Analysis of Influencing Factors in the Preparation Process of Solid Waste-Based Ternesite Sulphoaluminate Cement
Abstract
1. Introduction
2. Experiment
2.1. Experimental Materials
2.2. Experimental Design
- (1)
- Design of control group
- (2)
- Design of variable parameters
| Group Number | MC-1 | CP | MC-2 | |
|---|---|---|---|---|
| Mineral composition /% | 52.5 | 45 | 37.5 | |
| 35 | 35 | 35 | ||
| CaSO4 | 7.5 | 15 | 22.5 | |
| C4AF | 5 | 5 | 5 | |
| NO. | Petroleum Coke Ash /% | Fly Ash /% | Calcined Bauxite /% |
|---|---|---|---|
| MC-1 (1150.00 °C-42.12 min) | 71.43 | 15.67 | 12.90 |
| MC-1 (1162.50 °C-5.00 min) | 75.30 | 14.85 | 9.86 |
| MC-1 (1190.00 °C-60.00 min) | 71.13 | 15.25 | 13.62 |
| MC-1 (1190.00 °C-60.00 min) | 73.22 | 15.04 | 11.74 |
| MC-1 (1190.00 °C-60.00 min) | 72.75 | 14.50 | 12.75 |
| MC-1 (1207.50 °C-5.00 min) | 75.24 | 14.80 | 9.96 |
| MC-1 (1207.50 °C-50.37 min) | 71.43 | 15.67 | 12.90 |
| MC-1 (1207.50 °C-5.00 min) | 75.30 | 14.85 | 9.86 |
| MC-2 (1150.00 °C-42.12 min) | 74.14 | 9.77 | 16.09 |
| MC-2 (1162.50 °C-5.00 min) | 78.39 | 9.28 | 12.33 |
| MC-2 (1190.00 °C-60.00 min) | 73.59 | 9.48 | 16.93 |
| MC-2 (1190.00 °C-60.00 min) | 75.99 | 9.38 | 14.64 |
| MC-2 (1190.00 °C-60.00 min) | 75.17 | 9.00 | 15.83 |
| MC-2 (1207.50 °C-5.00 min) | 78.30 | 9.25 | 12.45 |
| MC-2 (1207.50 °C-50.37 min) | 74.14 | 9.77 | 16.09 |
| MC-2 (1207.50 °C-5.00 min) | 78.39 | 9.28 | 12.33 |
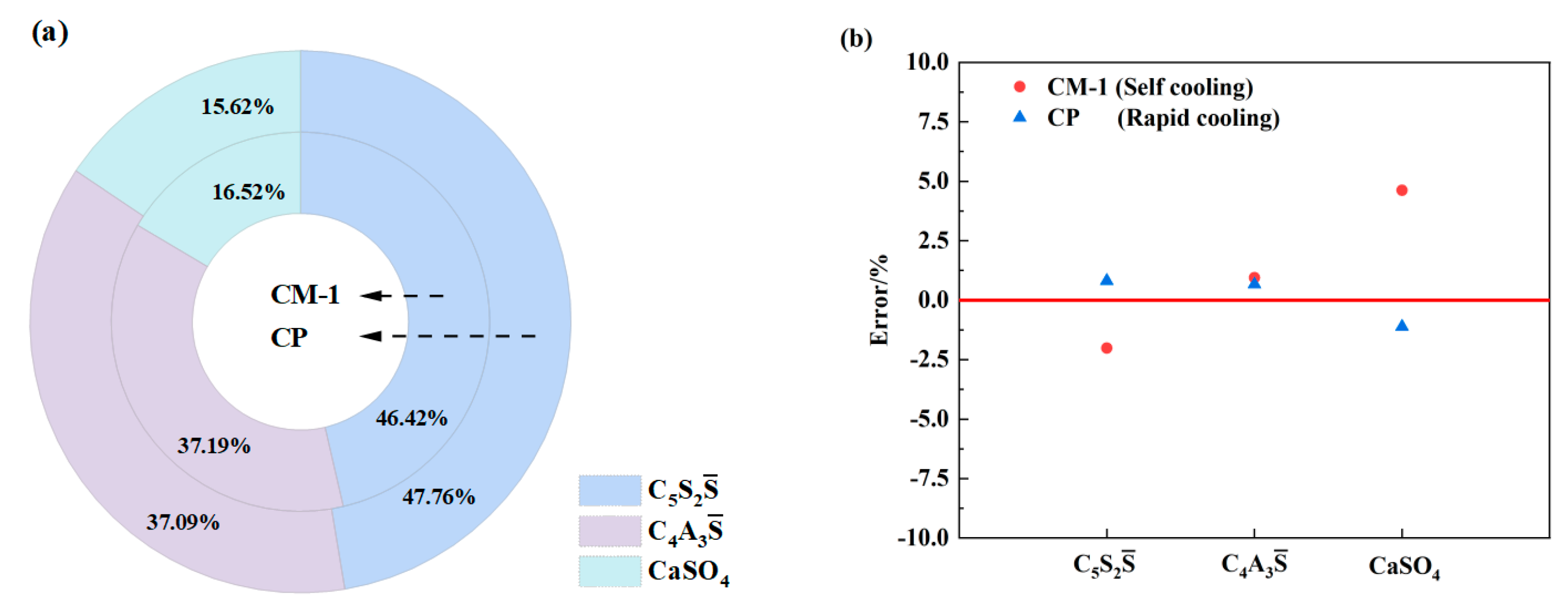
| Group Number | CM-1 | CP |
|---|---|---|
| Cooling method | Self cooling | Rapid cooling |
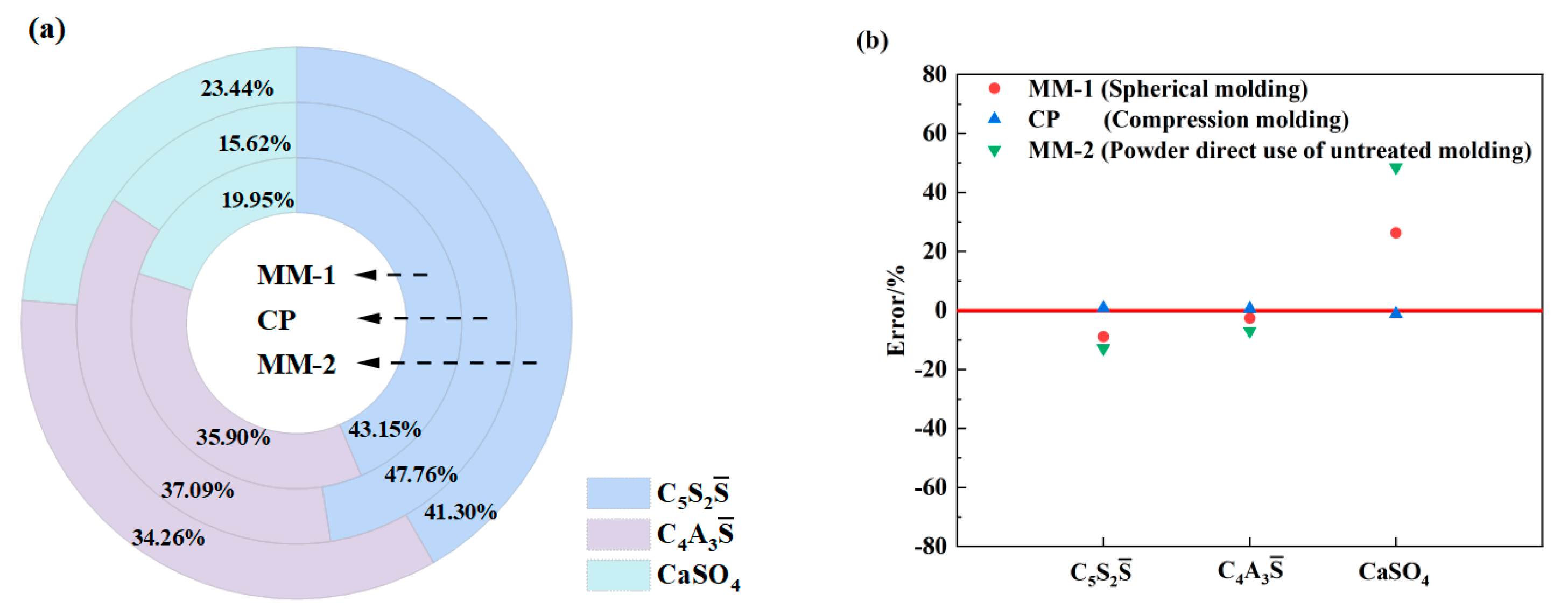
| Group Number | CP | MM-1 | MM-2 |
|---|---|---|---|
| Molding method | Compression molding | Spherical molding | Powder direct use of untreated molding |
| Sample |  |  |  |
| Group Number | MF-1 | MF-2 | CP | MF-3 |
|---|---|---|---|---|
| Molding pressure/MPa | 5 | 10 | 15 | 20 |
