Initial Stages of Al-AM60-Modified Surface of Magnesium Alloy Activity Exposed to Simulated Marine Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Model Solution
2.2. Coating Deposition
2.3. Surface Characterization and Immersion Test
2.4. Electrochemical Characterization
3. Results
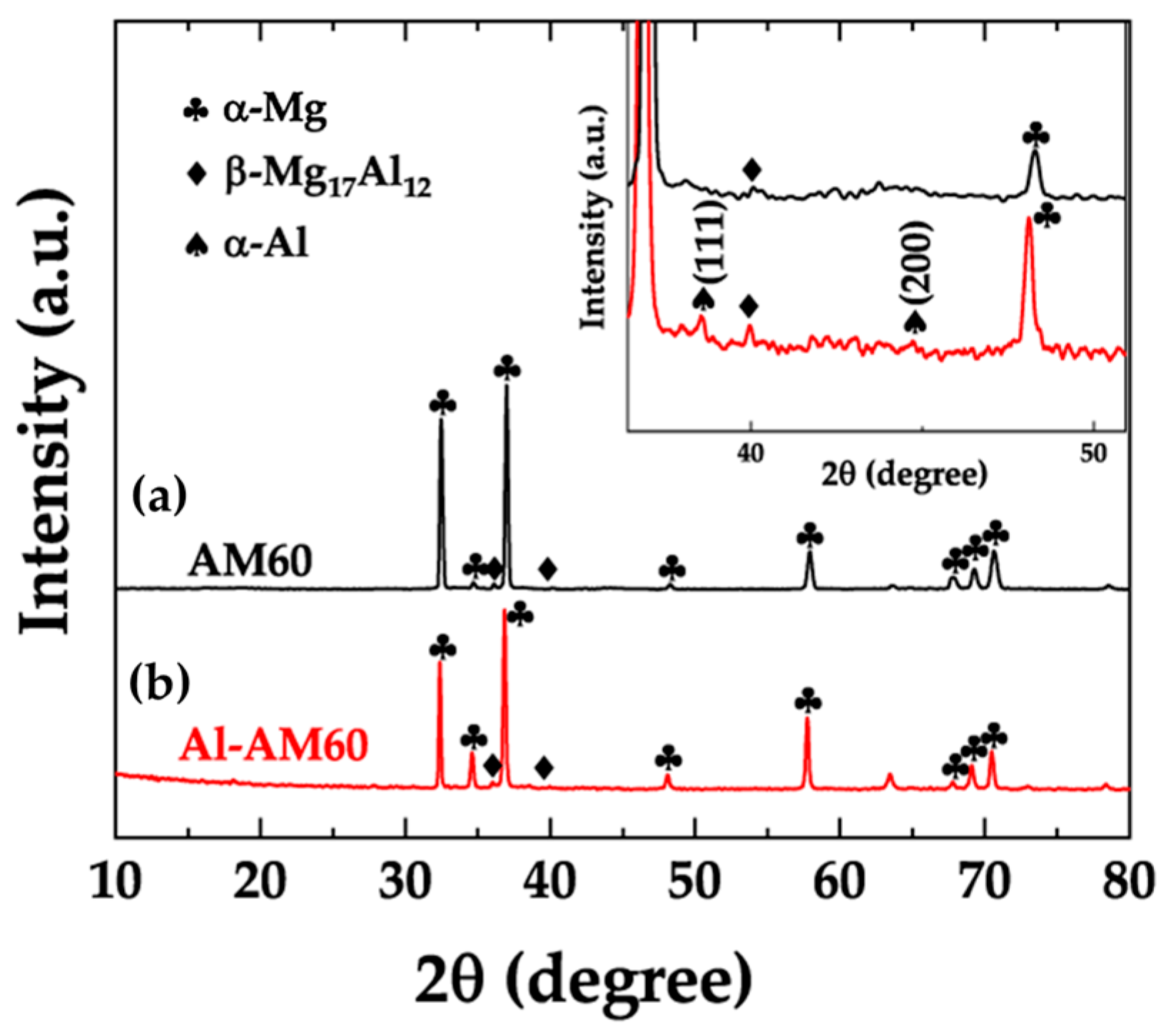
3.1. X-Ray Diffraction Analysis
3.2. SEM-EDS Characterization
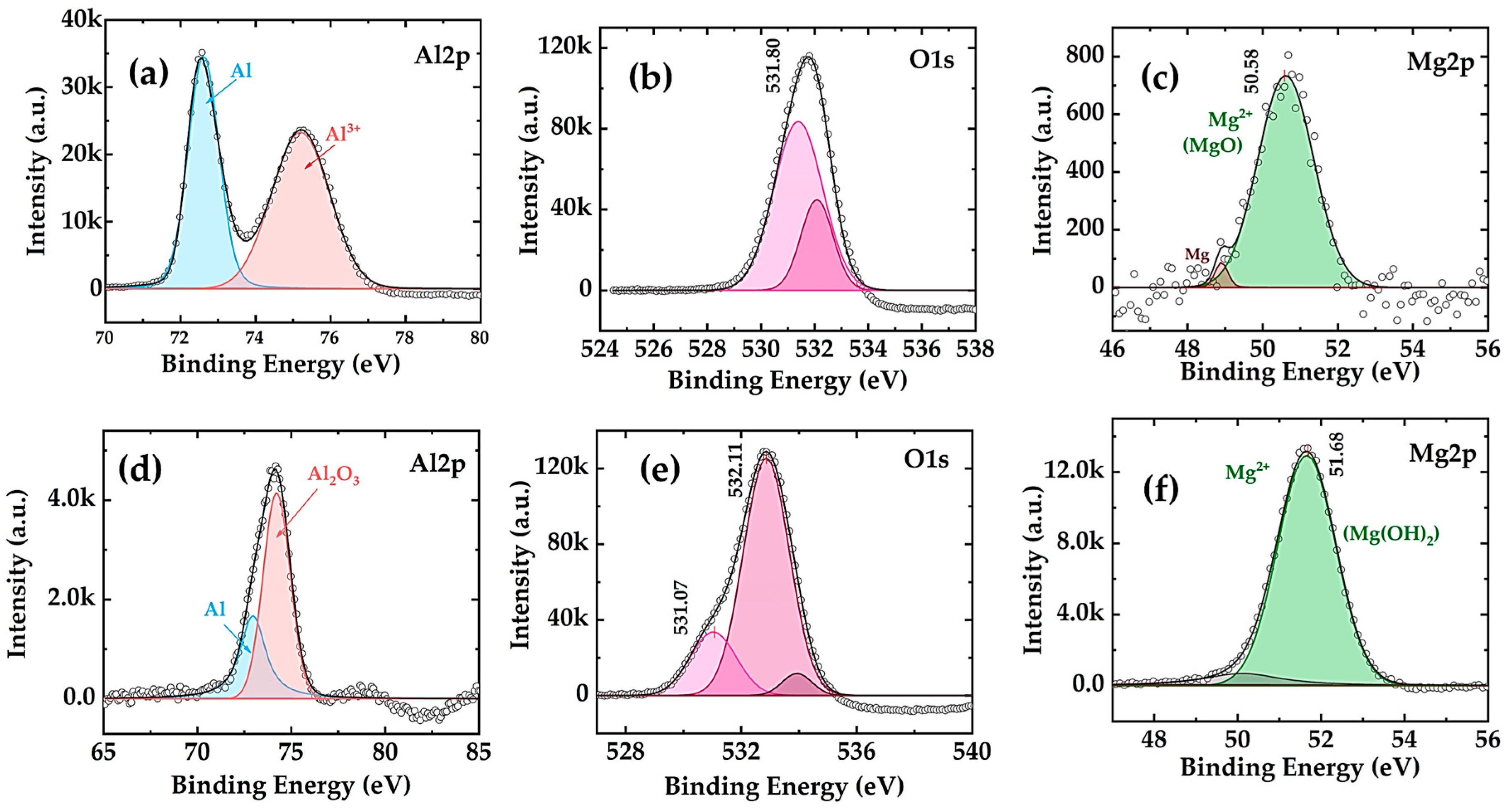
3.3. XPS Analysis of Al-AM60 Modified Alloy Surface
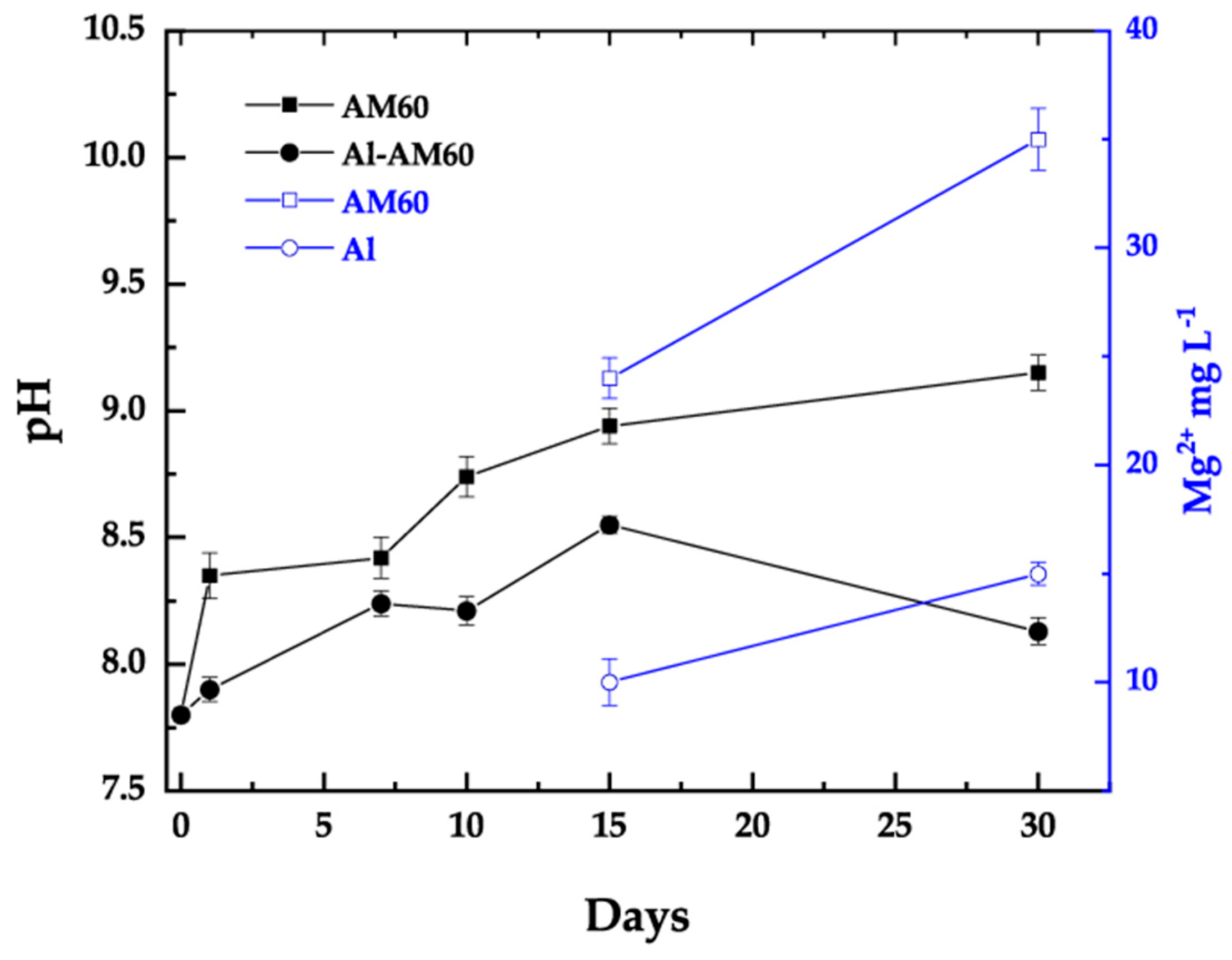
3.4. Immersion Test in Model Saline Solution (SME)
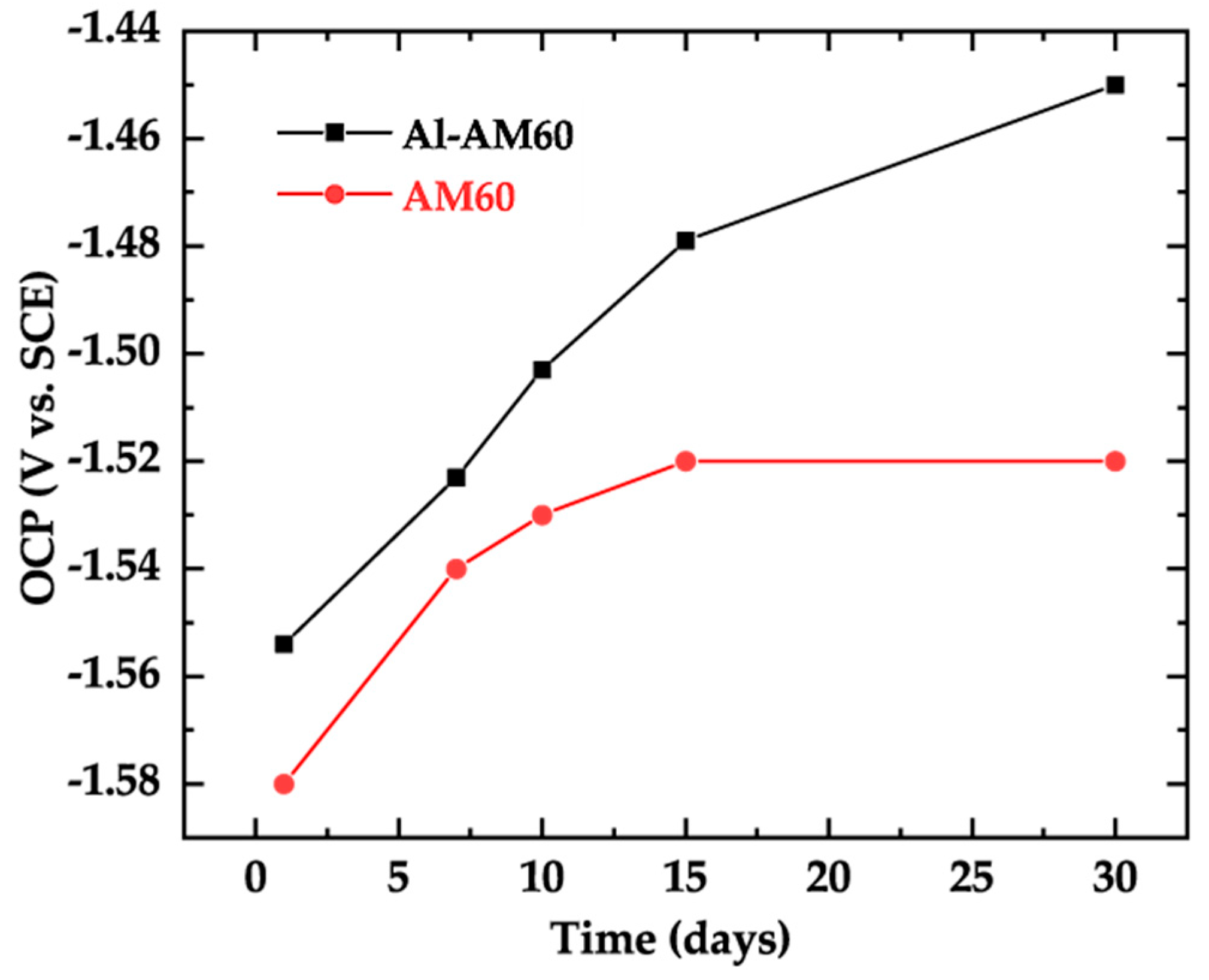
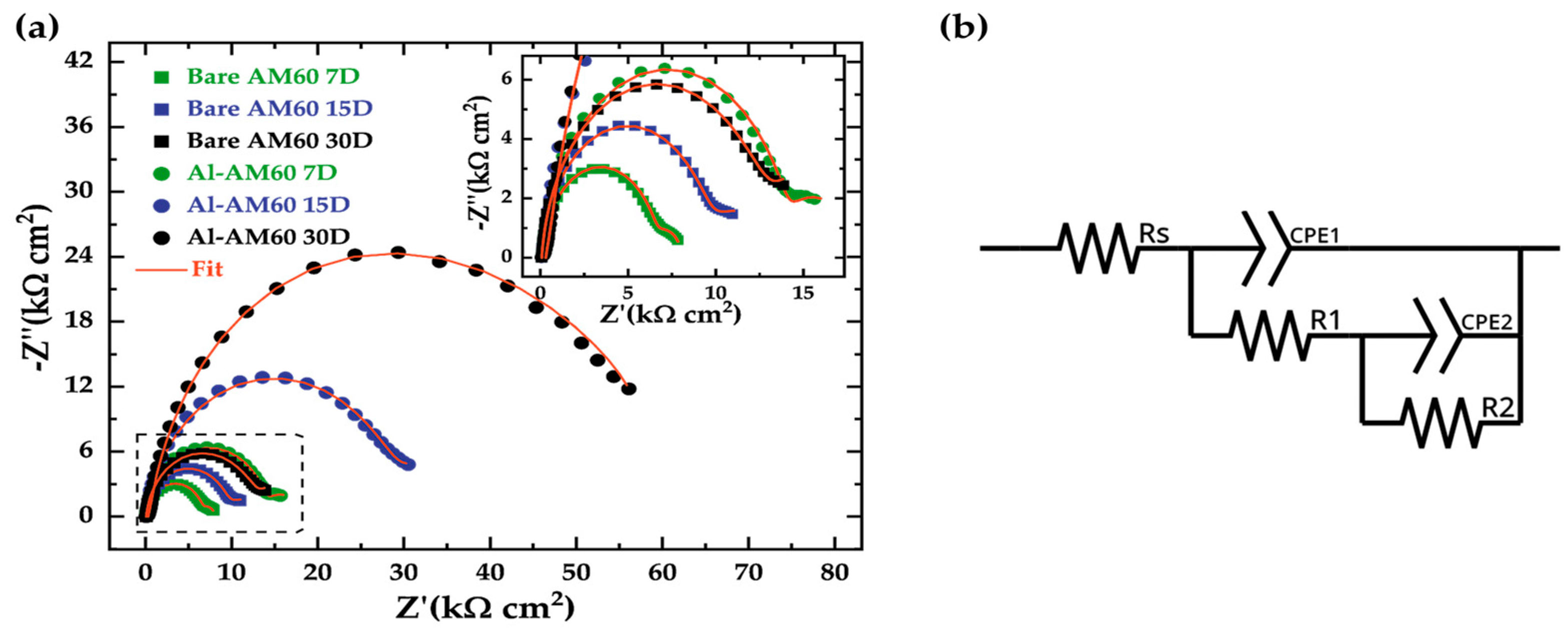
3.5. Electrochemical Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kojima, Y. Project of Platform Science and Technology for Advanced Magnesium Alloys. Mater. Trans. 2001, 42, 1154–1159. [Google Scholar] [CrossRef]
- Pollock, T.M. Weight Loss with Magnesium Alloys. Science 2010, 328, 986–987. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, S.K.; Kumar, K.; Kumar, N.; Yuan, W.; Mishra, R.S.; DeLorme, R.; Davis, B.; Howell, R.A.; Cho, K. Transition of Deformation Behavior in an Ultrafine Grained Magnesium Alloy. Mater. Sci. Eng. A 2012, 549, 123–127. [Google Scholar] [CrossRef]
- Luo, A.A. Magnesium Casting Technology for Structural Applications. J. Magnes. Alloys 2013, 1, 2–22. [Google Scholar] [CrossRef]
- You, S.; Huang, Y.; Kainer, K.U.; Hort, N. Recent Research and Developments on Wrought Magnesium Alloys. J. Magnes. Alloys 2017, 5, 239–253. [Google Scholar] [CrossRef]
- Cole, G.S. Issues That Influence Magnesium’s Use in the Automotive Industry. Mater. Sci. Forum 2003, 419–422, 43–50. [Google Scholar] [CrossRef]
