Interfacial Modulation of Laser-Deposited Ti6Al4V-TiC Wear-Resistant Coatings: Surface Ni-P Metallization of TiC Particles
Abstract
1. Introduction
2. Experiment
2.1. Chemical Deposition Process
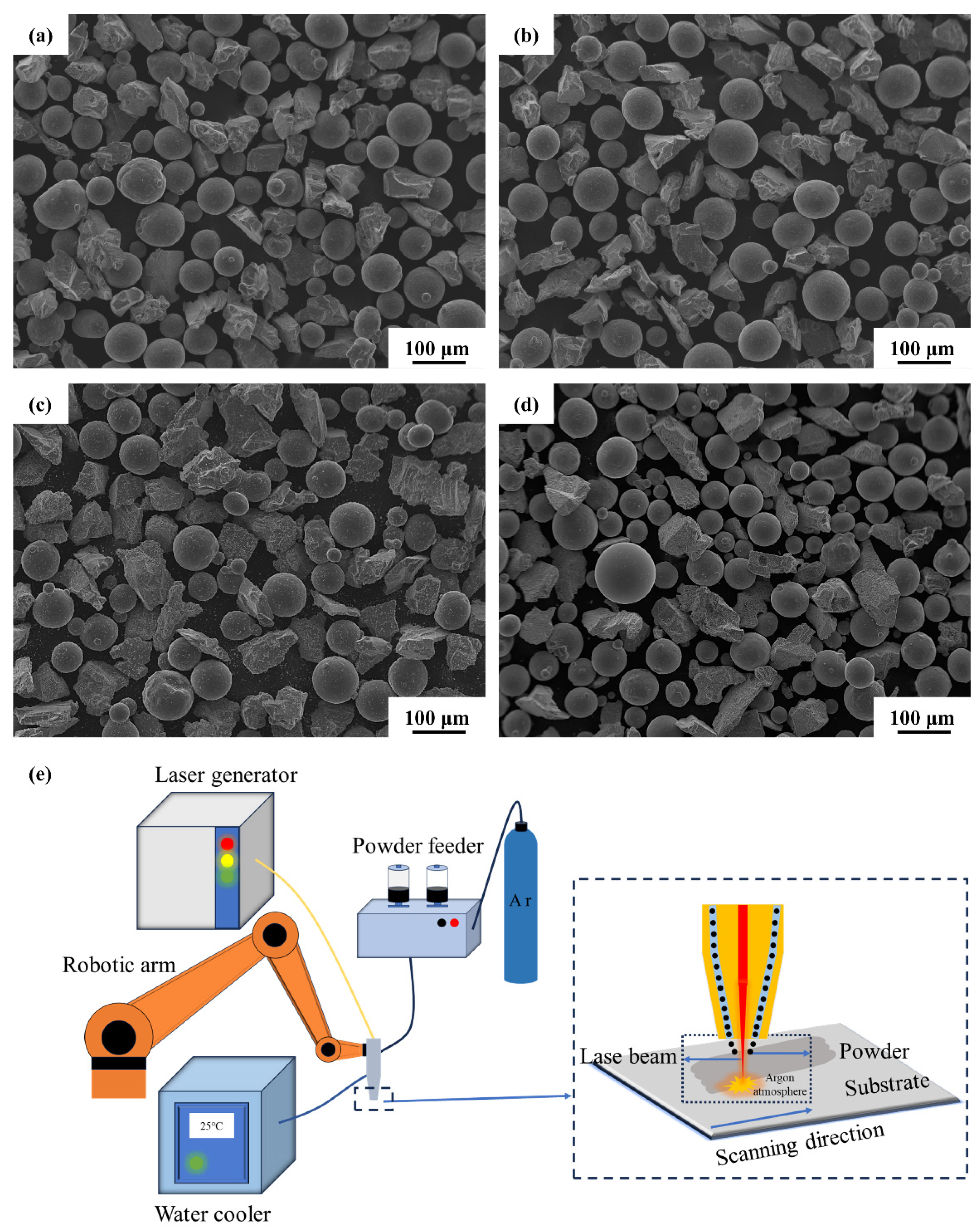
2.2. Laser Melting