1. Introduction
Zinc–nickel (Zn-Ni) alloy coatings are widely regarded as a cornerstone of modern corrosion protection technologies, offering exceptional performance in safeguarding ferrous substrates exposed to harsh environments, such as marine, automotive and industrial settings. The unique
γ-phase (Ni
5Zn
21) intermetallic structure that forms at nickel contents of 8–15 wt% contributes to the coatings’ outstanding barrier properties and sacrificial anode functionality [
1,
2]. In comparison to conventional zinc coatings, Zn-Ni alloys exhibit 5–10 times better resistance to neutral salt spray and 3–5 times greater durability in cyclic corrosion tests [
3,
4], making them particularly indispensable for critical components such as fasteners, brake lines and offshore infrastructure [
5]. Moreover, due to their environmental compliance, Zn-Ni alloys have emerged as a sustainable alternative to toxic cadmium and cyanide-based electroplating systems. This shift is driven by global regulatory measures such as the EU REACH Directive and the U.S. Clean Air Act Amendments, further accelerating the adoption of Zn-Ni alloys as a preferred choice in electroplating applications [
6].
The transition to alkaline Zn-Ni electroplating introduces complex challenges due to the antagonistic electrochemical behaviors of zinc and nickel. Zinc’s high reactivity in alkaline media necessitates strong complexation to prevent hydroxide precipitation, whereas nickel’s more noble electrochemical potential requires careful complexing agent design to facilitate selective co-deposition. Conventional alkaline zincate baths are prone to instability, manifested in issues such as pH drift, non-uniform current distribution and hydrogen embrittlement risks. These limitations become more pronounced in industrial-scale operations, where bath decomposition products, such as zinc oxide colloids and organic breakdown residues, accumulate, thus degrading coating quality and process reproducibility [
7]. A critical challenge in this area is the search for cyanide-free complexing agents that can replicate the dual functionality of cyanide—namely, stabilizing metal ions and controlling deposition kinetics [
8]. Recent research indicates that significant progress has been made in understanding the electrochemical and structural characteristics of Zn-Ni alloys in alkaline electrolytes. Studies on the electroplating of these alloys show that while zinc and nickel exhibit distinct deposition behaviors, co-deposition can be achieved with the proper electrolyte composition, involving additives that stabilize both metals without compromising deposition uniformity [
9]. Additionally, the use of alternative complexing agents, such as gluconate-based complexing agents, has shown promise in improving bath stability and promoting the uniform deposition of Zn-Ni alloys under more environmentally friendly conditions [
10]. At the same time, the development of non-cyanide baths with comparable efficiency to cyanide-based systems continues to be an important area of research. The need for cyanide-free processes has driven innovation in the design of complexing agents capable of achieving similar results in terms of plating rate and quality while avoiding the toxicity and environmental hazards associated with cyanide [
11]. Despite this progress, many of these alternative baths still face challenges such as pH instability and reduced process reproducibility under industrial conditions [
12].
The evolution of Zn-Ni electroplating research has progressed along three synergistic directions: complexing agent chemistry innovation, microstructure–property correlation and electrochemical mechanism elucidation. Traditional complexing agent systems, such as citrate–triethanolamine and polyamine derivatives, have been widely explored for their moderate coordination strength and influence on metal deposition. Citrate complexing agents, for instance, can enable 12–15 wt% Ni incorporation via carboxylate-O coordination, yet are prone to oxidative degradation at high pH, reducing bath lifespan and requiring frequent replenishment [
13]. Polyamines such as tetraethylenepentamine (TEPA) offer enhanced Ni reduction efficiency through strong N-donor chelation, but their linear geometry often promotes dendritic growth and increased surface roughness [
14]. Moreover, both complexing agent systems struggle to suppress hydrogen evolution reactions (HERs), which lead to porosity and hydrogen-induced substrate embrittlement—a limitation that remains critical for high-strength steel applications [
15]. Recent studies highlight that modifying complexing agent architecture—such as with thiosemicarbazone, S
2N
2 tetradentate or PS-chelated Ni complexes—can improve HER suppression and catalytic efficiency [
16,
17].
Hydrogen embrittlement (HE) poses a significant durability challenge in Zn-Ni electroplating systems, particularly for high-strength steel substrates subjected to cathodic polarization during deposition. In conventional Zn electroplating processes, hydrogen evolution reactions (HERs) at the cathode interface lead to substantial hydrogen absorption, with Zn-coated bolts exhibiting hydrogen uptake levels of 5.79 mass ppm in the as-electrodeposited state, compared to 1.06 mass ppm for ZnNi-coated systems [
18]. Despite the higher hydrogen content in Zn coatings, tensile tests reveal no HE-induced failure in these systems, as the hydrogen atoms remain trapped within the brittle δ-Zn phase or at the coating-substrate interface, preventing permeation into the steel matrix [
19]. This contrasts with ZnNi coatings, where microcracks in the γ-ZnNi phase facilitate hydrogen effusion but simultaneously reduce hydrogen retention. However, prolonged exposure to chloride-rich environments exacerbates hydrogen permeation coefficients by 2–3 orders of magnitude, highlighting the vulnerability of both systems under marine conditions. Recent studies emphasize the role of ligand engineering in modulating hydrogen pathways: π-backdonation interactions in advanced complexing agents stabilize intermediate metal–hydrogen bonds, decoupling HERs from deposition kinetics. For instance, pyridine-based ligands demonstrate hydrogen permeability reductions of 2–3 orders of magnitude compared to conventional citrate/TEPA systems by geometrically shielding cathodic active sites. These insights underscore the necessity of integrating HE mitigation strategies into Zn-Ni electroplating technologies, balancing microstructure control with hydrogen barrier properties. Future research directions should prioritize ligand architectures that simultaneously suppress HERs and enhance hydride resistance, particularly for automotive and marine applications where cyclic stresses and aggressive electrolytes accelerate delayed hydride cracking.
Further efforts have explored EDTA, citrate and zwitterionic supramolecular hydrogels to stabilize Zn
2+/Ni
2+ ions and buffer HERs through complexing agent–field tuning [
20,
21]. Electrochemical investigations confirm that suppressing HERs while promoting uniform deposition depends not only on complexing agent strength but also on the electronic modulation and spatial conformation around the Ni
2+ centers [
22]. The integration of HER inhibitors into Zn-Ni alloy systems continues to be essential for advancing durable and fine-grained coating technologies [
23]. Emerging complexing agent classes—including amino acids, imidazole derivatives and supramolecular hosts—have broadened the coordination landscape in Zn-Ni alloy electroplating. Histidine-based systems leverage zwitterionic structures and pH responsiveness to enhance nickel selectivity and γ-phase content, but are limited by narrow thermal operation windows and biological degradation risks, which hinder industrial scalability [
24,
25]. Heterocyclic complexing agents—especially those containing pyridine or imidazole cores—have emerged as strong N-donor systems, valued for their chelation geometry, redox stability and π-stacking interactions. Pyridine derivatives such as 2,2′-bipyridine have demonstrated strong Ni
2+ complexation (logβ ≈ 10), while effectively suppressing HERs by sterically blocking the cathode interface [
26,
27].
Coordination polymers constructed from imidazo[1,5-a]pyridine or bipyridine analogs exhibit tunable electronic and steric properties, promoting both supramolecular stability and metal selectivity [
28,
29]. Yet, despite this progress, a persistent trade-off remains: complexing agents designed for strong nickel coordination often destabilize zinc ions in the bath, whereas zinc-favoring complexing agents tend to reduce γ-phase purity—a critical structural parameter for corrosion resistance [
30]. To overcome this, complexing agent systems with dual affinity and electronic adaptability—such as pyridine–imidazole hybrids or functionalized supramolecular scaffolds—are being investigated for their ability to balance Zn/Ni coordination and phase-selective deposition [
31,
32].