- Polmear, I.J. Magnesium Alloys and Applications. Mater. Sci. Technol. 1994, 10, 1–16. [Google Scholar] [CrossRef]
- Bai, J.; Yang, Y.; Wen, C.; Chen, J.; Zhou, G.; Jiang, B.; Peng, X.; Pan, F. Applications of Magnesium Alloys for Aerospace: A Review. J. Magnes. Alloys 2023, 11, 3609–3619. [Google Scholar] [CrossRef]
- Aghion, E.; Bronfin, B. Magnesium Alloys Development towards the 21st Century. Mater. Sci. Forum 2000, 350–351, 19–30. [Google Scholar] [CrossRef]
- Cole, G.S.; Sherman, A.M. Lightweight Materials for Automotive Application. Mater. Charact. 1995, 35, 3–9. [Google Scholar] [CrossRef]
- Kulekci, M.K. Magnesium and Its Alloys Applications in Automotive Industry. Int. J. Adv. Manuf. Technol. 2008, 39, 851–865. [Google Scholar] [CrossRef]
- Armao, F.G. Design & Fabrication of Aluminum Automobiles. Weld. Innov. 2002, 19, 2–6. [Google Scholar]
- Powell, B.R.; Luo, A.A.; Krajewski, P.E. Magnesium Alloys for Lightweight Powertrains and Automotive Bodies. In Advanced Materials in Automotive Engineering; Rowe, J., Ed.; Woodhead Publishing: Sawston, UK, 2012; pp. 150–209. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, J. Advanced Lightweight Materials for Automobiles: A Review. Mater. Des. 2022, 221, 110994. [Google Scholar] [CrossRef]
- Polmear, I.; Stjohn, D.; Nie, J.-F.; Qian, M. Magnesium Alloys. In Light Alloys: Metallurgy of the Light Metals; Garcia, A.C., Ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 287–367. ISBN 978-0-08-099431-4. [Google Scholar]
- Kainer, K.U. The Current State of Technology and Potential for Further Development of Magnesium Applications. In Magnesium-Alloys and Technology; Kainer, K.U., Ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003; pp. 1–22. ISBN 352730570X. [Google Scholar]