| Sample |  |  |  |  |
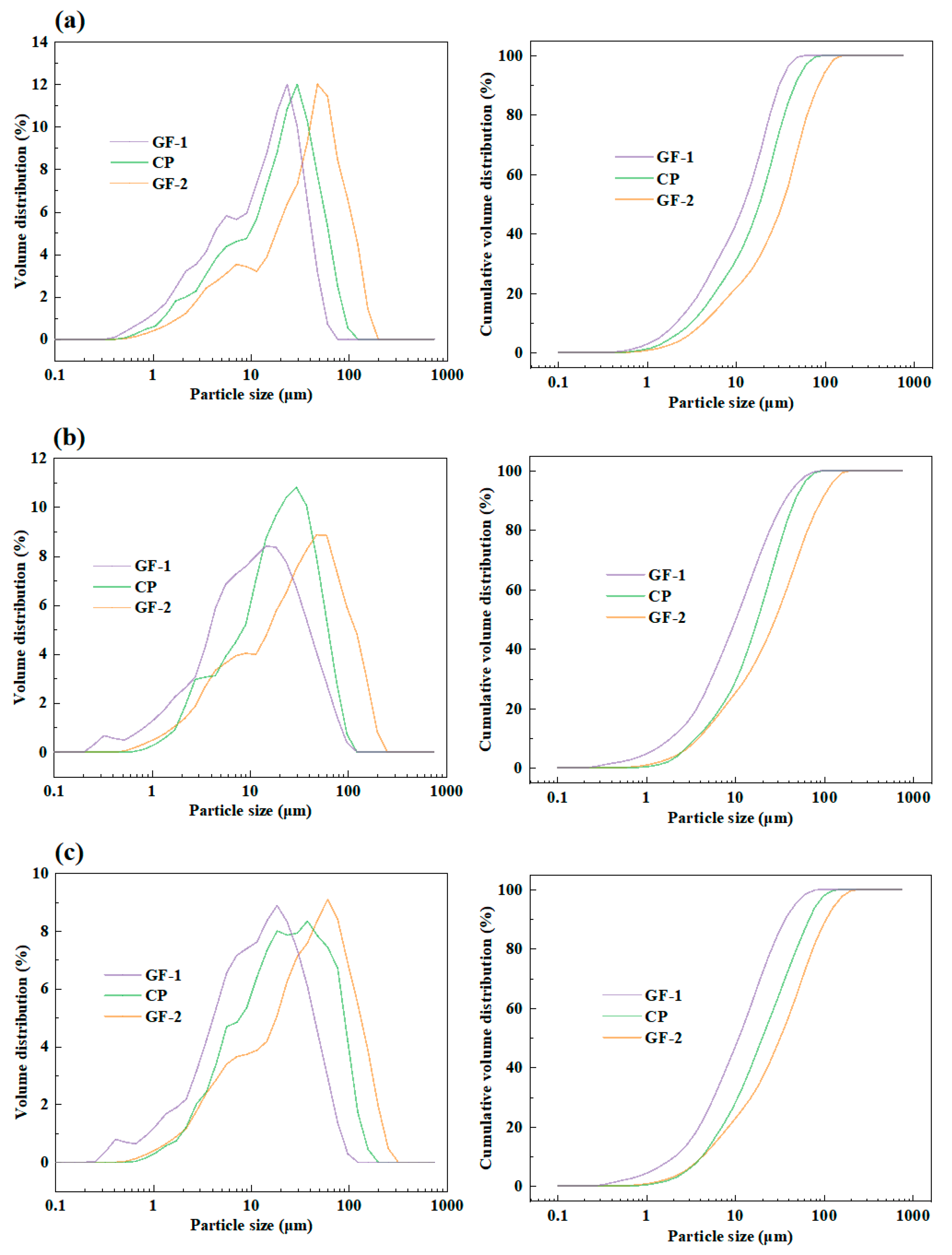
| Group Number | GF-1 | CP | GF-2 |
|---|---|---|---|
| Fineness/μm | 10 | 20 | 30 |
2.3. Test Methods
3. Results and Discussion
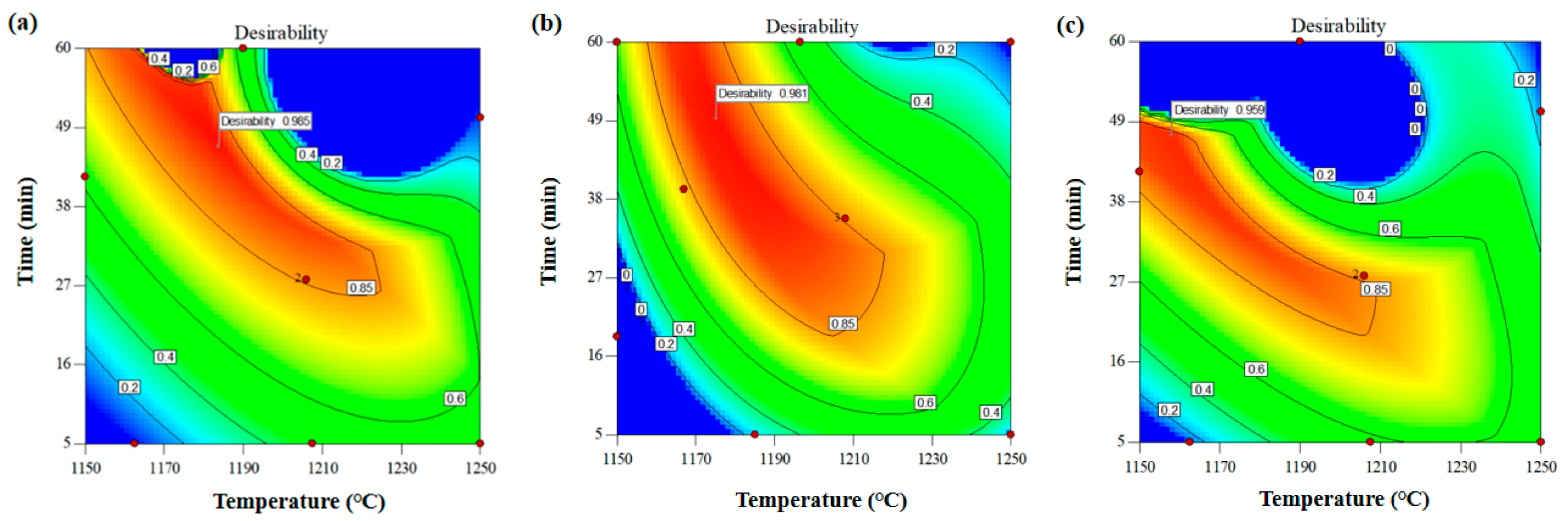
3.1. The Effect of Mineral Composition on Clinker Calcination
3.2. The Effect of Cooling Methods on Clinker Calcination
3.3. The Effect of Molding Technology of Samples on Clinker Calcination
3.3.1. Molding Method
3.3.2. Molding Pressure
3.4. The Effect of Grinding Fineness of Raw Materials on Clinker Calcination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Chemical Formula | Chemical Name | Mineral Name |
| C5S2 | 4CaO·2SiO2·CaSO4 | Calcium Sulfosilicate | Ternesite |
| C4A3 | 3CaO·3Al2O3·CaSO4 | Calcium Sulphoaluminate | Ye’elimite |
| C4AF | 4CaO·Al2O3·Fe2O3 | Tetracalcium Aluminoferrite | Brownmillerite |
| C2S | 2CaO·SiO2 | Dicalcium Silicate | Belite |
References
- Ige, O.E.; Olanrewaju, O.A.; Duffy, K.J.; Obiora, C. A review of the effectiveness of Life Cycle Assessment for gauging environmental impacts from cement production. J. Clean. Prod. 2021, 324, 129213. [Google Scholar] [CrossRef]
- Terán-Cuadrado, G.; Tahir, F.; Nurdiawati, A.; Almarshoud, M.A.; Al-Ghamdi, S.G. Current and potential materials for the low-carbon cement production: Life cycle assessment perspective. J. Build. Eng. 2024, 96, 110528. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Q.; Zhou, W.; Lu, Y.; Liu, X.; Chang, X. A fast-setting and eco-friendly superhydrophobic high belite sulphoaluminate cement mortar. J. Mater. Res. Technol. 2023, 23, 2690–2702. [Google Scholar] [CrossRef]
- Abdel-Gawwad, H.; Heikal, E.; El-Didamony, H.; Hashim, F.; Mohammed, A. Recycling of concrete waste to produce ready-mix alkali activated cement. Ceram. Int. 2018, 44, 7300–7304. [Google Scholar] [CrossRef]
