Sustainable Use of Expired Metoprolol as Corrosion Inhibitor for Carbon Steel in Saline Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrochemical Methods
2.3. Molecular Modelling
3. Results
3.1. Cyclic Voltammetry
3.2. Linear Sweep Voltammetry
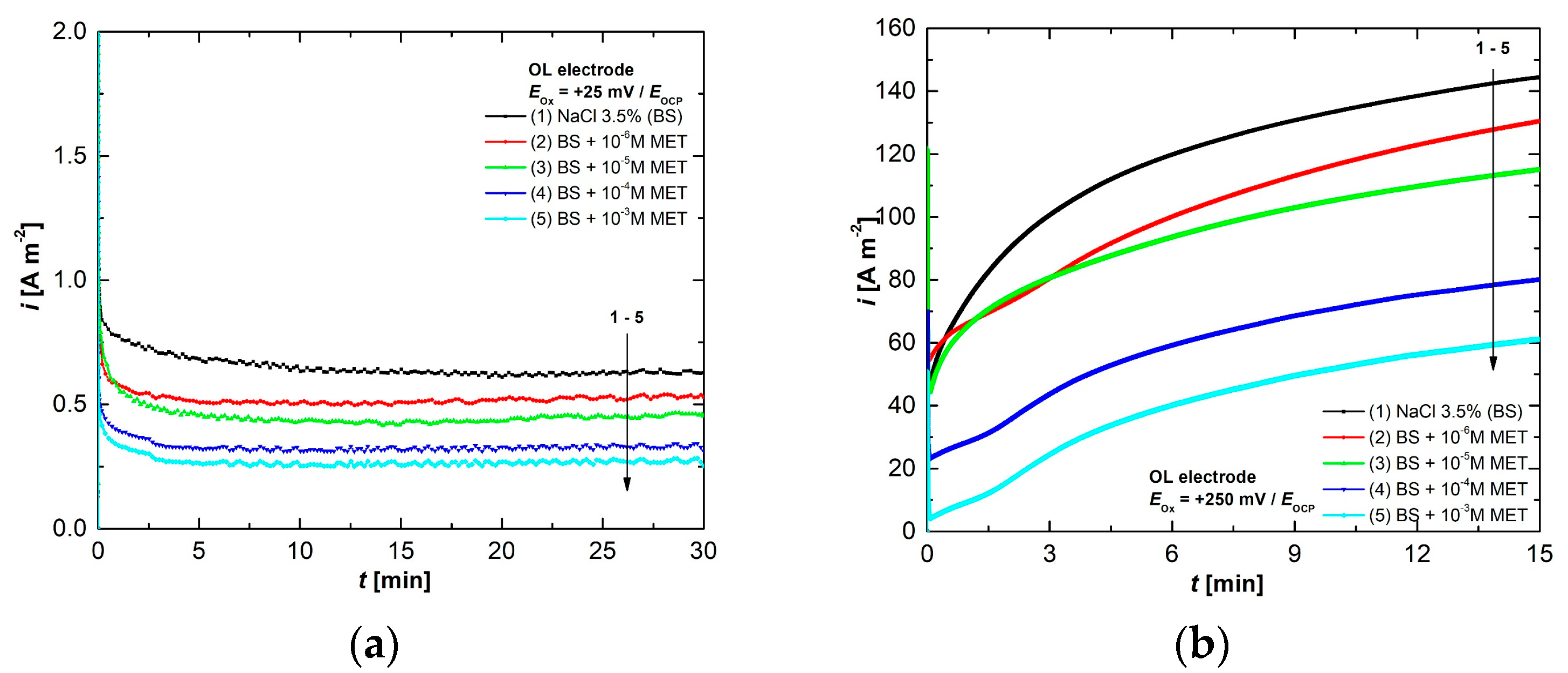
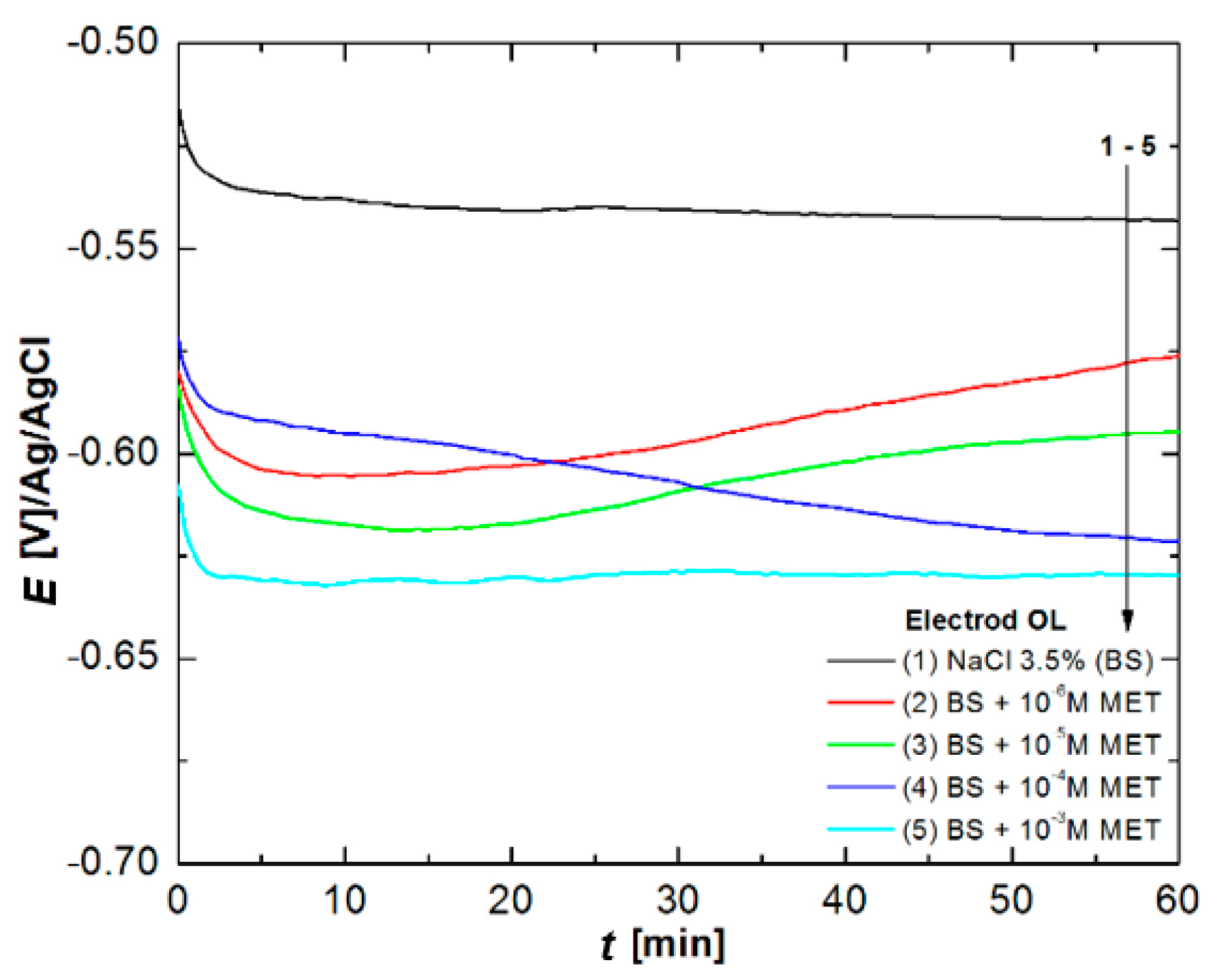
3.3. Chronoamperometry Studies
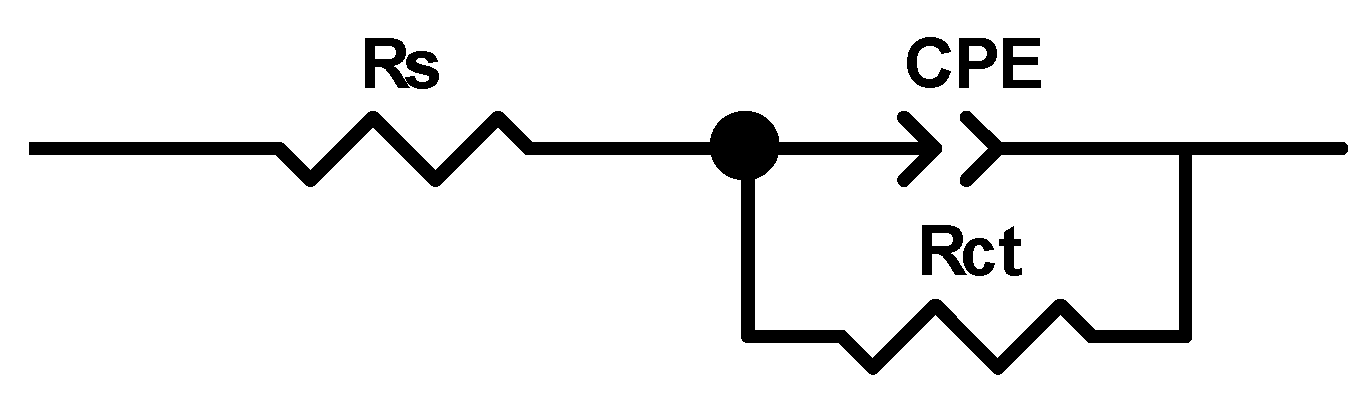