Microstructural studies have demonstrated that the performance of Zn–Ni coatings is strongly influenced by nanocrystalline grain refinement (typically < 30 nm) and crystallographic texture orientation. For instance, coatings with a high fraction of the (330)-oriented γ-phase exhibit superior barrier properties against chloride ions, attributed to denser passive films and reduced intergranular corrosion pathways [
26]. Studies have shown that coatings with a uniform γ-phase and grain sizes around 25 nm provide exceptional corrosion resistance, particularly when optimized deposition conditions and complexing agent coordination are employed [
33,
34]. Crystallographic texture also plays a crucial role in coating durability, with textured deposits exhibiting enhanced mechanical and thermal stability compared to randomly oriented structures [
35,
36].
Nevertheless, achieving such microstructural control in alkaline baths remains challenging due to competing side reactions such as hydrogen evolution and hydroxide entrapment. Recent electrochemical impedance spectroscopy (EIS) studies have identified diffusion-controlled Ni
2+ reduction as the rate-determining step in citrate-based systems, contrasting with the activation-controlled Zn
2+ reduction observed in amine-based electrolytes [
37,
38]. Tailored complexing agents, such as 2,2′-bipyridine or vanillin, have demonstrated the potential to decouple these redox pathways, improving both deposition selectivity and surface uniformity [
39,
40]. From an industrial standpoint, commercial Zn–Ni alkaline baths face additional complications. Their operational lifetimes are often limited to under six months, primarily due to the degradation of organic additives and zincate polymerization [
41]. Complexing agent decomposition results in significant residual carbon content (10–15 wt%) that not only deteriorates coating ductility but also increases the burden of industrial wastewater treatment [
42]. To address these systemic inefficiencies, research has emphasized the design of complexing agents that simultaneously offer thermodynamic robustness, redox selectivity and environmental safety [
43,
44].
This study introduces pyridine oxime-based complexing agents as a breakthrough solution to address the limitations of conventional systems. These agents synergize pyridine’s nitrogen-donor coordination with oxime’s oxygen functionality, enabling the dual stabilization of Zn2+ and Ni2+ through pH-responsive N,O-chelation. Unlike existing complexing agents, the molecular architecture of pyridine oximes suppresses hydrogen evolution and random nucleation while promoting diffusion-controlled deposition. This design balances bath stability, phase purity and nanocrystalline refinement, overcoming the trade-offs observed in traditional and emerging complexing agents. By aligning coordination chemistry with industrial requirements, this work advances the development of sustainable, high-performance Zn-Ni electroplating technologies.
2. Materials and Methods
2.1. Materials
All chemicals used in the experiment were analytically pure, including NaOH (analytically pure, Tianjin, China), ZnO (analytically pure, Tianjin, China), NiSO4∙6H2O (analytically pure, Tianjin, China), 2-pyridine-formaldehyde (analytically pure, Shanghai Aladdin Biochemical Technology Co., Ltd, Shanghai, China), 2-acetylpyridine (analytically pure, Aladdin, China), 2-cyanopyridine (analytically pure, Aladdin, China), sodium citrate (analytically pure, Tianjin, China), TEPA (analytically pure, Tianjin, China), triethylamine (99.5%, Beijing Innochem Technology Co., Ltd, Beijing, China), DPE-III (analytical purity, Jinan, China), BH-336 (analytical purity, Jinan), HNO3 (analytical purity, Qingdao, China), NaCl (analytical purity, Qingdao) and HCl (analytical purity, Qingdao).
DPE-III acts as the primary brightener in alkaline zincate galvanizing, primarily consisting of dimethylaminopropylamine. In contrast, BH-336 serves as the secondary brightener and includes components such as a carrier brightener, main brightener, auxiliary brightener, wetting agent and impurity removal agent. These two additives are introduced simultaneously into the plating solution, where they collaborate with other components to refine the coating grains, thereby achieving a more uniform, dense and smooth coating microstructure.
The performance of the coating was evaluated using a direct-current (DC) KSY-multi-function experimental rectifier. A 45# mild steel specimen measuring 45 mm × 40 mm × 2 mm was used as the cathode. The mild steel sheet underwent sequential surface preparation before electroplating. First, mechanical polishing was performed using 400#, 800#, 1000#, 1200# and 1500# sandpapers to achieve a smooth surface. The polished steel was then cleaned in absolute ethanol, acetone and deionized water for 3 min each to remove contaminants. Subsequently, the substrate was activated by immersion in 10% dilute nitric acid for 30 s to enhance surface reactivity, followed by thorough rinsing with deionized water. Finally, the steel was dried to complete the pre-treatment process. This procedure ensured contaminant removal and surface activation for effective electroplating.
2.2. Synthesis of Pyridine Oxime Complexing Angents
2.2.1. Synthesis of 2-Pyridinecarboxaldehyde Oxime
Dissolve 30.53 g of 2-pyridine-formaldehyde in methanol and transfer the solution to a round-bottom flask. Subsequently, dissolve 24.32 g of hydroxylamine hydrochloride in deionized water and add it to the same round-bottom flask, stirring continuously until complete dissolution is achieved. Then, prepare a sodium hydroxide solution by dissolving 28.00 g of sodium hydroxide in deionized water and slowly drip it into the reaction mixture in the round-bottom flask. During this process, a white precipitate will initially form but gradually disappear as the sodium hydroxide is added dropwise. Once the precipitate has completely disappeared, continue stirring for an additional 24 h to ensure the reaction reaches completion. Afterward, adjust the pH of the solution to 7 by slowly adding concentrated hydrochloric acid while monitoring the pH with pH test paper. Once the desired pH is reached, pour the solid–liquid mixture from the round-bottom flask into a Buchner funnel lined with filter paper and apply vacuum filtration to separate the solid product. Finally, dry the collected solid in a desiccator.
2.2.2. Synthesis of 2-Acetylpyridine Ketoxime
Following the procedure outlined in
Section 2.2.1, replace 2-pyridine-formaldehyde with 36.34 g of 2-acetylpyridine.
2.2.3. Synthesis of 2-Pyridine Amidoxime
Adopting the method described in
Section 2.2.1, substitute 2-pyridine-formaldehyde with 31.2 g of 2-cyanopyridine. Maintain the entire reaction process at a constant temperature of 45 °C to ensure optimal reaction conditions.
2.3. Formulation and Methodology
In alkaline Zn-Ni electroplating systems, the absence of complexing agents leads to rapid Ni(OH)2 precipitation due to the low-solubility product of nickel hydroxide. This precipitation disrupts ionic mobility, causing erratic current distribution and preferential Zn deposition. Comparative trials without complexing agents yielded porous, low-Ni coatings with rough surfaces, validating the necessity of coordinated metal stabilization for alloy formation.
The citrate/TEPA complexing agent system is widely adopted in cyanide-free plating facilities due to its moderate metal coordination capacity and compatibility with conventional zincate baths. Despite achieving baseline performance metrics such as moderate bath lifetimes and established corrosion resistance, this system suffers from three critical limitations in industrial applications: (1) the oxidative degradation of citrate at a high pH, leading to accelerated bath aging and frequent organic replenishment requirements; (2) dendritic growth induced by TEPA’s linear molecular geometry, resulting in an uneven current distribution and surface irregularities; and (3) insufficient hydrogen evolution suppression, causing hydrogen embrittlement in high-strength substrates. These systemic deficiencies contribute to elevated operational costs through weekly additive adjustments and stringent wastewater treatment demands to meet environmental regulations. The persistent industrial reliance on this obsolete system, despite its inherent shortcomings, underscores the urgent need for advanced complexing agents capable of combining pH stability with microstructural control.
Pyridine oxime complexing agents were employed in the Zn-Ni alloy electroplating solution and systematically compared with the conventional complexing agent system comprising sodium citrate and TEPA. Leveraging their multi-dentate coordination characteristics and robust metal ion complexation ability, pyridine oxime complexing agents were designed to enhance the stability of electroplating solutions and modulate the co-deposition rate of zinc and nickel ions, ultimately improving the performance of Zn-Ni alloy coatings.
