Research on High-Temperature Frictional Performance Optimization and Synergistic Effects of Phosphate-Based Composite Lubricating Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Coating
2.3. Coating Performance Test and Characterization
3. Results and Discussion
3.1. Influence of Filler on the Heat Resistance of the Binder Matrix
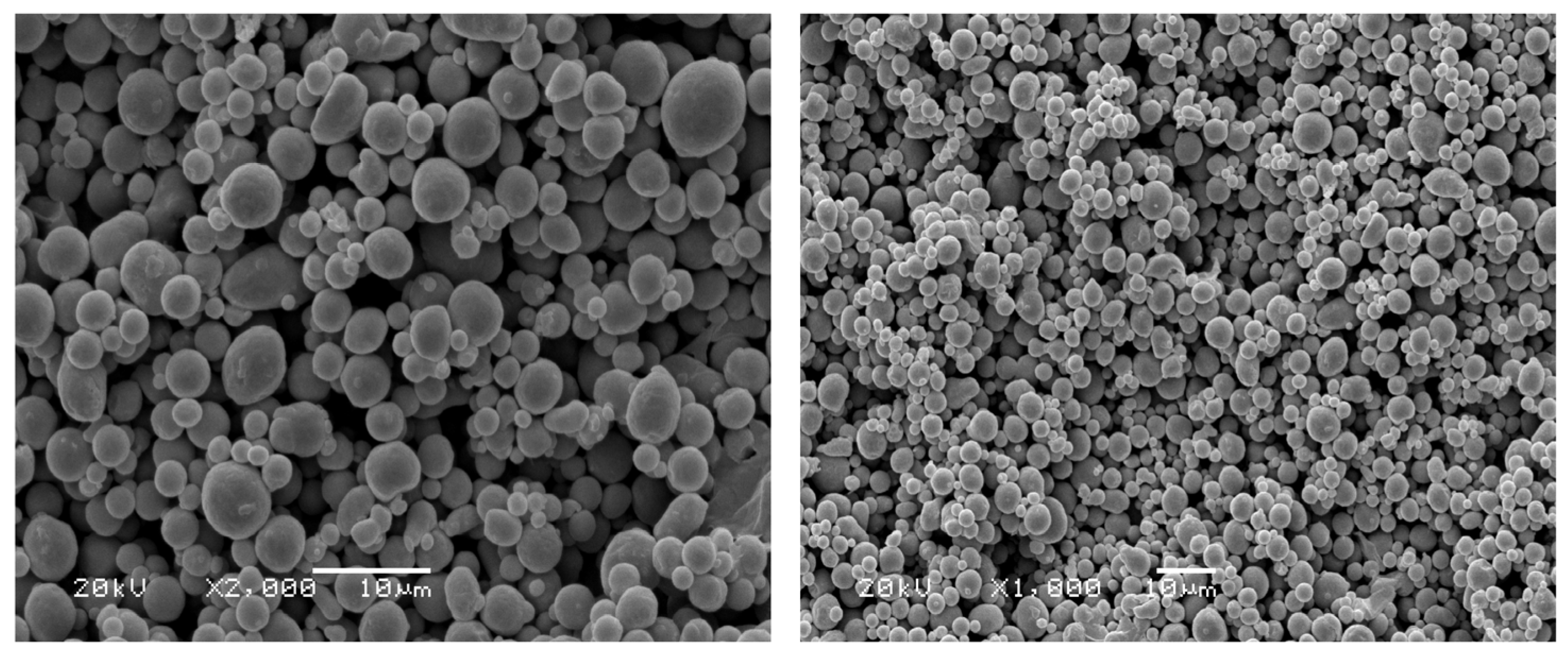
3.2. Effect of Aluminum Powder on Coating Adhesion
3.3. Effect of MoS2 Content on Coating Performance
3.3.1. Effect of MoS2 Content on Coating Microhardness
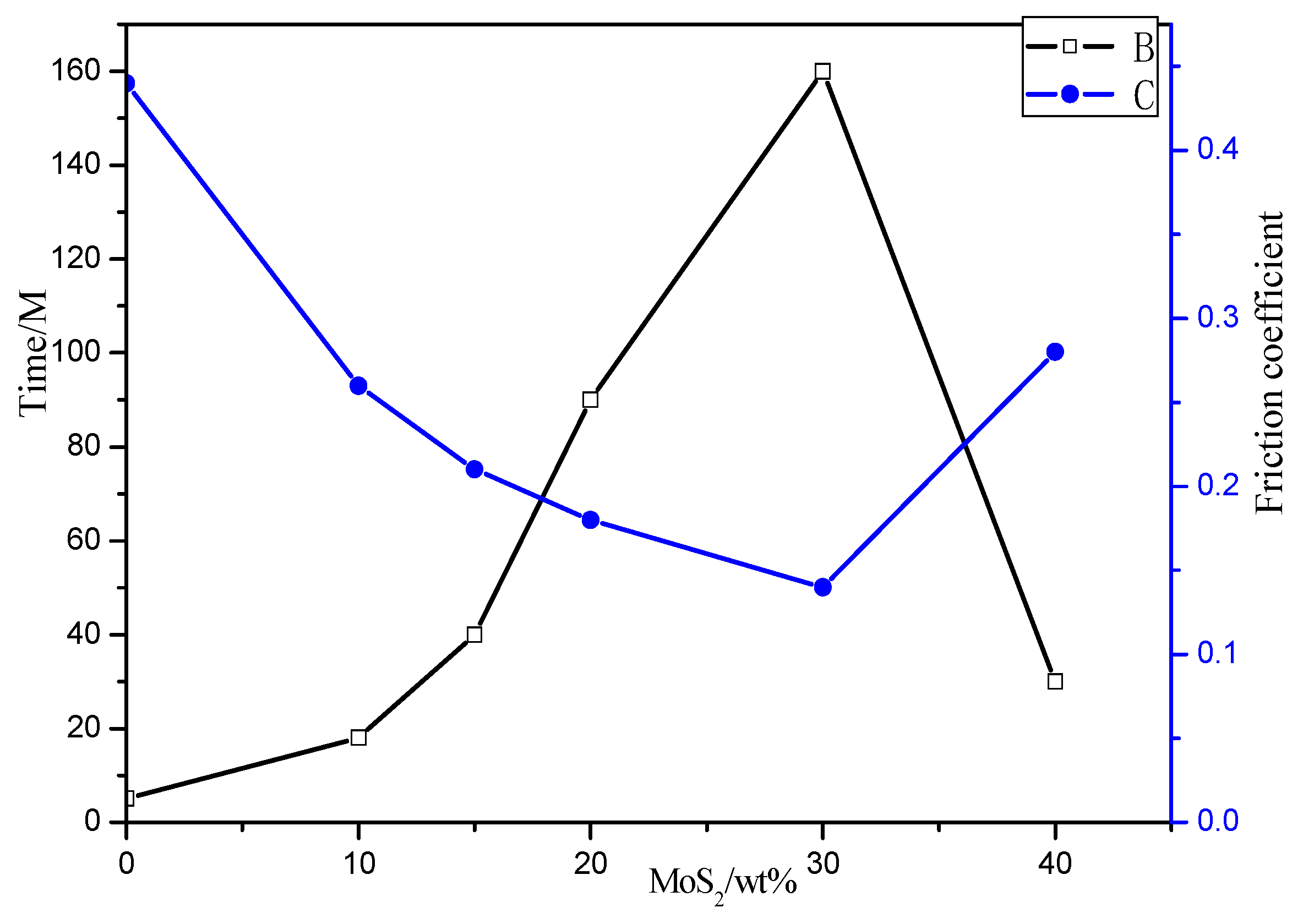
3.3.2. Effect of Different MoS2 Content on Coating Friction Performance
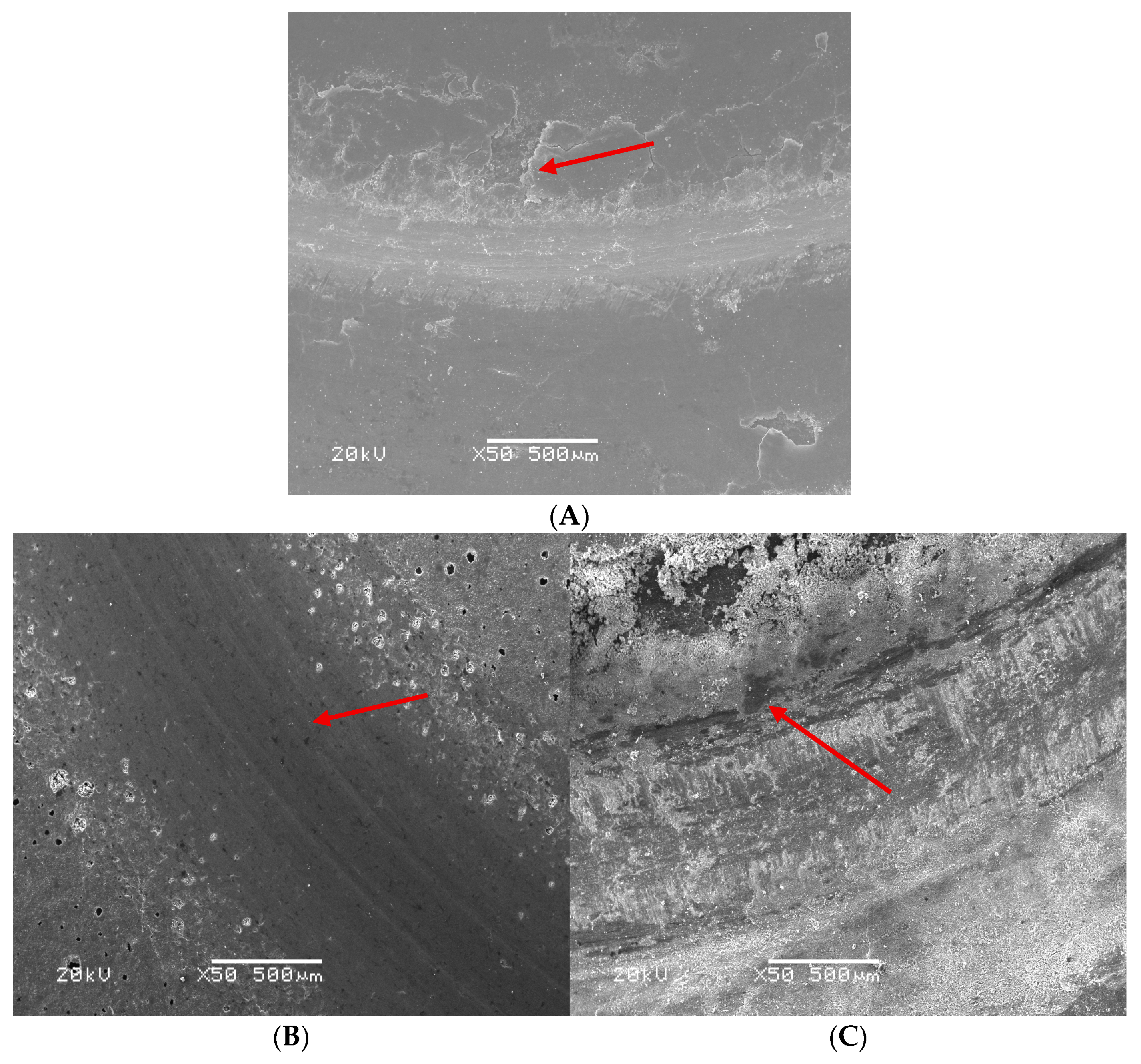
3.3.3. Friction Surface Analysis of Coatings with Different MoS2 Contents
3.4. Effect of Graphite Content on Coating Performance
3.4.1. Effect of Graphite Content on Coating Microhardness
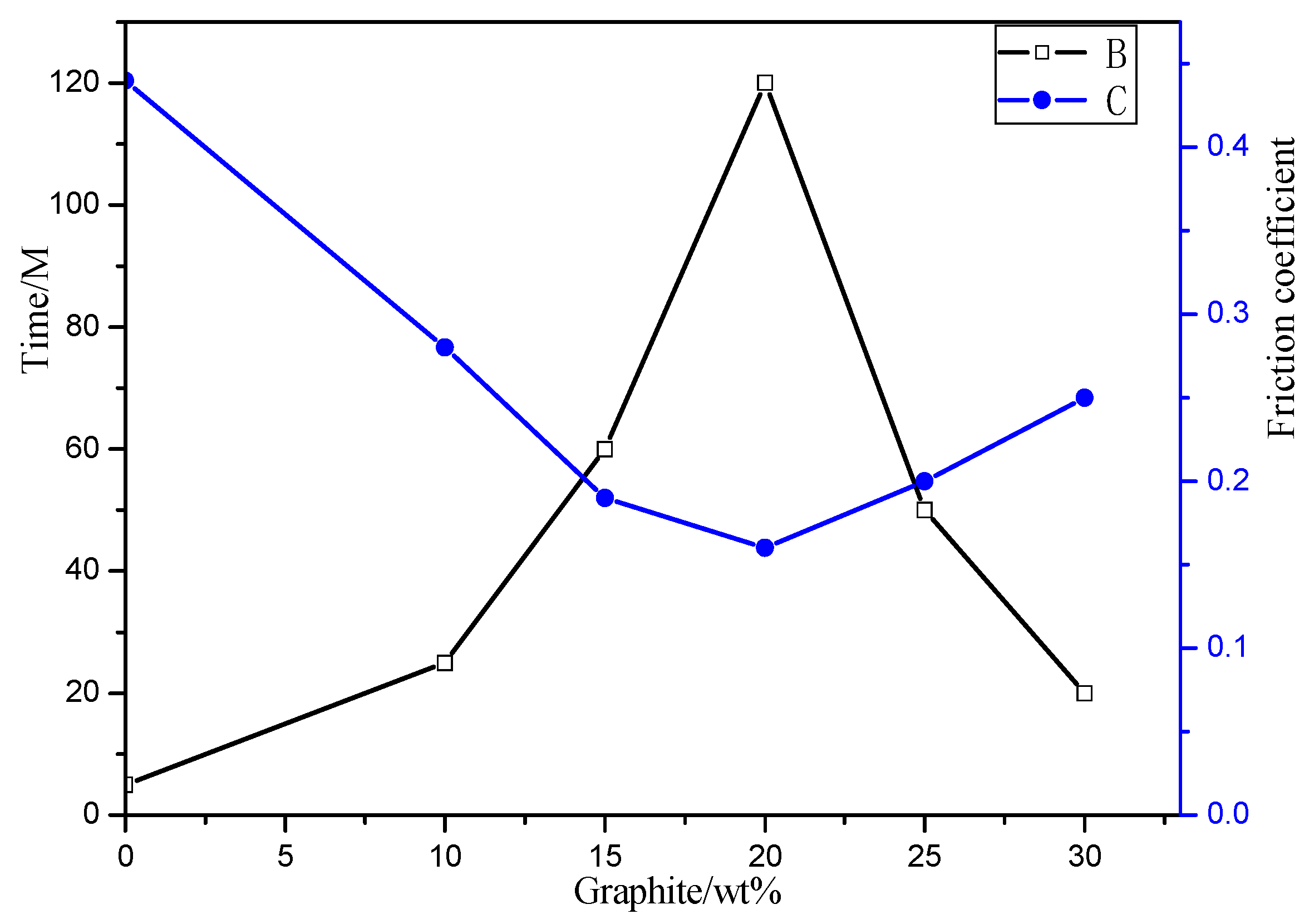
3.4.2. Effect of Graphite Content on Coating Friction Performance
3.4.3. Friction Surface Analysis of Graphite-Containing Coatings
3.5. Synergistic Effect of Graphite and MoS2 on Coating Friction Performance
3.5.1. Experimental Design
3.5.2. SEM Morphology Analysis of Composite Coatings
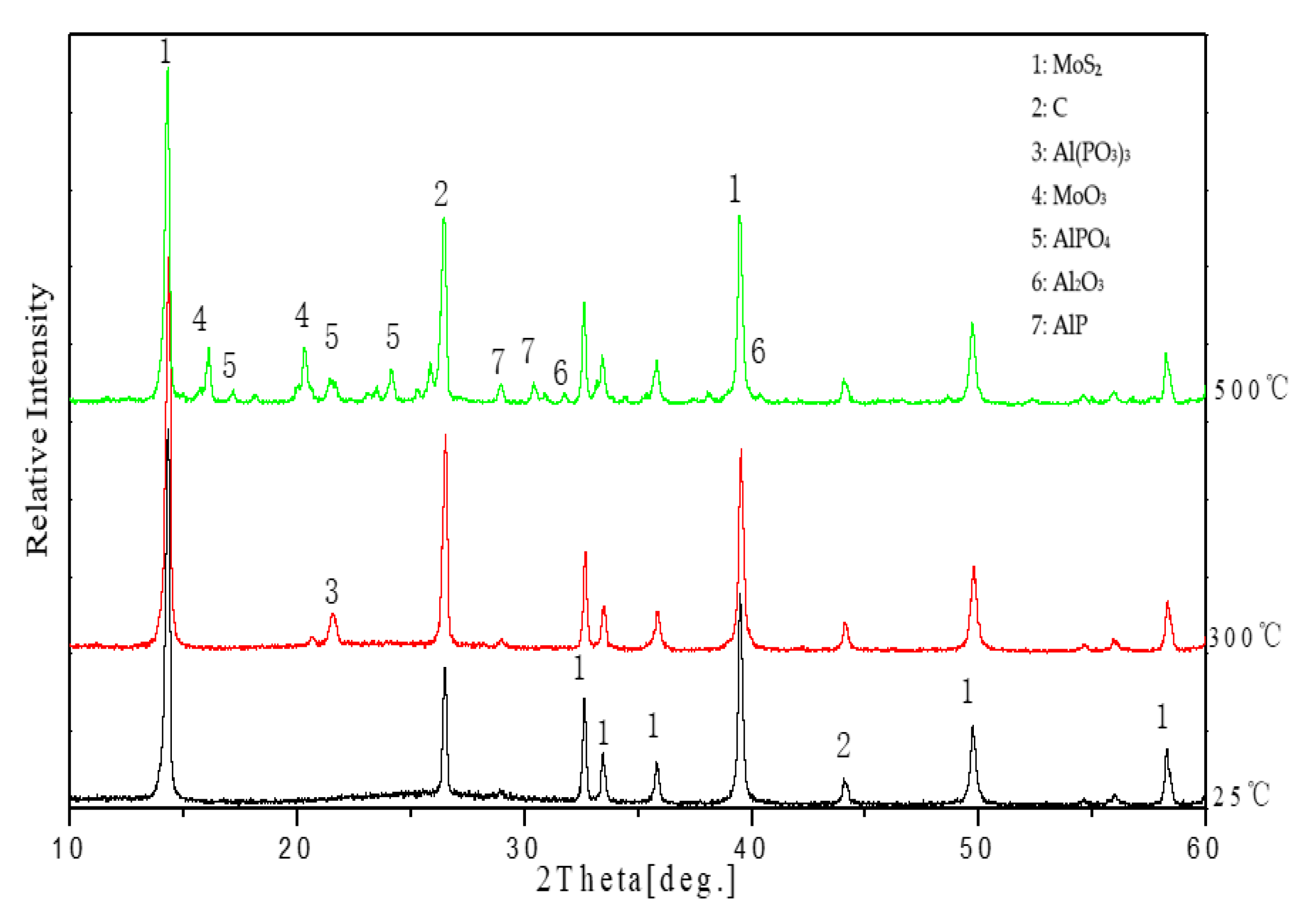
3.5.3. XRD Analysis of Coatings at Different Temperatures
3.6. Friction Performance of Composite Coatings at Different Temperatures
4. Conclusions
- The synergistic combination of MoS2 (30%) and graphite (20%) significantly enhances lubricating film continuity through shear slippage of layered materials and pore-filling effects, reducing the room-temperature friction coefficient by 20% compared to single-component coatings and extending wear resistance to 260 min. SEM analysis confirms that the formation of a continuous lubricating film effectively suppresses adhesive and abrasive wear.