- Gao, Y.F.; Li, Z.F.; Zhang, J.; Zhang, Q.S.; Wang, Y.S. Synergistic use of industrial solid wastes to prepare belite-rich sulphoaluminate cement and its feasibility use in repairing materials. Constr. Build. Mater. 2020, 264, 120201. [Google Scholar] [CrossRef]
- Wu, S.; Yao, Y.; Yao, X.; Ren, C.; Li, J.; Xu, D.; Wang, W. Co-preparation of calcium sulfoaluminate cement and sulfuric acid through mass utilization of industrial by-product gypsum. J. Clean. Prod. 2020, 265, 121801. [Google Scholar] [CrossRef]
- Tao, L.; Deng, F.; Xu, K.; Dong, X.; Xu, D.; Liao, Y.; Tang, S. Effect of calcium sulfoaluminate cement on the hydration process and water resistance of phosphogypsum slag cement. J. Mater. Res. Technol. 2024, 33, 5375–5383. [Google Scholar] [CrossRef]
- Canbek, O.; Shakouri, S.; Erdoğan, S.T. Laboratory production of calcium sulfoaluminate cements with high industrial waste content. Cem. Concr. Compos. 2020, 106, 103475. [Google Scholar] [CrossRef]
- Cai, X.; Yang, D.; Zhang, D.; Cui, J.; Wang, W.; Liu, L. Development of high-early-strength low-carbon engineered cementitious composites with calcium sulfoaluminate cement incorporating high-volume fly ash. Case. Stud. Constr. Mat. 2023, 18, e01959. [Google Scholar] [CrossRef]
- Isteri, V.; Ohenoja, K.; Hanein, T.; Kinoshita, H.; Kletti, H.; Rößler, C.; Tanskanen, P.; Illikainen, M.; Fabritius, T. Ferritic calcium sulfoaluminate belite cement from metallurgical industry residues and phosphogypsum: Clinker production, scale-up, and microstructural characterisation. Cem. Concr. Res. 2022, 154, 106715. [Google Scholar] [CrossRef]
- Zhang, T.; Gao, P.; Gao, P.; Wei, J.; Yu, Q. Effectiveness of novel and traditional methods to incorporate industrial wastes in cementitious materials—An overview. Resour., Conserv. Recycl. 2013, 74, 134–143. [Google Scholar] [CrossRef]