The selection of pyridine oxime derivatives (2-pyridinecarboxaldehyde oxime, 2-acetylpyridine ketoxime and 2-pyridine amidoxime) was guided by their structural adaptability to alkaline Zn-Ni co-deposition requirements. Unlike traditional pyridine complexing agents (e.g., 2,2′-bipyridine) that exhibit single N-donor coordination and limited pH stability, pyridine oximes combine redox-stable pyridine rings with oxime groups capable of pH-dependent N,O-chelation. Carboxaldehyde oxime provides accessible hydroxyl oxygen for Zn
2+ stabilization, while ketoxime’s methyl substituent enhances Ni
2+ affinity through inductive effects. Amidoxime extends coordination diversity via amino nitrogen, forming tridentate complexes that suppress hydrogen evolution. These derivatives address key limitations of citrate/TEPA systems, including dendritic growth and organic degradation, by establishing diffusion-controlled deposition pathways (
Table 1).
All chemicals used to prepare the electroplating samples were analytical grade chemicals and were acquired from different manufacturers in China. Electrolytes were prepared using ZnO (8–12 g/L), NaOH (80–120 g/L), NiSO4∙6H2O (8–12 g/L), DPE-III (6 mL/L) and BH-336 (6 mL/L). In terms of complexing agents, the pyridine oxime complexing agent was 24–32 g/L and the control groups of sodium citrate/TEPA complexing agents were 55 g/L and 6 mL/L, respectively. The weighed ZnO and NaOH were dissolved completely by stirring in deionized water. The NiSO4∙6H2O and the complexing agent were also dissolved in deionized water, followed by the addition of triethylamine. The mixture was stirred continuously until complete dissolution was achieved and the solution turned a wine-red color. The role of the trace amounts of triethylamine was to act as a deprotonating agent during the process of Ni2+ forming complexes with the complexing agents. Gradually, the two aforementioned solutions were combined, sequentially incorporating DPE-III and BH-336, to prepare the electroplating solution.
The cathode and anode were, respectively, made of 45 mm × 40 mm × 2 mm annealed low-carbon steel sheets and nickel sheets. The electrodes before electrode deposition were polished and cleaned before plating to ensure the combination of the coating and the substrate. Under the condition that the current density was 4 A/dm
2 and the room temperature was 25 °C, the DC rectifier was used for electrodeposition for 15 min each, respectively. The composition and parameter selections for DC electrodeposition are listed in
Table 2.
All coatings underwent ultrasonic cleaning in deionized water followed by drying to remove residual electrolytes. No passivation treatments were applied to ensure the tested corrosion performance exclusively reflected the intrinsic properties of the Zn-Ni alloy coatings.
The pyridine oxime concentrations (24–32 g/L) were optimized through an orthogonal experimental design, systematically evaluating their electrochemical performance in alkaline Zn-Ni alloy deposition. For 2-pyridinecarboxaldehyde oxime, the optimal concentration was determined as 24 g/L, 2-Acetylpyridine ketoxime exhibited ideal properties at 32 g/L and 2-pyridine amidoxime achieved optimal properties at 30 g/L.
2.4. Electrochemical Measurements
The Tafel and electrochemical impedance spectroscopy (EIS) measurements of the prepared samples were taken at 25 °C using a Chenhua CHI760E (Aoqikehua Medical Supply Chain Management Service Co., Ltd, Shanghai, China) electrochemical workstation, following the ASTM G5-14 and ASTM G106-89 standards. A standard three-electrode system was employed, with the sample (exposed area of 1 cm2) serving as the working electrode, a platinum electrode (Pt) as the auxiliary electrode and a saturated calomel electrode as the reference electrode. The electrolyte used for testing was a 3.5% NaCl solution. The open-circuit potential was recorded for 10 min prior to further measurements. Subsequently, EIS measurements were carried out with an amplitude of 5 mV over a frequency range of 100 kHz to 0.1 Hz. Following the EIS analysis, potentiodynamic polarization tests (Tafel) were performed at a scanning rate of 1 mV/s.
2.5. Microstructure Characterization
Using a Ultra™ Model 55 (Carl Zeiss Management Ltd, Shanghai, China). Scanning Electron Microscope (SEM) with a Secondary Electron (SE) signal, the surface morphology of the coatings was observed at an acceleration voltage ranging from 5 to 10 kV. The chemical compositions of the coatings were analyzed using Energy-Dispersive Spectroscopy (EDS) integrated with SEM, providing semiquantitative results. The phase composition of the samples was characterized by a D8 Advance (Bruker Company, Germany) X-Ray Diffractometer (XRD) using Cu Kα radiation (λ = 1.5406 Å), with a scanning range of 10° ≤ 2θ ≤ 90° and a scanning speed of 2°/min. The diffraction patterns were analyzed using Jade 6 software. Additionally, the surface morphology and roughness of the samples were evaluated using a iNanoScope 3D (Veeco, NY, USA) Atomic Force Microscope (AFM).
2.6. Thickness Measurements
A DJH-G multifunction electrolyte thickness gauge (Dongguan Kedi Instrument Co., LTD, Guangdong, China) was used to evaluate the thickness of the Zn-Ni alloy coatings (three-point average per sample). Meanwhile, a cross-sectional SEM analysis was performed to investigate the internal morphology of the coatings.
3. Results and Discussion
3.1. Electrochemical Corrosion Performance
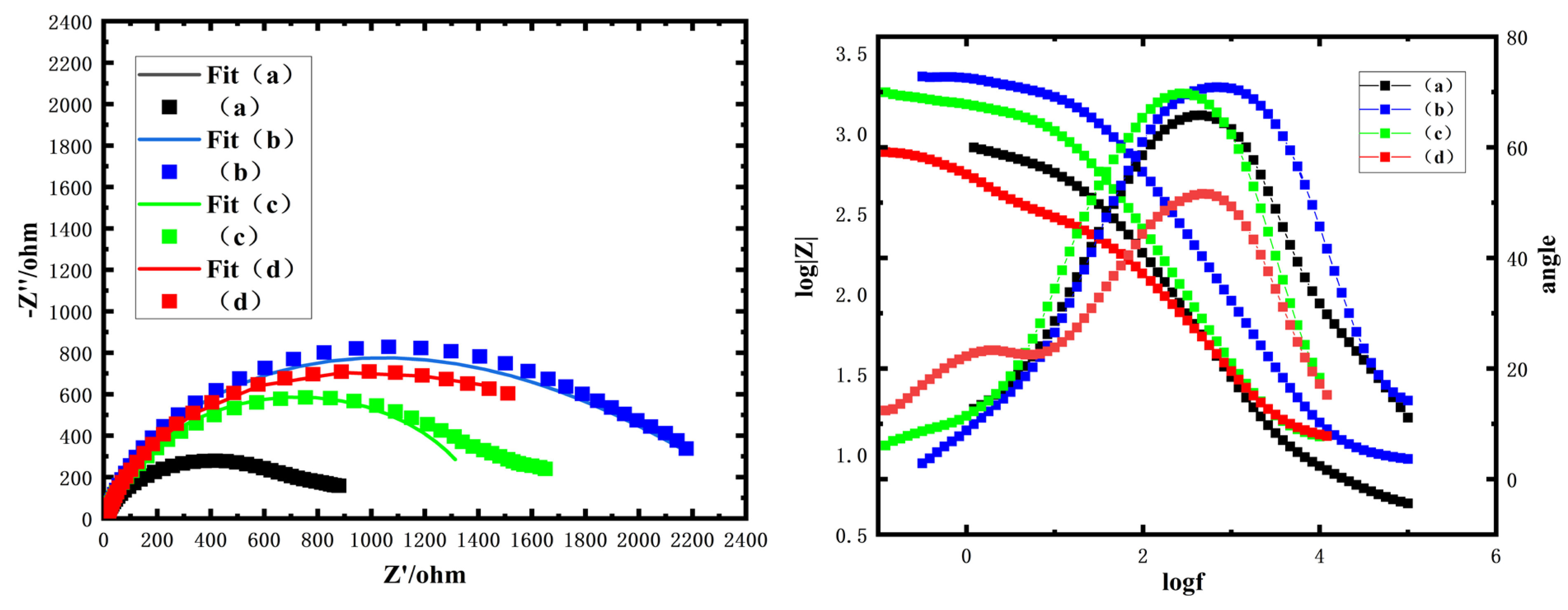
The electrochemical impedance spectroscopy (EIS) results of the Zn-Ni alloy coatings deposited with four complexing agents are presented in
Figure 1. Nyquist plots reveal distinct semicircular characteristics in the high-frequency region, corresponding to charge transfer resistance (
Rct) at the electrode/electrolyte interface. Notably, the coatings obtained with 2-pyridinecarboxaldehyde oxime and 2-pyridine amidoxime demonstrate superior capacitive behavior, as evidenced by their significantly larger impedance arc diameters (>2000 Ω), which exceed those of the conventional citrate/TEPA complexes (800 Ω) by 150% and 250%, respectively. The 2-acetylpyridine ketoxime system exhibits an intermediate arc diameter (≈1500 Ω), still maintaining an 87.5% improvement over traditional formulations.
