Sutherland, D.; Bozhevolnyi, S.; Ritter, M.; Pedersen, K.; et al. Unprecedented thermal stability of plasmonic titanium nitride films up to 1400 °C. Adv. Opt. Mater. 2021, 9, 2100323. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Qin, Y.; Lu, J.; Liu, W. Improvement of TiN coating on comprehensive performance of NiTi alloy braided vascular stent. Ceram. Int. 2023, 49, 13405–13413. [Google Scholar] [CrossRef]
- Saad, K.; Saba, T.; Rashid, A. Application of PVD coatings in medical implantology for enhanced performance, biocompatibility, and quality of life. Heliyon 2024, 10, e35541. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.; Li, H.; Benson, P.; Whitehead, K.; Verran, J.; Arnell, R.; Iordanova, I. Comparison of the tribological and antimicrobial properties of CrN/Ag, ZrN/Ag, TiN/Ag, and TiN/Cu nanocomposite coatings. Surf. Coat. Technol. 2010, 205, 1606–1610. [Google Scholar] [CrossRef]
- Rojas, N.; Sanchez-Molina, M.; Sevilla, G.; Amores, E.; Almandoz, E.; Esparza, J.; Vivas, M.; Colominas, C. Coated stainless steels evaluation for bipolar plates in PEM water electrolysis conditions. Int. J. Hydrogen Energy 2021, 46, 25929–25943. [Google Scholar] [CrossRef]
- Comakli, O. Improved structural, mechanical, corrosion and tribocorrosion properties of Ti45Nb alloys by TiN, TiAlN monolayers, and TiAlN/TiN multilayer ceramic films. Ceram. Int. 2021, 47, 4149–4156. [Google Scholar] [CrossRef]
- Liu, J.; Hao, Z.; Cui, Z.; Ma, D.; Lu, J.; Cui, Y.; Li, C.; Liu, W.; Xie, S.; Hu, P.; et al. Oxidation behavior, thermal stability, and the coating/substrate interface evolution of CrN-coated Zircaloy under high-temperature steam. Corros. Sci. 2021, 185, 109416. [Google Scholar] [CrossRef]
- Zhang, K.; Xin, L.; Ma, T.; Chang, H.; Lu, Y.; Feng, C.; Zhu, S.; Wang, F. Investigation of the role of silicon in TiAlSiN coating deposited on TiAl alloys during long-term oxidation. Corros. Sci. 2022, 204, 110394. [Google Scholar] [CrossRef]
- Cheng, Z.; Qi, W.; Pang, C.; Thomas, T.; Wu, T.; Liu, S.; Yang, M. Recent advances in transition metal nitride-based materials for photocatalytic applications. Adv. Funct. Mater. 2021, 31, 2100553. [Google Scholar] [CrossRef]
- Luo, Q.; Lu, C.; Liu, L.; Zhu, M. review on the synthesis of transition metal nitride nanostructures and their energy related applications. Green Energy Environ. 2023, 8, 406–437. [Google Scholar] [CrossRef]
- Wang, Z.; Inoue, Y.; Hisatomi, T.; Ishikawa, R.; Wang, Q.; Takata, T.; Chen, S.; Shibata, N.; Ikuhara, Y.; Domen, K. Overall water splitting by Ta3N5 nanorod single crystals grown on the edges of KTaO3 particles. Nat. Catal. 2018, 1, 756–763. [Google Scholar] [CrossRef]
- Ebaid, M.; Min, J.; Zhao, C.; Ng, T.; Idriss, H.; Ooi, B. Water splitting to hydrogen over epitaxially grown InGaN nanowires on metallic titanium/silicon template: Reduced interfacial transfer resistance and improved stability. J. Mater. Chem. A 2018, 16, 6922–6930. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, H.; Shuai, T.; Zhan, Q.; Zhang, Z.; Huang, K.; Dai, C.; Li, G. Recent progress in the synthesis of transition metal nitride catalysts and their applications in electrocatalysis. Nanoscale 2023, 15, 11777. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, M.; Lopez-Navarrete, G.; Osendi, M.; Miranzo, P. Heat dissipation in 3D printed cellular aluminum nitride structures. J. Eur. Ceram. Soc. 2021, 41, 2407–2414. [Google Scholar] [CrossRef]
