B2 NiAl Coatings Alloyed with Rare Earth Element Y: A First-Principles Study
Abstract
1. Introduction
2. Computational Method
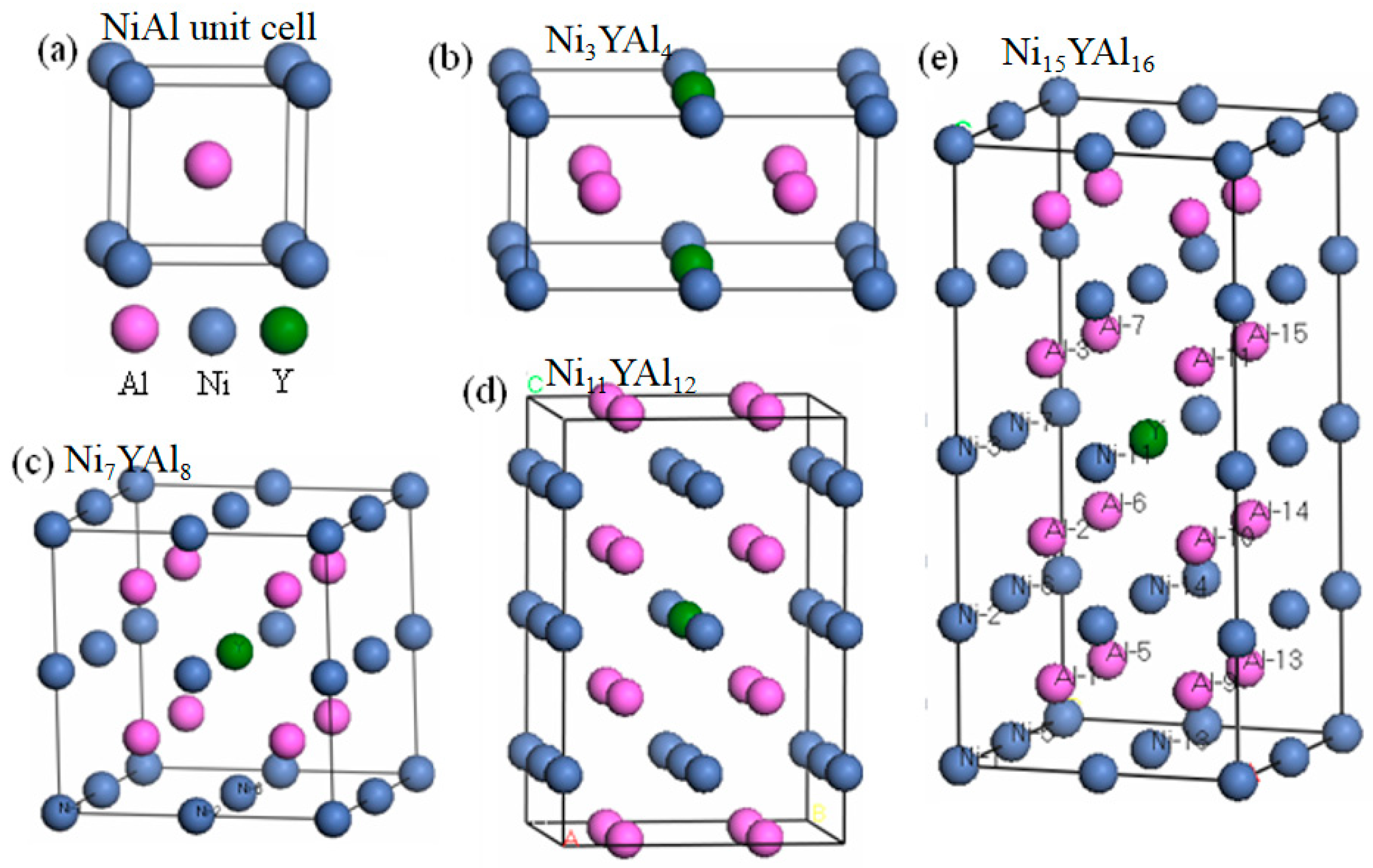
2.1. Model Structure
2.2. DFT Parameterization
2.3. Convergence Criteria
3. Results and Discussions
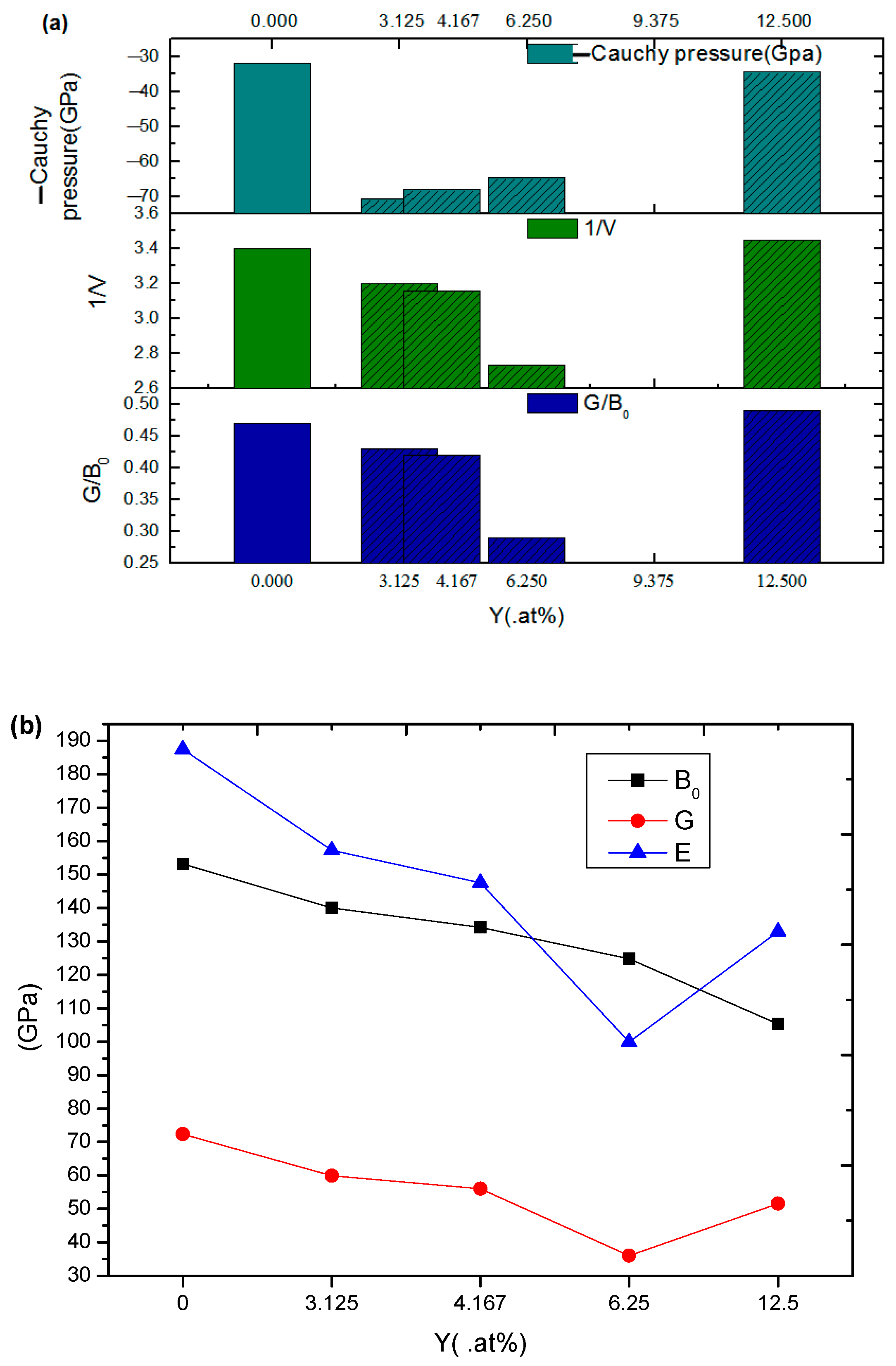
3.1. Elastic Properties
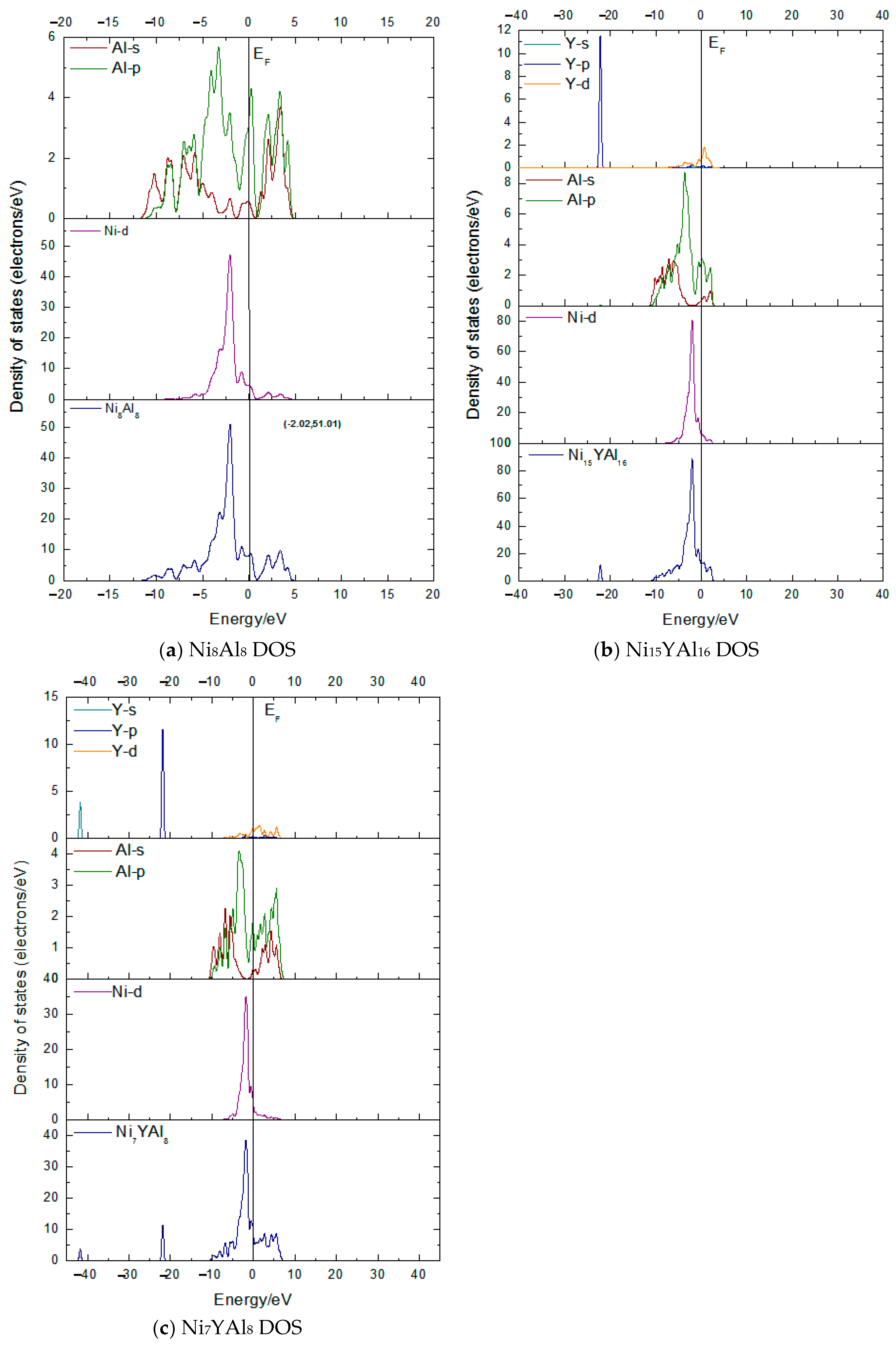
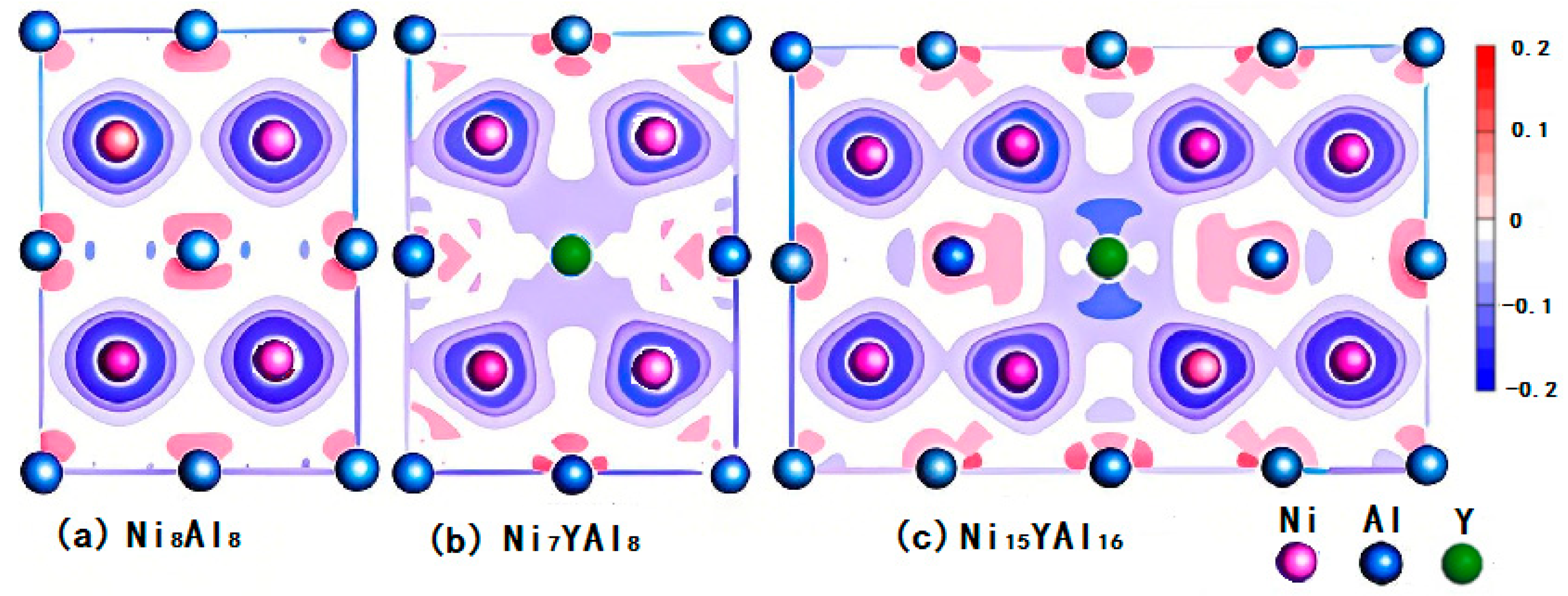
3.2. Density of States (DOSs) and Charge Density Contours
3.3. Population Analysis
3.3.1. Ni-Al Bonds
3.3.2. Ni-Ni Bonds and Al-Al Bonds
3.3.3. Y-Ni Bonds and Y-Al Bonds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| REE | Rare earth element |
| DFT | Density functional theory |
| CASTEP | Cambridge Sequential Total Energy Package |
| GGA | Generalized gradient approximation |
| Ecut | Kinetic energy cutoff |
| SCF | Self-consistent field |
References
- Liu, C.T.; Stringer, J.; Mundy, J.N.; Horton, L.L.; Angelini, P. Ordered. Intermetallic Alloys: An Assessment. Intermetallics 1997, 5, 579–596. [Google Scholar] [CrossRef]
- Sun, Y.W.; Yuan, J.H.; Wang, M.; Wang, H.Y.; Chen, Z.Y.; Liu, X.Y.; Li, S.W.; Zhao, W.W.; Yang, J.Y. Enhanced tribological performance of NiAl-based composite coatings: Design of the composition in coatings by addition of Mo and Nanodiamond. Tribol. Int. 2025, 203, 110405. [Google Scholar] [CrossRef]