- Vashistha, P.; Park, S.; Pyo, S. A review on sustainable fabrication of futuristic cementitious binders based on application of waste concrete powder, steel slags, and coal bottom ash. Int. J. Concr. Struct. Mater. 2022, 16, 51. [Google Scholar] [CrossRef]
- Ren, C.Z.; Wang, W.L.; Li, G.L. Preparation of high-performance cementitious materials from industrial solid waste. Constr. Build. Mater. 2017, 152, 39–47. [Google Scholar] [CrossRef]
- Arjunan, P.; Silsbee, M.R.; Roy, D.M. Sulfoaluminate-belite cement from low-calcium fly ash and sulfur-rich and other industrial by-products. Cem. Concr. Res. 1999, 29, 1305–1311. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, J.K. Fractal characteristics of pore structure of hardened cement paste prepared by pressurized compact molding. Constr. Build. Mater. 2020, 259, 119856. [Google Scholar] [CrossRef]
- Guo, Z.; Xu, J.; Xu, Z.; Gao, J.; Zhu, X. Performance of cement-based materials containing calcined coal gangue with different calcination regimes. J. Build. Eng. 2022, 56, 104821. [Google Scholar] [CrossRef]
- Emanuelson, A.; Hansen, S.; Viggh, E. A comparative study of ordinary and mineralised Portland cement clinker from two different production units: Part I: Composition and hydration of the clinkers. Cem. Concr. Res. 2003, 33, 1613–1621. [Google Scholar] [CrossRef]
- Ma, Z.; Yao, Y.; Liu, Z.; Wu, B.; Wen, Z. Effect of calcination and cooling conditions on mineral compositions and properties of high-magnesia and low-heat Portland cement clinker. Constr. Build. Mater. 2020, 260, 119907. [Google Scholar] [CrossRef]
- Ren, M.; Shen, P.; Tao, Y.; Poon, C.S. Development of highly carbonation-effective calcium silicates (β-C2S): Phase evolution, microstructure, and carbonation mechanisms. Constr. Build. Mater. 2024, 181, 107542. [Google Scholar] [CrossRef]
- Wang, X.; Xie, Z.; Huo, Z.; Shi, Q.; Luo, H.; Ji, S. Effect of boron doping and cooling condition on polymorph and early strength of dicalcium silicate. Constr. Build. Mater. 2025, 469, 140508. [Google Scholar] [CrossRef]