- At 500 °C, the coating undergoes sequential phase transitions (aluminum phosphate–aluminum metaphosphate–aluminum pyrophosphate), forming a three-dimensional Al–O–P cross-linked framework. Concurrently, the oxidation of MoS2 into MoO3 and aluminum into Al2O3 constructs a “ceramic–phosphate” composite phase, improving coating density and oxidation resistance.
- Orthogonal experiments confirmed that a binder/filler ratio of 6:4 balances mechanical strength and thermal stability, while 10% aluminum powder content optimizes adhesion and prevents brittleness caused by excessive addition.
- The preparation process of this coating is environmentally friendly, suitable for surface protection of high-temperature industrial equipment, and holds potential for large-scale application in fields such as the petrochemical and power industries.
- Future research could explore novel lubricants (e.g., h-BN, graphene) and advanced deposition technologies, combined with computational optimization of formulations to expand coating applications under extreme operating conditions. Additionally, validating long-term stability, environmental compatibility, and industrial adaptability would facilitate the engineering implementation of high-performance eco-friendly coatings.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiong, X.H.; Lv, Z.M.; Tan, H.Z.; Wei, B. A typical Super-Heater tube leakage and high temperature corrosion mechanism investigation in a 260 t/h circulated fluidized boiler. Eng. Fail. Anal. 2020, 109, 104255. [Google Scholar] [CrossRef]
- Zhang, X.J.; Liu, H.; Chen, T.Z.; Wang, G.Y.; Li, H.Y.; Hu, H.Y.; Yu, Y.; Yao, H. Application of coatings to alleviate fireside corrosion on heat transfer tubes during the combustion of Low-Grade solid fuels: A review. Energy. Fuels. 2020, 34, 11752–11770. [Google Scholar] [CrossRef]