- Kumaresan, V.; Sreekantan, S.; Devarajan, M.; Mohamed, K. Non- oil bleed two-part silicone dispensable thermal gap filler with Al2O3 and AlN filler for effective heat dissipation in electronics packaging. J. Adhes. 2020, 98, 855–870. [Google Scholar] [CrossRef]
- Wei, J.; Chen, N.; Li, L.; Liu, J.; Zhao, J.; Wang, C.; He, N. Effect of spoiler columns on heat transfer performance of aluminum nitride-based microchannel heat sink. Ceram. Int. 2022, 48, 36226–36237. [Google Scholar] [CrossRef]
- Chasnyk, V.; Chasnyk, D.; Fesenko, I.; Kaidash, O.; Turkevych, V. Dielectric characteristics of pressureless sintered AlN-based composites in the 3–37 GHz frequency range. J. Mater. Sci. Mater. Electron. 2021, 32, 2524–2534. [Google Scholar] [CrossRef]
- Radhika, E.; Samuel, T.; Dobbidi, P. A modified sintering method to prepare phase pure AlN ceramics: Structural and dielectric studies for microwave applications. Ceram. Int. 2022, 48, 29372–29385. [Google Scholar] [CrossRef]
- Su, Z.; Yang, H.; Wang, g.; Zhang, Y.; Zhang, J.; Lin, J.; Jia, D.; Wang, H.; Lu, Z.; Hu, P. Transparent and high-performance electromagnetic interference shielding composite film based on single-crystal graphene/hexagonal boron nitride heterostructure. J. Colloid Interface Sci. 2023, 640, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wu, Q.; Lu, L.; Chen, J. Preparation and properties of a super-hydrophobic, electromagnetic interference shielding titanium nitride film. Thin Solid Films 2023, 783, 140056. [Google Scholar] [CrossRef]
- Bi, J.; Yang, J.; Liu, X.; Wang, D.; Yang, Z.; Liu, G.; Wang, X. Development and evaluation of nitride coated titanium bipolar plates for PEM fuel cells. Int. J. Hydrogen Energy 2021, 46, 1144–1154. [Google Scholar] [CrossRef]
- Adalati, R.; Sharma, M.; Sharma, S.; Kumar, A.; Malik, G.; Boukherroub, R.; Chandra, R. Metal nitrides as efficient electrode material for supercapacitors: A review. J. Energy Storage 2022, 56, 105912. [Google Scholar] [CrossRef]
- Pramitha, A.; Raviprakash, Y. Recent developments and viable approaches for high-performance supercapacitors using transition metal-based electrode materials. J. Energy Storage 2022, 49, 104120. [Google Scholar] [CrossRef]
- Parveen, N.; Ansari, M.O.; Ansari, S.A.; Kumar, P. Nanostructured titanium nitride and its composites as high-performance supercapacitor electrode material. Nanomaterials 2023, 13, 105. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Guo, W.; Li, T. A review on transition metal nitrides as electrode materials for supercapacitors. Ceram. Int. 2019, 45, 21062–21076. [Google Scholar] [CrossRef]
- Idrees, M.; Mukhtar, A.; Rehman, A.; Abbas, S.; Zhang, Q.; Li, X. Transition metal nitride electrodes as future energy storage devices: A review. Mater. Today Commun. 2021, 27, 102363. [Google Scholar] [CrossRef]
- Ijaz, S.; Rana, A.; Ahmad, Z.; Rehman, B.; Zubair, M.; Mehmood, M. Exploiting zirconium nitride for an efficient heat-resistant absorber and emitter pair for solar thermophotovoltaic systems. Opt. Express 2021, 29, 31537–31548. [Google Scholar] [CrossRef] [PubMed]