- Pesterev, E.; Yakovlev, E.; Pichugin, N.; Petrov, V.; Maznoy, A. Application of vacuum plasma techniques for preparation of Ni-Cr-Dy powders proposed as masteralloys for combustion synthesis of Ni-Al intermetallics. Vacuum 2025, 234, 114054. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, J.; Ren, S.; Wang, L.; Xin, B.; Cao, S. Tribological properties of laser cladding NiAl intermetallic compound coatings at elevated temperatures. Tribol. Int. 2016, 104, 321–327. [Google Scholar] [CrossRef]
- Song, Y.; Guo, Z.X.; Yang, R.; Li, D. First principles study of site substitution of ternary elements in NiAl. Acta Mater. 2001, 49, 1647–1654. [Google Scholar] [CrossRef]
- Bozzolo, G.; Noebe, R.D.; Honecy, F. Modeling of ternary element site substitution in NiAl. Intermetallics 2000, 8, 7–18. [Google Scholar] [CrossRef]
- Serrano, M.; Molina, A.; Guardian, R.; Pozo, A.D.; Verduzco, J.A.; Sedano, A. Synthesis and microstructure characterization of nanocrystalline NiAl compound with copper addition by mechanical alloying. MRS Adv. 2023, 8, 1182–1187. [Google Scholar] [CrossRef]
- Zhao, C.S.; Zhou, Y.H.; Zou, Z.H.; Luo, L.R.; Zhao, X.F.; Guo, F.W.; Xiao, P. Effect of alloyed Lu, Hf and Cr on the oxidation and spallation behavior of NiAl. Corros. Sci. 2017, 126, 334–343. [Google Scholar] [CrossRef]
- Barth, T.L.; Marquis, E.A. Effects of Minor Alloying Elements on Alumina Transformation During the Transient Oxidation of β-NiAl. Oxid. Met. 2021, 95, 293–309. [Google Scholar] [CrossRef]