- Makar, G.L.; Kruger, J. Corrosion of Magnesium. Int. Mater. Rev. 1993, 38, 138–153. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A. Corrosion Mechanisms of Magnesium Alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- LeBozec, N.; Jönsson, M.; Thierry, D. Atmospheric Corrosion of Magnesium Alloys: Influence of Temperature, Relative Humidity, and Chloride Deposition. Corrosion 2004, 60, 356–361. [Google Scholar] [CrossRef]
- Song, G.L.; St John, D.H.; Abbott, T. Corrosion Behaviour of a Pressure Die Cast Magnesium Alloy. Int. J. Cast Met. Res. 2005, 18, 174–180. [Google Scholar] [CrossRef]
- Jönsson, M. Atmospheric Corrosion of Magnesium Alloys: Influence of Microstructure and Environment. Ph.D. Thesis, School of Chemical Science and Engineering, KTH, Stockholm, Sweden, 2007. [Google Scholar]
- Kainer, K.U.; Srinivasan, P.B.; Blawert, C.; Dietzel, W. Corrosion of Magnesium and Its Alloys. In Shreir’s Corrosion—Corrosion and Degradation of Engineering Materials; Cottis, B., Graham, M., Lindsay, R., Lyon, S., Richardson, T., Scantlebury, D., Stott, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 3, pp. 2011–2041. [Google Scholar] [CrossRef]
- Liu, F.; Song, Y.; Shan, D.; Han, E.-H. Corrosion Mechanism of AM50 Magnesium Alloy in Simulated Acid Rain. Recent Pat. Corros. Sci. 2013, 3, 47–57. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and Advances in Magnesium Alloy Corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Danaie, M.; Asmussen, R.M.; Jakupi, P.; Shoesmith, D.W.; Botton, G.A. The Cathodic Behaviour of Al–Mn Precipitates during Atmospheric and Saline Aqueous Corrosion of a Sand-Cast AM50 Alloy. Corros. Sci. 2014, 83, 299–309. [Google Scholar] [CrossRef]
