N-S Co-Doped WC Nanoparticles Show High Catalytic Activity in Hydrogen Evolution Reaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Materials
2.2. Characterization of Material Morphology and Structure
2.3. Electrode Preparation and Electrochemical Testing
3. Results and Analysis
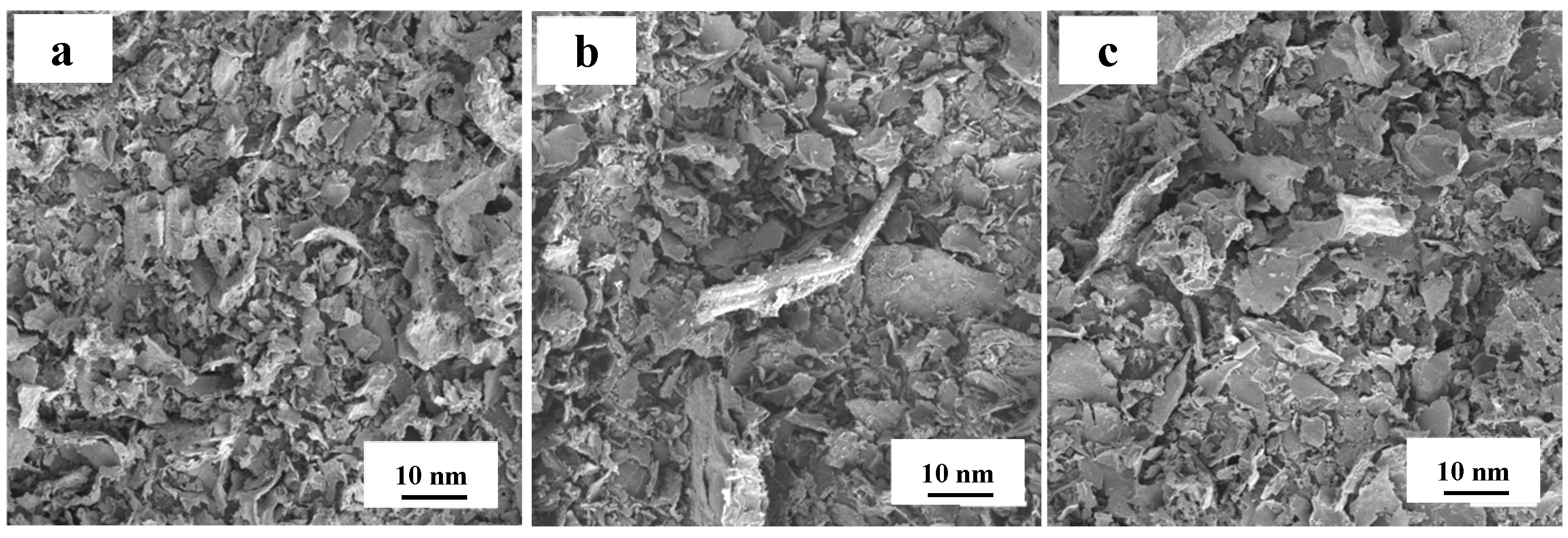
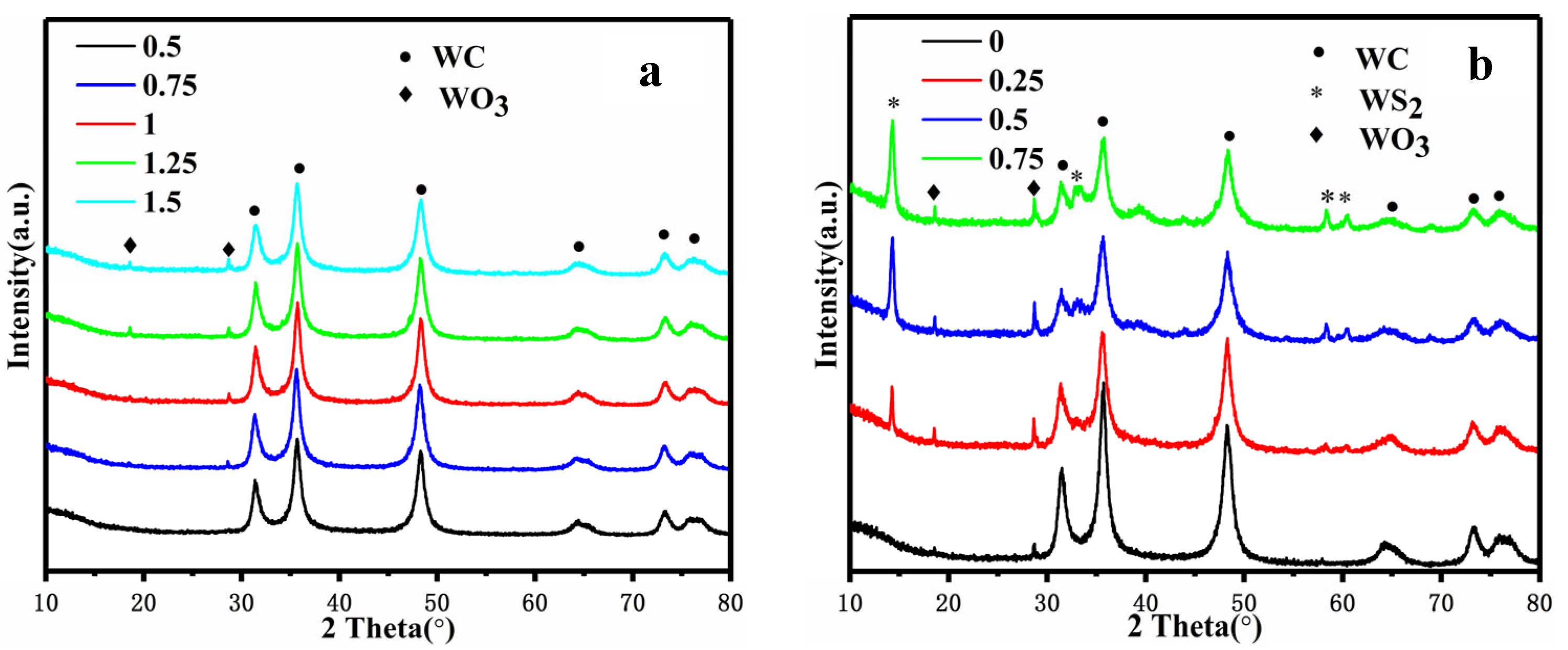
3.1. Physical Phase and Morphology Analysis of Biomass Carbon Carriers