- Lucio-Porto, R.; Bouhtiyya, S.; Pierson, J.; Morel, A.; Capon, F.; Boulet, P.; Brousse, T. VN thin films as electrode materials for electrochemical capacitors. Electrochimica Acta 2014, 141, 203–211. [Google Scholar] [CrossRef]
- Lebreton, A.; Barbe, J.; Lethien, C.; Coleman, J.; Brousse, T. Tuning Deposition Conditions for VN Thin Films Electrodes for Microsupercapacitors: Influence of the Thickness. J. Electrochem. Soc. 2024, 171, 090513. [Google Scholar] [CrossRef]
- Prakash, R.; Kumar, A.; Pandey, A.; Kaur, D. Binder free and high performance of sputtered tungsten nitride thin film electrode for supercapacitor device. Int. J. Hydrogen Energy 2019, 44, 10823–10832. [Google Scholar] [CrossRef]
- Qi, Z.; Wei, B.; Wang, J.; Yang, Y.; Wang, Z. Nanostructured porous CrN thin films by oblique angle magnetron sputtering for symmetric supercapacitors. J. Alloys Compd. 2019, 806, 953–959. [Google Scholar] [CrossRef]
- Sun, N.; Zhou, D.; Liu, W.; Shi, S.; Tian, Z.; Liu, F.; Li, S.; Wang, J.; Ali, F. Tailoring surface chemistry and morphology of titanium nitridee for on-chip supercapacitors. ACS Sustain. Chem. Eng. 2020, 8, 7869–7878. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, J.; Zhu, R.; Fu, S.; Yun, D. Effects of sputtering pressure on microstructure and mechanical properties of ZrN films deposited by magnetron sputtering. Mater. Res. Bull. 2018, 105, 231–236. [Google Scholar] [CrossRef]
- Aissani, L.; Alhussein, A.; Ayad, A.; Nouveau, C.; Zgheib, E.; Belgroune, A.; Zaabat, M.; Barille, R. Relationship between structure, surface topography and tribo-mechanical behavior of Ti-N thin films elaborated at different N2 flow rates. Thin Solid Films 2021, 724, 138598. [Google Scholar] [CrossRef]
- Aissani, L.; Fellah, M.; Chadli, A.; Samad, M.; Cheriet, A.; Salhi, F.; Nouveau, C.; Weiß, S.; Obrosov, A.; Alhussein, A. Investigating the effect of nitrogen on the structural and tribo-mechanical behavior of vanadium nitride thin films deposited using R.F. magnetron sputtering. J. Mater. Sci. 2021, 56, 17319–17336. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, W.; Wei, B.; Zheng, J.; Qi, Z.; Wang, Z. Freestanding Co3N thin film for high performance supercapacitors. Ceram. Int. 2021, 47, 3267–3271. [Google Scholar] [CrossRef]
- Adalati, R.; Kumar, A.; Sharma, M.; Tiwari, P.; Chandra, R. Catalyst free approach for the fabrication of CoN//Zn3N2 asymmetric configuration for highly efficient flexible supercapacitor. Appl. Phys. Lett. 2020, 117, 123904. [Google Scholar] [CrossRef]
- Durai, G.; Kuppusami, P.; Maiyalagan, T.; Ahila, M.; Vinoth kumar, P. Supercapacitive properties of manganese nitride thin film electrodes prepared by reactive magnetron sputtering: Effect of different electrolytes. Ceram. Int. 2019, 45, 17120–17127. [Google Scholar] [CrossRef]
- Adalati, R.; Kumar, A.; Sharma, M.; Chandra, R. Pt enhanced capacitive performance of Cr2N electrode toward flexible asymmetric supercapacitor. Appl. Phys. Lett. 2021, 118, 183901. [Google Scholar] [CrossRef]
- Achour, A.; Lucio-Porto, R.; Chaker, M.; Armanc, A.; Ahmadpourian, A.; Soussou, M.; Boujtita, M.; Le Brizoual, L.; Djouadi, M.; Brousse, T. Titanium vanadium nitride electrode for micro-supercapacitors. Electrochem. Commun. 2017, 77, 40–43. [Google Scholar] [CrossRef]
