Improved Adhesion of PTFE Surfaces via Low-Power DC Plasma and Fast Neutrals Flow
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Preparation
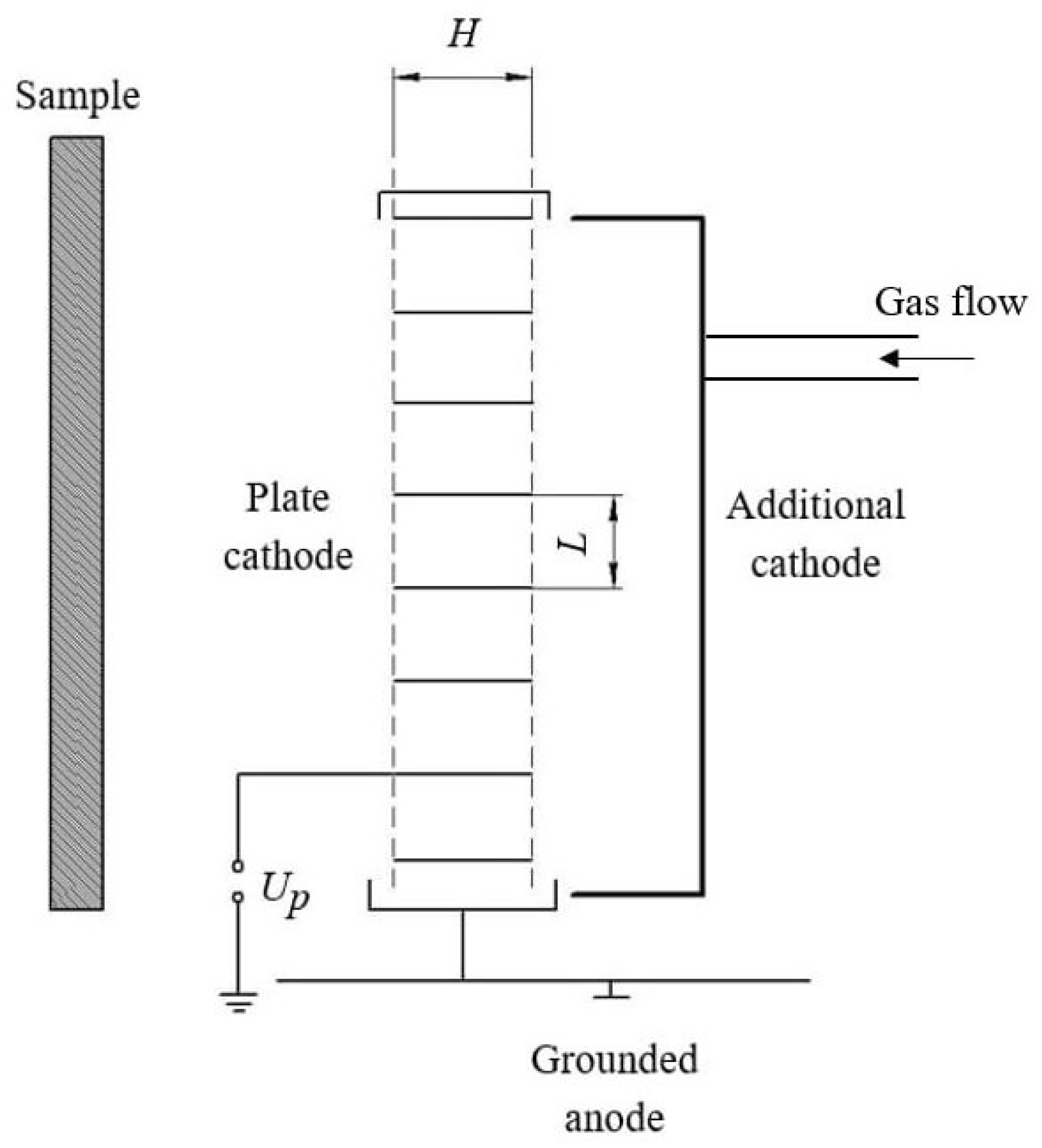
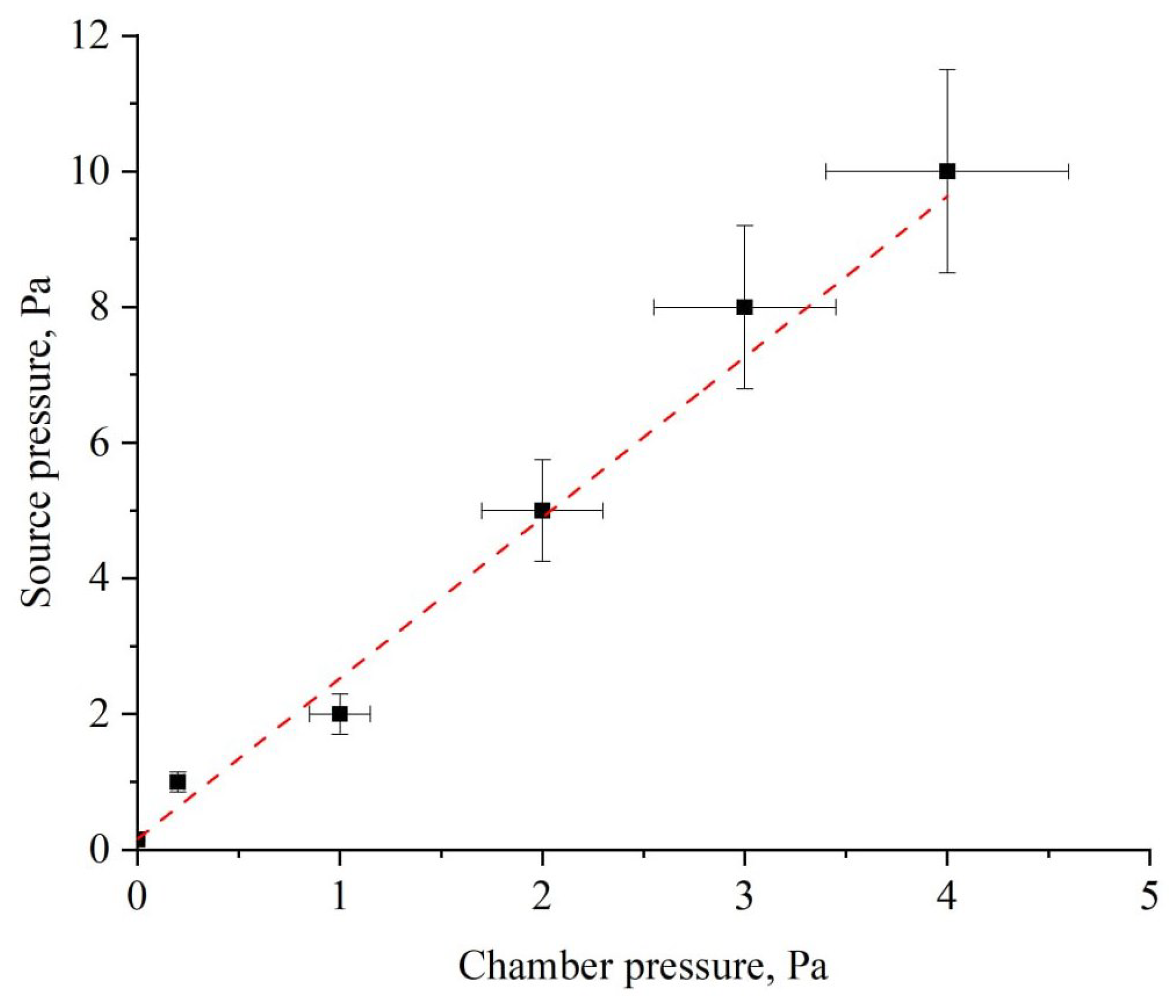
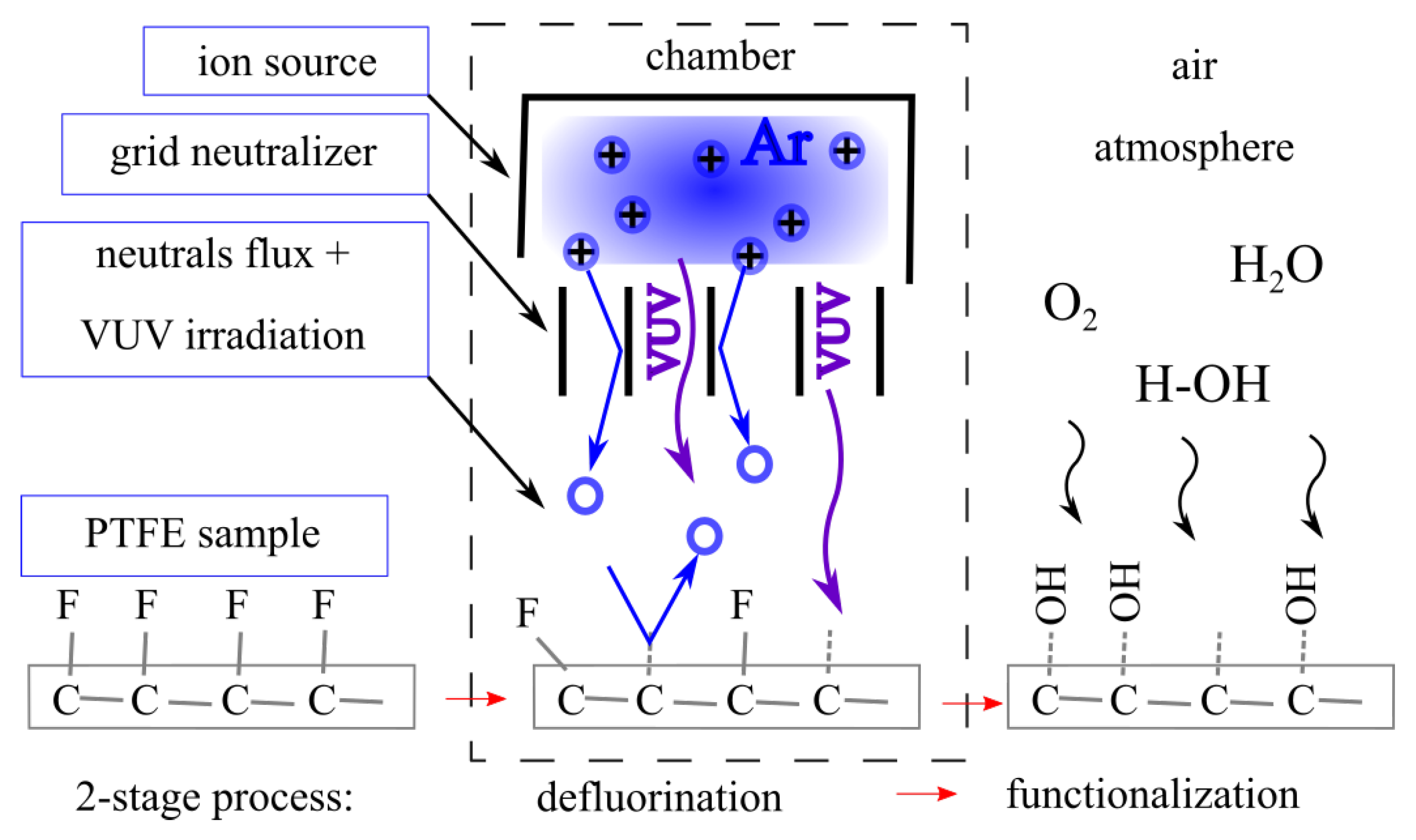
2.2. Fast-Neutrals Activation
2.3. Activation by the Techniques Used in Industrial Environments
2.3.1. Activation by Corona Discharge
2.3.2. Chemical Activation
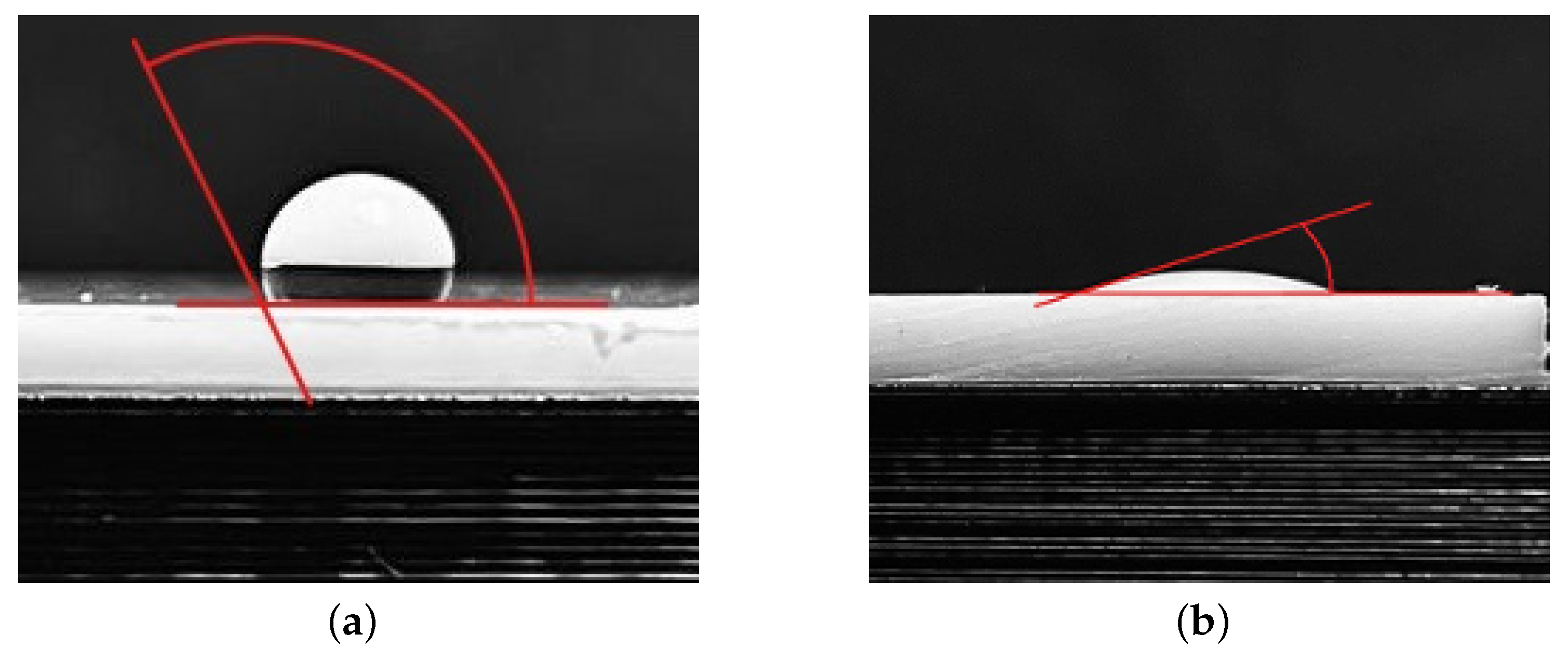
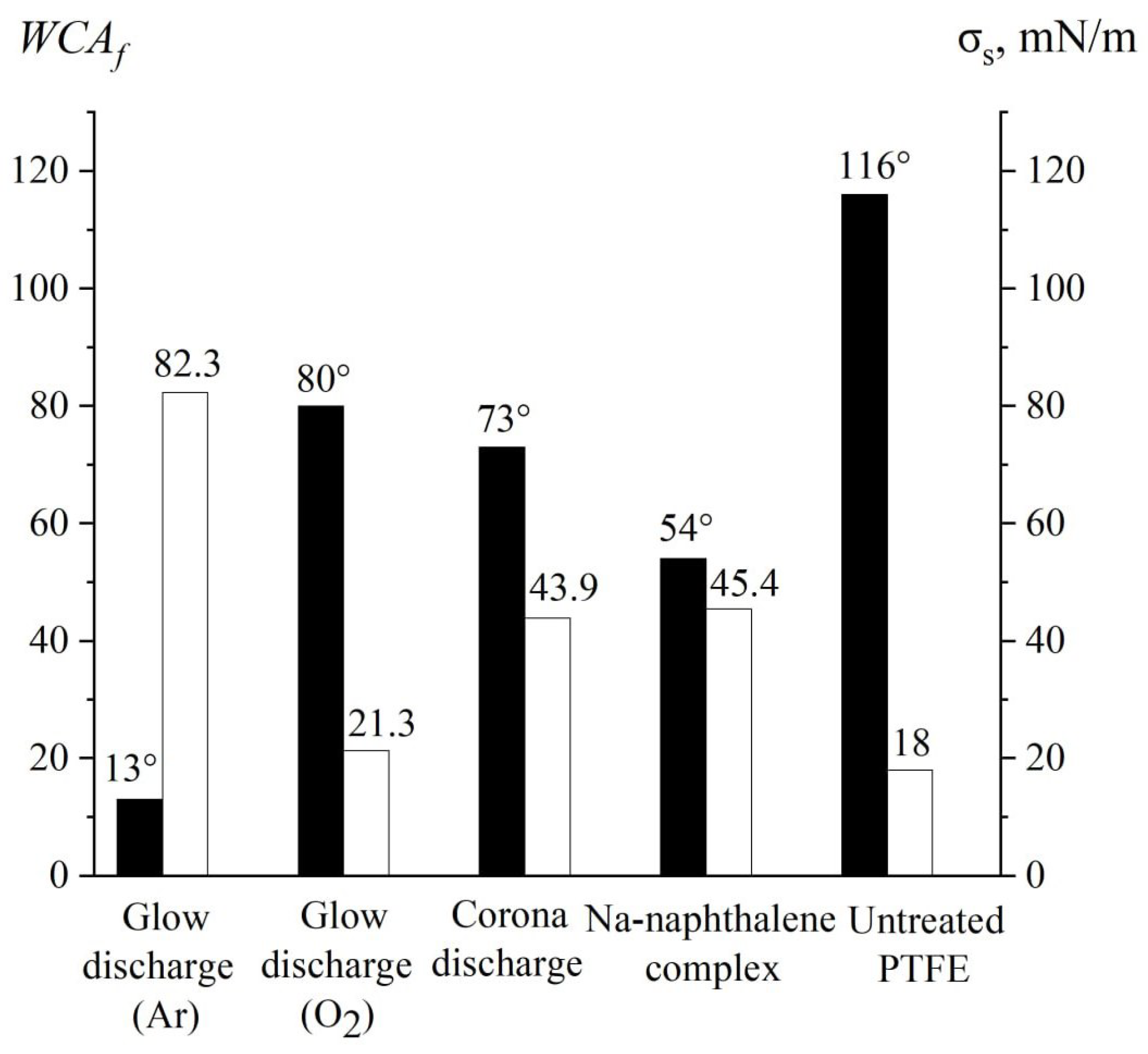