- Blawert, C.; Dietzel, W.; Ghali, E.; Song, G. Anodizing Treatments for Magnesium Alloys and Their Effect on Corrosion Resistance in Various Environments. Adv. Eng. Mater. 2006, 8, 511–533. [Google Scholar] [CrossRef]
- Ortiz, C.H.; Aperador, W.; Caicedo, J.C. Electrochemical Study of Anodized AZ31 Magnesium Alloy (Mg/MgO) Immersed under Watered Cementice Paste. J. Mater. Eng. Perform. 2022, 31, 8896–8905. [Google Scholar] [CrossRef]
- Rahimian, M.; Parvin, N.; Ehsani, N. The Effect of Production Parameters on Microstructure and Wear Resistance of Powder Metallurgy Al–Al2O3 Composite. Mater. Des. 2011, 32, 1031–1038. [Google Scholar] [CrossRef]
- Li, Z.; Jing, X.; Yuan, Y.; Zhang, M. Composite Coatings on a Mg–Li Alloy Prepared by Combined Plasma Electrolytic Oxidation and Sol–Gel Techniques. Corros. Sci. 2012, 63, 358–366. [Google Scholar] [CrossRef]
- Salami, B.; Afshar, A.; Mazaheri, A. The Effect of Sodium Silicate Concentration on Microstructure and Corrosion Properties of MAO-Coated Magnesium Alloy AZ31 in Simulated Body Fluid. J. Magnes. Alloys 2014, 2, 72–77. [Google Scholar] [CrossRef]
- Surmeneva, M.A.; Surmenev, R.A. Microstructure Characterization and Corrosion Behaviour of a Nano-Hydroxyapatite Coating Deposited on AZ31 Magnesium Alloy Using Radio Frequency Magnetron Sputtering. Vacuum 2015, 117, 60–62. [Google Scholar] [CrossRef]
- Wu, H.; Qasim, A.M.; Xiao, S.; Huang, Q.; Zhang, F.; Wu, Z.; Fu, R.K.Y.; Wu, G.; Chu, P.K. Magnetron-Sputtered Fluorocarbon Polymeric Film on Magnesium for Corrosion Protection. Surf. Coat. Technol. 2018, 352, 437–444. [Google Scholar] [CrossRef]
- Zhang, Z.; Kitada, A.; Fukami, K.; Murase, K. Aluminum Electroplating on AZ31 Magnesium Alloy with Acetic Anhydride Pretreatment. Acta Metall. Sin. 2022, 35, 1996–2006. [Google Scholar] [CrossRef]