- Jiang, C. Site preference of transition-metal elements in B2 NiAl: A comprehensive study. Acta Mater. 2007, 55, 4799–4806. [Google Scholar] [CrossRef]
- Frommeyer, G.; Fischer, R.; Deges, J.; Rablbauer, R.; Schneider, A. APFIM investigations on site occupancies of the ternary alloying elements Cr, Fe and Re in NiAl. Ultramicroscopy 2004, 101, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.T.; Huai, K.W.; Gao, Q.; Ren, W.L.; Li, G.S. Effects of rare earth elements on the microstructure and mechanical properties of NiAl-based eutectic alloy. Intermetallics 2007, 15, 727–733. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, C.C.; Chen, H.; He, J. Pinning effect of reactive elements on structural stability and adhesive strength of environmental sulfur segregation on Al2O3/NiAl interface. Scr. Mater. 2020, 188, 174–178. [Google Scholar] [CrossRef]
- Niu, X.F.; Huang, W.; Wang, B.J.; Wang, C.C.; Song, Z.L. Effects of Point Defects on Properties of B2 NiAl: A First-principles Study. Rare Met. Mater. Eng. 2018, 47, 2687–2692. [Google Scholar]
- Wang, X.L.; Cai, C.Y.; Zhou, G.W. First-principles study on the NiAl/Al2O3 interfacial segregation of Hf during the oxidation of Hf-modified NiAl. Appl. Surf. Sci. 2022, 578, 151917. [Google Scholar] [CrossRef]
- He, J.Q.; Wang, Y.; Yan, M.F.; Yang, Y.; Wang, L. First-principles study of NiAl microalloyed with Sc, Y, La and Nd. Comp. Mater. Sci. 2010, 50, 545–549. [Google Scholar] [CrossRef]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 1990, 41, 7892. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar] [CrossRef]