2.4. Water Contact Angle Measurement
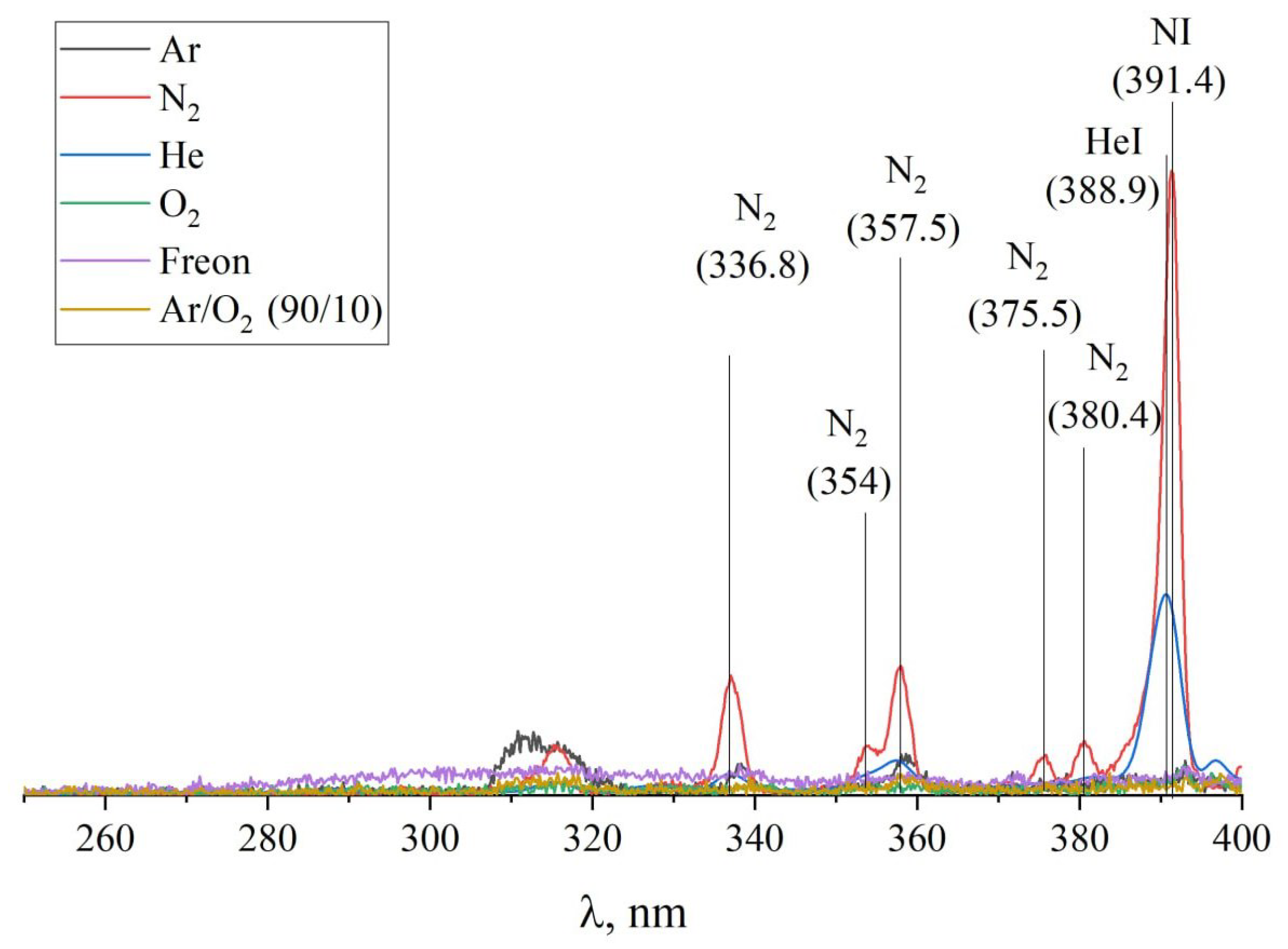
2.5. Plasma Optical Spectra Measurements
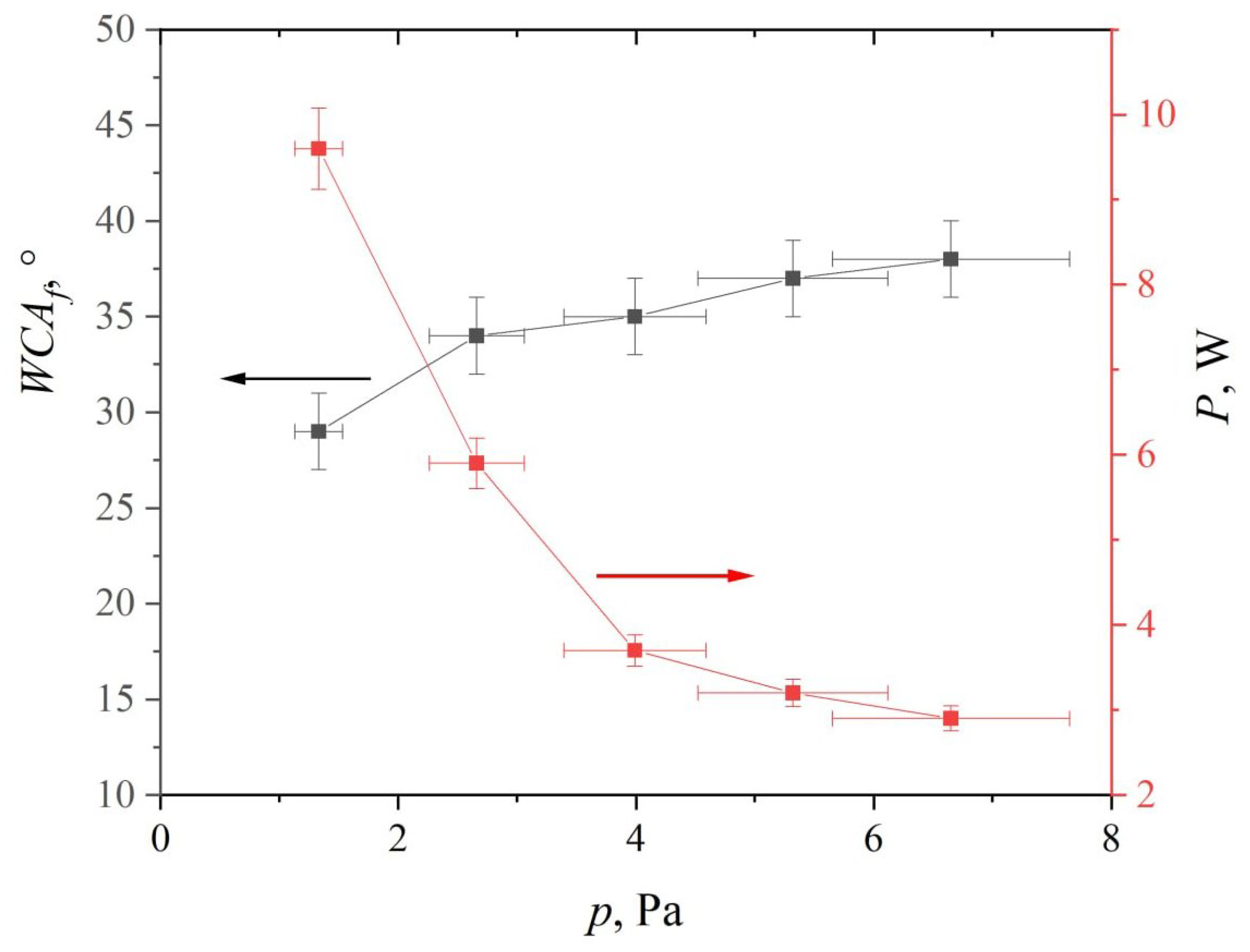
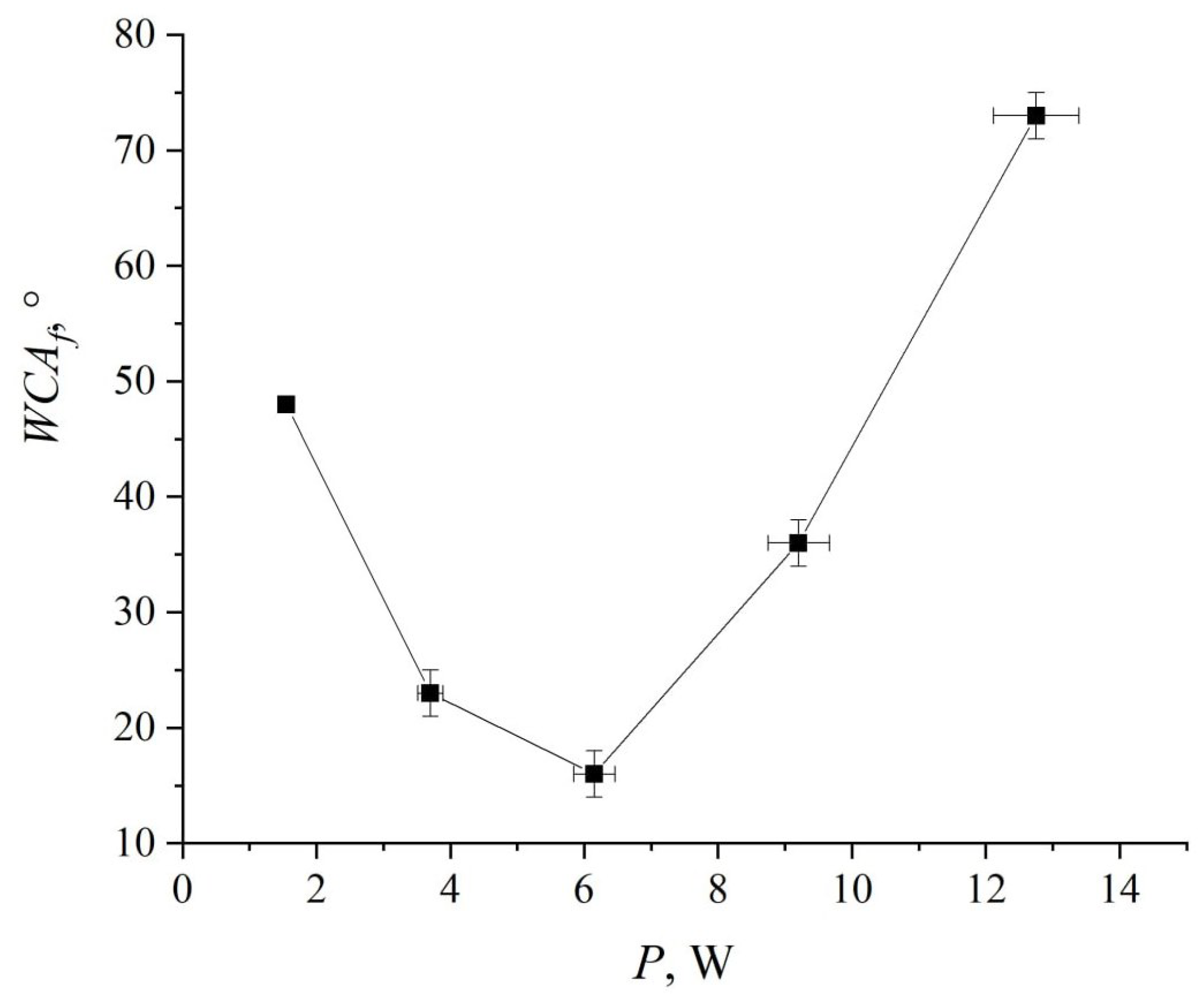
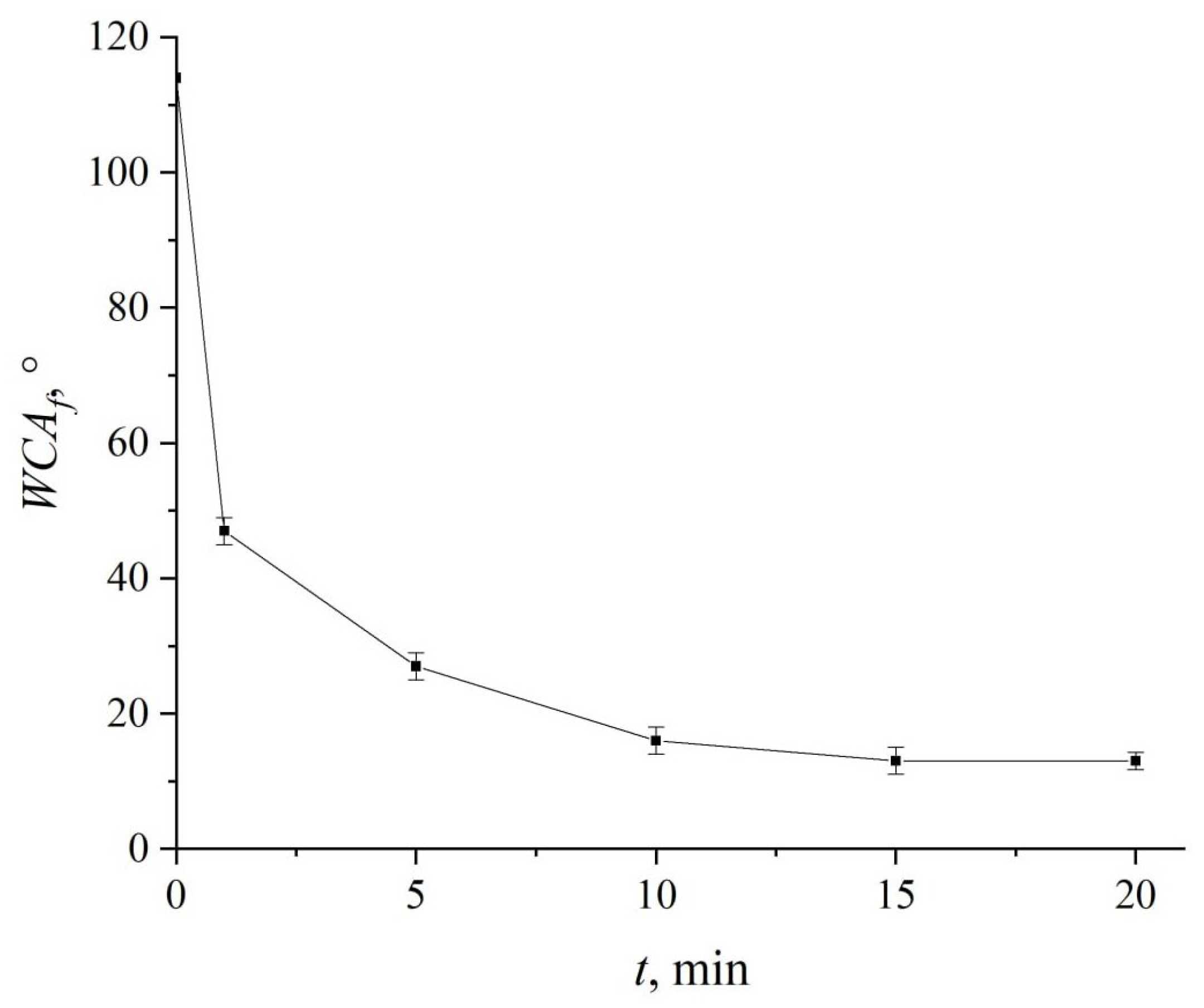
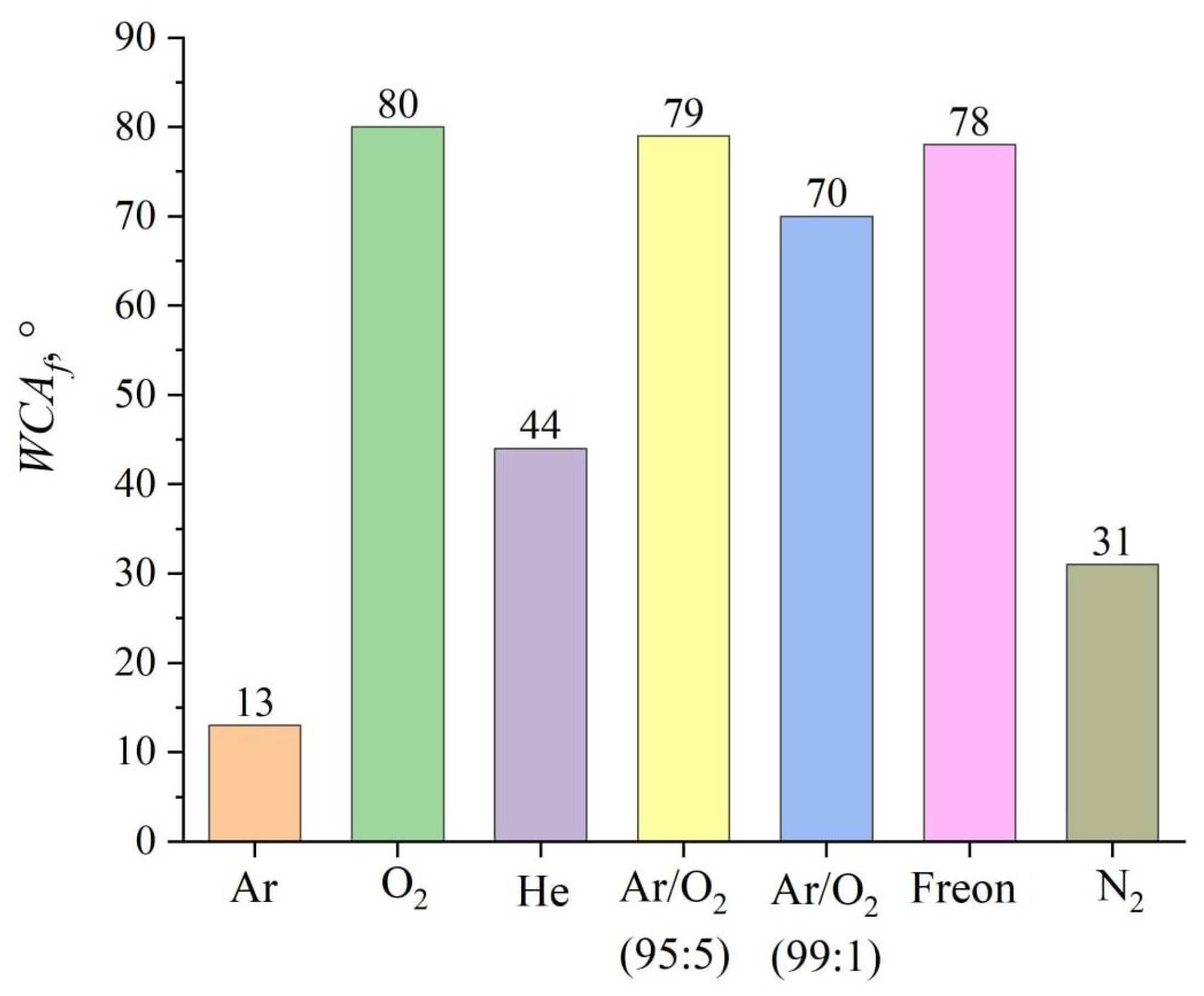
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
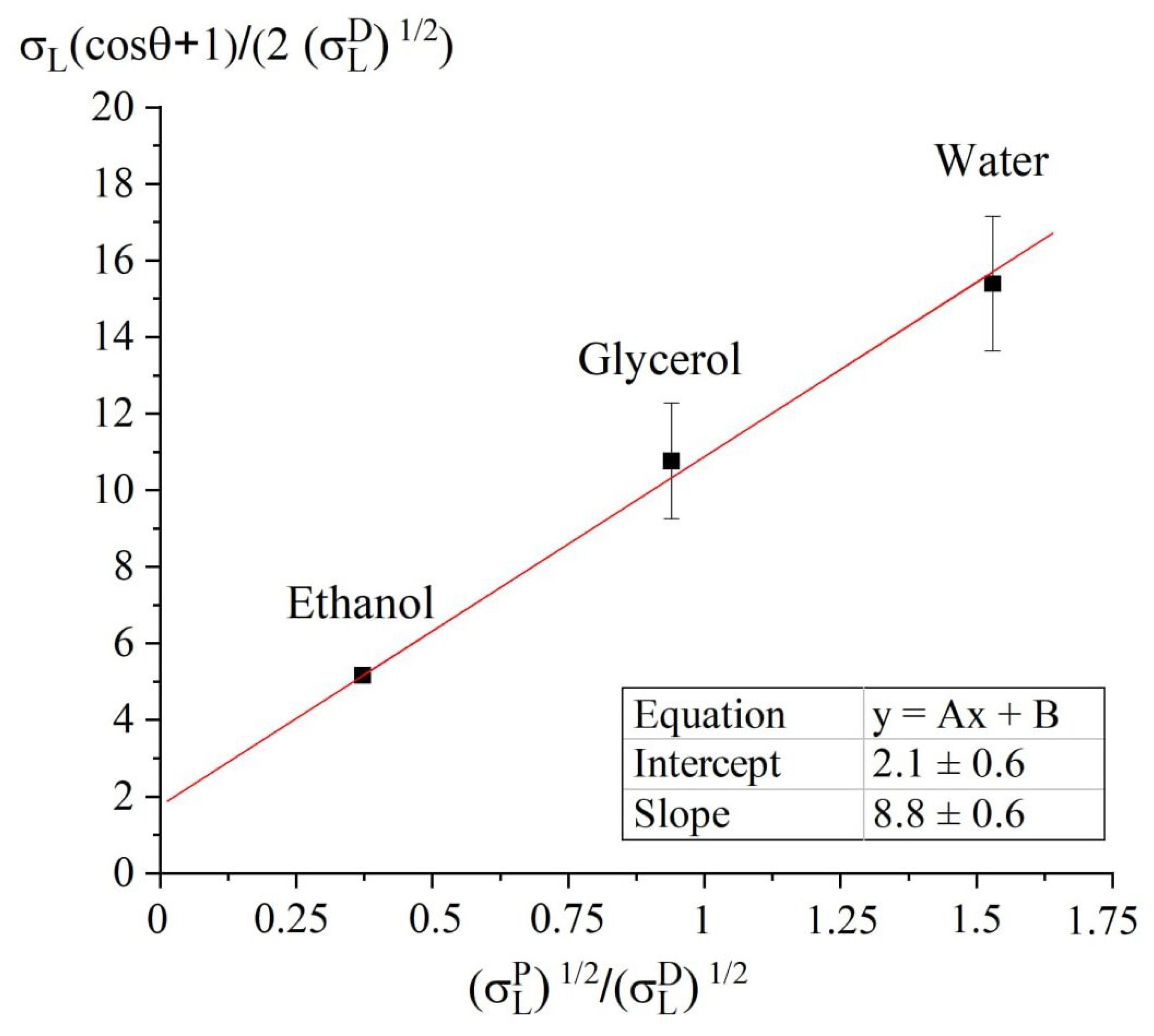
Appendix A. Estimation of the Surface Energy by Owens-Wendt-Rabel- Kaelble Model
| Liquid | , mN/m | , mN/m | , mN/m |