- Zhang, M.; Yu, X.; Sheng, M.; Chen, H.; Chen, B. Preparation of a Mussel-Inspired Supramolecular Polymer Coating Containing Graphene Oxide on Magnesium Alloys with Anti-Corrosion and Self-Healing Properties. Int. J. Mol. Sci. 2023, 24, 4981. [Google Scholar] [CrossRef]
- Peng, F.; Li, H.; Wang, D.; Tian, P.; Tian, Y.; Yuan, G.; Xu, D.; Liu, X. Enhanced Corrosion Resistance and Biocompatibility of Magnesium Alloy by Mg-Al-Layered Double Hydroxide. ACS Appl. Mater. Interfaces 2016, 8, 35033–35044. [Google Scholar] [CrossRef]
- Kumar, V.C.; Rajyalakshmi, G.; Kartha, J. Insights on Anti-Corrosion Coating of Magnesium Alloy: A Review. J. Bio Tribo Corros. 2023, 9, 1–21. [Google Scholar] [CrossRef]
- Tan, J.; Liu, L.; Wang, H.; Luo, J. Advances in Anti-Corrosion Coatings on Magnesium Alloys and Their Preparation Methods. J. Coat. Technol. Res. 2024, 21, 811–825. [Google Scholar] [CrossRef]
- Hoche, H.; Groß, S.; Oechsner, M. Development of New PVD Coatings for Magnesium Alloys with Improved Corrosion Properties. Surf. Coat. Technol. 2014, 259, 102–108. [Google Scholar] [CrossRef]
- Zhang, D.; Wei, B.; Wu, Z.; Qi, Z.; Wang, Z. A Comparative Study on the Corrosion Behaviour of Al, Ti, Zr and Hf Metallic Coatings Deposited on AZ91D Magnesium Alloys. Surf. Coat. Technol. 2016, 303, 94–102. [Google Scholar] [CrossRef]
- Carboneras, M.; López, M.D.; Rodrigo, P.; Campo, M.; Torres, B.; Otero, E.; Rams, J. Corrosion Behaviour of Thermally Sprayed Al and Al/SiCp Composite Coatings on ZE41 Magnesium Alloy in Chloride Medium. Corros. Sci. 2010, 52, 761–768. [Google Scholar] [CrossRef]