- Su, D.L. Synthesis and Application Fundamental Research of High Belite Sulphoaluminate Cement Based on Comprehensive Disposal of Various Solid Wastes. Ph.D. Thesis, Qingdao Technological University, Qingdao, China, 2021. [Google Scholar]
- Chen, H.M. Internal burning and clinkering behaviours of dry compact carbon-contained cement raw meal pellets. J. Chin. Ceram. Soc. 2004, 32, 39–51. [Google Scholar]
- Vizcaino Andres, L.M.; Antoni, M.G.; Alujas Diaz, A.; Martirena Hernandez, J.F.; Scrivener, K.L. Effect of fineness in clinker-calcined clays-limestone cements. Adv. Cem. Res. 2015, 27, 546–556. [Google Scholar] [CrossRef]
- Briki, Y.; Zajac, M.; Haha, M.B.; Scrivener, K. Impact of limestone fineness on cement hydration at early age. Cem. Concr. Res. 2021, 147, 106515. [Google Scholar] [CrossRef]
- Su, D.; Yue, G.; Li, Q.; Guo, Y.; Gao, S.; Wang, L. Research on the preparation and properties of high belite sulphoaluminate cement (HBSAC) based on various industrial solid wastes. Materials 2019, 12, 1510. [Google Scholar] [CrossRef]
- Su, D.; Hao, Y.; Wang, J.; Liu, H.; Tang, H.; Yang, M.; Xing, D. Study on preparation and high temperature reaction kinetics of a new ternesite sulphoaluminate cement based on solid waste. Constr. Build. Mater. 2025, 458, 139525. [Google Scholar] [CrossRef]
- Wei, Y.; Yao, W.; Xing, X.; Wu, M. Quantitative evaluation of hydrated cement modified by silica fume using QXRD, 27Al MAS NMR, TG–DSC and selective dissolution techniques. Constr. Build. Mater. 2012, 36, 925–932. [Google Scholar] [CrossRef]
- Fan, D.; Yu, R.; Shui, Z.; Wu, C.; Song, Q.; Liu, Z.; Sun, Y.; Gao, X.; He, Y. A new design approach of steel fibre reinforced ultra-high performance concrete composites: Experiments and modeling. Cem. Concr. Compos. 2020, 110, 103597. [Google Scholar] [CrossRef]