- Niu, Y.Q.; Tan, H.Z.; Hui, S.E. Ash-Related issues during biomass combustion: Alkali-Induced slagging, silicate Melt-Induced slagging (ash fusion), agglomeration, corrosion, ash utilization, and related countermeasures. Prog. Energ. Combust. 2016, 52, 1–61. [Google Scholar] [CrossRef]
- Nagarajan, R.; Ambedkar, B.; Gowrisankar, S.; Somasundaram, S. Development of predictive model for Fly-Ash erosion phenomena in Coal-Burning boilers. Wear 2009, 267, 122–128. [Google Scholar] [CrossRef]
- Ramteke, M.S.; Walczak, M.; Stefano, D.M.; Ruggiero, A.; Rosenkranz, A.; Marian, M. 2D materials for Tribo-corrosion and oxidation protection. Adv. Colloid. Interface 2024, 331, 103243. [Google Scholar] [CrossRef]
- Lv, Y.Z.; Sun, Y.N.; Deng, C.M.; Fan, X.J.; Li, S.J.; Wang, C.; Yang, Y.Q.; Zhang, Y.H.; Chun, Y. Preparation, corrosion and tribological properties evolution of Self-Lubricating Al2O3-TiO2 coatings with superior Anti-Corrosion. Surf. Coat. Technol. 2025, 498, 131849. [Google Scholar] [CrossRef]
- Zhu, H.M.; Li, B.C.; Chen, M.H.; Qiu, C.J.; Tang, Z.F. Improvement of Corrosion Resistance of Hastelloy-N Alloy in LiF-NaF-KF Molten Salt by Laser Cladding Pure Metallic Coatings. Coatings 2018, 8, 322. [Google Scholar] [CrossRef]
- Vasile, S.B.; Birca, C.A.; Surdu, A.V.; Surdu, A.V.; Neacsu, A.I.; Nicoară, A.I. Ceramic Composite Materials Obtained by Electron-Beam Physical Vapor Deposition Used as Thermal Barriers in the Aerospace Industry. Nanomaterials 2020, 10, 370. [Google Scholar] [CrossRef]
- Li, C.Y.; Quan, G.N.; Zhang, Q.; Wang, X.H.; Li, X.C.; Kou, S.Z. A Novel Amorphous Alloy Coating for Elevating Corrosion Resistance of X70 Pipeline Steel. Therm. Spray. Technol. 2024, 33, 1612–1629. [Google Scholar] [CrossRef]
- Patel, D.; Bhatt, P.; Bateriwala, R. Thermal Spray Techniques for Developing a Carbide Coating. Int. J. Innov. Technol. Explor. Eng. 2019, 8, 692–700. [Google Scholar]