This order in Rct magnitudes directly correlates with the coordination stability constants of the complexing agents, where stronger complexing agent–metal interactions (particularly through the N-O donor atoms in pyridine oximes) effectively suppress charge transfer processes associated with metal dissolution. The depressed semicircle characteristics suggest non-ideal capacitor behavior, possibly arising from surface roughness or heterogeneous reaction distributions.
Corresponding Bode plots reveal distinct time constants in the intermediate frequency range (1–100 Hz), corresponding to charge transfer dynamics, and a low-frequency plateau (<0.1 Hz) indicative of diffusion-limited processes. The phase angle maxima of 70.81° and 69.72° observed in the 2-pyridinecarboxaldehyde oxime and 2-acetylpyridine ketoxime systems suggest reduced surface heterogeneity compared to the citrate/TEPA control (68.80°), aligning with their superior corrosion resistance. This dual-plot analysis reinforces the critical role of coordination chemistry in modulating interfacial stability and mass transport behavior. However, the 2-pyridine amidoxime system features the lowest phase angle (51.60°) despite its elevated Rct, indicating compromised surface film stability. The impedance modulus |Z| and phase angle collectively demonstrate that 2-pyridinecarboxaldehyde oxime achieves optimal interfacial characteristics, with |Z| exceeding 104 Ω·cm2 and the phase angle approaching ideal capacitive behavior (70.81°), confirming that it is the most stable coating/surface oxidation film system.
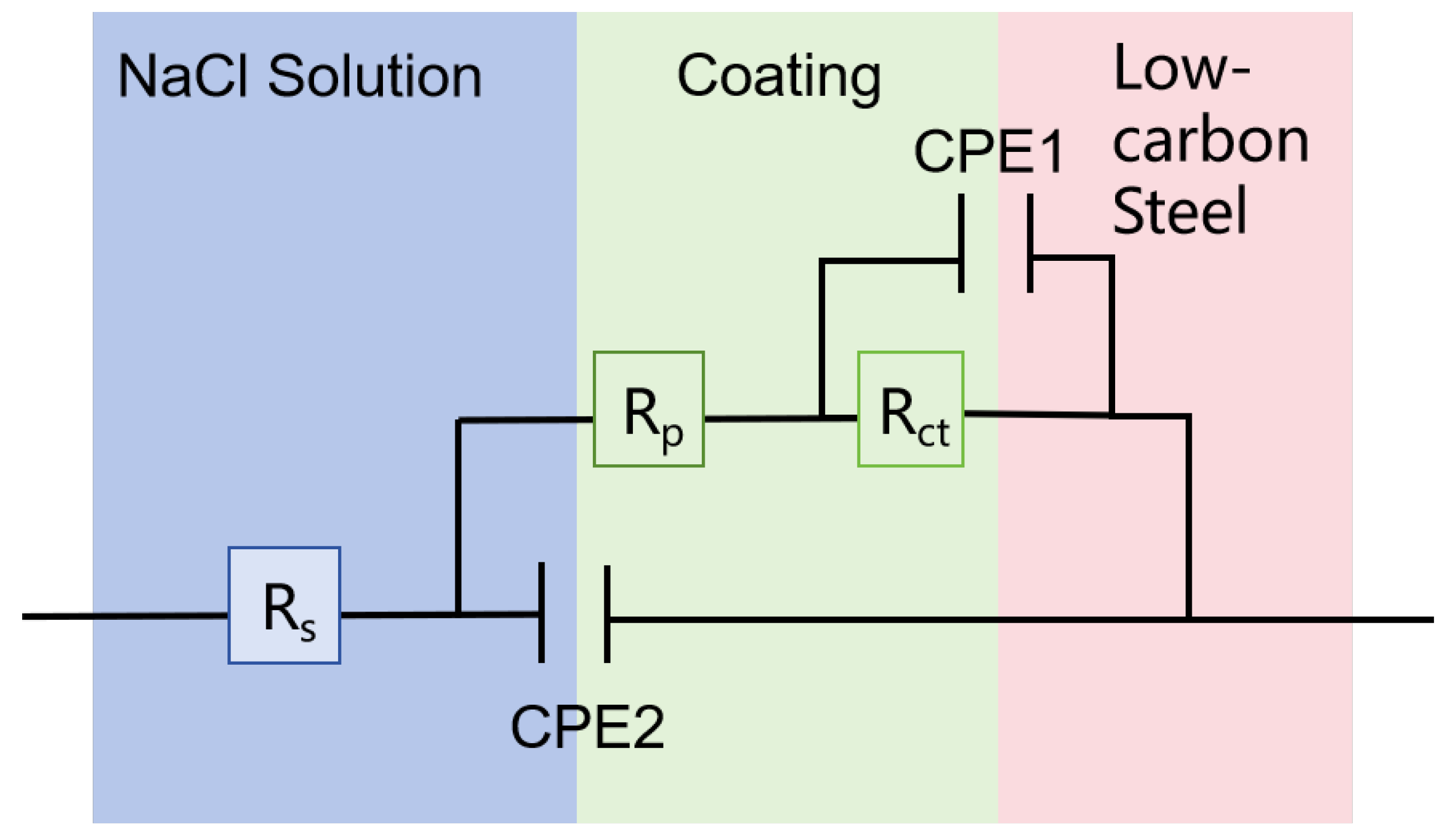
The EIS data were modeled using the equivalent circuit shown in
Figure 2, with their key parameters summarized in
Table 3.
Rs represents the solution resistance between the working and reference electrodes, governed by the electrolyte’s ionic conductivity. CPE (constant phase element) replaces the ideal capacitor to account for non-ideal capacitive behavior caused by surface heterogeneity, such as porosity or uneven current distribution. Its impedance is defined as
, where
Y0 is the admittance magnitude,
w is the angular frequency and
n quantifies surface roughness (with
n = 1 for an ideal capacitor). CPE1 denotes the bilayer capacitance of the coating/metal, and CPE2 denotes the constant phase element of the bilayer capacitance of the solution/coating.
Rct (charge transfer resistance) reflects the kinetic barrier for electrochemical reactions at the electrode/electrolyte interface, inversely proportional to the corrosion rates.
R1 represents the resistance of the surface oxidation film.
