Unveiling the Role of Hydrogel Stiffness Threshold in Schwann Cell Context: Regulating Adhesion Through TRIP6 Gene Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PAM/CS Hydrogels with Different Moduli
2.3. Measurement of the Elastic Modulus of Hydrogels
2.4. Cell Culture and Transfection
2.5. Cell Viability Assay
2.6. Cell Proliferation Assay
2.7. Cell Adhesion Assay
2.8. Immunofluorescence Staining Experiment
2.9. Real-Time Quantitative Polymerase Chain Reaction
2.10. Statistical Analysis
3. Results and Discussion
3.1. Modulation of Cell Adhesion by PAM/CS Hydrogels with Different Stiffness
3.2. Substrate Stiffness Controls RSC96 Cell Phenotype and Proliferative Capacity In Vitro
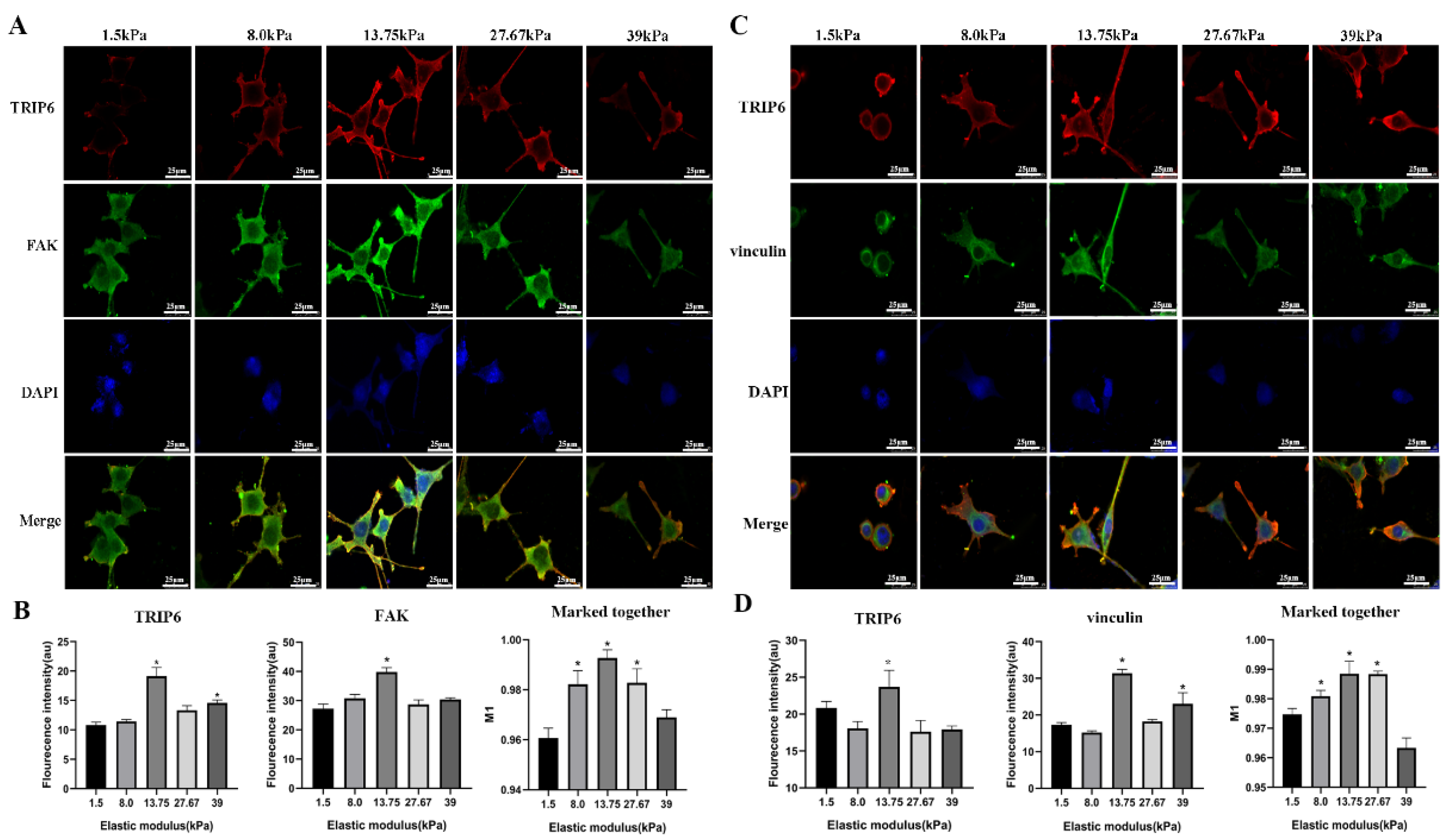
3.3. Important Relevance of TRIP6 for Matrix Stiffness-Induced RSC9 Cell Adhesion
3.4. Inhibition of TRIP6 Expression Attenuated RSC96 Cell Adhesion on 1.5 kPa and 13.75 kPa Substrates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosso, G.; Wehner, D.; Schweitzer, C.; Mollmert, S.; Sock, E.; Guck, J.; Shahin, V. Matrix stiffness mechanosensing modulates the expression and distribution of transcription factors in Schwann cells. Bioeng. Transl. Med. 2022, 7, e10257. [Google Scholar] [CrossRef] [PubMed]
- Bunge, M.B.; Monje, P.V.; Khan, A.; Wood, P.M. From transplanting Schwann cells in experimental rat spinal cord injury to their transplantation into human injured spinal cord in clinical trials. Prog. Brain Res. 2017, 231, 107–133. [Google Scholar]
- Monje, P.V. Schwann Cell Cultures: Biology, Technology and Therapeutics. Cells 2020, 9, 1848. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The origin and development of glial cells in peripheral nerves. Nat. Rev. Neurosci. 2005, 6, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Arthur-Farraj, P. Repair Schwann cell update: Adaptive reprogramming, EMT, and stemness in regenerating nerves. Glia. 2019, 67, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Belaadi, N.; Pernet, L.; Aureille, J.; Chadeuf, G.; Rio, M.; Vaillant, N.; Vitiello, E.; Lafanechere, L.; Loirand, G.; Guilluy, C. SUN2 regulates mitotic duration in response to extracellular matrix rigidity. Proc. Natl. Acad. Sci. USA 2022, 119, e2116167119. [Google Scholar] [CrossRef]
- Takizawa, N.; Smith, T.C.; Nebl, T.; Crowley, J.L.; Palmieri, S.J.; Lifshitz, L.M.; Ehrhardt, A.G.; Hoffman, L.M.; Beckerle, M.C.; Luna, E.J. Supervillin modulation of focal adhesions involving TRIP6/ZRP-1. J. Cell Biol. 2006, 174, 447–458. [Google Scholar] [CrossRef]
- Ridley, A.J.; Schwartz, M.A.; Burridge, K.; Firtel, R.A.; Ginsberg, M.H.; Borisy, G.; Parsons, J.T.; Horwitz, A.R. Cell migration: Integrating signals from front to back. Science 2003, 302, 1704–1709. [Google Scholar] [CrossRef]
- Discher, D.E.; Janmey, P.; Wang, Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef]