3.4. Electrochemical Impedance Spectroscopy Studies
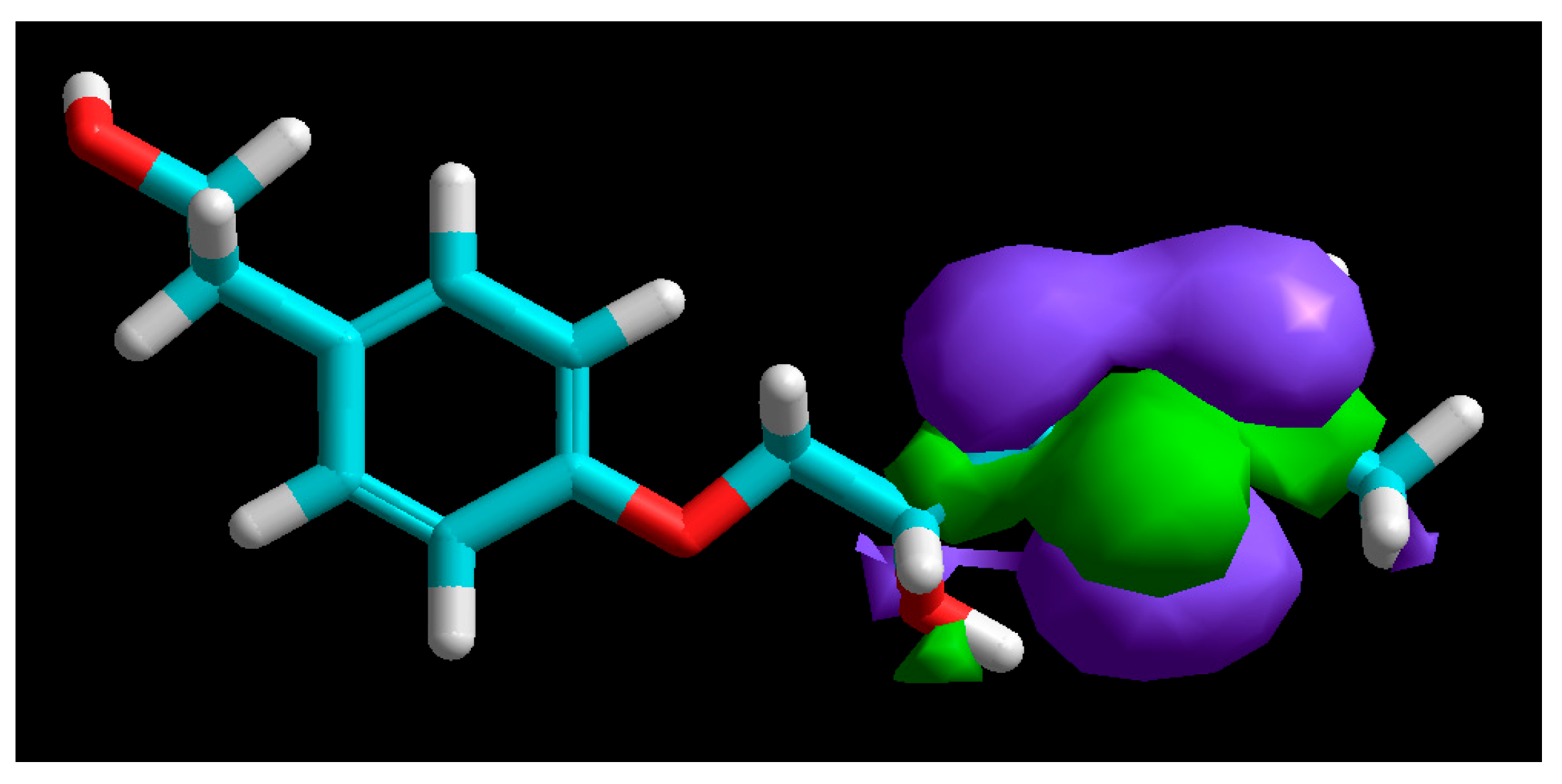
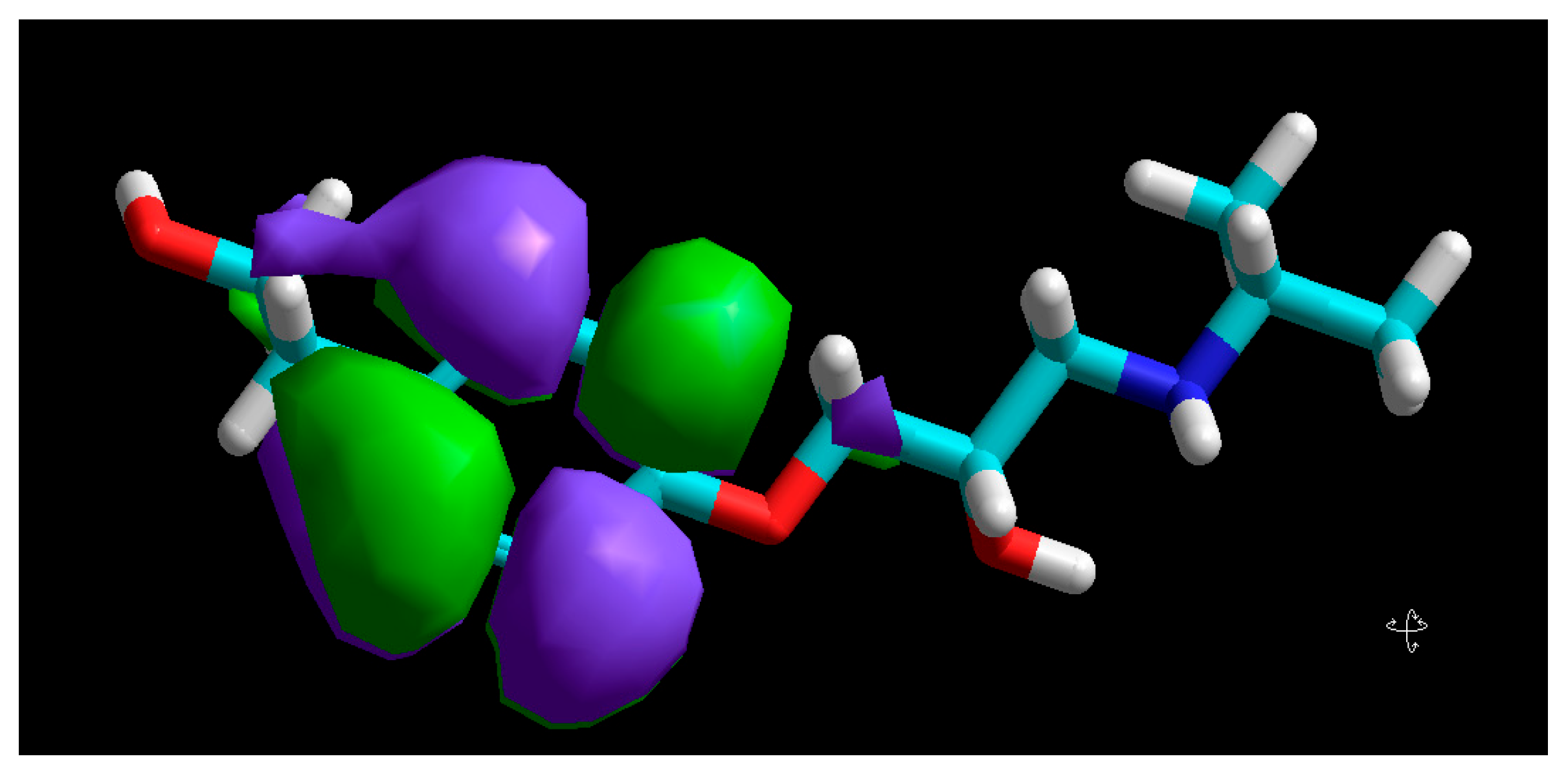
3.5. Molecular Modelling
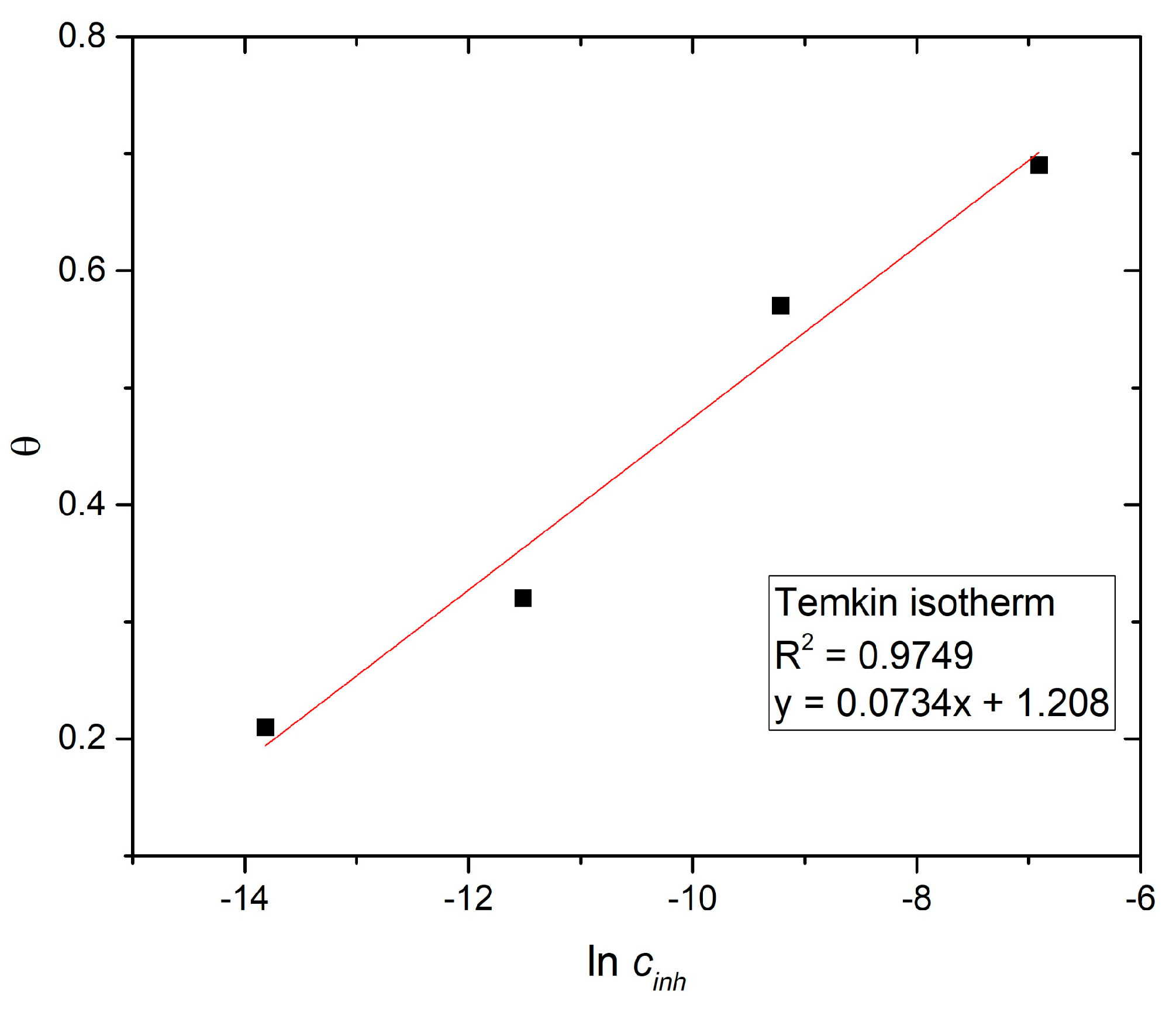
4. Adsorption Isotherms
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vaszilcsin, N.; Kellenberger, A.; Dan, M.L.; Duca, D.A.; Ordodi, V.L. Efficiency of Expired Drugs Used as Corrosion Inhibitors: A Review. Materials 2023, 16, 5555. [Google Scholar] [CrossRef]
- Vaszilcsin, C.G.; Putz, M.V.; Kellenberger, A.; Dan, M.L. On the Evaluation of Metal-Corrosion Inhibitor Interactions by Adsorption Isotherms. J. Mol. Struct. 2023, 1286, 135643. [Google Scholar] [CrossRef]
- Chen, L.; Lu, D.; Zhang, Y. Organic Compounds as Corrosion Inhibitors for Carbon Steel in HCl Solution: A Comprehensive Review. Materials 2022, 15, 2023. [Google Scholar] [CrossRef] [PubMed]
- Aljibori, H.S.; Alamiery, A.; Kadhum, A.A.H. Advances in corrosion protection coatings: A comprehensive review. Int. J. Corros. Scale Inhib. 2023, 12, 1476–1520. [Google Scholar]
- Matcor. Cathodic Protection for Underground Piping-3 Methods Explained. Available online: https://www.matcor.com/cathodic-protection-underground-piping-3-methods (accessed on 8 June 2025).