Deposition Process
2.3. Microstructural Characterization and Mechanical Properties
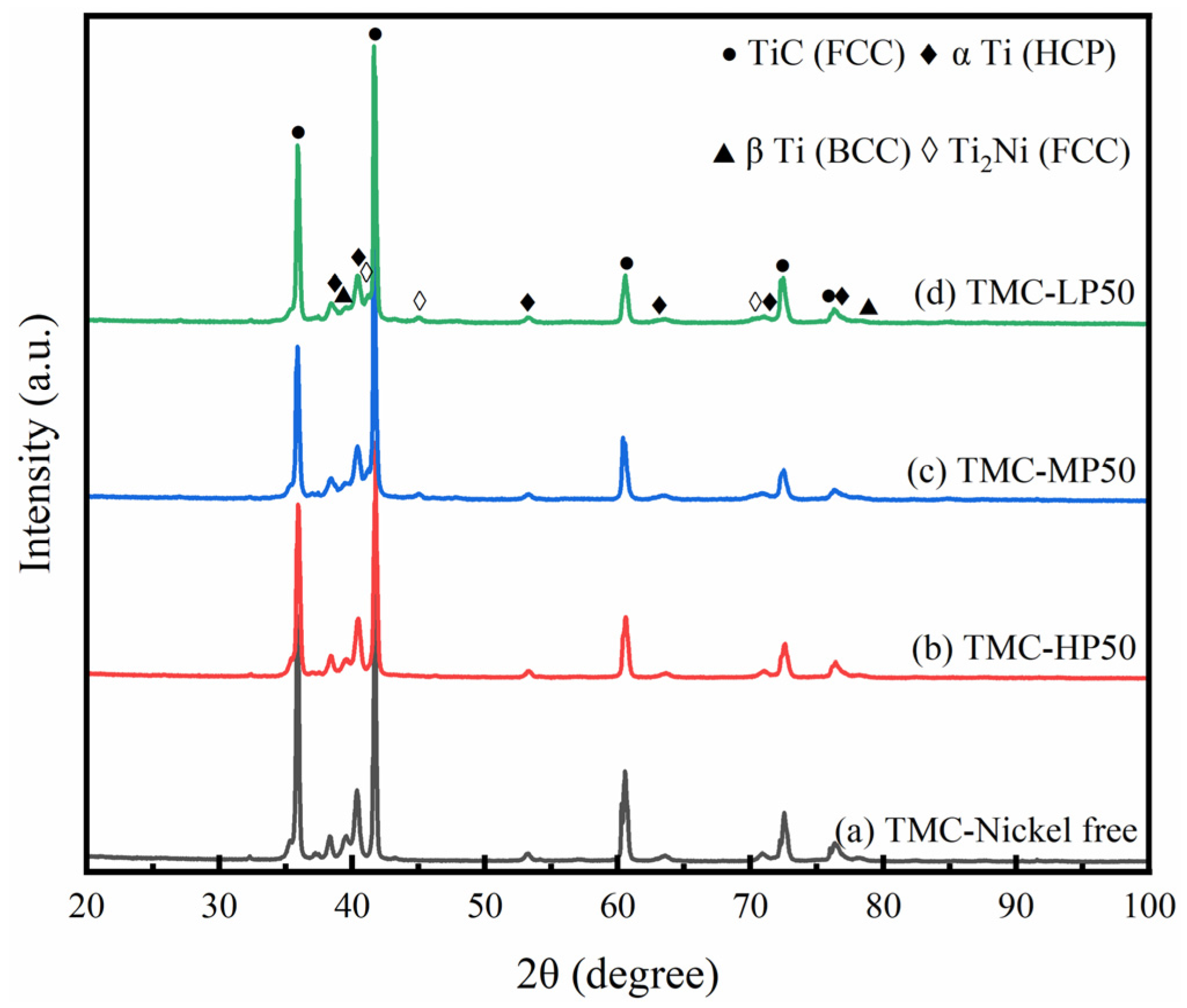
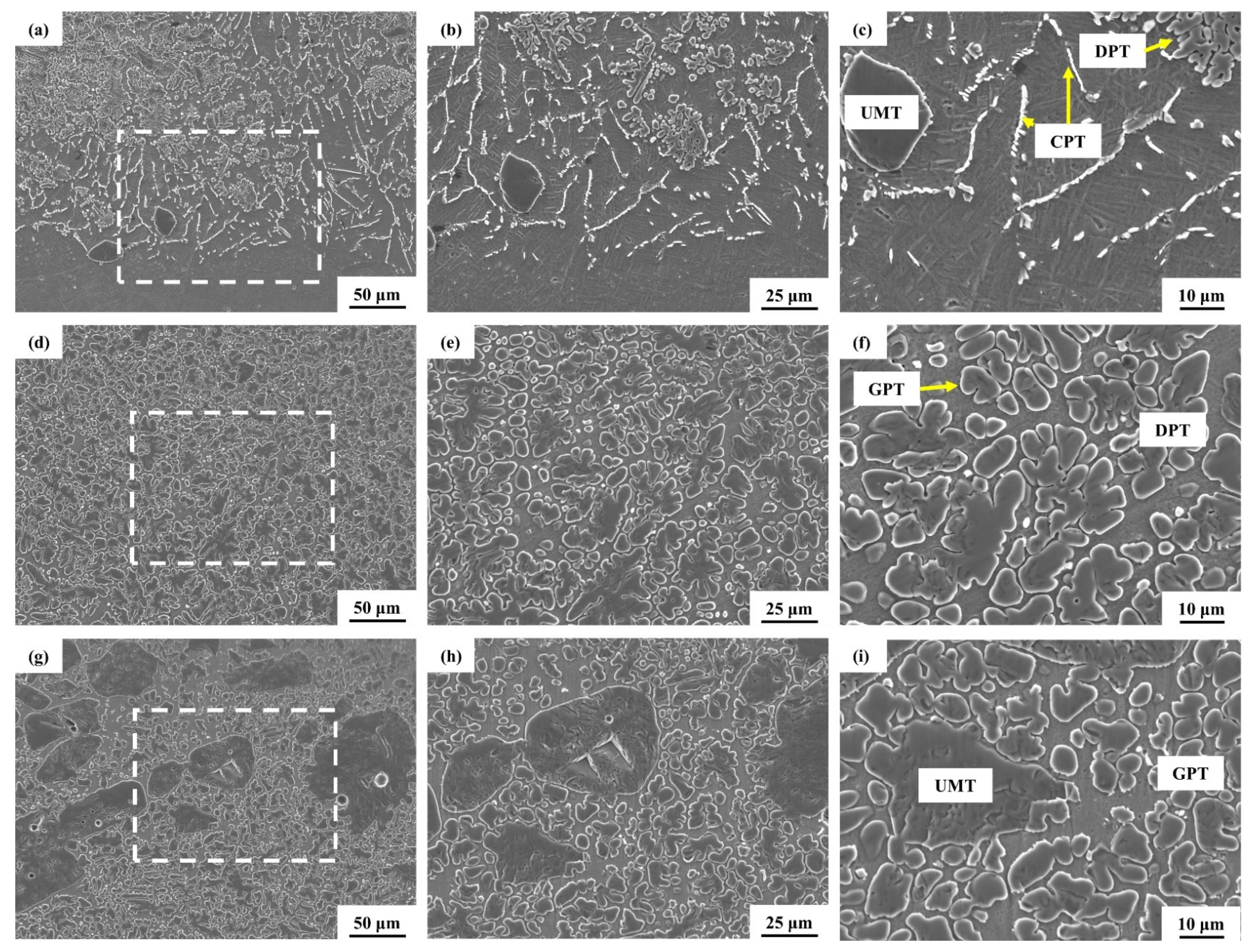