- Tao, Y.; Xiong, T.; Sun, C.; Kong, L.; Cui, X.; Li, T.; Song, G.L. Microstructure and Corrosion Performance of a Cold Sprayed Aluminium Coating on AZ91D Magnesium Alloy. Corros. Sci. 2010, 52, 3191–3197. [Google Scholar] [CrossRef]
- Yang, H.; Guo, X.; Wu, G.; Ding, W.; Birbilis, N. Electrodeposition of Chemically and Mechanically Protective Al-Coatings on AZ91D Mg Alloy. Corros. Sci. 2011, 53, 381–387. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, D.; Yan, B.; Kong, D. Effects of Laser Remelting on Microstructures and Immersion Corrosion Performance of Arc Sprayed Al Coating in 3.5% NaCl Solution. Opt. Laser Technol. 2018, 99, 282–290. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Ma, D.; Jiang, X.; Xie, D.; Leng, Y. Influence of Interlayers on Adhesion Strength of TiN Film on Mg Alloy. Coatings 2024, 14, 121. [Google Scholar] [CrossRef]
- Chávez, L.; Veleva, L.; Sánchez, G.; Dieringa, H. AM60-AlN Nanocomposite and AM60 Alloy Corrosion Activity in Simulated Marine-Coastal Ambience. Metals 2022, 12, 1997. [Google Scholar] [CrossRef]
- Chávez, L.; Veleva, L.; Sánchez-Ahumada, D.; Ramírez-Bon, R. Hybrid Coating of Polystyrene–ZrO2 for Corrosion Protection of AM Magnesium Alloys. Coatings 2023, 13, 1059. [Google Scholar] [CrossRef]
- Sánchez, G.; Veleva, L.; Flores, E. Surface Modification of AM60 Mg-Al Alloy with Vanadium and V2O5 Sputtered Deposits: Activity in Marine Ambience. Coatings 2024, 14, 955. [Google Scholar] [CrossRef]
- Chávez, L.; Veleva, L.; Castillo-Atoche, A. Electroless ZnO Deposition on Mg-Al Alloy for Improved Corrosion Resistance to Marine Environments. Coatings 2024, 14, 1192. [Google Scholar] [CrossRef]
- ASTM G31-21; Standard Guide for Laboratory Immersion Corrosion Testing of Metals. ASTM International: West Con-shohocken, PA, USA, 2021.