- Raghavendra, N.; Chapi, S.; Murugendrappa, M.V.; Pawlak, M.; Saeb, M.R. Sustainable corrosion inhibitors from pharmaceutical wastes: Advancing energy-efficient chemistry with green solutions. Energies 2025, 18, 224. [Google Scholar] [CrossRef]
- Njoku, C.N.; Enendu, B.N.; Okechukwu, S.J.; Igboko, N.; Anyikwa, S.O.; Ikeuba, A.I.; Onyeachu, I.B.; Etim, I.I.N.; Njoku, D.I. Review on Anti-Corrosion Properties of Expired Antihypertensive Drugs as Benign Corrosion Inhibitors for Metallic Materials in Various Environments. Results Eng. 2023, 18, 101183. [Google Scholar] [CrossRef]
- Vaszilcsin, N.; Duca, D.A.; Flueras, A.; Dan, M.L. Expired Drugs as Inhibitors in Electrochemical Processes—A Mini-Review. Stud. UBB Chem. 2019, 3, 17–32. [Google Scholar] [CrossRef]
- Radjenovic, J.; Esher, B.I.; Rabaey, K. Electrochemical Degradation of the β-Blocker Metoprolol by Ti/RuO2 and Ti/SnO2 Electrodes. Water Res. 2011, 45, 3205–3214. [Google Scholar] [CrossRef]
- Kumar, S.A.; Rameshkumar, S.; Velumani, K.; Subramanian, S.S. Experimental and Theoretical Studies on Corrosion Inhibi-tion Performance of an Expired Drug. ScieXplore Int. J. Res. Sci. 2023, 10, 22–30. [Google Scholar]
- Isarain-Chávez, E.; Rodríguez, R.M.; Cabot, P.L.; Centellas, F.; Arias, C.; Garrido, J.A.; Brillas, E. Degradation of Pharmaceutical Beta-Blockers by Electro-chemical Advanced Oxidation Processes Using a Flow Plant with a Solar Compound Parabolic Collector. Water Res. 2011, 45, 4119–4130. [Google Scholar] [CrossRef]
- Mohammadinejad, F.; Hosseini, S.M.A.; Shahidi, M.; Bahrami, M.j.; Golshani, Z. Metoprolol: New and Efficient Corrosion Inhibitor for Mild Steel in Hydrochloric and Sulfuric Acid Solutions. Acta Chim. Slov. 2020, 67, 710–719. [Google Scholar] [CrossRef]
- Sheit, H.M.K.; Kani, S.M.; Sathiq, M.A.; Abuthahir, S.S.S.; Subhapriya, P.; Nivedhitha, K.S.; Umarfarooq, M.A.; Badruddin, I.A.; Kamangar, S.; Shaik, A.S. Experimental Studies on the Effect of Expired Amiodarone Drug (EAD) as a Corrosion Inhibitor on Mild Steel in 1 M HCl. Materials 2024, 17, 751. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Lee, D. Unused medications: A global perspective on the ex-tent and impact. WMR 2018, 36, 865–871. [Google Scholar]
- Gros, M.; Petrovic, M.; Barceló, D. Analysis of emerging contaminants in environmental samples. Environ. Pollut. 2012, 163, 267–273. [Google Scholar]
- Fent, K.; Weston, A.A.; Caminada, D. Ecotoxicology of human pharma-ceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef]
- Hughes, S.R.; Kay, P.; Brown, L.E. Global synthesis and critical evalua-tion of pharmaceutical data sets collected from river systems. Environ. Sci. Technol. 2013, 47, 661–677. [Google Scholar] [CrossRef]
- Bound, J.P.; Voulvoulis, N. Household disposal of pharmaceuticals as a pathway for aquatic contamination in the United Kingdom. Environ. Health Perspect. 2005, 113, 1705–1711. [Google Scholar] [CrossRef] [PubMed]
- Borkar, R.M.; Raju, B.; Srinivas, R.; Patel, P.; Shetty, S.K. Identification and characterization of stressed degradation products of metoprolol using LC/Q-TOF-ESI-MS/MS and MS(n) experiments. Biomed. Chromatogr. 2012, 26, 720–736. [Google Scholar] [CrossRef]