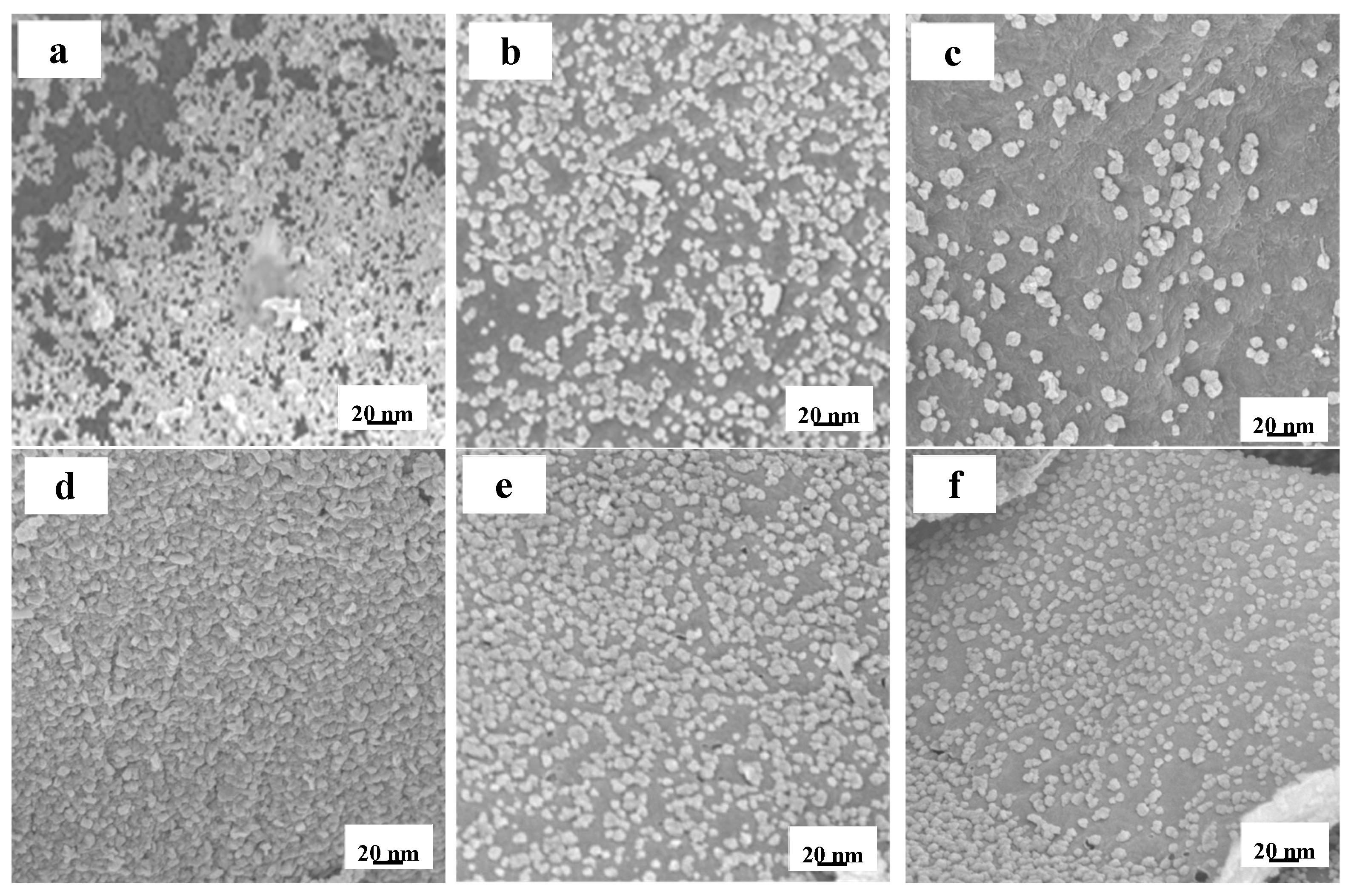
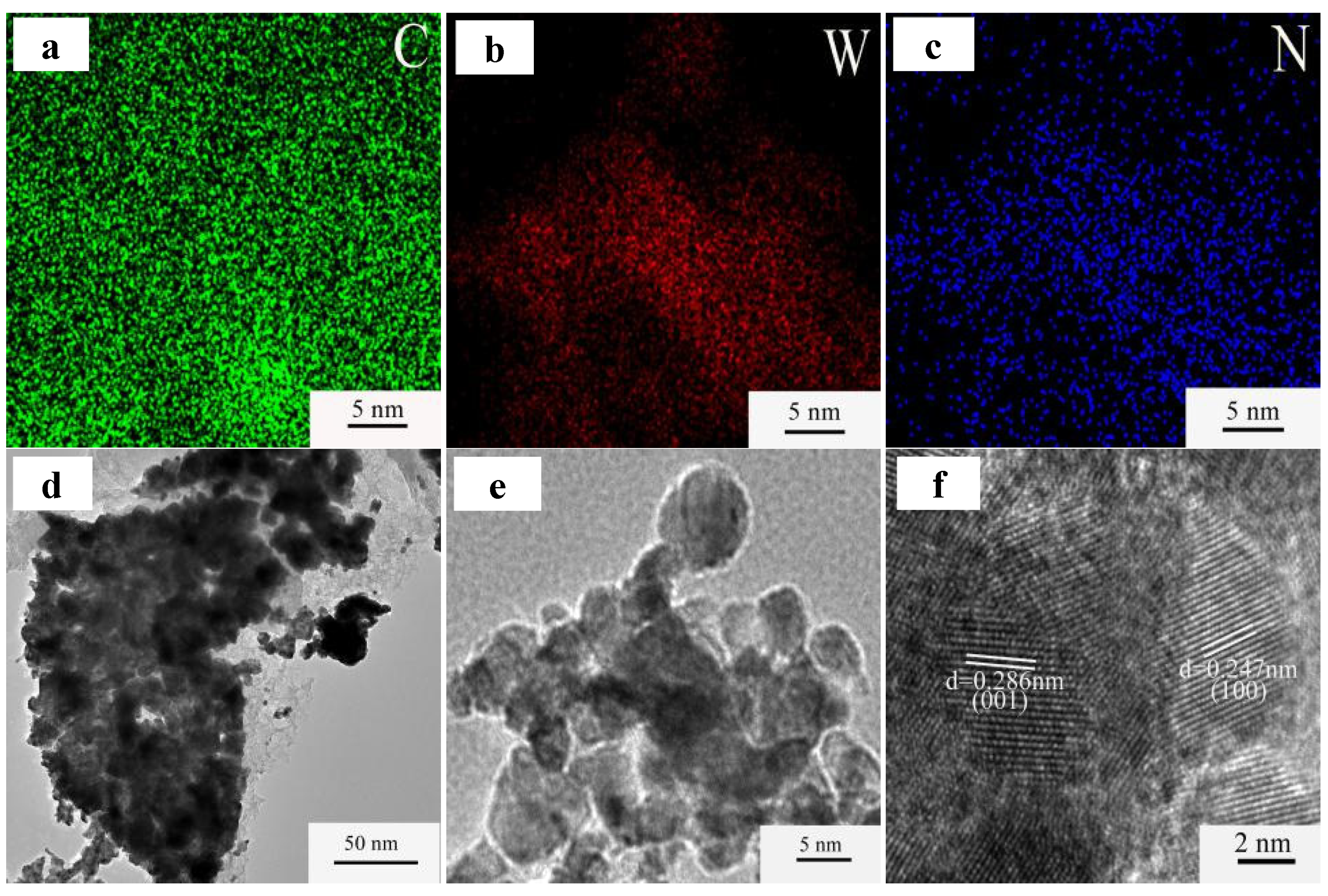
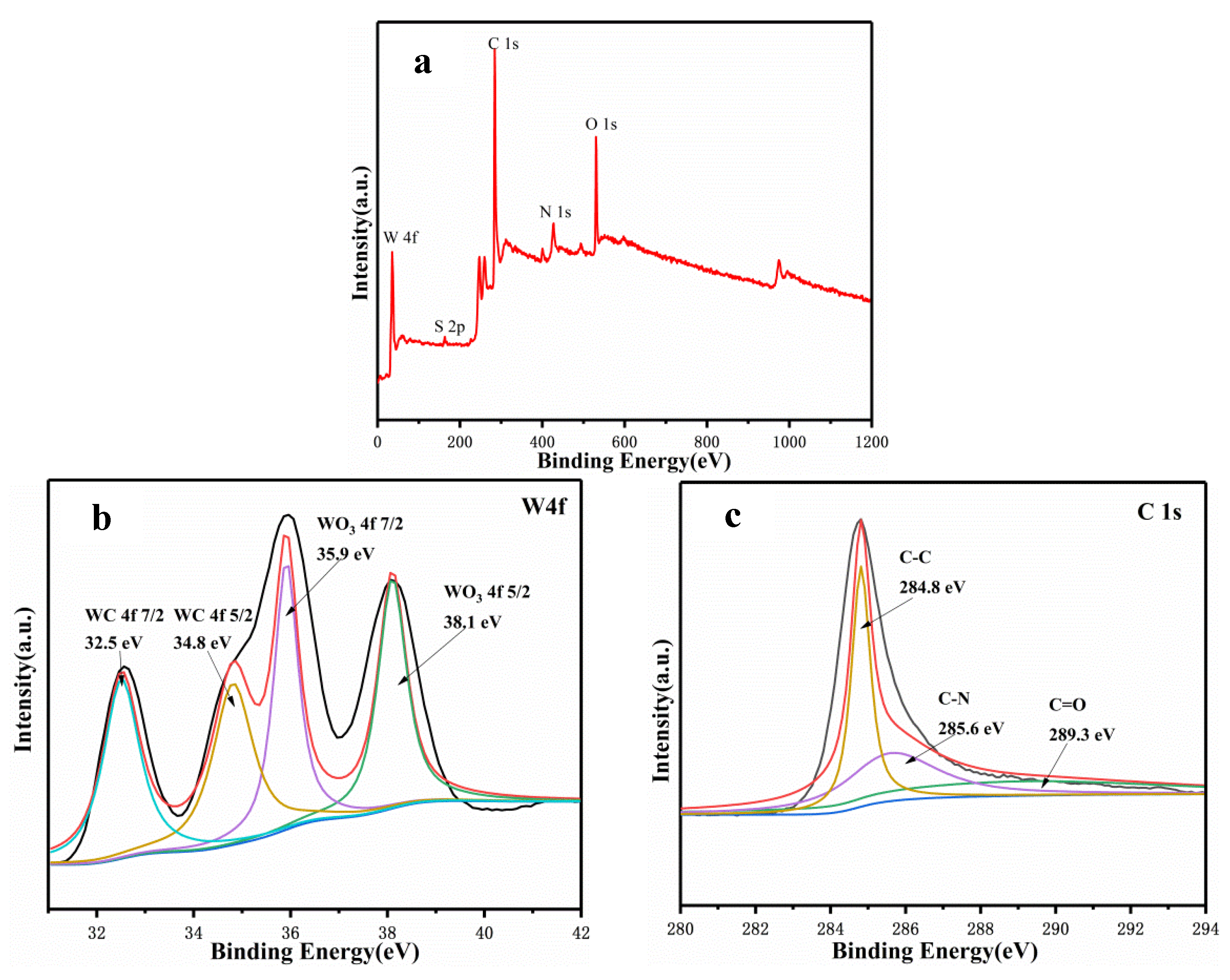
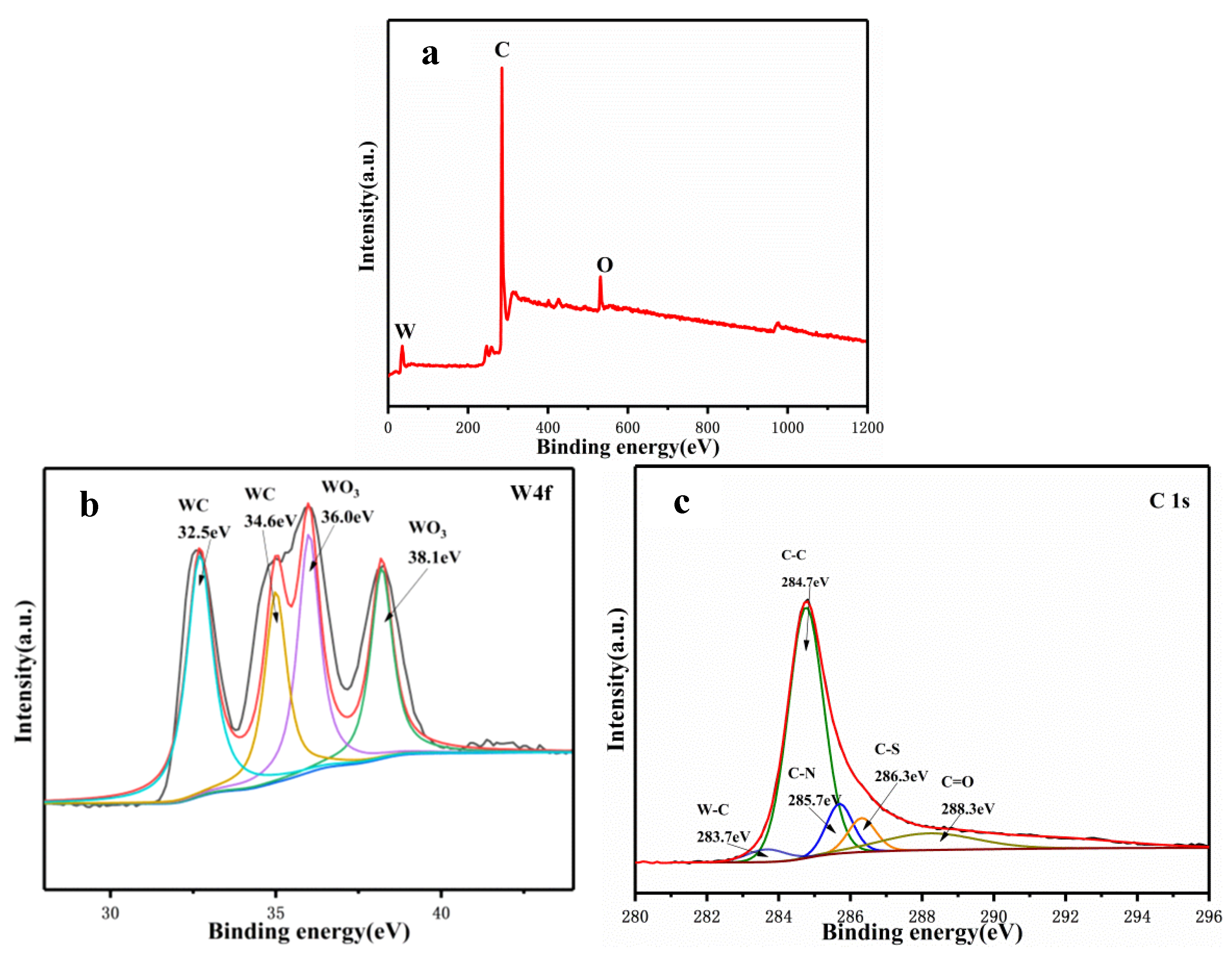
3.2. Elementally Doped WC/C Catalysts and Electrochemical Activities
4. Conclusions
- (1)
- The molten salt method can be used to successfully carbonize pomelo peel powder into flaked and pure biomass carbon with a good specific surface. The sheet structure of biomass carbon doped by N-S remains unchanged.