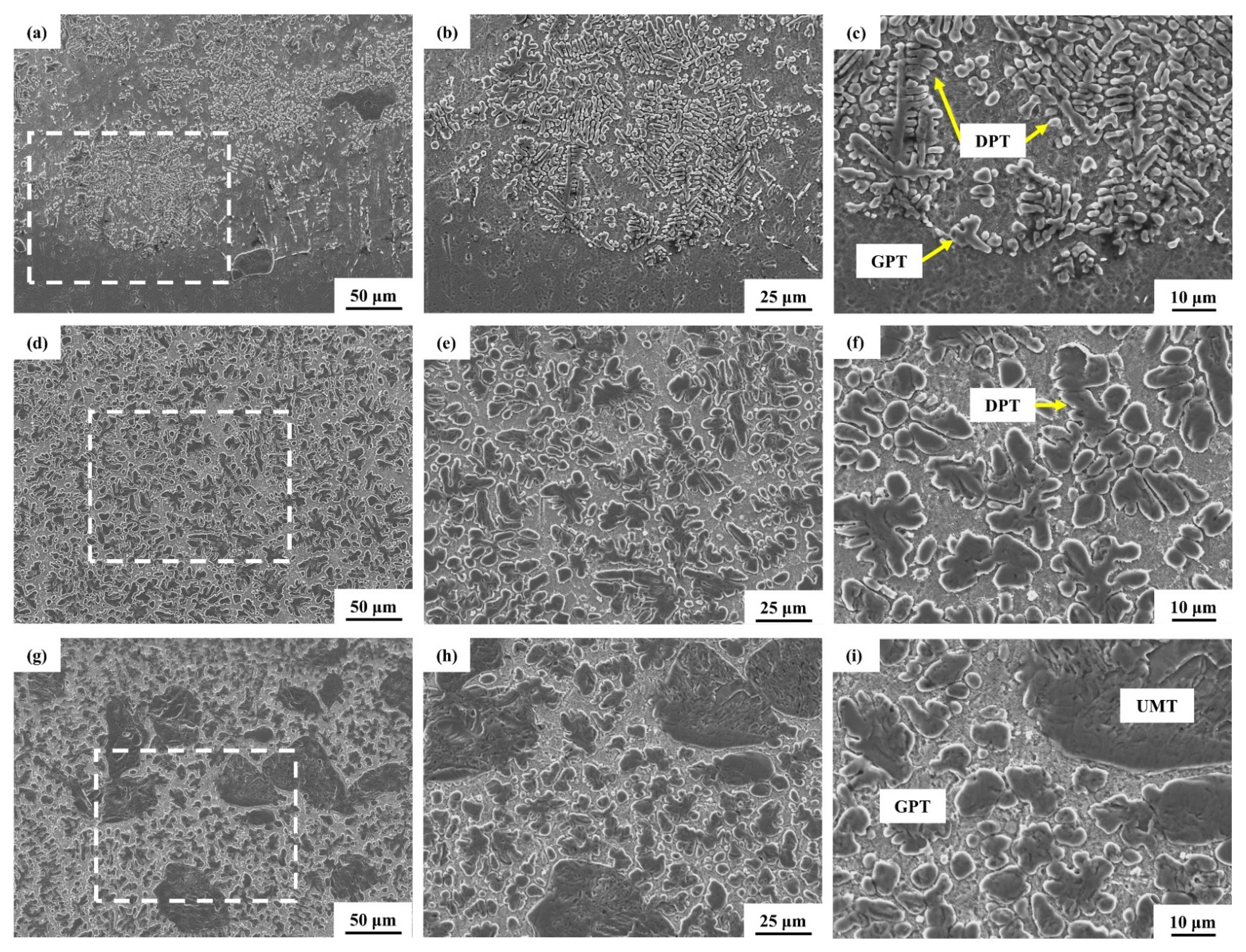
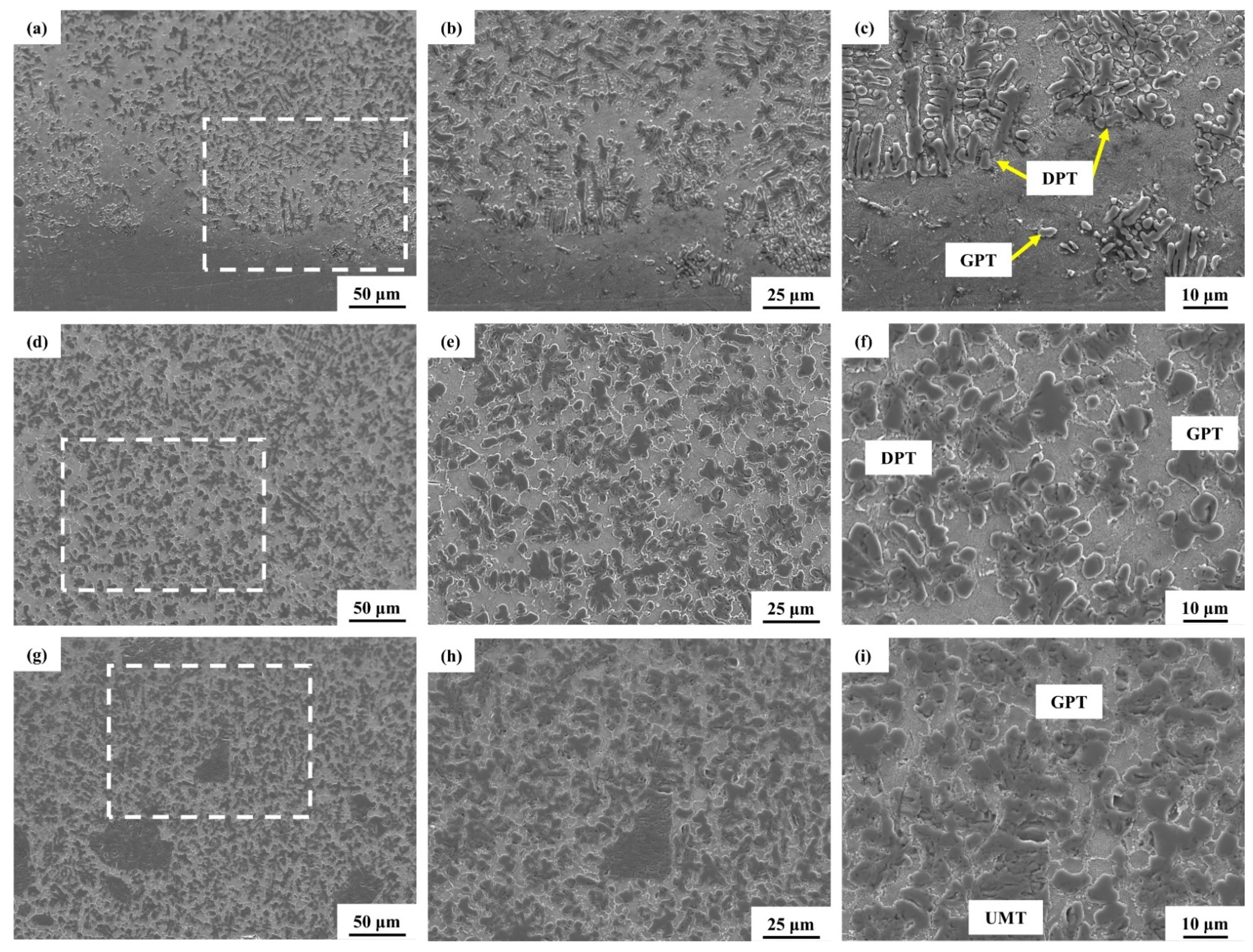
3. Results and Discussion