- Hamrapurkar, P.; Patil, P.; Phale, M.; Pawar, S. Optimization and estab-lishment of a validated stability-indicating HPLC method for study of the stress degrada-tion behavior of metoprolol succinate. J. AOAC Int. 2010, 93, 800–808. [Google Scholar]
- Ciciliati, M.A.; Cavalheiro, É.T.G. Studies of thermal behavior of metoprolol tartrate. J. Therm. Anal. Calorim. 2019, 138, 3653–3663. [Google Scholar] [CrossRef]
- Tay, K.S.; Rahman, N.A.; Abas, M.R.B. Ozonation of metoprolol in aqueous solution: Ozonation by-products and mechanisms of degradation. Environ. Sci. Pollut. Res. 2013, 20, 3115–3121. [Google Scholar] [CrossRef] [PubMed]
- Karthik, G.; Sundaravadivelu, M. Studies on the inhibition of mild steel corrosion in hydrochloric acid solution by atenolol drug. Egypt. J. Pet. 2016, 25, 183–191. [Google Scholar] [CrossRef]
- Fouda, A.S.; Shalabi, K. Effect of β-Blocker Inhibitors on Aluminum Corrosion. J. Korean Chem. Soc. 2011, 55, 268. [Google Scholar] [CrossRef]
- Dima, G.; Șerboiu, O.T.; Dan, M.; Costea, L. Valerian Extract Used as Potential Corrosion Inhibitor for Carbon Steel in Different Media. Stud. UBB Chem. 2023, 68, 99–114. [Google Scholar] [CrossRef]
- Vaszilcsin, C.G.; Putz, M.V.; Dan, M.L.; Medeleanu, M. Myricetin as Corrosion Inhibitor for Metals in Alcoholic Solutions. Stud. UBB Chem. 2023, 68, 23–36. [Google Scholar] [CrossRef]
- Berkowitz, B.A. Basic and Clinical Pharmacology; McGraw-Hill Education: New York, NeY, USA, 2020. [Google Scholar]
- Williams, D.A.; Lemke, T.L. Foye′s Principles of Medicinal Chemistry; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2019. [Google Scholar]
- Popov, B.N. Chapter 5—Basics of Corrosion Measurements. In Corros. Eng.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 181–237. [Google Scholar]
- Ho, M.Y.; Geddes, J.; Hughes, T.L.; Barmatov, E. Effect of copper content of AISI 1018 mild carbon steel on inhibition efficiency of imidazoline-based corrosion inhibitor in CO2-saturated brine. Corros. Sci. 2023, 224, 111478. [Google Scholar] [CrossRef]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical Impedance Spectroscopy (EIS): Principles, Construction, and Biosensing Applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef] [PubMed]
- Gutowska, I.; Machoy, Z.; Machaliński, B. The role of bivalent metals in hydroxyapatite structures as revealed by molecular modeling with the HyperChem software. J. Biomed. Mater. Res. A 2005, 75, 788–793. [Google Scholar] [CrossRef]
- Pal, B.; Mishra, M.; Singh, D.; Kumar, D. A theoretical investigation of nonlinear optical and electronic molecular parameters of hexabutyloxytryphenylene and halogenated hexabutyloxytryphenylene molecules using density functional theory (DFT) for nonlinear device applications. Phys. Scr. 2022, 97, 065808. [Google Scholar] [CrossRef]