- (2)
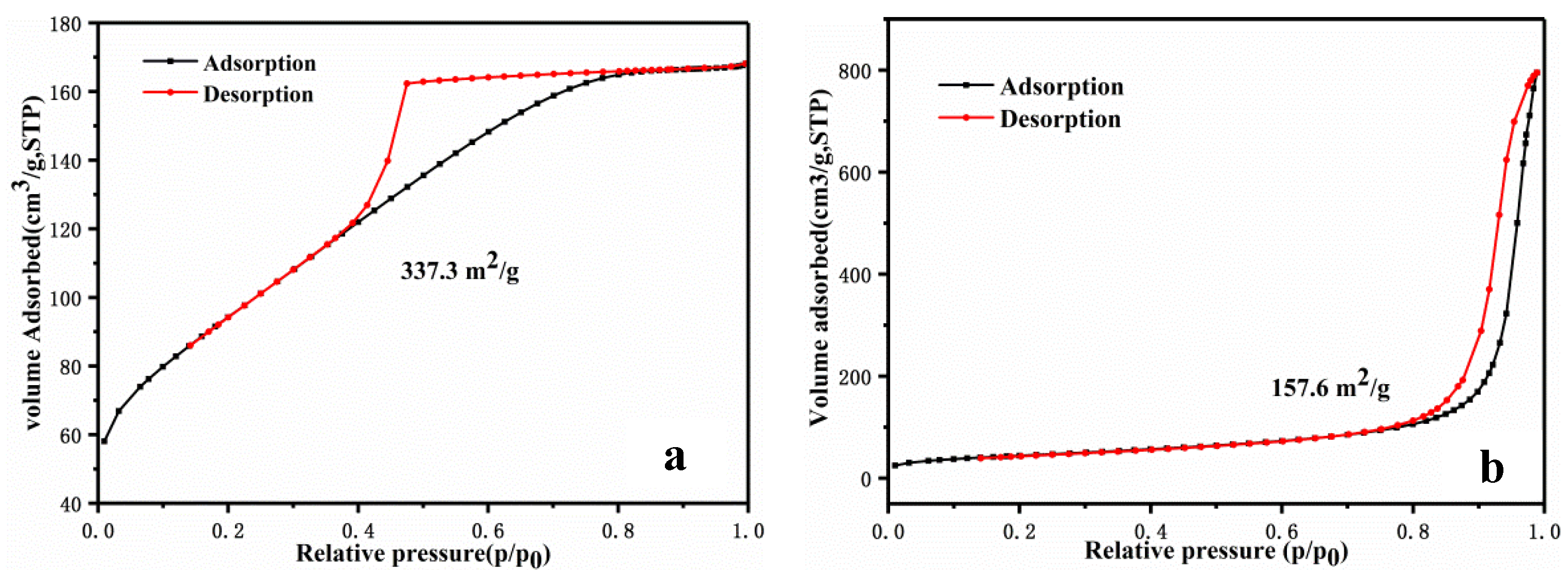
- The N-S-doped WC/C@N-S catalyst, composed of WC, WS2, and WO3, inhibited the agglomeration of surface WC particles and increased the specific surface area from 157.6 m2/g to 337.2 m2/g, thereby improving the HER performance of the catalyst.
- (3)
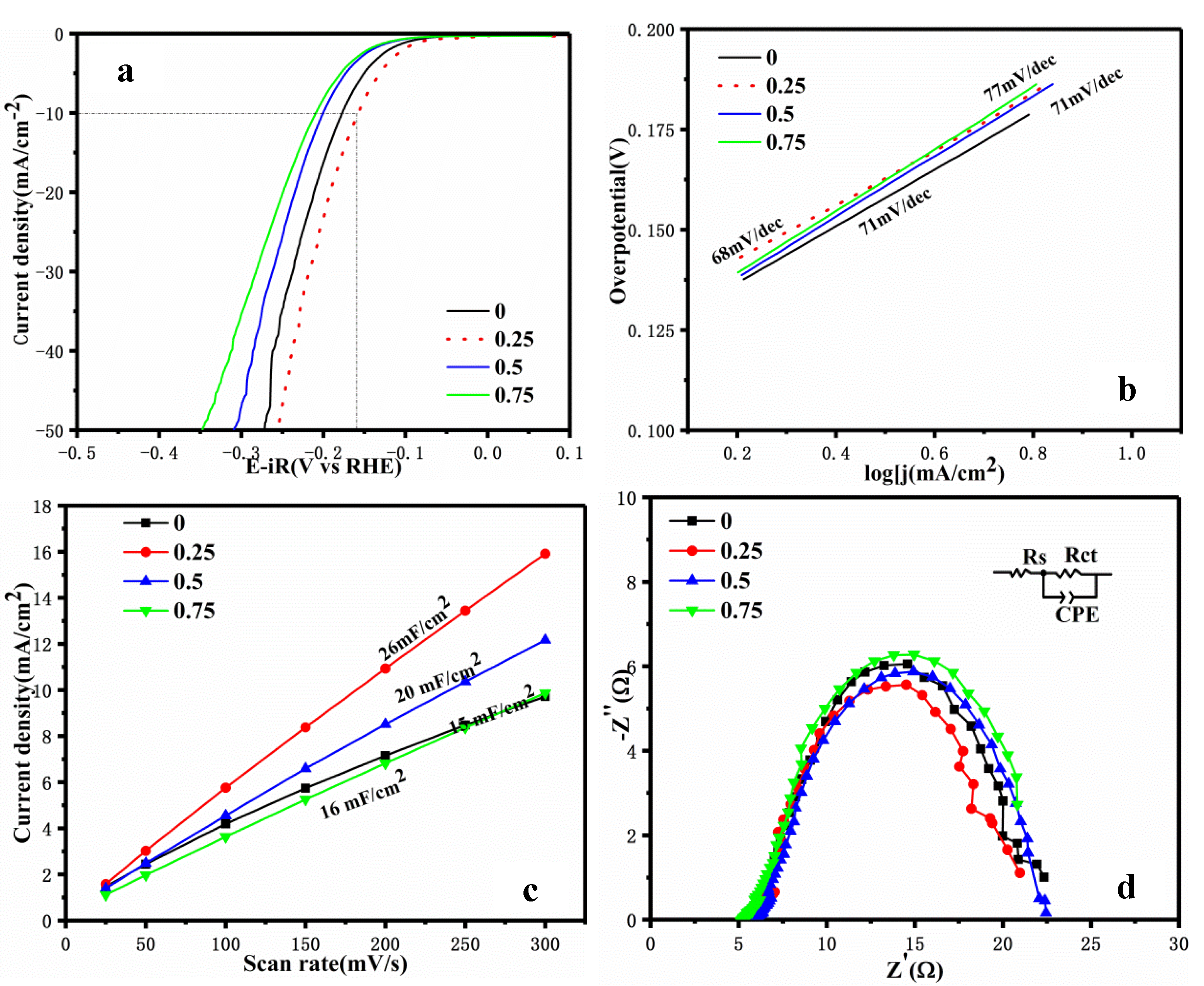
- N-S doping improves the HER activity of WC/C@N-S catalysts by changing the electronic structure of WC and producing lattice defects, promoting electron transfer and increasing the specific surface area. The hydrogen evolution performance of the N-S-doped WC/C@N-S catalyst was evaluated in a 0.5 mol/L H2SO4 solution. The results showed that when the cathodic current density reached 10 mA/cm2, the overpotential was 158 mV and the Tafel slope was 68 mV/dec, indicating good electrochemical activity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laimon, M.; Yusaf, T. Towards energy freedom: Exploring sustainable solutions for energy independence and self-sufficiency using integrated renewable energy-driven hydrogen system. Renew. Energy 2024, 222, 119948. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Li, J.K.; Wang, Y.X.; Lei, L.F.; Zhuang, L.Z. Controlled Aggregation of Cobalt and Platinum Atoms via Plasma Treatment for Exceptional Hydrogen Evolution Reaction Activity. Coatings 2024, 14, 1569. [Google Scholar] [CrossRef]
- Kannaiyan, K.; Lekshmi, G.S.; Ramakrishna, S.; Kang, M.; Kumaravel, V. Perspectives for the green hydrogen energy-based economy. Energy 2023, 284, 129358. [Google Scholar] [CrossRef]
- Murugesan, R.; Yesupatham, M.S.; Agamendran, N.; Sekar, K.; Neethinathan, C.S.S.; Maruthapillai, A. Recent advances in biomass-derived carbon-based nanostructures for electrocatalytic reduction reactions: Properties-performance correlation. Energy Technol. 2024, 12, 882. [Google Scholar] [CrossRef]
- Sun, J.M.; Wu, Z.W.; Ma, C.H.; Xu, M.C.; Luo, S.; Li, W.; Liu, S.X. Biomass-derived tubular carbon materials: Progress in synthesis and applications. J. Mater. Chem. A 2021, 9, 13822–13850. [Google Scholar] [CrossRef]
- Li, L.; Lu, S.; Fang, L.; Wei, Y.J.; Yang, S.L. Review on Biomass-derived carbon-based materials for electrocatalytic hydrogen production: State of the art and outlook. Energy Fuels 2023, 37, 18485–18501. [Google Scholar] [CrossRef]
- Alorku, K.D.; Shen, C.; Li, Y.H.; Xu, Y.; Wang, C.G.; Liu, Q.Y. Biomass-derived 2-methyltetrahydrofuran platform: A focus on precious and non-precious metal-based catalysts for the biorefinery. Green Chem. 2022, 24, 4210–4236. [Google Scholar] [CrossRef]
- Zhang, M.M.; Li, P.F.; Wu, D.P. Synthesis and application of biomass-derived carbon-based nanomaterial. Nanomaterials 2023, 13, 2020. [Google Scholar] [CrossRef]
- Yan, P.P.; Wu, Y.C.; Wei, X.F.; Zhu, X.W.; Su, W. Preparation of robust hydrogen evolution reaction electrocatalyst WC/C by molten salt. Nanomaterials 2020, 10, 1621. [Google Scholar] [CrossRef]
- Xiao, J.J.; Chen, Q.F.; Yu, R.; Qian, J.Y.; Liu, Z.H.; Li, T.; Yang, H.P.; Shao, J.A.; Yang, W.R.; Chen, H.P. Enhanced stability of tungsten carbide catalysts via superhydrophobic modification for one-pot conversion of biomass-derived fructose involving water and iodine. Appl. Surf. Sci. 2021, 565, 150523. [Google Scholar] [CrossRef]