|---|---|---|---|
| Water | 51 | 21.8 | 72.8 |
| Glycerol | 30 | 34 | 64 |
| Ethanol | 2.6 | 18.8 | 22.4 |
| Treatment | WCA , ° | ||
|---|---|---|---|
| Water | Glycerol | Ethanol | |
| untreated PTFE | |||
| – | 116 | 145 | 9 |
| Glow discharge | |||
| Ar | 13 | 16 | 0 |
| O2 | 80 | 99 | 6 |
| He | 44 | 54 | 3 |
| 99%/1% O2 | 79 | 97 | 6 |
| 95%/5% O2 | 70 | 86 | 5 |
| Freon | 78 | 96 | 6 |
| N2 | 31 | 38 | 2 |
| Corona discharge | |||
| Air | 73 | 90 | 2 |
| Chemical treatment | |||
| Na-naphtalene complex | 54 | 67 | 4 |
| Treatment | , mN/m | , mN/m | , mN/m |
|---|---|---|---|
| untreated PTFE | |||
| – | |||
| Glow discharge | |||
| Ar | |||
| O2 | |||
| He | |||
| 99%/1% O2 | |||
| 95%/5% O2 | |||
| Freon | |||
| N2 | |||
| Corona discharge | |||
| Air | |||
| Chemical treatment | |||
| Na-naphtalene complex | |||
References
- Dhanumalayan, E.; Joshi, G.M. Performance properties and applications of polytetrafluoroethylene (PTFE)—A review. Adv. Compos. Hybrid Mater. 2018, 1, 247–268. [Google Scholar] [CrossRef]
- Ebnesajjad, S. Expanded PTFE Applications Handbook: Technology, Manufacturing and Applications; William Andrew: Norwich, NY, USA, 2016. [Google Scholar]
- Domalanta, M.R.B.; Caldona, E.B. Toward Enhancing the Surface Adhesion of Fluoropolymer-Based Coating Materials. Polym. Rev. 2024, 64, 980–1030. [Google Scholar] [CrossRef]
- Schonhorn, H.; Ryan, F.W. Adhesion of polytetrafluoroethylene. J. Adhes. 1969, 1, 43–47. [Google Scholar] [CrossRef]
- Lee, K.W.; Viehbeck, A. Wet-process surface modification of dielectric polymers: Adhesion enhancement and metallization. IBM J. Res. Dev. 1994, 38, 457–474. [Google Scholar] [CrossRef]
- Rojas, G. Recent Advances in Fluoropolymers: Copolymers of Tetrafluoroethylene and Related Fluorinated Molecules. Available online: http://www.researchgate.net/publication/242686971_Recent_Advances_in_Fluoropolymers_Copolymers_of_Tetrafluoroethylene_and_Related_Fluorinated_Molecules (accessed on 22 May 2025).