- Iordanova, I.; Kelly, P.; Burova, M.; Andreeva, A.; Stefanova, B. Influence of thickness on the crystallography and surface topography of TiN nano-films deposited by reactive DC and pulsed magnetron sputtering. Thin Solid Films 2012, 520, 5333–5339. [Google Scholar] [CrossRef]
- Iordanova, I.; Kelly, P.; Mirchev, R.; Antonov, V. Crystallography of magnetron sputtered TiN coatings on steel substrates. Vacuum 2007, 81, 830–842. [Google Scholar] [CrossRef]
- Petrov, P.; Dechev, D.; Ivanov, N.; Hikov, T.; Valkov, S.; Nikolova, M.; Yankov, E.; Parshorov, S.; Bezdushnyi, R.; Andreeva, A. Strudy of the influence on electron beam treatment of Ti5Al4V substrate on the mechanical properties and surface topography of multilayer TiN/TiO2 coatings. Vacuum 2018, 154, 264–271. [Google Scholar] [CrossRef]
- Darjanov, P.; Marinova, I.; Darjanova, D. Electrical Engineering II; Amadeus’ Company Ltd.: Sofia, Bulgaria, 2006; p. 223. ISBN 954-9897-14-1. [Google Scholar]
- Oliveira, A.; Brito, G.; Teixeira, M. Mechanism of nanocomposite formation in the layer-by-layer single-step electropolymerization of π-conjugated azopolymer and reduced graphene oxide: An electrochemical impedance spectroscopy study. ACS Omega 2020, 5, 25954–25967. [Google Scholar] [CrossRef] [PubMed]
- Hauff, E. Impedance spectroscopy for emerging photovoltaics. J. Phys. Chem. C 2019, 123, 11329–11346. [Google Scholar] [CrossRef]
- Li, B.; Zhang, S.; Xia, F.; Huang, Y.; Ran, X.; Xia, Y.; Chen, Y.; Huang, W. Insights into the hole transport properties of LiTFSI-doped spiro-OMeTAD films through impedance spectroscopy. J. Appl. Phys. 2020, 128, 085501. [Google Scholar] [CrossRef]
- Szendrei, A.; Sparks, T.; Virkar, A. Three and Four-Electrode Electrochemical Impedance Spectroscopy Studies Using Embedded Composite Thin Film Pseudo-Reference Electrodes in Proton Exchange Membrane Fuel Cells. J. Electrochem. Soc. 2019, 166, 784–795. [Google Scholar] [CrossRef]
- Yoo, S.; Yoon, S.; Anta, J.; Lee, H.; Boix, P.; Sero, I. An equivalent circuit for perovskite solar cell bridging sensitized to thin film architectures. Joule 2019, 3, 2535–2549. [Google Scholar] [CrossRef]
- Nara, H.; Yokoshima, T.; Osaka, T. Technology of electrochemical impedance spectroscopy for an energy-sustainable society. Curr. Opin. Electrochem. 2020, 20, 66–77. [Google Scholar] [CrossRef]
- Magar, H.; Hassan, R.; Mulchandani, A. Electrochemical impedance spectroscopy (EIS): Principles, construction, and biosensing applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dai, Y.; Li, C.; Dang, Y.; Zheng, R.; Wang, Z.; Wang, Y.; Cui, Y.; Arandiyan, H.; Shao, Z.; et al. Recent advances in electrochemical impedance spectroscopy for solid-state batteries. Energy Storage Mater. 2024, 69, 103378. [Google Scholar] [CrossRef]
- Lazanas, A.; Prodromidis, M. Electrochemical impedance spectroscopy—A tutorial. ACD Meas. Sci. Au 2023, 3, 162–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, J.; Gharbi, O.; Vivier, V.; Gao, M.; Orazem, M. Electrochemical impedance spectroscopy. Nat. Rev. Methods Primers 2021, 1, 41. [Google Scholar] [CrossRef]