- Jiang, W.Y.; Gao, Z.H.; Shen, M.; Zhou, J.; Tang, R.; Zhang, L.J.; Wang, J.Q. Molten salt N-modified Mo2CTx as a non-precious metal catalyst for efficient hydrogen evolution reaction. Int. J. Hydrogen Energy 2024, 57, 1–7. [Google Scholar] [CrossRef]
- Eunsu, J.; Cho, J.W.; Kim, J.; Kim, J. WC nanoparticles and NiFe alloy co-encapsulated in N-doped carbon nanocage for exceptional OER and ORR bifunctional electrocatalysis. Appl. Surf. Sci. 2024, 663, 160201–160210. [Google Scholar] [CrossRef]
- Qu, K.G.; Chen, Z.F.; Wang, L.H.; Li, H.B.; Zeng, S.Y.; Li, R.; Meng, L.J.; Chen, H.Y.; Yao, Q.X. Covalent organic framework assisted low-content ultrafine Ru on porous N-doped carbon for efficient hydrogen evolution reaction. Rare Met. 2025, 44, 2094–2102. [Google Scholar] [CrossRef]
- Li, G.X.; Yu, J.Y.; Yu, W.Q.; Yang, L.J.; Zhang, X.L.; Liu, X.Y.; Liu, H.; Zhou, W.J. Phosphorus-doped iron nitride nanoparticles encapsulated by nitrogen-doped carbon nanosheets on iron foam in situ derived from saccharomycetes cerevisiae for electrocatalytic overall water splitting. Small 2020, 16, 2001980. [Google Scholar] [CrossRef]
- Jiang, Q.C.; Li, J.; Gao, J.Y.; Zhu, W.J.; Liu, H.H.; Yang, Y.J.; Ren, Y.J.; Lv, Y.R.; Wang, L.; He, Z.X. Marine biomass–derived nitrogen-doped carbon microsphere electrocatalyst for vanadium redox flow battery. Ionics 2023, 29, 259–269. [Google Scholar] [CrossRef]
- Wang, Y.H.; Li, R.Q.; Li, H.B.; Huang, H.L.; Guo, Z.J.; Chen, H.Y.; Zheng, Y.; Qu, K.G. Controlled synthesis of ultrasmall RuP2 particles on N, P-codoped carbon as superior pH-wide electrocatalyst for hydrogen evolution. Rare Met. 2021, 40, 1040–1047. [Google Scholar] [CrossRef]
- Zhao, G.Y.; Yu, D.F.; Zhang, H.; Sun, F.F.; Li, J.W.; Zhu, L.; Sun, L.; Yu, M.; Besenbacher, F.; Sun, Y. Sulphur-doped carbon nanosheets derived from biomass as high-performance anode materials for sodium-ion batteries. Nano Energy 2020, 67, 104219. [Google Scholar] [CrossRef]
- Levinas, R.; Tsyntsaru, N.; Cesiulis, H.; Viter, R.; Grundsteins, K.; Tamasauskaite-Tamasiunaite, L.; Norkus, E. Electrochemical Synthesis of a WO3/MoSx Heterostructured Bifunctional Catalyst for Efficient Overall Water Splitting. Coatings 2023, 13, 673. [Google Scholar] [CrossRef]
- Prasert, K.; Sanglaow, T.; Liangruksa, M.; Sutthibutpong, T. Effects of stacking layers and different doping elements on the electronic structures and quantum capacitance of graphene: A DFT study. J. Phys. Chem. Solids 2024, 185, 111758. [Google Scholar] [CrossRef]
- Wu, H.Y.; Zhao, Q.Q.; Jiang, S.; Liu, W.; Xiao, H.N.; Wu, W.B. Research advances in doped carbon electrocatalysts derived from biomass. Chem. Eng. J. 2025, 505, 159694. [Google Scholar] [CrossRef]
- Su, W.; Yan, P.P.; Wei, X.F.; Zhu, X.W.; Zhou, Q.Y. Facile one-step synthesis of nitrogen-doped carbon sheets supported tungsten carbide nanoparticles electrocatalyst for hydrogen evolution reaction. Int. J. Hydrogen Energy 2020, 45, 33430–33439. [Google Scholar] [CrossRef]
- Zhang, B.; Luo, C.; Deng, Y.Q.; Huang, Z.J.; Zhou, G.M.; Lv, W.; He, Y.B.; Wan, Y.; Kang, F.Y.; Yang, Q.H. Optimized catalytic WS2-WO3 heterostructure design for accelerated polysulfide conversion in lithium-sulfur batteries. Adv. Energy Mater. 2020, 10, 2000091. [Google Scholar] [CrossRef]