- Sun, Z.; Guo, S.S.; Fassler, R. Integrin-mediated mechanotransduction. J. Cell Biol. 2016, 215, 445–456. [Google Scholar] [CrossRef]
- Elosegui-Artola, A.; Trepat, X.; Roca-Cusachs, P. Control of Mechanotransduction by Molecular Clutch Dynamics. Trends Cell Biol. 2018, 28, 356–367. [Google Scholar] [CrossRef]
- Seetharaman, S.; Etienne-Manneville, S. Integrin diversity brings specificity in mechanotransduction. Biol. Cell 2018, 110, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Yoshigi, M.; Hoffman, L.M.; Jensen, C.C.; Yost, H.J.; Beckerle, M.C. Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal reinforcement. J. Cell Biol. 2005, 171, 209–215. [Google Scholar] [CrossRef]
- Colombelli, J.; Besser, A.; Kress, H.; Reynaud, E.G.; Girard, P.; Caussinus, E.; Haselmann, U.; Small, J.V.; Schwarz, U.S.; Stelzer, E.H. Mechanosensing in actin stress fibers revealed by a close correlation between force and protein localization. J. Cell Sci. 2009, 122, 1665–1679. [Google Scholar] [CrossRef]
- Oakes, P.W.; Wagner, E.; Brand, C.A.; Probst, D.; Linke, M.; Schwarz, U.S.; Glotzer, M.; Gardel, M.L. Optogenetic control of RhoA reveals zyxin-mediated elasticity of stress fibres. Nat. Commun. 2017, 8, 15817. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, L.M.; Jensen, C.C.; Chaturvedi, A.; Yoshigi, M.; Beckerle, M.C. Stretch-induced actin remodeling requires targeting of zyxin to stress fibers and recruitment of actin regulators. Mol. Biol. Cell 2012, 23, 1846–1859. [Google Scholar] [CrossRef] [PubMed]
- Lin, V.T.; Lin, F.T. TRIP6: An adaptor protein that regulates cell motility, antiapoptotic signaling and transcriptional activity. Cell Signal. 2011, 23, 1691–1697. [Google Scholar] [CrossRef]
- Lai, Y.J.; Li, M.Y.; Yang, C.Y.; Huang, K.H.; Tsai, J.C.; Wang, T.W. TRIP6 regulates neural stem cell maintenance in the postnatal mammalian subventricular zone. Dev. Dyn. 2014, 243, 1130–1142. [Google Scholar] [CrossRef]
- Schiller, H.B.; Fassler, R. Mechanosensitivity and compositional dynamics of cell-matrix adhesions. EMBO Rep. 2013, 14, 509–519. [Google Scholar] [CrossRef]
- Liu, F.; Xu, J.; Liu, A.; Wu, L.; Wang, D.; Han, Q.; Zheng, T.; Wang, F.; Kong, Y.; Li, G.; et al. Development of a polyacrylamide/chitosan composite hydrogel conduit containing synergistic cues of elasticity and topographies for promoting peripheral nerve regeneration. Biomater. Sci. 2022, 10, 4915–4932. [Google Scholar] [CrossRef]
- Gu, Y.; Ji, Y.; Zhao, Y.; Liu, Y.; Ding, F.; Gu, X.; Yang, Y. The influence of substrate stiffness on the behavior and functions of Schwann cells in culture. Biomaterials 2012, 33, 6672–6681. [Google Scholar] [CrossRef]
- Xu, H.; Duan, S.; Hu, Y.; Ding, X.; Xu, F.J. Rapid Regulation of Cardiomyocytes Adhesion on Substrates with Varied Modulus via Mechanical Cues. Biomacromolecules 2023, 24, 5847–5858. [Google Scholar] [CrossRef]