- Roina, Y.; Gonçalves, A.M.; Fregnaux, M.; Auber, F.; Herlem, G. Sodium naphthalenide diglyme solution for etching ptfe, characterizations and molecular modelization. ChemistrySelect 2022, 7, e202200153. [Google Scholar] [CrossRef]
- Gabriel, M.; Dahm, M.; Vahl, C.F. Wet-chemical approach for the cell-adhesive modification of polytetrafluoroethylene. Biomed. Mater. 2011, 6, 035007. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.G.; Khang, G.S.; Lee, H.B.; Jhon, M.S. Characterization of wettability gradient surfaces prepared by corona discharge treatment. J. Colloid Interface Sci. 1992, 151, 563–570. [Google Scholar] [CrossRef]
- Markgraf, D.A. Corona treatment: An overview. In Polymers Laminations and Coatings Conference; TAPPI Press: Atlanta, GA, USA, 1994; p. 159. [Google Scholar]
- Olariu, M.A.; Herrero, R.; Astanei, D.G.; Jofré, L.; Morentin, J.; Filip, T.A.; Burlica, R. Improving Printability of Polytetrafluoroethylene (PTFE) with the Help of Plasma Pre-Treatment. Polymers 2023, 15, 3348. [Google Scholar] [CrossRef]
- Jie-Rong, C.; Wakida, T. Studies on the surface free energy and surface structure of PTFE film treated with low temperature plasma. J. Appl. Polym. Sci. 1997, 63, 1733–1739. [Google Scholar] [CrossRef]
- Liu, C.; Arnell, R.; Gibbons, A.; Green, S.; Ren, L.; Tong, J. Surface modification of PTFE by plasma treatment. Surf. Eng. 2000, 16, 215–217. [Google Scholar] [CrossRef]
- Liu, C.; Wu, J.; Ren, L.; Tong, J.; Li, J.; Cui, N.; Brown, N.; Meenan, B. Comparative study on the effect of RF and DBD plasma treatment on PTFE surface modification. Mater. Chem. Phys. 2004, 85, 340–346. [Google Scholar] [CrossRef]
- Wilson, D.; Williams, R.; Pond, R. Plasma modification of PTFE surfaces. Part I: Surfaces immediately following plasma treatment. Surf. Interface Anal. 2001, 31, 385–396. [Google Scholar] [CrossRef]
- Bruce, R.; Weilnboeck, F.; Lin, T.; Phaneuf, R.; Oehrlein, G.; Long, B.; Willson, C.; Vegh, J.; Nest, D.; Graves, D. Relationship between nanoscale roughness and ion-damaged layer in argon plasma exposed polystyrene films. J. Appl. Phys. 2010, 107, 084310. [Google Scholar] [CrossRef]
- Zanini, S.; Bami, R.; Della Pergola, R.; Riccardi, C. Development of super-hydrophobic PTFE and PET surfaces by means of plasma processes. J. Phys. 2014, 550, 012029. [Google Scholar] [CrossRef]
- Hai, W.; Hi, T.; Shimizu, K.; Yajima, T. Preparation of a super hydrophilic polytetrafluoroethylene surface using a gaseous ammonia-water low-temperature plasma. J. Photopolym. Sci. Technol. 2015, 28, 479–483. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Yajima, T. A spectroscopic study on argon-ammonia water gaseous plasma super-hydrophilizing polytetrafluoroethylene. J. Photopolym. Sci. Technol. 2017, 30, 325–330. [Google Scholar] [CrossRef]
- Vesel, A.; Zaplotnik, R.; Primc, G.; Mozetič, M.; Katan, T.; Kargl, R.; Mohan, T.; Kleinschek, K.S. Rapid functionalization of polytetrafluorethylene (PTFE) surfaces with nitrogen functional groups. Polymers 2021, 13, 4301. [Google Scholar] [CrossRef]
- Lojen, D.; Primc, G.; Mozetič, M.; Vesel, A. Optimization of surface wettability of polytetrafluoroethylene (PTFE) by precise dosing of oxygen atoms. Appl. Surf. Sci. 2022, 598, 153817. [Google Scholar] [CrossRef]
- Zhang, Y.; Ishikawa, K.; Mozetič, M.; Tsutsumi, T.; Kondo, H.; Sekine, M.; Hori, M. Polyethylene terephthalate (PET) surface modification by VUV and neutral active species in remote oxygen or hydrogen plasmas. Plasma Process. Polym. 2019, 16, 1800175. [Google Scholar] [CrossRef]
- Károly, Z.; Kalácska, G.; Zsidai, L.; Mohai, M.; Klébert, S. Improvement of adhesion properties of polyamide 6 and polyoxymethylene-copolymer by atmospheric cold plasma treatment. Polymers 2018, 10, 1380. [Google Scholar] [CrossRef]
- Hunke, H.; Soin, N.; Shah, T.H.; Kramer, E.; Pascual, A.; Karuna, M.S.L.; Siores, E. Low-Pressure H2, NH3 microwave plasma treatment of polytetrafluoroethylene (PTFE) powders: Chemical, thermal and wettability analysis. Materials 2015, 8, 2258–2275. [Google Scholar] [CrossRef]
- Károly, Z.; Kalácska, G.; Sukumaran, J.; Fauconnier, D.; Kalácska, Á.; Mohai, M.; Klébert, S. Effect of atmospheric cold plasma treatment on the adhesion and tribological properties of polyamide 66 and poly (tetrafluoroethylene). Materials 2019, 12, 658. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Kim, T.H.; Choi, S. Hydrophilic surface modification of polytetrafluoroethylene film with gliding arc plasma. Appl. Sci. Converg. Technol. 2019, 28, 101–106. [Google Scholar] [CrossRef]
- Kolská, Z.; Řezníčková, A.; Hnatowicz, V.; Švorčík, V. PTFE surface modification by Ar plasma and its characterization. Vacuum 2012, 86, 643–647. [Google Scholar] [CrossRef]
- Rychkov, D.; Yablokov, M.; Rychkov, A. Chemical and physical surface modification of PTFE films—An approach to produce stable electrets. Appl. Phys. A 2012, 107, 589–596. [Google Scholar] [CrossRef]
- Carbone, E.; Verhoeven, M.; Keuning, W.; Van Der Mullen, J. PTFE treatment by remote atmospheric Ar/O2 plasmas: A simple reaction scheme model proposal. J. Phys. 2016, 715, 012011. [Google Scholar]
- Yablokov, M.Y.; Kuznetsov, A.A. Electret properties and wettability of polymer materials treated by DC glow discharge. Phys. Complex Syst. 2024, 5, 202–204. [Google Scholar] [CrossRef]
- Heo, W.; Han, D.H.; Oh, S.J.; Yoon, J.U.; Woo, I.; Choi, S.E.; Yoon, J.M.; Bae, J.W. Enhancing polymer electrolyte membrane fuel cell performance and efficiency: Plasma-treated effects of expanded polytetrafluoroethylene in reinforced composite membranes. J. Power Sources 2025, 642, 237010. [Google Scholar] [CrossRef]
- Takahashi, T.; Hirano, Y.; Takasawa, Y.; Gowa, T.; Fukutake, N.; Oshima, A.; Tagawa, S.; Washio, M. Change in surface morphology of polytetrafluoroethylene by reactive ion etching. Radiat. Phys. Chem. 2011, 80, 253–256. [Google Scholar] [CrossRef]
- Esrom, H.; Zhang, J.Y.; Kogelschatz, U. Photochemical modification and etching of PTFE with excimer VUV/UV radiation. In Polymer Surfaces and Interfaces: Characterization, Modification and Application; CRC Press: Boca Raton, FL, USA, 2023; pp. 27–35. [Google Scholar]
- Egitto, F.; Matienzo, L. Modification of polytetrafluoroethylene and polyethylene surfaces downstream from helium microwave plasmas. Polym. Degrad. Stab. 1990, 30, 293–308. [Google Scholar] [CrossRef]