- Zhang, C.L.; Han, P.D.; Li, J.M.; Chi, M.; Yan, L.Y.; Liu, Y.P.; Liu, X.G.; Xu, B.S. First-principles study of the mechanic properties of NiAl microalloyed by M (Y, Zr, Nb, Mo, Tc, Ru, Rh, Pd, Ag, Cd). J. Phys. D Appl. Phys. 2008, 41, 1–5. [Google Scholar]
- Hammer, B.; Hansen, L.B.; Nrskov, J.K. Improved adsorption energetics within density-functional theory using revised perdew-burke-ernzerhof functionals. Phys. Rev. B 1999, 59, 7413. [Google Scholar] [CrossRef]
- Nye, J.F. Physical Properties of Crystals; Oxford University Press: Oxford, UK, 1985. [Google Scholar]
- Karki, B.B.; Ackland, G.J.; Crain, J.; Phys, J. Elastic instabilities in crystals from ab initio stress—Strain relations. J. Phys. Condens. Matter 1997, 9, 8579–8589. [Google Scholar] [CrossRef]
- Rusovi, N.; Warlimont, H. The elastic behaviour of β2-NiAl alloys. Phys. Status Solidi (a) 1977, 44, 609–619. [Google Scholar] [CrossRef]
- Beckstein, O.; Klepeis, J.E.; Hart, G.L.W.; Pankratov, O. First principles elastic constants and electronic structure of α-Pt2Si and PtSi. Phys. Rev. B 2001, 63, 1–12. [Google Scholar] [CrossRef]
- Pettifor, D.G. Theoretical predictions of structure and related properties of intermetallics. Mater. Sci. Technol. 1992, 8, 345–349. [Google Scholar] [CrossRef]
- Born, M.; Huang, K. Dynamical Theory of Crystal Lattices; Oxford University Press: Oxford, UK, 1954. [Google Scholar]