- Montazerozohori, M.; Masoudiasl, A.; Doert, T.; Seykens, H. Structural and computational study of some new nano-structured Hg(II) compounds: A combined X-ray, Hirshfeld surface and NBO analyses. RSC Adv. 2023, 2016, 21396–21412. [Google Scholar] [CrossRef]
- Parr, R.; Pearson, R. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Kumer, A.; Sarker, N.; Paul, S.; Zannat, A. The theoretical prediction of thermophysical properties, HOMO, LUMO, QSAR and biological indices of cannabinoids (CBD) and tetrahydrocannabinol (THC) by computational chemistry. J. Adv. Chem. Chem. Sci. 2019, 2, 190–202. [Google Scholar]
- Duca, D.A.; Dan, M.L.; Vaszilcsin, N. Expired domestic drug—Paracetamol—As corrosion inhibitor for carbon steel in acid media. IOP Conf. Ser. Mater. Sci. Eng. 2018, 416, 012118. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Kadhum, A.A.H.; Kadihum, A.; Mohamad, A.B.; How, C.K.; Junaedi, S. Corrosion inhibition of carbon steel in acidic medium by some drugs. Materials 2014, 7, 787–804. [Google Scholar] [CrossRef]
- Galai, M.; El Gouri, M.; Dagdag, B.; El Kacimi, Y.; Elharfi, A.; Ebn Touhami, M. Evaluation of some drugs as corrosion inhibitors for carbon steel in hydrochloric acid solution. J. Mater. Environ. Sci. 2016, 7, 1562–1575. [Google Scholar]
- Kellenberger, A.; Duca, D.A.; Dan, M.L.; Medeleanu, M. Recycling unused midazolam drug as efficient corrosion inhibitor for copper in nitric acid solution. Materials 2022, 15, 2918. [Google Scholar] [CrossRef]
- Froimowitz, M. HyperChem: A software package for computational chemistry and molecular modeling. Biotechniques 1993, 14, 1010–1013. [Google Scholar] [PubMed]
- Letícia, B.S.; Daniel, L.H.S.; Kamil, K.; Ramalho, C.T. Perspectives on the role of the frontier effective-for-reaction molecular orbital (FERMO) in the study of chemical reactivity: An updated review. Curr. Org. Chem. 2020, 24, 314–331. [Google Scholar]
- Erazua, E.A.; Adeleke, B.B. A computational study of quinoline derivatives as corrosion inhibitors for mild steel in acidic medium. J. Appl. Sci. Environ. Manag. 2019, 23, 1819–1824. [Google Scholar] [CrossRef]
- Malinowski, S.; Wróbel, M.; Woszuk, A. Quantum chemical analysis of the corrosion inhibition potential by aliphatic amines. Materials 2021, 14, 6197. [Google Scholar] [CrossRef]
- Ahmed, L.; Bulut, N.; Kaygili, O.; Omer, R. Quantum chemical study of some basic organic compounds as the corrosion inhibitors. J. Phys. Chem. Funct. Mater. 2023, 6, 34–42. [Google Scholar] [CrossRef]
- Masoud, M.S.; Awad, M.K.; Shaker, M.A.; El-Tahawy, M.M.T. The role of structural chemistry in the inhibitive performance of some aminopyrimidines on the corrosion of steel. Corros. Sci. 2010, 52, 2387–2396. [Google Scholar] [CrossRef]
- Kokalj, A. On the Use of the Langmuir and Other Adsorption Isotherms in Corrosion Inhibition. Corros. Sci. 2023, 217, 111112. [Google Scholar] [CrossRef]
- Kokalj, A. A General-Purpose Adsorption Isotherm for Improved Estimation of Standard Adsorption Free Energy. Corros. Sci. 2023, 217, 111124. [Google Scholar] [CrossRef]
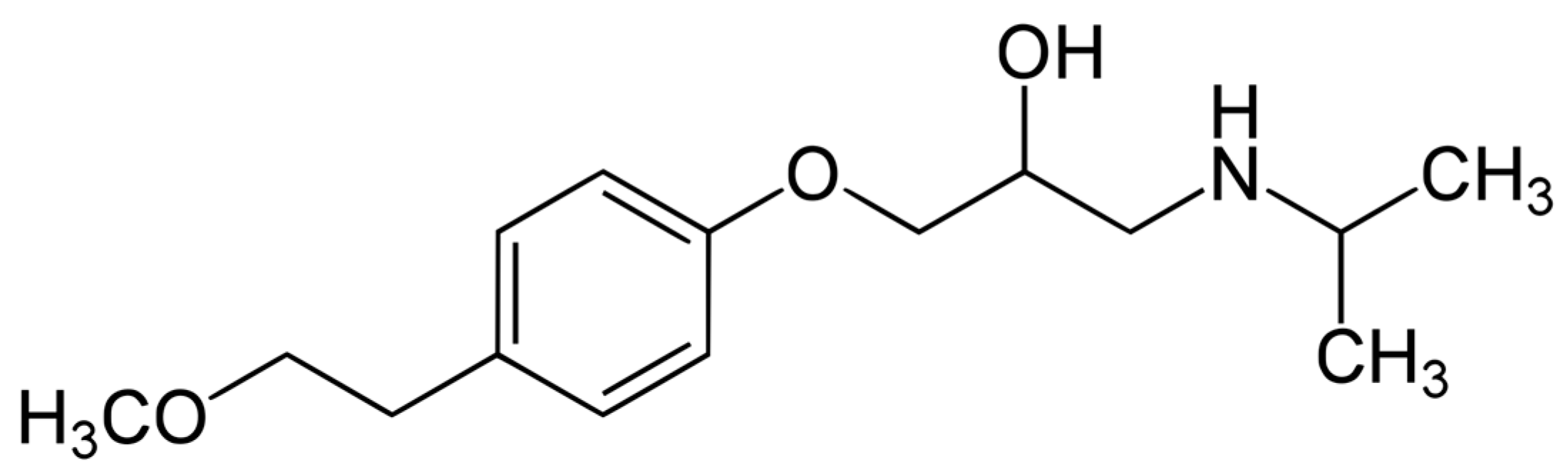
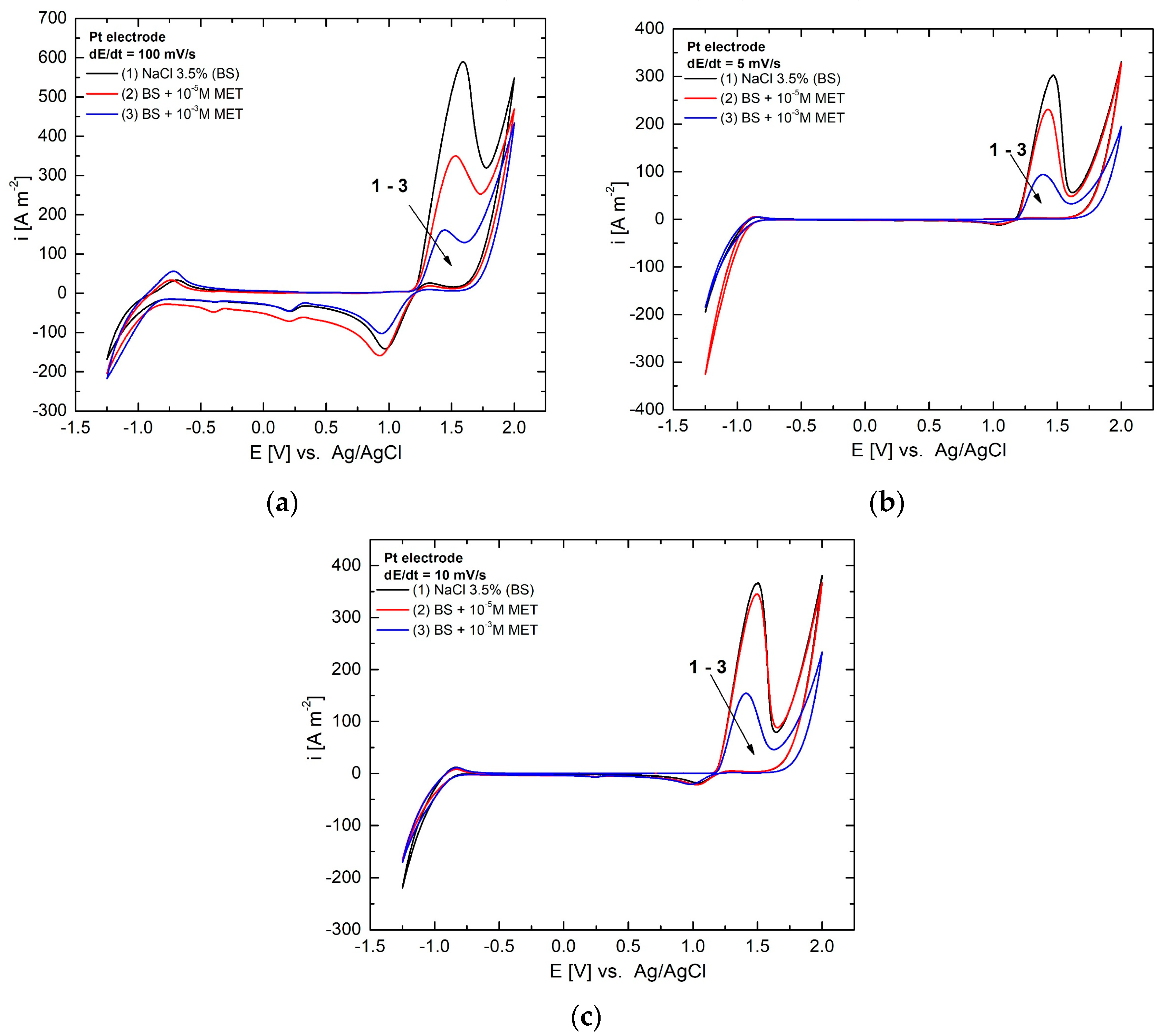