- Bedolla, E.; Lemus-Ruiz, J.; Contreras, A. Synthesis and Characterization of Mg-AZ91/AlN Composites. Mater. Des. 2012, 38, 91–98. [Google Scholar] [CrossRef]
- Esmaily, M.; Shahabi-Navid, M.; Mortazavi, N.; Svensson, J.E.; Halvarsson, M.; Wessén, M.; Jarfors, A.E.W.; Johansson, L.G. Microstructural Characterization of the Mg–Al Alloy AM50 Produced by a Newly Developed Rheo-Casting Process. Mater. Charact. 2014, 95, 50–64. [Google Scholar] [CrossRef]
- Heczel, A.; Akbaripanah, F.; Salevati, M.A.; Mahmudi, R.; Vida; Gubicza, J. A Comparative Study on the Microstructural Evolution in AM60 Alloy Processed by ECAP and MDF. J. Alloys Compd. 2018, 763, 629–637. [Google Scholar] [CrossRef]
- Bu, H.; Yandouzi, M.; Lu, C.; Macdonald, D.; Jodoin, B. Cold Spray Blended Al+Mg17Al12 Coating for Corrosion Protection of AZ91D Magnesium Alloy. Suf. Coat. Technol. 2012, 207, 155–162. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, J.; Claypool, J.; O’Keefe, M.J. Structure and Corrosion Behavior of Sputter Deposited Ce-Al-O Coating on Al 2024-T3 Alloy Substrate. J. Electrochem. Soc. 2016, 163, 198. [Google Scholar] [CrossRef]
- Mraied, H.; Cai, W. The Effects of Mn Concentration on the Tribocorrosion Resistance of Al–Mn Alloys. Wear 2017, 380–381, 191–202. [Google Scholar] [CrossRef]
- Mraied, H.; Cai, W.; Sagüés, A.A. Corrosion Resistance of Al and Al–Mn Thin Films. Thin Solid Films 2016, 615, 391–401. [Google Scholar] [CrossRef]
- Reffass, M.; Berziou, C.; Rébéré, C.; Billard, A.; Creus, J. Corrosion Behaviour of Magnetron-Sputtered Al1−x–Mnx Coatings in Neutral Saline Solution. Corros. Sci. 2010, 52, 3615–3623. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, J.; Poplawsky, J.; Michel, F.M.; Deng, C.; Cai, W. The Origin of Passivity in Aluminum-Manganese Solid Solutions. Corros. Sci. 2020, 173, 108749. [Google Scholar] [CrossRef]
- Moon, S.M.; Pyun, S.I. The Corrosion of Pure Aluminium during Cathodic Polarization in Aqueous Solutions. Corros. Sci. 1997, 39, 399–408. [Google Scholar] [CrossRef]
- Van de Ven, E.P.G.T.; Koelmans, H. The Cathodic Corrosion of Aluminum. J. Elchem. Soc. 1976, 123, 143–145. [Google Scholar] [CrossRef]
- Asmussen, R.M.; Binns, W.J.; Partovi-Nia, R.; Jakupi, P.; Shoesmith, D.W. The Stability of Aluminum-Manganese Intermetallic Phases under the Microgalvanic Coupling Conditions Anticipated in Magnesium Alloys. Mater. Corros. 2016, 67, 39–50. [Google Scholar] [CrossRef]
- Wang, L.; Shinohara, T.; Zhang, B.P. XPS Study of the Surface Chemistry on AZ31 and AZ91 Magnesium Alloys in Dilute NaCl Solution. Appl. Surf. Sci. 2010, 256, 5807–5812. [Google Scholar] [CrossRef]
- Zähr, J.; Oswald, S.; Türpe, M.; Ullrich, H.J.; Füssel, U. Characterisation of Oxide and Hydroxide Layers on Technical Aluminum Materials Using XPS. Vacuum 2012, 86, 1216–1219. [Google Scholar] [CrossRef]
- Zhou, W.; Xue, F.; Li, M. Corrosion Behavior of Al-Mg Alloys with Different Alloying Element Contents in 3.5% NaCl Solution. Metals 2025, 15, 327. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Duong, L.V.; Wood, B.J.; Frost, R.L. XPS Study of the Major Minerals in Bauxite: Gibbsite, Bayerite and (Pseudo-) Boehmite. J. Colloid Interface Sci. 2006, 296, 572–576. [Google Scholar] [CrossRef]