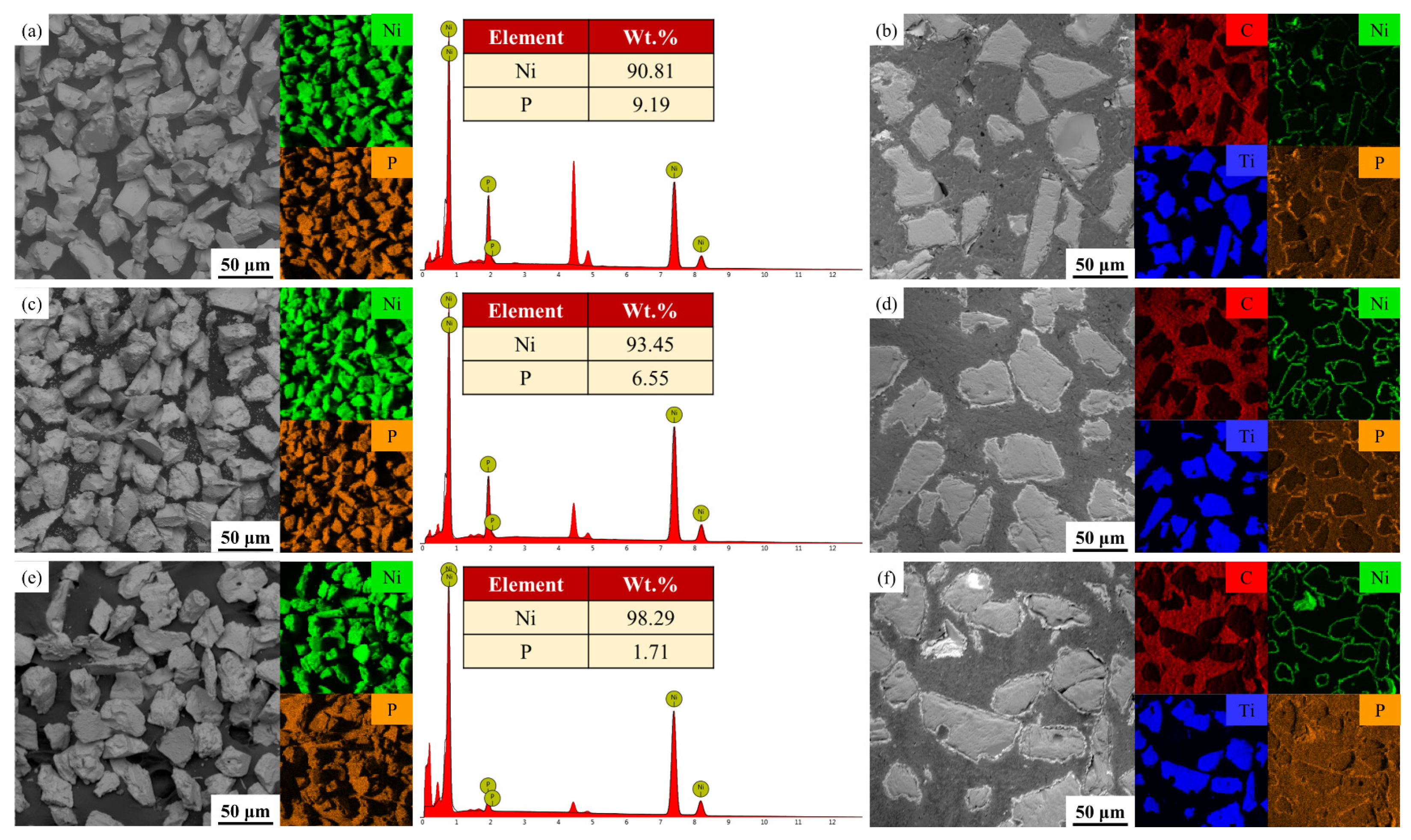
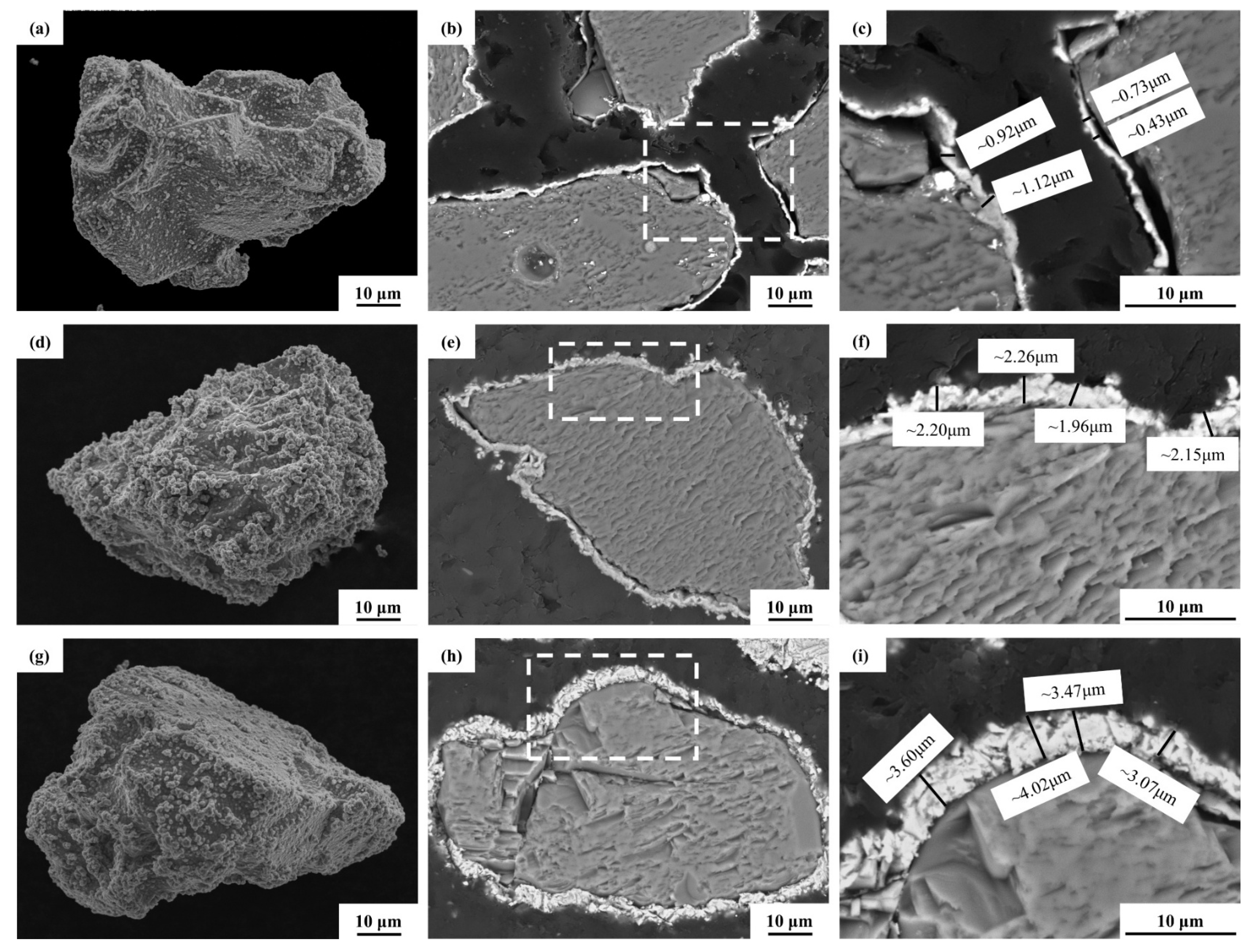