- Vadhva, P.; Hu, J.; Johnson, M.; Stocker, R.; Braglia, M.; Brett, D.; Rettie, A. Electrochemical impedance spectroscopy for all-solid-state batteries: Theory, methods and future outlook. ChemElectroChem 2021, 8, 1930–1947. [Google Scholar] [CrossRef]
- Freger, V.; Bason, S. Characterization of ion transport in thin films using electrochemical impedance spectroscopy I. Principles and theory. J. Membr. Sci. 2007, 302, 1–9. [Google Scholar] [CrossRef]
- Jeong, D.; Schroeder, H.; Waser, R. Impedance spectroscopy of TiO2 thin films showing resistive switching. Appl. Phys. Lett. 2006, 89, 082909. [Google Scholar] [CrossRef]
- Larfaillou, S.; Guy-Bouyssou, D.; Cras, F.; Franger, S. Comprehensive characterization of all-sloid-state thin films commercial microbatteries by electrochemical impedance spectroscopy. J. Power Sources 2016, 319, 139–146. [Google Scholar] [CrossRef]
- Ciucci, F. Modeling electrochemical impedance spectroscopy. Curr. Opin. Electrochem. 2019, 13, 132–139. [Google Scholar] [CrossRef]
- Erinmwingbovo, C.; Siller, V.; Nunez, M.; Trocloli, R.; Brogioli, D.; Morata, A.; Mantia, F. Dynamic impedance spectroscopy of LiMn2O4 thin films made by multi-layer pulsed laser deposition. Electrochim. Acta 2020, 331, 135385. [Google Scholar] [CrossRef]
- Cesiulis, H.; Tsyntsaru, N.; Ramanavicius, A.; Ragoisha, G. The study of thin films by electrochemical impedance spectroscopy. In Nanostructures and Thin Films for Multifunctional Applications; Springer Nature: Berlin, Germany, 2016; Chapter 1; pp. 3–42. [Google Scholar]
- Krammer, M.; Schmid, A.; Nenning, A.; Bumberger, A.; Siebenhofer, M.; Herzig, C.; Limbeck, A.; Rameshan, C.; Kubicek, M.; Fleig, J. Closed-pore formation in oxygen electrodes for solid oxide electrolysis cells investigated by impedance spectroscopy. ACS Appl. Mater. Interfaces 2023, 15, 8076–8092. [Google Scholar] [CrossRef] [PubMed]
- Pehlivan, E.; Granqvist, C.; Niklasson, G. Electrochromic nickel-oxide-based thin films in KOH electrolyte: Ionic and electronic effects elucidated by impedance spectroscopy. Sol. Energy Mater. Sol. Cells 2024, 269, 112795. [Google Scholar] [CrossRef]
- Pehlivan, E.; Granqvist, C.; Niklasson, G. Impedance Spectroscopy of Electrochromic Hydrous Tungsten Oxide Films. Electron. Mater. 2021, 2, 312–323. [Google Scholar] [CrossRef]
- Shahkhatuni, G.; Aroutiounian, V.; Arakelyan, V.; Aleksanyan, M.; Shahnazaryan, G. Investigation of sensor made of ZnO:La for detection of hydrogen peroxide vapours by impedance spectroscopy method. J. Contemp. Phys. 2019, 54, 188–195. [Google Scholar] [CrossRef]
- Kiew, L.; Chang, C.; Huang, S.; Wang, P.; Heh, C.; Liu, C.; Cheng, C.; Lu, Y.; Chen, Y.; Huang, Y.; et al. Development of flexible electrochemical impedance spectroscopy-based biosensing platform for rapid screening of SARS-CoV-2 inhibitors. Biosens. Bioelectron. 2021, 183, 113213. [Google Scholar] [CrossRef] [PubMed]
- Jokar, A.; Torabi, S.; Mirzaei, M.; Dabir, D.; Bakhtiargonbadi, F.; Esfahani, H. Arrangement of n-type ZnO and p-type NiO nanofibrous thin films on FTO electrode for electrochemical impedance spectroscopy of glucose biosensors. Surf. Interfaces 2025, 56, 105714. [Google Scholar] [CrossRef]