- Esfandiari, N.; Aliofkhazraei, M.; Colli, A.N.; Walsh, F.C.; Cherevko, S.; Kibler, L.A.; Elnagar, M.M.; Lund, P.D.; Zhang, D.; Omanovic, S.; et al. Metal-Based Cathodes for Hydrogen Production by Alkaline Water Electrolysis: Review of Materials, Degradation Mechanism, and Durability Tests. Prog. Mater. Sci. 2024, 144, 101254. [Google Scholar] [CrossRef]
- Nordlien, J.H.; NişancioǦu, K.; Ono, S.; Masuko, N. Morphology and Structure of Oxide Films Formed on MgAl Alloys by Exposure to Air and Water. J. Electrochem. Soc. 1996, 143, 2564–2572. [Google Scholar] [CrossRef]
- Delgado, M.C.; García-Galvan, F.R.; Barranco, V.; Feliu, S. A Measuring Approach to Asses the Corrosion Rate of Magnesium Alloys Using Electrochemical Impedance Spectroscopy. In Magnesium Alloys; Aliofkhazraei, M., Ed.; IntechOpen: Rijeka, Croatia, 2017; pp. 129–160. [Google Scholar] [CrossRef]
- Shkirskiy, V.; King, A.D.; Gharbi, O.; Volovitch, P.; Scully, J.R.; Ogle, K.; Birbilis, N. Revisiting the Electrochemical Impedance Spectroscopy of Magnesium with Online Inductively Coupled Plasma Atomic Emission Spectroscopy. ChemPhysChem 2015, 16, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Curioni, M.; Scenini, F.; Monetta, T.; Bellucci, F. Correlation between Electrochemical Impedance Measurements and Corrosion Rate of Magnesium Investigated by Real-Time Hydrogen Measurement and Optical Imaging. Electrochim. Acta 2015, 166, 372–384. [Google Scholar] [CrossRef]
- Brooks, E.K.; Der, S.; Ehrensberger, M.T. Corrosion and Mechanical Performance of AZ91 Exposed to Simulated Inflammatory Conditions. Mater. Sci. Eng. C 2016, 60, 427–436. [Google Scholar] [CrossRef]

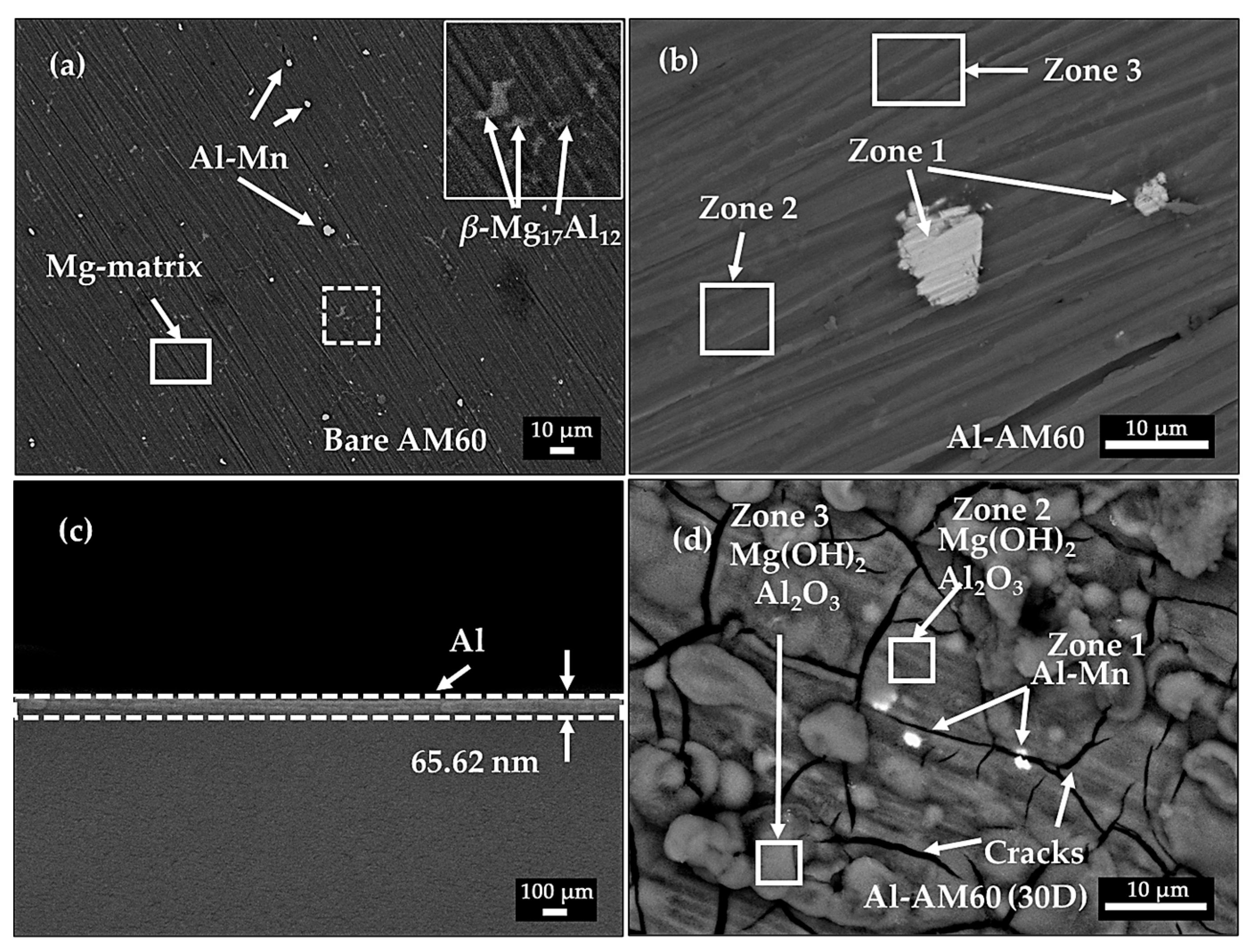
| Element | O | Mg | Al | Mn | |
|---|---|---|---|---|---|
| AM60 | Al-Mn | 2.52 | 26.26 | 34.17 | 37.05 |
| Mg matrix | 2.21 | 95.20 | 2.59 | - | |
| 1.43 | 79.65 | 18.92 | - | ||
| Al-AM60 | Zone 1 | 5.67 | 3.56 | 38.88 | 51.89 |
| Zone 2 | 7.38 | 78.99 | 13.63 | - | |
| Zone 3 | 5.49 | 81.86 | 12.65 | - | |
| Al-AM60 (30 days exposure) | Zone 1 | 45.06 | 21.43 | 18.55 | 14.96 |
| Zone 2 | 58.63 | 31.66 | 9.23 | - | |
| Zone 3 | 64.68 | 28.21 | 6.70 | - |
| Rs (Ω cm2) | CPE1 (µS sn cm−2) | n1 | R1 (kΩ cm2) | CPE2 (µS sn cm−2) | n2 | R2 (kΩ cm2) | Rp (kΩ cm2) |
|---|---|---|---|---|---|---|---|
| AM60 | |||||||
| Al-AM60 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, G.; Veleva, L.; Flores, E. Initial Stages of Al-AM60-Modified Surface of Magnesium Alloy Activity Exposed to Simulated Marine Environment. Coatings 2025, 15, 661. https://doi.org/10.3390/coatings15060661
Sánchez G, Veleva L, Flores E. Initial Stages of Al-AM60-Modified Surface of Magnesium Alloy Activity Exposed to Simulated Marine Environment. Coatings. 2025; 15(6):661. https://doi.org/10.3390/coatings15060661
Chicago/Turabian StyleSánchez, Gerardo, Lucien Veleva, and Eduardo Flores. 2025. "Initial Stages of Al-AM60-Modified Surface of Magnesium Alloy Activity Exposed to Simulated Marine Environment" Coatings 15, no. 6: 661. https://doi.org/10.3390/coatings15060661
APA StyleSánchez, G., Veleva, L., & Flores, E. (2025). Initial Stages of Al-AM60-Modified Surface of Magnesium Alloy Activity Exposed to Simulated Marine Environment. Coatings, 15(6), 661. https://doi.org/10.3390/coatings15060661