Rp (polarization resistance) combines the contributions from the charge transfer and surface oxidation film, representing the coating’s overall corrosion resistance. This CPE behavior aligns with the nanocrystalline microstructure of the Zn-Ni coatings, where grain boundaries and defects disrupt ideal capacitive responses.
The solution resistance Rs ranges from 6.25 to 11.64 Ω·cm2, governed by ionic conductivity variations in the electrolyte microenvironment. Constant phase elements (CPEs) replace the ideal capacitors to account for surface heterogeneity, with CPE1 (coating/metal interface) showing admittance values of 2.06–9.77 × 10−6 Ω−1·cm−2·sn and CPE2 (solution/coating interface) ranging 20.73–91.73 × 10−6 Ω−1·cm−2·sn. The 2-pyridinecarboxaldehyde oxime system demonstrates superior charge transfer resistance (Rct = 2647.02 Ω·cm2) and polarization resistance (Rp = 2684.31 Ω·cm2), exceeding conventional citrate/TEPA formulations by 214%, respectively. Notably, the CPE-T parameters reveal microstructural differences: 2-acetylpyridine ketoxime exhibits maximum dispersion at the solution/coating interface (CPE2-T = 91.73 × 10−6 Ω−1·cm−2·sn), while 2-pyridinecarboxaldehyde oxime balances optimal charge transfer inhibition with moderate surface homogeneity (CPE2-T = 33.07 × 10−6 Ω−1·cm−2·sn). The conventional system’s intermediate CPE-T value (46.94 × 10−6 Ω−1·cm−2·sn) correlates with its compromised surface morphology, while 2-pyridinecarboxaldehyde oxime’s 3.3-fold higher CPE-T compared to 2-acetylpyridine ketoxime suggests enhanced time constant dispersion from microstructural defects. These systematic correlations establish quantitative structure–property relationships between ligand architecture (particularly N-O donor configurations), interfacial charge transfer dynamics and macroscopic corrosion resistance in Zn-Ni alloy electrodeposition systems.
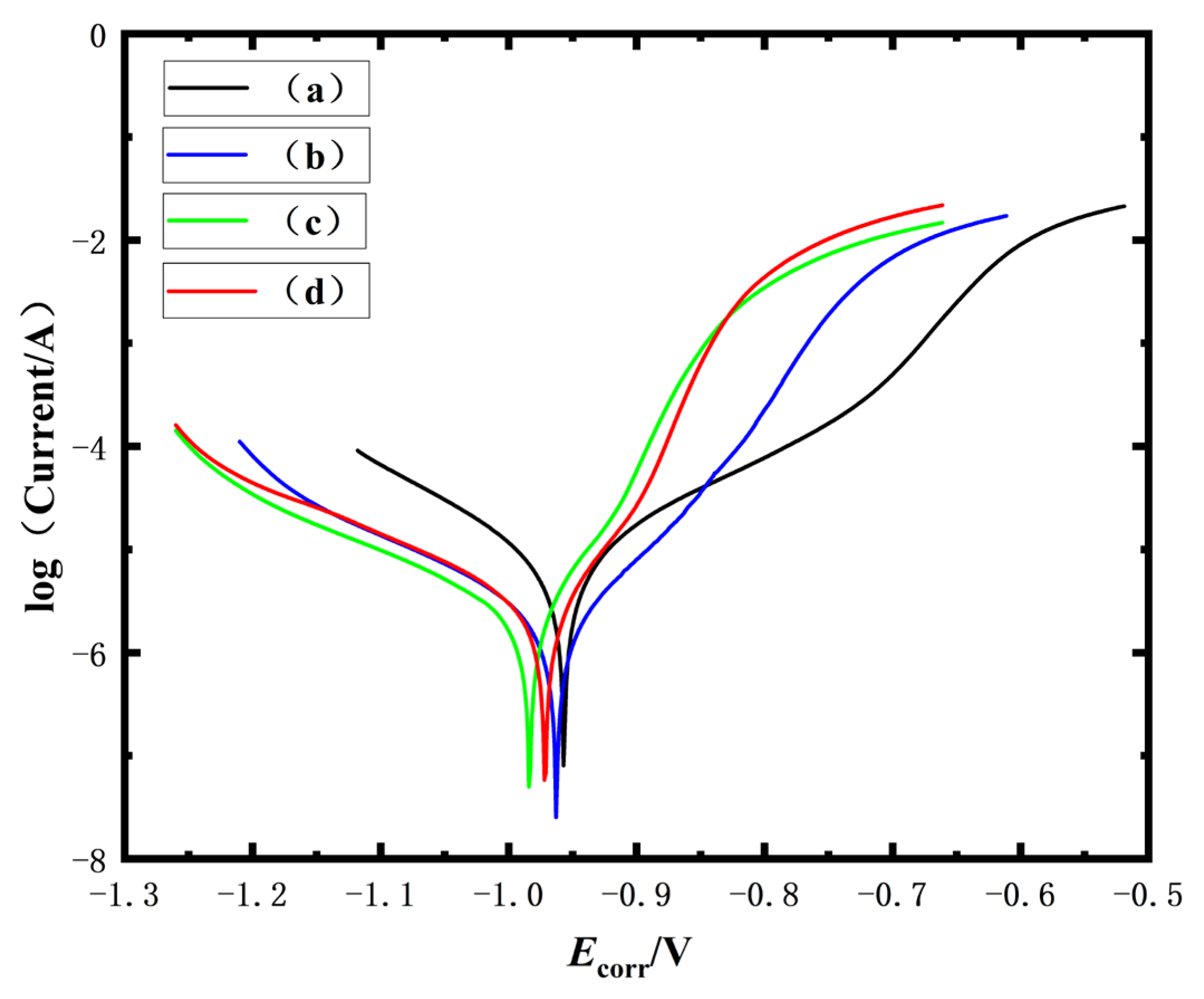
The Tafel polarization analysis (
Figure 3) reveals that 2-acetylpyridine ketoxime achieves the lowest corrosion current density (
icorr = 1.686 μA·cm
−2), demonstrating an 82.4% reduction compared to the conventional citrate/TEPA systems (8.530 μA·cm
−2). As detailed in
Table 4, the pyridine oxime derivatives exhibit comparable corrosion potentials (
Ecorr = −0.963 to −0.984 V) with nuanced kinetic differences: 2-pyridinecarboxaldehyde oxime shows superior anodic inhibition (βa = 13.1 mV/dec) versus 2-acetylpyridine ketoxime (βa = 25.1 mV/dec), suggesting distinct passivation mechanisms. Notably, the 11.3%
icorr differential between 2-acetylpyridine ketoxime and 2-pyridine amidoxime (1.775 μA·cm
−2) contrasts with their 23.5% difference in
Rct from the EIS analysis, indicating 2-acetylpyridine ketoxime’s compromised charge transfer resistance despite optimal active corrosion suppression. The conventional system’s 4.8-fold higher
icorr aligns with its minimal
Rct, confirming deficient barrier protection.
Synergistic analysis reveals 2-pyridinecarboxaldehyde oxime achieves optimal balance between kinetic control (icorr = 1.997 μA·cm−2) and thermodynamic stability (Rct = 2647.02 Ω·cm2), attributed to its dual N,O-chelation enhancing coating stability. Among the pyridine oximes, performance follows the following order: 2-acetylpyridine ketoxime (best icorr), 2-pyridine amidoxime, 2-pyridinecarboxaldehyde oxime (best Rct) and finally citrate/TEPA. There is <15% variation among the pyridine derivatives versus an 82% improvement over the conventional system.