- Monette, Z.; Kasar, A.K.; Daroonparvar, M.; Menezes, P.L. Supersonic particle deposition as an additive technology: Methods, challenges, and applications. Int. J. Adv. Manuf. Technol. 2020, 106, 2079–2099. [Google Scholar] [CrossRef]
- Ralls, A.M.; Daroonparvar, M.; Sikdar, S.; Rahman, M.H.; Monwar, M.; Watson, K.; Kay, C.M.; Menezes, P.L. Tribological and corrosion behavior of high pressure cold sprayed duplex 316 L stainless steel. Tribol. Int. 2022, 169, 107471. [Google Scholar] [CrossRef]
- Ralls, A.M.; Kasar, A.K.; Daroonparvar, M.; Siddaiah, A.; Kumar, P.; Kay, C.M.; Misra, M.; Menezes, P.L. Effect of gas propellant temperature on the microstructure, friction, and wear resistance of High-Pressure cold sprayed Zr7O2 coatings on Al6061 alloy. Coatings 2022, 12, 263. [Google Scholar] [CrossRef]
- Chen, P.J.; Xiao, P.; Li, Z.; Li, J.W. Oxidation properties of Tri-Layer Ytterbium-Disilicate/mullite/Silicon-Carbide environment barrier coatings for Cf/SiC composites. Surf. Coat. Technol. 2020, 402, 126329. [Google Scholar] [CrossRef]
- Fogliati, M.; Fontana, D.; Garbero, M.; Vanni, M.; Baldi, G.; Dondè, R. CFD simulation of paint deposition in an air spray process. JCT Res. 2006, 3, 117–125. [Google Scholar] [CrossRef]
- Zhang, S.M.; Wang, W.J.; Gao, Y.; Liang, K.; Cui, J.Y.; Yang, D.Y.; Zhu, K.; Ma, C.L.; Yuan, H.Y. Air pressure spray deposition of layer-by-layer calcium niobate nanosheets for efficient thermal insulation coatings. Ceram. Int. 2024, 50, 53899–53906. [Google Scholar] [CrossRef]
- Shi, M.J.; Li, X.H.; Jiang, Y.D.; Li, S.X.; Li, B.W.; Zhang, S.J.; Nan, C.W. 2D Nanosheet Spray Coating for Scalable Processing of High-Energy-Density Dielectric Polymer Films. Adv. Electron. Mater. 2023, 9, 53899–53906. [Google Scholar] [CrossRef]