- Takacs, G.; Vukanovic, V.; Tracy, D.; Chen, J.; Egitto, F.; Matienzo, L.; Emmi, F. Photoetching and modification of organic polymer surfaces with vacuum UV Radiation. Polym. Degrad. Stab. 1993, 40, 73–81. [Google Scholar] [CrossRef]
- López, C.D.; Cedeño-Mata, M.; Dominguez-Pumar, M.; Bermejo, S. Surface modification of polytetrafluoroethylene thin films by non-coherent UV light and water treatment for electrowetting applications. Prog. Org. Coat. 2020, 149, 105593. [Google Scholar] [CrossRef]
- Heitz, J.; Niino, H.; Yabe, A. Chemical surface modification on polytetrafluoroethylene films by vacuum ultraviolet excimer lamp irradiation in ammonia gas atmosphere. Appl. Phys. Lett. 1996, 68, 2648–2650. [Google Scholar] [CrossRef]
- Takacs, G.A.; Miri, M.J.; Kovach, T. Vacuum UV surface photo-oxidation of polymeric and other materials for improving adhesion: A critical review. Prog. Adhes. Adhes. 2021, 6, 559–585. [Google Scholar]
- Takacs, G.A.; Miri, M.J. Vacuum UV (VUV) Photo-Oxidation of Polymer Surfaces to Enhance Adhesion. In Polymer Surface Modification to Enhance Adhesion: Techniques and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2024; pp. 119–154. [Google Scholar]
- Primc, G. Recent advances in surface activation of polytetrafluoroethylene (PTFE) by gaseous plasma treatments. Polymers 2020, 12, 2295. [Google Scholar] [CrossRef]
- Matienzo, L.; Zimmerman, J.; Egitto, F. Surface modification of fluoropolymers with vacuum ultraviolet irradiation. J. Vac. Sci. Technol. A Vac. Surfaces Film. 1994, 12, 2662–2671. [Google Scholar] [CrossRef]
- Lojen, D.; Primc, G.; Mozetič, M.; Vesel, A. Effect of VUV radiation and reactive hydrogen atoms on depletion of fluorine from polytetrafluoroethylene surface. Appl. Surf. Sci. 2020, 533, 147356. [Google Scholar] [CrossRef]
- Dasilva, W.; Entenberg, A.; Kahn, B.; Debies, T.; Takacs, G. Surface modification of Teflon® PFA with vacuum UV photo-oxidation. J. Adhes. Sci. Technol. 2006, 20, 437–455. [Google Scholar] [CrossRef]
- Shimokawa, F.; Tanaka, H.; Uenishi, Y.; Sawada, R. Reactive–fast-atom beam etching of GaAs using Cl2 gas. J. Appl. Phys. 1989, 66, 2613–2618. [Google Scholar] [CrossRef]
- Maishev, Y.P.; Shevchuk, S.; Kudrya, V. Generation of fast neutral beams based on closed drift ion sources. Russ. Microelectron. 2014, 43, 345–351. [Google Scholar] [CrossRef]
- Mizutani, T.; Nishimatsu, S. Sputtering yield and radiation damage by neutral beam bombardment. J. Vac. Sci. Technol. A Vac. Surfaces Film. 1988, 6, 1417–1420. [Google Scholar] [CrossRef]
- Panda, S.; Economou, D.J.; Chen, L. Anisotropic etching of polymer films by high energy (100 s of eV) oxygen atom neutral beams. J. Vac. Sci. Technol. A Vac. Surfaces Film. 2001, 19, 398–404. [Google Scholar] [CrossRef]
- Samukawa, S.; Sakamoto, K.; Ichiki, K. Generating high-efficiency neutral beams by using negative ions in an inductively coupled plasma source. J. Vac. Sci. Technol. A Vac. Surfaces Film. 2002, 20, 1566–1573. [Google Scholar] [CrossRef]
- Ranjan, A.; Donnelly, V.M.; Economou, D.J. Energy distribution and flux of fast neutrals and residual ions extracted from a neutral beam source. J. Vac. Sci. Technol. A 2006, 24, 1839–1846. [Google Scholar] [CrossRef]
- Economou, D.J. Modeling and simulation of fast neutral beam sources for materials processing. Plasma Process. Polym. 2009, 6, 308–319. [Google Scholar] [CrossRef]
- Kostrin, D.; Oukhov, A. Hardware-Software Spectrometric Complex for Research of the Parameters of Light-Emitting Diodes. Biotekhnosphera 2013, 21–25. (In Russian) [Google Scholar]
- Uhov, A.; Gerasimov, V.; Kostrin, D.; Selivanov, L. Use of compact spectrometer for plasma emission qualitative analysis. J. Phys. 2014, 567, 012039. [Google Scholar] [CrossRef]
- Golda, J.; Biskup, B.; Layes, V.; Winzer, T.; Benedikt, J. Vacuum ultraviolet spectroscopy of cold atmospheric pressure plasma jets. Plasma Process. Polym. 2020, 17, 1900216. [Google Scholar] [CrossRef]
- Kramida, A.; Ralchenko, Y.; Reader, J.; Team, N.A. NIST Atomic Spectra Database. Version 5.12. 2024. Available online: https://physics.nist.gov/asd (accessed on 18 April 2025).
- Qayyum, A.; Zeb, S.; Ali, S.; Waheed, A.; Zakaullah, M. Optical emission spectroscopy of abnormal glow region in nitrogen plasma. Plasma Chem. Plasma Process. 2005, 25, 551–564. [Google Scholar] [CrossRef]
- Suraj, K.; Bharathi, P.; Prahlad, V.; Mukherjee, S. Near cathode optical emission spectroscopy in N2–H2 glow discharge plasma. Surf. Coat. Technol. 2007, 202, 301–309. [Google Scholar] [CrossRef]
- Fierro, A.; Laity, G.; Neuber, A. Optical emission spectroscopy study in the VUV–VIS regimes of a developing low-temperature plasma in nitrogen gas. J. Phys. D Appl. Phys. 2012, 45, 495202. [Google Scholar] [CrossRef]
- Young, T. An essay on the cohesion of fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar]
- Owens, D.K.; Wendt, R. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Palencia, M. Surface free energy of solids by contact angle measurements. J. Sci. Technol. Appl 2017, 2, 84–93. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komlev, A.; Kudryavtseva, D.; Neustroev, I.; Sudalenko, Y.; Altynnikov, A.; Tsymbalyuk, A.; Gagarin, A.; Platonov, R. Improved Adhesion of PTFE Surfaces via Low-Power DC Plasma and Fast Neutrals Flow. Coatings 2025, 15, 644. https://doi.org/10.3390/coatings15060644
Komlev A, Kudryavtseva D, Neustroev I, Sudalenko Y, Altynnikov A, Tsymbalyuk A, Gagarin A, Platonov R. Improved Adhesion of PTFE Surfaces via Low-Power DC Plasma and Fast Neutrals Flow. Coatings. 2025; 15(6):644. https://doi.org/10.3390/coatings15060644
Chicago/Turabian StyleKomlev, Andrey, Darya Kudryavtseva, Ilya Neustroev, Yaroslava Sudalenko, Andrey Altynnikov, Andrey Tsymbalyuk, Alexander Gagarin, and Roman Platonov. 2025. "Improved Adhesion of PTFE Surfaces via Low-Power DC Plasma and Fast Neutrals Flow" Coatings 15, no. 6: 644. https://doi.org/10.3390/coatings15060644
APA StyleKomlev, A., Kudryavtseva, D., Neustroev, I., Sudalenko, Y., Altynnikov, A., Tsymbalyuk, A., Gagarin, A., & Platonov, R. (2025). Improved Adhesion of PTFE Surfaces via Low-Power DC Plasma and Fast Neutrals Flow. Coatings, 15(6), 644. https://doi.org/10.3390/coatings15060644