- Lang, L.; Duan, H.; Chen, B. Properties of pervious concrete made from steel slag and magnesium phosphate cement. Constr. Build. Mater. 2019, 209, 95–104. [Google Scholar] [CrossRef]
- Garboczi, E.J.; Bullard, J.W. Shape analysis of a reference cement. Cem. Concr. Res. 2004, 34, 1933–1937. [Google Scholar] [CrossRef]
- Liu, C.; Qian, C.; Qian, R.; Liu, Z.; Qiao, H.; Zhang, Y. Numerical prediction of effective diffusivity in hardened cement paste between aggregates using different shapes of cement powder. Constr. Build. Mater. 2019, 223, 806–816. [Google Scholar] [CrossRef]
- Qiaodi, Y.U.; Canhua, L.I.; Wenzhen, X.U.; Yuhong, Z.H.A. Analysis and research on influencing factors of compressive strength of red mud-fly ash sintered brick. New. Build. Mater. 2021, 48, 29. [Google Scholar]
- Shen, Y.; Qian, J.S.; Chai, J.Q.; Fan, Y.Y. Calcium sulphoaluminate cements made with phosphogypsum: Production issues and material properties. Cem. Concr. Compos. 2014, 48, 67–74. [Google Scholar] [CrossRef]




| Raw Materials | CaO/% | Al2O3/% | SiO2/% | SO3/% | Fe2O3/% | TiO2/% | MgO/% | Others/% | LOI/% |
|---|---|---|---|---|---|---|---|---|---|
| Petroleum coke ash | 62.36 | 0.45 | 0.85 | 26.09 | 0.24 | 0.00 | 1.07 | 0.36 | 8.58 |
| Fly ash | 3.44 | 30.35 | 52.14 | 0.79 | 5.63 | 1.22 | 0.73 | 3.25 | 2.45 |
| Calcined bauxite | 1.07 | 75.14 | 14.84 | 0.18 | 1.53 | 4.50 | 0.48 | 1.63 | 0.63 |
| Group Number | Calcination System | Cooling Method | Molding Method | Molding Pressure | Fineness of Raw Material | Mineral Composition | Raw Material Mix Ratio/% | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Design Value/% | Actual Value/% | ||||||||||
| CP | 1175 °C -49 min | Rapid cooling | Compression molding | 15 MPa | 20 μm | 45 | 47.76 | Petroleum coke ash | 74.42 | ||
| 35 | 37.09 | Fly ash | 12.35 | ||||||||
| CaSO4 | 15 | CaSO4 | 15.62 | Calcined bauxite | 12.93 | ||||||