- Johnson, R.A. Analytic nearest-neighbor model for fcc metals. Phys. Rev. B 1988, 37, 3924–3931. [Google Scholar] [CrossRef]
- Pugh, S.F. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Philos. Mag. 1954, 45, 823–843. [Google Scholar] [CrossRef]
- Frantsevich, I.N.; Voronov, F.F.; Bokuta, S.A. Elastic Constants and Elastic Moduli of Metals and Insulators; Naukova Dumka: Kiev, Ukraine, 1983; pp. 60–180. [Google Scholar]
| Y(.at%) | C11(GPa) | C33(GPa) | C44(GPa) | C66(GPa) | C12(GPa) | C13(GPa) |
|---|---|---|---|---|---|---|
| 0 | 173.48 | 173.48 | 117.41 | 117.41 | 149.37 | 149.37 |
| 3.125 | 138.01 | 174.31 | 95.47 | 93.44 | 164.24 | 120.27 |
| 4.167 | 117.62 | 165.22 | 92.40 | 96.48 | 160.48 | 121.64 |
| 6.25 | 77.93 | 77.93 | 83.54 | 83.54 | 148.30 | 148.30 |
| 12.5 | 126.00 | 138.52 | 77.61 | 65.17 | 99.64 | 89.34 |
| Bond (Ni15YAl16) | Population | Length (Å) | p (/Å) |
|---|---|---|---|
| Ni1–Al1 | 0.16 | 2.53 | 0.063 |
| Ni2–Al1 | 0.15 | 2.48 | 0.060 |
| Ni5–Al1 | 0.16 | 2.52 | 0.063 |
| Ni14–Al1 | 0.16 | 2.59 | 0.062 |
| Ni6–Al1 | 0.17 | 2.52 | 0.067 |
| Ni2–Al2 | 0.18 | 2.46 | 0.073 |
| Ni6–Al2 | 0.19 | 2.52 | 0.075 |
| Ni7–Al2 | 0.19 | 2.71 | 0.070 |
| Ni14–Al2 | 0.22 | 2.55 | 0.086 |
| Ni3–Al2 | 0.16 | 2.60 | 0.06 |
| Bond (Ni7YAl8) | Population | Length (Å) | p (/Å) |
|---|---|---|---|
| Ni1–Al | 0.18 | 2.43 | 0.074 |
| Ni2–Al | 0.18 | 2.55 | 0.071 |
| Ni6–Al | 0.21 | 2.67 | 0.079 |