| Fe (%) | C (%) | Cu (%) | Ni (%) | P (%) | S (%) | Mn (%) | Cr (%) | Si (%) |
|---|---|---|---|---|---|---|---|---|
| 97.465 | 0.43 | 0.30 | 0.30 | 0.040 | 0.045 | 0.75 | 0.30 | 0.37 |
| Metal | Metoprolol conc. | icorr | Ecorr | −bc | ba | Rp | vcorr | IE | θ |
|---|---|---|---|---|---|---|---|---|---|
| (μA cm−2) | (mV) | (V dec−1) | (V dec−1) | (Ω) | (mm y−1) | (%) | |||
| OLC45 | BS (NaCl 3.5%) | 19.38 | −548 | 216 | 126 | 1.78 | 0.282 | - | - |
| 10−6 M | 15.35 | −578 | 144 | 158 | 2.13 | 0.222 | 20.7 | 0.21 | |
| 10−5 M | 13.12 | −601 | 146 | 142 | 2.38 | 0.191 | 32.3 | 0.32 | |
| 10−4 M | 8.28 | −624 | 119 | 116 | 3.09 | 0.120 | 57.2 | 0.57 | |
| 10−3 M | 5.97 | −636 | 108 | 95 | 3.68 | 0.086 | 69.1 | 0.69 |
| Metal | Metoprolol Concentration | Ecorr | Eox | |
|---|---|---|---|---|
| 25 mv/EOCP | 250 mv/EOCP | |||
| icorr | ||||
| (mV)/Ref | (A m−2) | |||
| OLC45 | BS (NaCl 3.5%) | −545 | 0.63 | 144 |
| 10−6 M | −576 | 0.53 | 130 | |
| 10−5 M | −595 | 0.45 | 115 | |
| 10−4 M | −619 | 0.31 | 80 | |
| 10−3 M | −630 | 0.25 | 61.5 | |
| MET Conc. (M) | RS (Ω) | CPE-T (F cm−2 sn−1) | n | Rct (Ω cm2) | Chi2 · 103 | Cdl · 104 (µF cm−2) | E (%) | θ |
|---|---|---|---|---|---|---|---|---|
| BS | 8.77 (0.25%) | 5.88·10−3 (0.53%) | 0.67 (0.27%) | 424 (1.05%) | 1.55 | 13.5 | – | – |
| 10−6 | 8.55 (0.22%) | 4.46·10−3 (0.40%) | 0.63 (0.20%) | 525 (0.69%) | 0.69 | 6.94 | 19.2 | 0.19 |
| 10−5 | 8.43 (0.28%) | 4.20·10−3 (0.48%) | 0.61 (0.24%) | 639 (0.91%) | 0.92 | 5.00 | 33.6 | 0.34 |
| 10−4 | 8.36 (0.35%) | 2.98·10−3 (0.55%) | 0.60 (0.25%) | 1020 (1.06%) | 1.07 | 2.55 | 58.4 | 0.58 |
| 10−3 | 8.17 (0.24%) | 2.21·10−3 (0.36%) | 0.59 (0.18%) | 1313 (1.01%) | 0.87 | 1.36 | 67.7 | 0.68 |
| Molecular Descriptor | Value |
|---|---|
| EHOMO (eV) | −9.12 |
| ELUMO (eV) | 0.21 |
| ΔE (eV) | 9.33 |
| µ (Debye) | 3.95 |
| χ (eV) | 4.45 |
| η (eV) | 4.56 |
| σ (eV−1) | 0.22 |
| V (Å3) | 855.05 |
| S (Å2) | 529.18 |
| V/S (Å) | 1.62 |
| T [K] | a | b | r2 | Kads | ∆Go (kJ/mol) | f |
|---|---|---|---|---|---|---|
| 298 | 1.21 | 0.0734 | 0.9749 | 1.4 ∙ 107 | −50.7 | 13.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dan, M.L.; Rudenko, N.; Vaszilcsin, C.G.; Dima, G.-D. Sustainable Use of Expired Metoprolol as Corrosion Inhibitor for Carbon Steel in Saline Solution. Coatings 2025, 15, 742. https://doi.org/10.3390/coatings15070742
Dan ML, Rudenko N, Vaszilcsin CG, Dima G-D. Sustainable Use of Expired Metoprolol as Corrosion Inhibitor for Carbon Steel in Saline Solution. Coatings. 2025; 15(7):742. https://doi.org/10.3390/coatings15070742
Chicago/Turabian StyleDan, Mircea Laurențiu, Nataliia Rudenko, Cristian George Vaszilcsin, and George-Daniel Dima. 2025. "Sustainable Use of Expired Metoprolol as Corrosion Inhibitor for Carbon Steel in Saline Solution" Coatings 15, no. 7: 742. https://doi.org/10.3390/coatings15070742
APA StyleDan, M. L., Rudenko, N., Vaszilcsin, C. G., & Dima, G.-D. (2025). Sustainable Use of Expired Metoprolol as Corrosion Inhibitor for Carbon Steel in Saline Solution. Coatings, 15(7), 742. https://doi.org/10.3390/coatings15070742