- Schmidt, R.; Erenstein, W.; Winiecki, T.; Morrison, F.; Midgley, P. Impedance spectroscopy of epitaxial multiferroic thin films. Phys. Rev. B 2007, 75, 245111. [Google Scholar] [CrossRef]
- Stoeva, D.; Kotlarski, G.; Dechev, D.; Ivanov, N.; Ormanova, M.; Mateev, V.; Marinova, I. Formation and electrical conductance of TiO2 coatings on Cu substrates. J. Phys. Conf. Ser. 2025, 2994, 012025. [Google Scholar] [CrossRef]



| Evaporation Technique | Advantages | Disadvantages | References |
|---|---|---|---|
| Vacuum evaporation |
|
| [26] |
| Electron beam-physical vapor deposition (EBPVD) |
|
| [29,30,31] |
| Pulsed laser deposition (PLD) |
|
| [32,33,34,35,36,37] |
| Cathodic arc evaporation (CAE) |
|
| [38,39,40,41,42,43] |
| Sputtering Technique | Advantages | Disadvantages | References |
|---|---|---|---|
| Ion sputtering |
|
| [44] |
| Direct current (DC) magnetron sputtering |
|
| [13,45,46,47,48,49,50,51] |
| Radio-frequency (RF) magnetron sputtering |
|
| [51,52,53,54,55,56,57] |
| High-power impulse magnetron sputtering (HiPIMS) |
|
| [58,59,60,61,62,63,64,65] |
| Metal-Oxide-Based Film | Synthesis Method | Properties | Applications | References |
|---|---|---|---|---|
| TiO2 | Magnetron sputtering | High hardness, high refractive index and extinction coefficient | Optical instruments | [98] |
| TiO2 | RFMS | High conductivity, low defect density, reduction in series resistance, improved crystallinity | Photovoltaic devices | [97] |
| CuO | DCMS | Good antibacterial and corrosion activities | Antibacterial films | [95] |
| Cu-doped TiO2 | Glow discharge deposition | Good biocompatible, non-cytotoxic and antimicrobial activity | Biomedical films | [92] |
| Si/ZrO2 | EBPVD | Improved corrosion resistance, improved fibroblast cells vitality and wettability | Biomedical films | [96] |
| TiO2 | Reactive DCMS | Improved photocatalytic effect | Self-cleaning surfaces | [89] |
| WO3 | RFMS | Increased photocurrent density, improved charge transfer efficiency | Photoelectrochemical devices | [88] |
| HfO2 | DCMS | High smoothness, high uniformity and density, excellent transmittance | Laser and optoelectronics equipment | [86] |
| SnO2:F | DCMS | High optical transparency, low electrical conductivity | Optical instruments | [83] |
| ZTO/Ti:ZTO | RFMS | Improved field-effect mobility, enhanced bias-stress stability | Thin-film transistors | [81] |
| Metal-Nitride-Based Film | Synthesis Method | Properties | Applications | References |
|---|---|---|---|---|
| ZrN | RFMS | High hardness, high elastic modulus, and low surface roughness | Protective films | [143] |
| TiN | Reactive MS | High wettability, high hardness and Young’s modulus, low coefficient of friction | Protective films for cutting tools and wear resistant parts, decorative films | [144] |
| VN | RFMS | High hardness, low friction coefficient and wear rate | Hard coating for cutting tools, superconductors, decorative films, microelectronics | [138,145] |
| CrAlN + TiAlN | HiPIMS + DCMS | Dense and fine crystalline structure, phase stability and oxidation resistance (T ≤ 700 °C) | Thin-film thermocouples | [100] |
| (CrAlTiNbV)Nx | Reactive MS | High hardness and elastic modulus, low fiction coefficient and wear rate | Protective films for aviation transmission components | [102] |