- Hussain, S.; Akbar, K.; Vikraman, D.; Afzal, R.A.; Song, W.; An, K.S.; Farooq, A.; Park, J.Y.; Chun, S.H.; Jung, J. WS(1-x)Sex nanoparticles decorated three-dimensional graphene on nickel foam: A robust and highly efficient electrocatalyst for the hydrogen evolution reaction. Nanomaterials 2018, 8, 929. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Chae, J.; Akbar, K.; Vikraman, D.; Linh, L.; Naqvi, B.A.; Abbas, Y.; Kim, H.S.; Chun, S.H.; Kim, G.; et al. Fabrication of robust hydrogen evolution reaction electrocatalyst using Ag2Se by vacuum evaporation. Nanomaterials 2019, 9, 1460. [Google Scholar] [CrossRef]
- Zeng, M.Q.; Luo, R.; Deng, X.Y.; Song, Y.L.; Hao, W.J.; Fan, J.C.; Bi, Q.Y.; Li, G.S. Molten-salt-chemistry-assisted synthesis of layered N-doped tungsten carbide with enhanced electrical conductivity for efffcient electrocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2024, 92, 1021–1029. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Do, H.H.; Tekalgne, M.; Le, Q.V.; Nguyen, T.P.; Hong, S.H.; Cho, J.H.; Dao, D.V.; Ahn, S.H.; Kim, S.Y. WS2-WC-WO3 nano-hollow spheres as an efficient and durable catalyst for hydrogen evolution reaction. Nano Converg. 2021, 8, 28. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Nguyen, T.P.; Le, Q.V.; Dao, D.V.; Ahn, S.H.; Kim, S.Y. Facile route for synthesizing WS2/W2C nano hollow flowers and their application for hydrogen evolution reaction. J. Alloys Compd. 2023, 955, 170231. [Google Scholar] [CrossRef]
- Wang, Y.N.; Zhang, L.P.; Meng, X.X.; Feng, L.; Wang, T.; Zhang, W.M.; Yang, N.T. Scalable processing hollow tungsten carbide spherical superstructure as an enhanced electrocatalyst for hydrogen evolution reaction over a wide pH range. Electrochim. Acta 2019, 319, 775–782. [Google Scholar] [CrossRef]
- Wei, X.D.; Li, N. Tungsten carbide/carbon composites coated with little platinum nano particles derived from the redox reaction between in-situ synthesized WC1-x and chloroplatinic acid as the electrocatalyst for hydrogen evolution reaction. Appl. Surf. Sci. 2019, 463, 1154–1160. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, L.S.; Zhao, P.X.; Lee, L.Y.S.; Wong, K.Y. Recent advances in electrocatalytic hydrogen evolution using nanoparticles. Chem. Rev. 2020, 120, 851–918. [Google Scholar] [CrossRef]
- Lin, L.X.; Sherrell, P.; Liu, Y.Q.; Lei, W.; Zhang, S.W.; Zhang, H.J.; Wallace, G.G.; Chen, J. Engineered 2D transition metal dichalcogenides—A vision of viable hydrogen evolution reaction catalysis. Adv. Energy Mater. 2020, 10, 1903870. [Google Scholar] [CrossRef]
- Liu, Y.P.; Yu, G.T.; Li, G.D.; Sun, Y.H.; Asefa, T.; Chen, W.; Zou, X.X. Coupling Mo2C with nitrogen-rich nanocarbon leads to efficient hydrogen-evolution electrocatalytic sites. Angew. Chem. Int. Ed. 2015, 127, 10752–10757. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.T.; Xiao, X.F.; Ye, Z.M.; Zhao, S.L.; Shen, R.A.; He, C.T.; Zhang, J.P.; Li, Y.D.; Chen, X.M. Cage-confinement pyrolysis route to ultrasmall tungsten carbide nanoparticles for efficient electrocatalytic hydrogen evolution. J. Am. Chem. Soc. 2017, 139, 5285–5288. [Google Scholar] [CrossRef]
- Qu, K.G.; Zheng, Y.; Da, S.; Qiao, S.Z. Graphene oxide-polydopamine derived N, S-codoped carbon nanosheets as superior bifunctional electrocatalysts for oxygen reduction and evolution. Nano Energy 2016, 19, 373–381. [Google Scholar] [CrossRef]
- Ito, Y.; Cong, W.T.; Fujita, T.; Tang, Z.; Chen, M.W. High Catalytic Activity of Nitrogen and Sulfur Co-Doped Nanoporous Graphene in the Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2015, 54, 2131–2136. [Google Scholar] [CrossRef]