- Donnet, C.; Erdemir, A. Historical developments and new trends in tribological and solid lubricant coatings. Surf. Coat. Technol. 2003, 180, 76–84. [Google Scholar] [CrossRef]
- Bartuli, C.; Valente, T.; Casadei, F.; Tului, M. Advanced thermal spray coatings for tribological applications. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2007, 221, 175–185. [Google Scholar] [CrossRef]
- Manh, C.L.; Huong, T.L. The Study’s Chemical Interaction of the Sodium Silicate Solution with Extender Pigments to Investigate High Heat Resistance Silicate Coating. J. Anal. Methods. Chem. 2021, 2021, 5510193. [Google Scholar]
- Cao, X.Q.; Zhao, K.L.; Wang, L.; Wang, T.T.; Wu, Y.Z.; Tang, X.P.; Bai, J.W.; Zhang, C.H.; Liu, W.X.; Cao, W.X.; et al. Robust, hermally insulating, and High-Temperature resistant Phosphate-Enhanced mullite fiber porous ceramic composites. Ceram.Int. 2024, 50, 23733–23743. [Google Scholar] [CrossRef]
- Chung, D.D.L. Acid aluminum phosphate for the binding and coating of materials. J. Mater. Sci. 2003, 38, 2785–2791. [Google Scholar] [CrossRef]
- Ye, Y.P.; Zhou, H.D.; Jia, J.H.; Chen, J.M. High temperature Anti-Sticking lubricating coating. CN98123517.4, 6 December 2000. (In Chinese). [Google Scholar]
- Yuan, H.; Wang, Y.S.; Liu, Z.Y.; Li, S.Y. A study on the properties and working mechanism of a waterborne Polyurethane-Modified Silicate-Based coating. RSC Adv. 2019, 9, 26817–26824. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.H.; Li, H.; Li, W.; Fan, X.Q.; He, J.F.; Cai, Z.B.; Fu, W.; Zhang, G.G.; Wan, S.H.; Zhu, M.H. Probing fretting performance of DLC and MoS2 films under fluid lubrication. Appl. Surf. Sci. 2019, 478, 661–679. [Google Scholar] [CrossRef]
- Kong, X.; Liu, Y.; Chen, M.H.; Zhang, T.; Wang, T.H. High Temperature Oxidation and Tribological Behaviors of NiCrAl-Graphite Self-Lubricating Composite. Acta. Metal. Sin. 2021, 34, 900–912. [Google Scholar] [CrossRef]