| TiN | Cathodic arc evaporation | High hardness and elastic modulus, excellent adhesion, low wear | Wear protection | [108] |
| TiN/TiCrN | Cathodic arc evaporation | Improved hardness and elastic modulus compared to TiN, low coefficient of friction and wear rate | Tool protection | [109] |
| CrN/TiN | Cathodic arc evaporation/Magnetron sputtering | Good corrosion resistance | Proton exchange membrane water electrolysis | [115] |
| TiAlN/TiN | Cathodic arc evaporation | Low coefficient of friction and wear rate, excellent corrosion resistance | Surface protection in terms of wear and corrosion | [116] |
| CrN | Cathodic arc evaporation | Excellent oxidation resistance, high-temperature stability | Accident-tolerant fuel materials | [117] |
| Co3N | RFMS | Long-term cycling stability and high-capacity retention | High-performance supercapacitors | [146] |
| CoN/Zn3N2 | Reactive MS | High specific capacitance, excellent capacitance retention, high specific energy | High-performance supercapacitors | [147] |
| Mn3N2 | DCMS | High areal capacitance, excellent cycling stability with capacitance retention | Electrochemical energy storage devices | [148] |
| TiN | Reactive MS | High surface roughness, low electrical resistivity, good hydrophobic properties | Super-hydrophobic and electromagnetic shielding material | [130] |
| Cr2N | Reactive DCMS | High specific capacitance, excellent capacitance retention, high specific energy | High-performance supercapacitors | [149] |
| W2N | Reactive DCMS | High specific capacitance, excellent capacitance retention, high specific energy, excellent cycling stability, high energy and high power density | Supercapacitor and high-energy storage devices | [139] |
| TiVN | Reactive DCMS | High specific capacitance, excellent capacitance retention, high specific energy, excellent cycling stability | Micro-supercapacitor electrodes | [150] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotlarski, G.; Stoeva, D.; Dechev, D.; Ivanov, N.; Ormanova, M.; Mateev, V.; Marinova, I.; Valkov, S. Review on Metal (-Oxide, -Nitride, -Oxy-Nitride) Thin Films: Fabrication Methods, Applications, and Future Characterization Methods. Coatings 2025, 15, 869. https://doi.org/10.3390/coatings15080869
Kotlarski G, Stoeva D, Dechev D, Ivanov N, Ormanova M, Mateev V, Marinova I, Valkov S. Review on Metal (-Oxide, -Nitride, -Oxy-Nitride) Thin Films: Fabrication Methods, Applications, and Future Characterization Methods. Coatings. 2025; 15(8):869. https://doi.org/10.3390/coatings15080869
Chicago/Turabian StyleKotlarski, Georgi, Daniela Stoeva, Dimitar Dechev, Nikolay Ivanov, Maria Ormanova, Valentin Mateev, Iliana Marinova, and Stefan Valkov. 2025. "Review on Metal (-Oxide, -Nitride, -Oxy-Nitride) Thin Films: Fabrication Methods, Applications, and Future Characterization Methods" Coatings 15, no. 8: 869. https://doi.org/10.3390/coatings15080869
APA StyleKotlarski, G., Stoeva, D., Dechev, D., Ivanov, N., Ormanova, M., Mateev, V., Marinova, I., & Valkov, S. (2025). Review on Metal (-Oxide, -Nitride, -Oxy-Nitride) Thin Films: Fabrication Methods, Applications, and Future Characterization Methods. Coatings, 15(8), 869. https://doi.org/10.3390/coatings15080869