The superior icorr of the coatings deposited with 2-acetylpyridine ketoxime (1.686 μA·cm−2) compared to the other pyridine oximes (icorr = 1.775–1.997 μA·cm−2) suggests enhanced active corrosion inhibition, likely attributed to its ketoxime group’s preferential adsorption at anodic sites, disrupting metal dissolution pathways. However, its relatively lower Rct (1403.13 Ω·cm2 vs. 2647.02 Ω·cm2 for 2-pyridinecarboxaldehyde oxime) indicates weaker passive barrier protection, potentially due to its reduced γ-phase crystallinity (21.71 nm grain size vs. 24.02 nm for aldehyde oxime) and increased microstructural heterogeneity (Ra = 8.20 nm). This dichotomy implies that 2-acetylpyridine ketoxime primarily suppresses localized anodic activity through dynamic adsorption–desorption processes, whereas systems with a higher Rct rely on stable oxide/hydroxide interlayers for long-term protection. The intermediate CPE-T value further supports this mechanism, reflecting a balance between surface coverage efficiency and defect density.
3.2. Surface Morphology and Composition Analysis
3.2.1. Surface Morphology
The SEM analysis (
Figure 4 and
Figure 5) demonstrates significant morphological variations among Zn-Ni coatings deposited with different complexing agents. At 5 k× magnification (
Figure 4), the 2-pyridine amidoxime-derived coating exhibits optimal surface integrity with defect-free morphology, featuring only minor surface depressions. In contrast, 2-pyridinecarboxaldehyde oxime shows substantial porosity with an irregular cavity distribution, while 2-acetylpyridine ketoxime maintains moderate surface quality. The conventional citrate/TEPA system displays severe topographic irregularities, including ridge-like protrusions and linear striations aligned parallel to substrate polishing marks.
The comparative SEM analysis at 30 k× magnification (
Figure 5) reveals a distinct morphological evolution governed by complexing agent coordination chemistry. The citrate/TEPA system exhibits discontinuous grain growth with pronounced intergranular voids, indicative of activation-controlled deposition kinetics where weak complexation fails to suppress preferential nucleation. In contrast, the pyridine amidoxime system demonstrates densely packed hemispherical nodules with minimal boundary defects, suggesting strong N,O-chelating interactions that promote diffusion-limited growth through enhanced cathodic polarization. The aldehyde oxime and acetylpyridine ketoxime systems display intermediate textures—the former shows elongated columnar grains reflecting anisotropic growth under moderate coordination strength, while the latter achieves quasi-spherical grains through improved surface diffusion enabled by optimal complexing agent adsorption.
The pit formation observed in citrate/TEPA and aldehyde oxime derivatives may stem from two competing mechanisms: localized dezincification at coordination-deficient sites due to complexing agent desorption fluctuations, and hydrogen evolution-induced microporosity arising from insufficient overpotential regulation. The superior surface leveling in the amidoxime and ketoxime systems qualitatively correlates with their stronger metal–complexing agent binding constants, which restrict three-dimensional island growth via the interfacial stabilization of adatoms. These morphological differences, while visually apparent in high-resolution imaging, require complementary electrochemical characterization to quantitatively resolve the underlying coordination effects on deposition kinetics.
3.2.2. Composition Analysis
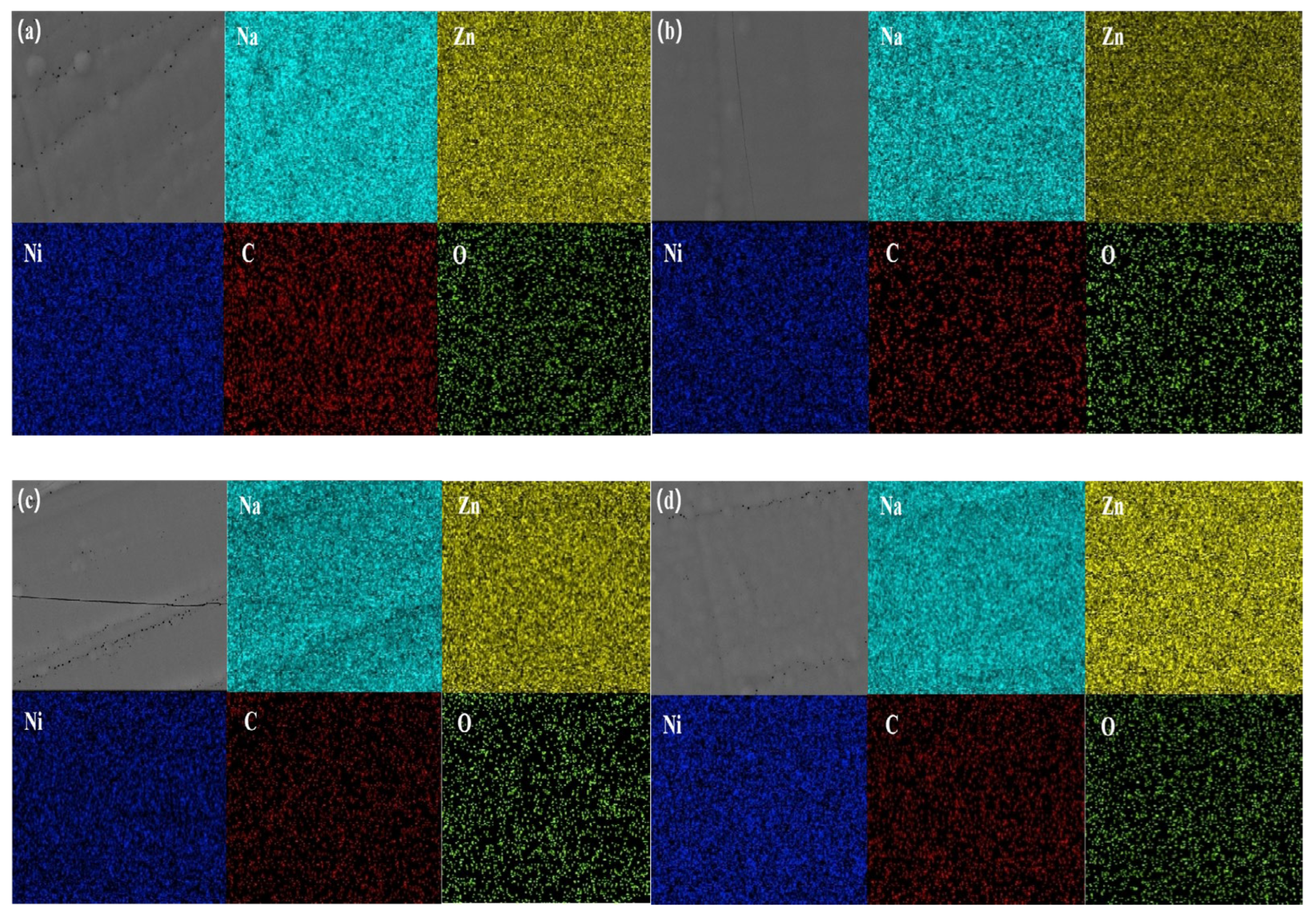
The EDS analysis (
Figure 6) confirms the homogeneous elemental distribution of Zn and Ni without segregation in all coatings, as evidenced by their spatial mapping correlation coefficients exceeding 0.95. The quantitative results (
Figure 7 and
Table 5) reveal complexing agent-dependent Ni incorporation efficiencies, with 2-pyridinecarboxaldehyde oxime achieving maximum Ni content (11.8 wt%, Zn/Ni = 5.0:1), followed by citrate/TEPA (10.8 wt%, Zn/Ni = 6.3:1) and 2-acetylpyridine ketoxime (10.4 wt%, Zn/Ni = 6.4:1), while 2-pyridine amidoxime shows marginally reduced Ni incorporation (9.8 wt%, Zn/Ni = 7.1:1). Notably, when calculated as the mass ratio within the Zn-Ni matrix, the Ni percentages range from 12.36% (2-pyridine amidoxime) to 14.29% (2-pyridinecarboxaldehyde oxime), demonstrating effective alloying control across all systems.