- Yin, H.; Li, H.Y.; Chen, J.D.; Wang, Y.Y.; Zhang, S.W.; Xu, L.L.; Li, T.; Zeng, Z.W.; Tang, J.L. Research Progress and Application of Phosphate Coatings. Surf. Technol. 2021, 50, 232–241. [Google Scholar]
- Sarkar, M.; Mandal, N. Solid lubricant materials for high temperature application: A review. Mater. Today Proc. 2022, 66, 3762–3768. [Google Scholar] [CrossRef]
- Kumar, R.; Hussainova, I.; Rahmani, R.; Antonov, M. Solid Lubrication at High-Temperatures A review. Materials 2022, 15, 1695. [Google Scholar] [CrossRef]
- Dienwiebel, M.; Verhoeven, G.S.; Pradeep, N.; Frenke, J.W.M.; Heimberg, J.A.; Zandbergen, H.W. Superlubricity of graphite. Phys. Rev. Lett. 2004, 92, 126101. [Google Scholar] [CrossRef]
- De Lima, G.A.; Klein, A.N.; Furlan, K.P. Controlling the Solid-State Reaction in Fe-MoS2 Self-Lubricating Composites for Optimized Tribological Properties. Lubricants 2022, 10, 142. [Google Scholar] [CrossRef]
- Cho, M.H.; Ju, J.; Kim, S.J.; Jang, H. Tribological properties of solid lubricants (graphite, Sb2S3, MoS2) for automotive brake friction materials. Wear 2005, 260, 855–860. [Google Scholar] [CrossRef]
- Li, J.L.; Xiong, D.S. Tribological properties of Nickel-Based Self-Lubricating composite at elevated temperature and counterface material selection. Wear 2008, 265, 533–539. [Google Scholar] [CrossRef]
- Vippola, M.; Vuorinen, J.; Vuoristo, P.; Lepistö, T.; Mäntylä, T. Thermal analysis of plasma sprayed oxide coatings sealed with aluminium phosphate. J. Eur. Ceram. Soc. 2002, 22, 1937–1946. [Google Scholar] [CrossRef]
- Han, H.J.; Kim, D.P. Studies on Curing Chemistry of Aluminum-Chromium-Phosphates as Low Temperature Curable Binders. J. Sol-Gel. Sci. Techn. 2003, 26, 223–228. [Google Scholar] [CrossRef]