- Uynuk-Ool, T.; Rothdiener, M.; Walters, B.; Hegemann, M.; Palm, J.; Nguyen, P.; Seeger, T.; Stockle, U.; Stegemann, J.P.; Aicher, W.K.; et al. The geometrical shape of mesenchymal stromal cells measured by quantitative shape descriptors is determined by the stiffness of the biomaterial and by cyclic tensile forces. J. Tissue Eng. Regen. Med. 2017, 11, 3508–3522. [Google Scholar] [CrossRef] [PubMed]
- Melica, M.E.; La Regina, G.; Parri, M.; Peired, A.J.; Romagnani, P.; Lasagni, L. Substrate Stiffness Modulates Renal Progenitor Cell Properties via a ROCK-Mediated Mechanotransduction Mechanism. Cells 2019, 8, 1561. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Haenold, R.; Urbanek, P.; Frappart, L.; Monajembashi, S.; Grigaravicius, P.; Nagel, S.; Min, W.K.; Tapias, A.; Kassel, O.; et al. TRIP6 functions in brain ciliogenesis. Nat. Commun. 2021, 12, 5887. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Wan, W.; Huang, G.; Li, Y.; Genin, G.M.; Mofrad, M.R.K.; Lu, T.J.; Xu, F.; Lin, M. Nanoscale integrin cluster dynamics controls cellular mechanosensing via FAKY397 phosphorylation. Sci. Adv. 2020, 6, eaax1909. [Google Scholar] [CrossRef]
- Critchley, D.R. Biochemical and structural properties of the integrin-associated cytoskeletal protein talin. Annu. Rev. Biophys. 2009, 38, 235–254. [Google Scholar] [CrossRef]
- Bays, J.L.; DeMali, K.A. Vinculin in cell-cell and cell-matrix adhesions. Cell Mol. Life Sci. 2017, 74, 2999–3009. [Google Scholar] [CrossRef]
- Demali, K.A. Vinculin—A dynamic regulator of cell adhesion. Trends Biochem. Sci. 2004, 29, 565–567. [Google Scholar] [CrossRef]
- Kanchanawong, P.; Shtengel, G.; Pasapera, A.M.; Ramko, E.B.; Davidson, M.W.; Hess, H.F.; Waterman, C.M. Nanoscale architecture of integrin-based cell adhesions. Nature 2010, 468, 580–584. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Xu, M.; Cao, Y.; Wu, W.; Jiang, C.; Li, F.; Li, Y.; Yang, Y.; He, J. Unveiling the Role of Hydrogel Stiffness Threshold in Schwann Cell Context: Regulating Adhesion Through TRIP6 Gene Expression. Coatings 2025, 15, 753. https://doi.org/10.3390/coatings15070753
Liu F, Xu M, Cao Y, Wu W, Jiang C, Li F, Li Y, Yang Y, He J. Unveiling the Role of Hydrogel Stiffness Threshold in Schwann Cell Context: Regulating Adhesion Through TRIP6 Gene Expression. Coatings. 2025; 15(7):753. https://doi.org/10.3390/coatings15070753
Chicago/Turabian StyleLiu, Fang, Mengjie Xu, Yi Cao, Weiyan Wu, Chunzhen Jiang, Feng Li, Yifan Li, Yumin Yang, and Jianghong He. 2025. "Unveiling the Role of Hydrogel Stiffness Threshold in Schwann Cell Context: Regulating Adhesion Through TRIP6 Gene Expression" Coatings 15, no. 7: 753. https://doi.org/10.3390/coatings15070753
APA StyleLiu, F., Xu, M., Cao, Y., Wu, W., Jiang, C., Li, F., Li, Y., Yang, Y., & He, J. (2025). Unveiling the Role of Hydrogel Stiffness Threshold in Schwann Cell Context: Regulating Adhesion Through TRIP6 Gene Expression. Coatings, 15(7), 753. https://doi.org/10.3390/coatings15070753