| X-Direction | Y-Direction | Z-Direction | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bond (Ni15YAl16) | Population | Length (Å) | p (/Å) | Bond (Ni15YAl16) | Population | Length (Å) | p (/Å) | Bond (Ni15YAl16) | Population | Length (Å) | p (/Å) |
| Ni5-Ni13 | −0.01 | 2.90 | −0.003 | Ni1-Ni5 | −0.01 | 2.90 | −0.003 | Ni1-Ni2 | −0.04 | 2.82 | −0.014 |
| Ni6-Ni14 | −0.02 | 2.91 | −0.007 | Ni2-Ni6 | 0.04 | 2.90 | 0.014 | Ni5-Ni6 | 0 | 2.92 | 0 |
| - | - | - | - | Ni3-Ni7 | 0.18 | 2.90 | 0.062 | - | - | - | - |
| Al1-Al9 | 0.23 | 2.88 | 0.080 | Al1-Al5 | 0.23 | 2.88 | 0.080 | Al1-Al2 | 0.09 | 2.91 | 0.031 |
| Al2-Al10 | 0.39 | 2.71 | 0.144 | Al2-Al6 | 0.39 | 2.71 | 0.144 | Al5-Al6 | 0.09 | 2.91 | 0.031 |
| Al5-Al13 | 0.23 | 2.88 | 0.080 | Al9-Al13 | 0.23 | 2.88 | 0.080 | Al9-Al10 | 0.09 | 2.91 | 0.031 |
| Al6-Al14 | 0.39 | 2.71 | 0.144 | Al10-Al14 | 0.39 | 2.71 | 0.144 | Al13-Al14 | 0.09 | 2.91 | 0.031 |
| X-Direction | Y-Direction | Z-Direction | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bond (Ni16Al16) | Population | Length (Å) | p (/Å) | Bond (Ni16Al16) | Population | Length (Å) | p (/Å) | Bond (Ni16Al16) | Population | Length (Å) | p (/Å) |
| Ni-Ni | 0 | 2.90 | 0 | Ni-Ni | 0 | 2.90 | 0 | Ni-Ni | −0.02 | 2.90 | −0.007 |
| Al-Al | 0.21 | 2.90 | 0.072 | Al-Al | 0.21 | 2.90 | 0.072 | Al-Al | 0.11 | 2.90 | 0.038 |