- Maier, C.R.; Jones, L.E. The influence of aluminum phosphates on graphite oxidation. Carbon 2005, 43, 2272–2276. [Google Scholar] [CrossRef]
- Sliney, H.E. Solid lubricant materials for high temperatures-a review. Tribol. Int. 1982, 15, 303–315. [Google Scholar] [CrossRef]
- Serpini, E.; Rota, A.; Ballestrazzi, A.; Marchetto, D.; Gualtieri, E.; Valeri, S. The role of humidity and oxygen on MoS2 thin films deposited by RF PVD magnetron sputtering. Surf. Coat. Technol. 2017, 319, 345–352. [Google Scholar] [CrossRef]
- Jeurgens, L.P.H.; Sloof, W.G.; Tichelaar, F.D.; Mittemeijer, E.J. Growth kinetics and mechanisms of Aluminum-Oxide films formed by thermal oxidation of aluminum. J. Appl. Phys. 2002, 92, 1649–1656. [Google Scholar] [CrossRef]








| Sample | Phosphate Binder Content (%) | Aluminum Content (%) | Adhesion |
|---|---|---|---|
| 1 | 100 | 0 | - |
| 2 | 99 | 1 | Level 4 |
| 3 | 95 | 5 | Level 3 |
| 4 | 90 | 10 | Level 1 |
| 5 | 85 | 15 | Level 3 |
| 6 | 80 | 20 | Level 5 |
| Sample | Phosphate Binder Content (%) | MoS2 Content (%) | Average Microhardness of Coating (HV) |
|---|---|---|---|
| 1 | 100 | 0 | - |
| 2 | 90 | 10 | 38.26 |
| 3 | 85 | 15 | 45.53 |
| 4 | 80 | 20 | 58.18 |
| 5 | 70 | 30 | 79.23 |
| 6 | 60 | 40 | 24.36 |
| Sample | Phosphate Binder Content (%) | Graphite Content (%) | Average Microhardness of Coating (HV) |
|---|---|---|---|
| 1 | 100 | 0 | - |
| 2 | 90 | 10 | 46.52 |
| 3 | 85 | 15 | 57.12 |
| 4 | 80 | 20 | 81.51 |
| 5 | 75 | 25 | 53.38 |
| 6 | 70 | 30 | 36.28 |
| Level | Factors | |||
|---|---|---|---|---|
| A (Binders:Fillers) | B (Graphite %) | C (MoS2%) | D (Aluminum Powder %) | |
| 1 | 7:3 | 5 | 10 | 5 |
| 2 | 6:4 | 10 | 15 | 10 |
| 3 | 5:5 | 15 | 20 | 15 |
| Number | A (Binders:Fillers) | B (Graphite %) | C (MoS2%) | D (Aluminum Powder %) | Friction Time yi (min) |
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| 1 | 1 | 1 | 1 | 1 | y1 = 30 |