Residual element analysis identifies three characteristic impurities: sodium (5.7–7.3 wt%) primarily originating from NaOH electrolyte residues in the alkaline zincate bath, carbon (10.5–15.0 wt%) arising from the incomplete combustion of organic additives (complexing agents/brighteners) and oxygen (0.6–1.2 wt%) associated with surface oxide/hydroxide formation. The residual carbon content (10.5–15.0 wt%) observed in all coatings primarily originates from two sources: (1) the incomplete decomposition of organic additives (complexing agents and brighteners) during electrodeposition and (2) electrolyte residues entrapped within the nanocrystalline matrix. Specifically, DPE-III and BH-336 undergo partial electrochemical reduction at cathodic potentials, generating carbonaceous byproducts that co-deposit with Zn-Ni alloys. The citrate/TEPA system exhibits the highest carbon content (15.0 wt%), consistent with its higher additive decomposition propensity under alkaline conditions, whereas 2-pyridinecarboxaldehyde oxime achieves the lowest carbon retention (10.5 wt%) due to its stronger metal-binding stability and reduced side reactions. Oxygen content shows complexing agent-specific variation, with 2-pyridinecarboxaldehyde oxime containing 1.2 wt% O versus 0.6–0.8 wt% in other systems, potentially linked to its aldehyde group’s enhanced surface oxidation tendency. Total impurity levels (17.3–23.1 wt%) remain consistent with the characteristic co-deposition artifacts in alkaline zincate systems operated at pH > 13.5.
3.3. Surface Morphology Characterized by AFM
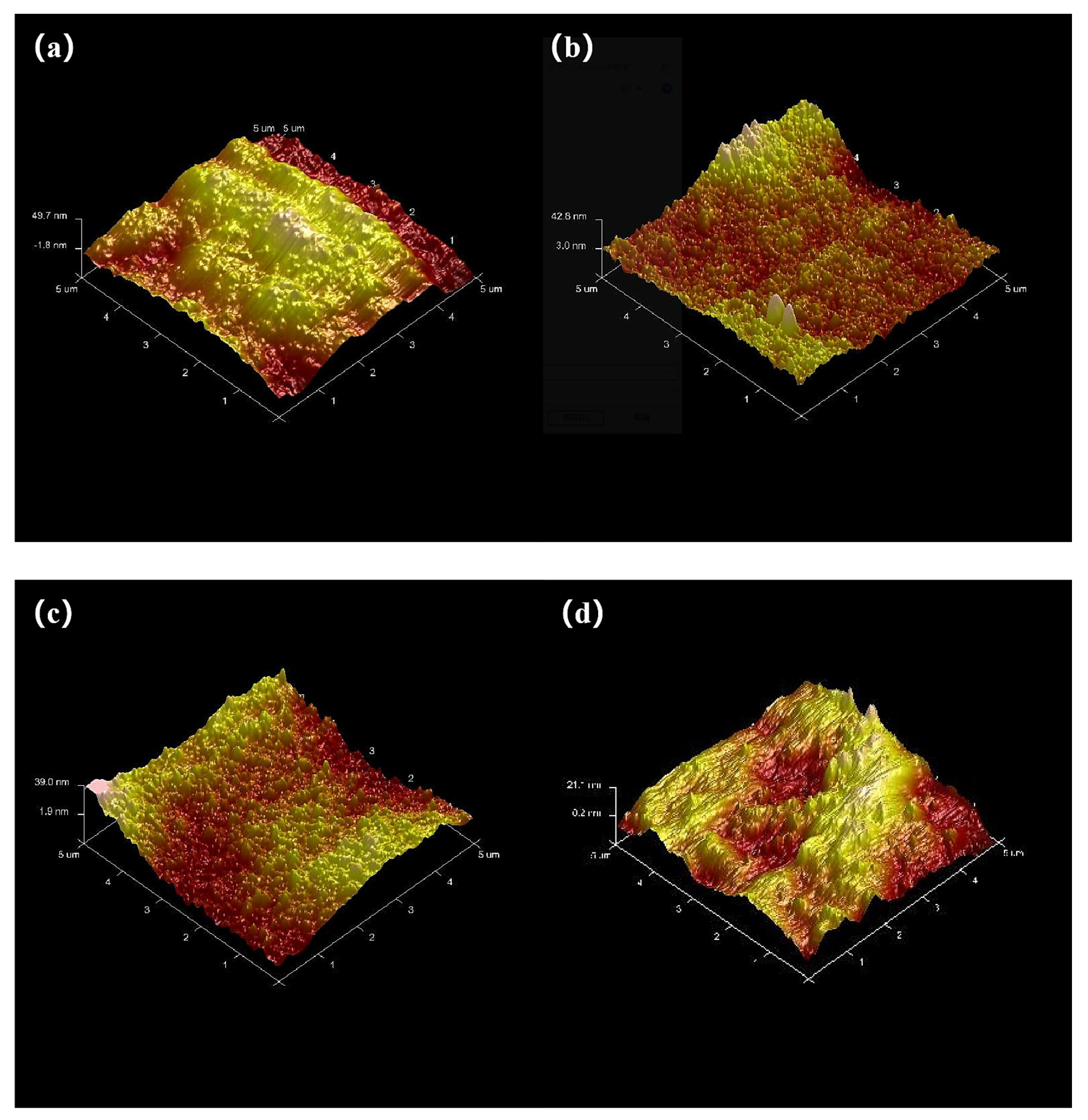
The AFM analysis (
Figure 8) reveals a distinct morphological evolution in Zn-Ni coatings mediated by different complexing agents. The citrate/TEPA system exhibits coarse crystallites (lateral size 1.2–1.8 μm) with directional alignment along the deposition axis, accompanied by substantial height variations (Rq = 16.4 nm, Ra = 13.4 nm). In contrast, oxime-based complexing agents demonstrate improved surface control: 2-pyridinecarboxaldehyde oxime produces mixed nanostructures containing both 300–500 nm spherical domains and sporadic micron-scale crystallites (Rq = 8.90 nm, Ra = 6.53 nm), while 2-acetylpyridine ketoxime forms continuous nanoaggregates (400–700 nm) with moderate surface undulation (Rq = 10.50 nm, Ra = 8.20 nm). The 2-pyridine amidoxime system achieves optimal planarization, featuring defect-free nanoclusters (200–300 nm) embedded in a smooth matrix (Rq = 6.10 nm, Ra = 4.76 nm), representing 62.8% and 64.5% reductions in Rq/Ra values, respectively, compared to the citrate-based system.
The surface roughness parameters (
Table 6) follow the following order: 2-pyridine amidoxime, 2-pyridinecarboxaldehyde oxime, 2-acetylpyridine ketoxime and finally citrate/TEPA. The oxime derivatives collectively demonstrate 37–63% lower roughness metrics than the conventional system. This improved surface topology correlates with the enhanced coordination capability of oxime complexing agents, which elevates the activation energy for metal reduction. This increased energy barrier facilitates diffusion-controlled deposition kinetics, effectively suppressing random nucleation events and promoting oriented grain growth. Simultaneously, enhanced cathodic polarization enables a uniform current distribution across the electrode surface, as described by the mixed potential model for alloy electrodeposition.
3.4. XRD Diffraction Pattern Analysis
The XRD analysis (
Figure 9) identifies γ-phase Ni
5Zn
21 intermetallic compounds (body-centered cubic, (330) plane at 2
θ = 43.25°) as the dominant crystalline component across all Zn-Ni coatings, with the
η-phase (hexagonal close-packed, (112) plane at 82.5°) and minor ZnO ((103) plane at 65.2°) as secondary phases. The oxime-derived coatings exhibit an 18–27% higher γ-phase diffraction intensity compared to the citrate/TEPA system, indicating the enhanced formation of this corrosion-resistant intermetallic. Among oxime complexing agents, 2-pyridinecarboxaldehyde oxime and 2-acetylpyridine ketoxime demonstrate marginally stronger
γ-phase signals than 2-pyridine amidoxime, despite the latter’s superior surface planarization observed in AFM analysis. This crystallographic order explains the comparable corrosion resistance among oxime derivatives despite their morphological differences.