- Deng, J.; Ren, P.J.; Deng, D.H.; Bao, X.H. Enhanced electron penetration through an ultrathin graphene layer for highly efficien catalysis of the hydrogen evolution reaction. Angew. Chem. Int. Ed. 2015, 54, 2100–2104. [Google Scholar] [CrossRef]
- Liu, Y.P.; Li, G.D.; Yuan, L.; Ge, L.; Ding, H.; Wang, D.J.; Zou, X.X. Carbon-protected bimetallic carbide nanoparticles for a highly efficient alkaline hydrogen evolution reaction. Nanoscale 2015, 7, 3130–3136. [Google Scholar] [CrossRef]
- Zou, X.X.; Huang, X.X.; Goswami, A.; Silva, R.; Sathe, B.R.; Mikmeková, E.; Asefa, T. Cobalt-embedded nitrogen-rich carbon nanotubes efficiently catalyze hydrogen evolution reaction at all pH values. Angew. Chem. Int. Ed. 2014, 53, 4372–4376. [Google Scholar] [CrossRef]
- Wang, S.P.; Wang, J.; Zhu, M.L.; Bao, X.B.; Xiao, B.Y.; Su, D.F.; Li, H.R.; Wang, Y. Molybdenum carbide modified nitrogen-doped carbon vesicle encapsulating nickel nano-particles: A highly efficient, low-cost catalyst for hydrogen evolution reaction. J. Am. Chem. Soc. 2015, 137, 15753–15759. [Google Scholar] [CrossRef]


| Reagent Name | Chemical Formula and Specifications | Manufacturer |
|---|---|---|
| Amine metatungstate | (NH4)6H2W12O40·xH2O/analytical purity | Sinopharm Chemical Reagent Co., Ltd., Shanghai, China |
| Pomelo peel powder | - | Self-prepared, Yangling, China |
| Dicyandiamide | C2H4N4/analytical purity | Sinopharm Chemical Reagent Co., Ltd., Shanghai, China |
| L-cysteine | C3H7NO2S/analytical purity | Sinopharm Chemical Reagent Co., Ltd., Shanghai, China |
| Salt | NaCl/KCl/analytical purity | Sinopharm Chemical Reagent Co., Ltd., Shanghai, China |
| Naphthol | C10H8O/analytical purity | Sigma-Aldrich Reagents Ltd., Shanghai, China |
| Acid/base | H2SO4/KOH/analytical purity | Sinopharm Chemical Reagent Co., Ltd., Shanghai, China |
| Anhydrous ethanol | C2H5OH/analytical purity | Sinopharm Chemical Reagent Co., Ltd., Shanghai, China |
| Deionized water | H2O | Self-prepared, Yangling, China |
| Sample Number | Pomelo Peel Powder Content | Cysteine Content | Dicyandiamide Content |
|---|---|---|---|
| 1 | 0.5 g | 0 g | 2.25 g |
| 2 | 0.75 g | 0 g | 2.25 g |
| 3 | 1 g | 0 g | 2.25 g |
| 4 | 1 g | 0.25 g | 2.25 g |
| 5 | 1 g | 0.5 g | 2.25 g |
| 6 | 1 g | 0.75 g | 2.25 g |
| 7 | 1.25 g | 0 g | 2.25 g |
| 8 | 1.5 g | 0 g | 2.25 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Wang, B.; Li, S.; Pan, F.; Zhu, X.; Wei, X. N-S Co-Doped WC Nanoparticles Show High Catalytic Activity in Hydrogen Evolution Reaction. Coatings 2025, 15, 630. https://doi.org/10.3390/coatings15060630
Lu Z, Wang B, Li S, Pan F, Zhu X, Wei X. N-S Co-Doped WC Nanoparticles Show High Catalytic Activity in Hydrogen Evolution Reaction. Coatings. 2025; 15(6):630. https://doi.org/10.3390/coatings15060630
Chicago/Turabian StyleLu, Zhaobin, Baoxin Wang, Shengtao Li, Feiyan Pan, Xuewei Zhu, and Xiaofeng Wei. 2025. "N-S Co-Doped WC Nanoparticles Show High Catalytic Activity in Hydrogen Evolution Reaction" Coatings 15, no. 6: 630. https://doi.org/10.3390/coatings15060630
APA StyleLu, Z., Wang, B., Li, S., Pan, F., Zhu, X., & Wei, X. (2025). N-S Co-Doped WC Nanoparticles Show High Catalytic Activity in Hydrogen Evolution Reaction. Coatings, 15(6), 630. https://doi.org/10.3390/coatings15060630