| C4AF | 5 | C4AF | -- | ||||||||
| Group Number | Data | Calcination Temperature /°C | Holding Time /min | /% | /% | CaSO4 /% |
|---|---|---|---|---|---|---|
| MC-1 | Wish value | -- | -- | 55.26 | 36.84 | 7.89 |
| Predictive value | 1183.77 | 47.38 | 52.72 | 36.84 | 7.89 | |
| Actual value | 1183.77 | 47.38 | 53.34 | 37.95 | 8.03 | |
| CP | Wish value | -- | -- | 47.37 | 36.84 | 15.79 |
| Predictive value | 1175.15 | 49.27 | 47.40 | 38.77 | 15.75 | |
| Actual value | 1175.15 | 49.27 | 47.76 | 37.09 | 15.62 | |
| MC-2 | Wish value | -- | -- | 39.47 | 36.84 | 23.68 |
| Predictive value | 1157.65 | 47.91 | 39.47 | 39.76 | 23.68 | |
| Actual value | 1157.65 | 47.91 | 38.98 | 38.25 | 22.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, D.; Liu, X.; Tang, H.; Hao, Y.; Wang, J.; Xing, D.; Liu, H.; Yang, M.; Kong, W. Analysis of Influencing Factors in the Preparation Process of Solid Waste-Based Ternesite Sulphoaluminate Cement. Coatings 2025, 15, 773. https://doi.org/10.3390/coatings15070773
Su D, Liu X, Tang H, Hao Y, Wang J, Xing D, Liu H, Yang M, Kong W. Analysis of Influencing Factors in the Preparation Process of Solid Waste-Based Ternesite Sulphoaluminate Cement. Coatings. 2025; 15(7):773. https://doi.org/10.3390/coatings15070773
Chicago/Turabian StyleSu, Dunlei, Xin Liu, Haojian Tang, Yani Hao, Jiahui Wang, Dejin Xing, Hongxing Liu, Mingxin Yang, and Weiyi Kong. 2025. "Analysis of Influencing Factors in the Preparation Process of Solid Waste-Based Ternesite Sulphoaluminate Cement" Coatings 15, no. 7: 773. https://doi.org/10.3390/coatings15070773
APA StyleSu, D., Liu, X., Tang, H., Hao, Y., Wang, J., Xing, D., Liu, H., Yang, M., & Kong, W. (2025). Analysis of Influencing Factors in the Preparation Process of Solid Waste-Based Ternesite Sulphoaluminate Cement. Coatings, 15(7), 773. https://doi.org/10.3390/coatings15070773