| X-direction | Y-Direction | Z-Direction | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bond (Ni7YAl)8 | Population | Length (Å) | p /Å) | Bond (Ni7YAl8) | Population | Length (Å) | p (/Å) | Bond (Ni7YAl8) | Population | Length (Å) | p (/Å) |
| Al-Al | 0.28 | 2.81 | 0.100 | Al-Al | 0.28 | 2.81 | 0.100 | Al-Al | 0.28 | 2.81 | 0.100 |
| X-direction | Y-direction | Z-direction | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bond (Ni8Al8) | Population | Length (Å) | p (/Å) | Bond (Ni8Al8) | Population | Length (Å) | p (/Å) | Bond (Ni8Al8) | Population | Length (Å) | p (/Å) |
| Ni-Ni | −0.03 | 2.90 | −0.010 | Ni-Ni | −0.03 | 2.90 | −0.010 | Ni-Ni | −0.03 | 2.90 | −0.010 |
| Al-Al | 0.22 | 2.90 | 0.076 | Al-Al | 0.22 | 2.90 | 0.076 | Al-Al | 0.22 | 2.90 | 0.076 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Yu, L.; Zhang, J. B2 NiAl Coatings Alloyed with Rare Earth Element Y: A First-Principles Study. Coatings 2025, 15, 671. https://doi.org/10.3390/coatings15060671
He J, Yu L, Zhang J. B2 NiAl Coatings Alloyed with Rare Earth Element Y: A First-Principles Study. Coatings. 2025; 15(6):671. https://doi.org/10.3390/coatings15060671
Chicago/Turabian StyleHe, Junqi, Ligang Yu, and Jinfeng Zhang. 2025. "B2 NiAl Coatings Alloyed with Rare Earth Element Y: A First-Principles Study" Coatings 15, no. 6: 671. https://doi.org/10.3390/coatings15060671
APA StyleHe, J., Yu, L., & Zhang, J. (2025). B2 NiAl Coatings Alloyed with Rare Earth Element Y: A First-Principles Study. Coatings, 15(6), 671. https://doi.org/10.3390/coatings15060671