| 2 | 1 | 2 | 2 | 2 | y2 = 80 |
| 3 | 1 | 3 | 3 | 3 | y3 = 40 |
| 4 | 2 | 1 | 2 | 3 | y4 = 260 |
| 5 | 2 | 2 | 3 | 1 | y5 = 180 |
| 6 | 2 | 3 | 1 | 2 | y6 = 200 |
| 7 | 3 | 1 | 3 | 2 | y7 = 140 |
| 8 | 3 | 2 | 1 | 3 | y8 = 90 |
| 9 | 3 | 3 | 2 | 1 | y9 = 110 |
| Statistical Measure | A (Binders:Fillers) | B (Graphite %) | C (MoS2%) | D (Aluminum Powder %) |
|---|---|---|---|---|
| K1 (Level 1 Sum) | 150 | 430 | 320 | 320 |
| K2 (Level 2 Sum) | 640 | 350 | 450 | 420 |
| K3 (Level 3 Sum) | 340 | 350 | 360 | 390 |
| 50 | 143 | 107 | 107 | |
| 213 | 117 | 150 | 140 | |
| 113 | 117 | 120 | 130 | |
| Rj (Range) | 163 | 26 | 43 | 33 |
| Optimal Level | A2 (6:4) | B1 (5%) | C2 (15%) | D2 (10%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Wang, S.; Zhou, Y.; Lv, H.; Yang, B. Research on High-Temperature Frictional Performance Optimization and Synergistic Effects of Phosphate-Based Composite Lubricating Coatings. Coatings 2025, 15, 704. https://doi.org/10.3390/coatings15060704
Ding Y, Wang S, Zhou Y, Lv H, Yang B. Research on High-Temperature Frictional Performance Optimization and Synergistic Effects of Phosphate-Based Composite Lubricating Coatings. Coatings. 2025; 15(6):704. https://doi.org/10.3390/coatings15060704
Chicago/Turabian StyleDing, Yong, Shengjun Wang, Youxin Zhou, Hongmei Lv, and Baoping Yang. 2025. "Research on High-Temperature Frictional Performance Optimization and Synergistic Effects of Phosphate-Based Composite Lubricating Coatings" Coatings 15, no. 6: 704. https://doi.org/10.3390/coatings15060704
APA StyleDing, Y., Wang, S., Zhou, Y., Lv, H., & Yang, B. (2025). Research on High-Temperature Frictional Performance Optimization and Synergistic Effects of Phosphate-Based Composite Lubricating Coatings. Coatings, 15(6), 704. https://doi.org/10.3390/coatings15060704