The 2-pyridinecarboxaldehyde oxime system exhibits the highest Iγ/Iη ratio of 4.7, followed by 2-acetylpyridine ketoxime (3.9) and 2-pyridine amidoxime (3.5), all significantly exceeding the citrate/TEPA control system (2.1). This order aligns with the enhanced coordination stability of pyridine oximes, which promotes γ-phase nucleation by stabilizing Ni2+ intermediates during electrodeposition. The inverse correlation between the Iγ/Iη ratios and η-phase grain sizes (25.71 nm for citrate/TEPA vs. 18.87–24.02 nm for oximes) further corroborates the role of oxime ligands in suppressing Zn-dominated η-phase growth. Notably, the 124% increase in Iγ/Iη for 2-pyridinecarboxaldehyde oxime over the conventional system directly explains its superior charge transfer resistance and reduced corrosion current density observed in the electrochemical tests.
The crystallite size can be determined using the Scherrer formula, as presented in Formula 1. In this equation,
D denotes the grain size,
β represents the full width at half maximum (FWHM),
λ corresponds to the X-ray wavelength and
K stands for the Scherrer constant. As shown in
Table 7, The analysis of (330) peak broadening reveals that oxime complexing agents produce refined crystallite sizes: 2-pyridine amidoxime achieves the smallest grains (18.87 nm), followed by 2-acetylpyridine ketoxime (21.71 nm) and 2-pyridinecarboxaldehyde oxime (24.02 nm), while the citrate-based system yields coarser microstructures (25.71 nm). The inverse correlation between grain size and γ-phase content suggests oxime complexing agents promote both phase purity and crystallographic refinement through enhanced metal coordination. The observed ZnO formation likely originates from post-deposition surface oxidation rather than bulk phase segregation, as evidenced by its consistent presence across all systems. These structural features collectively underpin the oxime complexing agents’ corrosion performance advantages, where
γ-phase abundance governs bulk stability while nanocrystalline grain boundaries contribute to coating integrity.
While instrumental broadening was not explicitly corrected in this study, the relative differences in grain sizes between the coatings remain valid for comparative analysis. Uncorrected instrumental contributions to peak broadening may lead to a slight underestimation of the absolute crystallite dimensions, as the measured FWHM (β) inherently includes both sample-related and equipment-induced broadening effects.
3.5. Thickness Analysis
The thickness analysis by the electrolytic thickness measurement of the Zn-Ni alloy coatings electrodeposited with different complexing agents revealed significant variations influenced by ligand chemistry. As quantified in
Table 8, the conventional citrate/TEPA system produced the thickest deposit (10.56 μm), while the pyridine oxime-based complexants generated comparatively thinner coatings: 2-pyridinecarboxaldehyde oxime (6.87 μm), 2-acetylpyridine ketoxime (7.81 μm) and 2-pyridine amidoxime (5.32 μm). A cross-sectional SEM characterization confirms the thickness measurements and demonstrates coherent interfacial adhesion without observable defects (
Figure 10), with coating morphology governed by deposition-controlled grain growth rather than substrate replication effects.
The thickness reduction in pyridine oxime systems correlates with their strong N,O-bidentate chelation capability, which enhances cathodic polarization to decelerate deposition kinetics while promoting optimized atomic rearrangement. This coordination chemistry prioritizes structural refinement over volumetric accumulation, as evidenced by the inverse relationship between coating thickness and microstructural quality. Thinner oxime-derived coatings exhibit increased γ-phase crystallinity and reduced surface roughness, consistent with the manuscript’s previous findings on coordination-controlled nanocrystallization and hydrogen evolution suppression.
The cross-sectional SEM characterization reveals distinct morphological differences between the coatings obtained from the pyridine oxime complexants and the sodium citrate/TEPA system. The pyridine oxime-derived coatings exhibit compact morphology with coherent grain packing and minimal intergranular fissures, reflecting optimized atomic rearrangement under strong N,O-chelation conditions. In contrast, the citrate/TEPA system displays intermittent microcracks along the coating cross-section (
Figure 10), indicative of stress accumulation during rapid deposition kinetics. This structural disparity aligns with the cathodic polarization data, where the oxime ligands’ superior complexation strength promotes cohesive columnar growth through controlled ion discharge, while the citrate/TEPA’s weaker coordination permits faster but less orderly metal deposition. The observed crack propagation in the citrate/TEPA coatings likely originates from hydrogen entrapment and differential shrinkage stresses, factors mitigated in oxime systems through their hydrogen evolution suppression capability, as previously discussed. These cross-sectional observations corroborate the inverse thickness–structure relationship, confirming that pyridine oximes achieve defect density reduction at the expense of deposition rate—a critical trade-off for applications requiring both corrosion resistance and mechanical durability.
Notably, all coating thicknesses exceeded the critical threshold (≈5 µm) required for substrate-independent electrochemical behavior, validating the corrosion resistance data despite thickness variations. The pyridine amidoxime system demonstrated particular efficiency in balancing thickness reduction (5.32 μm) with structural integrity, suggesting its potential for applications demanding both compact coatings and enhanced crystallographic perfection. This thickness–structure–performance relationship underscores the dominance of coordination chemistry in determining deposition outcomes, where ligand selection directly mediates the competition between growth rate modulation and defect minimization.
4. Conclusions
This study systematically demonstrates that pyridine oxime derivatives serve as superior complexing agents for alkaline Zn-Ni alloy electrodeposition through synergistic coordination chemistry mechanisms. The N,O-bidentate chelation capability fundamentally regulates metal reduction kinetics by establishing diffusion-controlled deposition pathways, which concurrently enhance γ-phase intermetallic formation and nanocrystalline refinement. The optimized coordination environment suppresses random nucleation while promoting (330)-oriented Ni5Zn21 crystallite growth, achieving an optimal balance between phase purity and surface defect minimization. Electrochemical performance enhancements arise from the coupled effects of increased charge transfer resistance and cathodic polarization modulation, establishing a self-consistent corrosion protection mechanism involving both barrier layer formation and active dissolution suppression.
While the SEM and AFM analyses reveal distinct morphological features across the complexing agent systems, the nucleation mechanism interpretations derive from established correlations between coordination chemistry and deposition kinetics. The citrate/TEPA system’s discontinuous grain structure aligns with the literature reports of activation-controlled nucleation under weak chelation, whereas pyridine oxime-induced nanocrystalline uniformity corresponds to the diffusion-limited growth patterns observed in strongly coordinated systems. These mechanistic inferences will be systematically validated in future studies through in situ electrochemical quartz crystal microbalance and time-resolved atomic force microscopy to directly monitor nucleation dynamics.
Future investigations will also focus on the molecular-scale tailoring of oxime complexing agents to decouple crystallographic orientation control from hydrogen evolution side reactions. Advanced alloy design strategies incorporating ternary metallic components could exploit lattice strain effects to further improve coating stability. Industrial-scale validation requires the systematic evaluation of coating durability under complex service environments, particularly addressing the organic decomposition byproducts identified in the impurity analyses. The fundamental insights into coordination-controlled deposition kinetics provide a universal framework for developing next-generation electroplating systems with customized microstructure–property relationships.
Comparative evaluations demonstrate that the novel oxime ligands achieve cost parity with conventional citrate/TEPA systems through their streamlined synthesis process and reduced purification requirements, while their compatibility with existing alkaline zincate bath infrastructure eliminates retrofitting costs. The observed enhanced bath stability and refined nanocrystalline morphology synergistically reduce operational expenses and improve coating durability, positioning this approach as both technically and economically viable for industrial-scale corrosion-resistant Zn-Ni alloy production. This advancement underscores the potential of coordination chemistry-driven design in bridging academic innovation with the industrial demands for sustainable and high-performance electroplating solutions.