3.1. Metallization of TiC Ceramic Surface
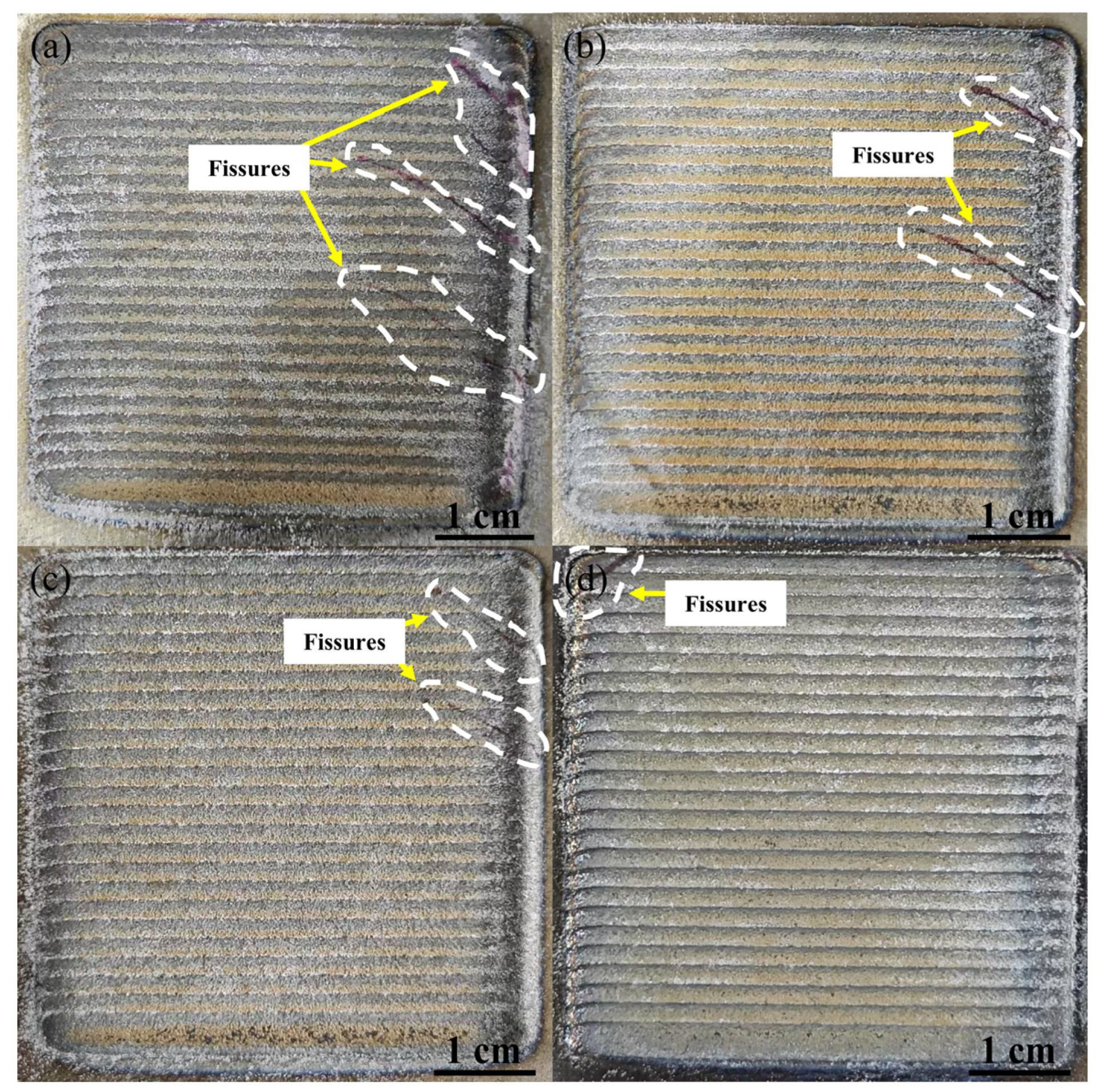
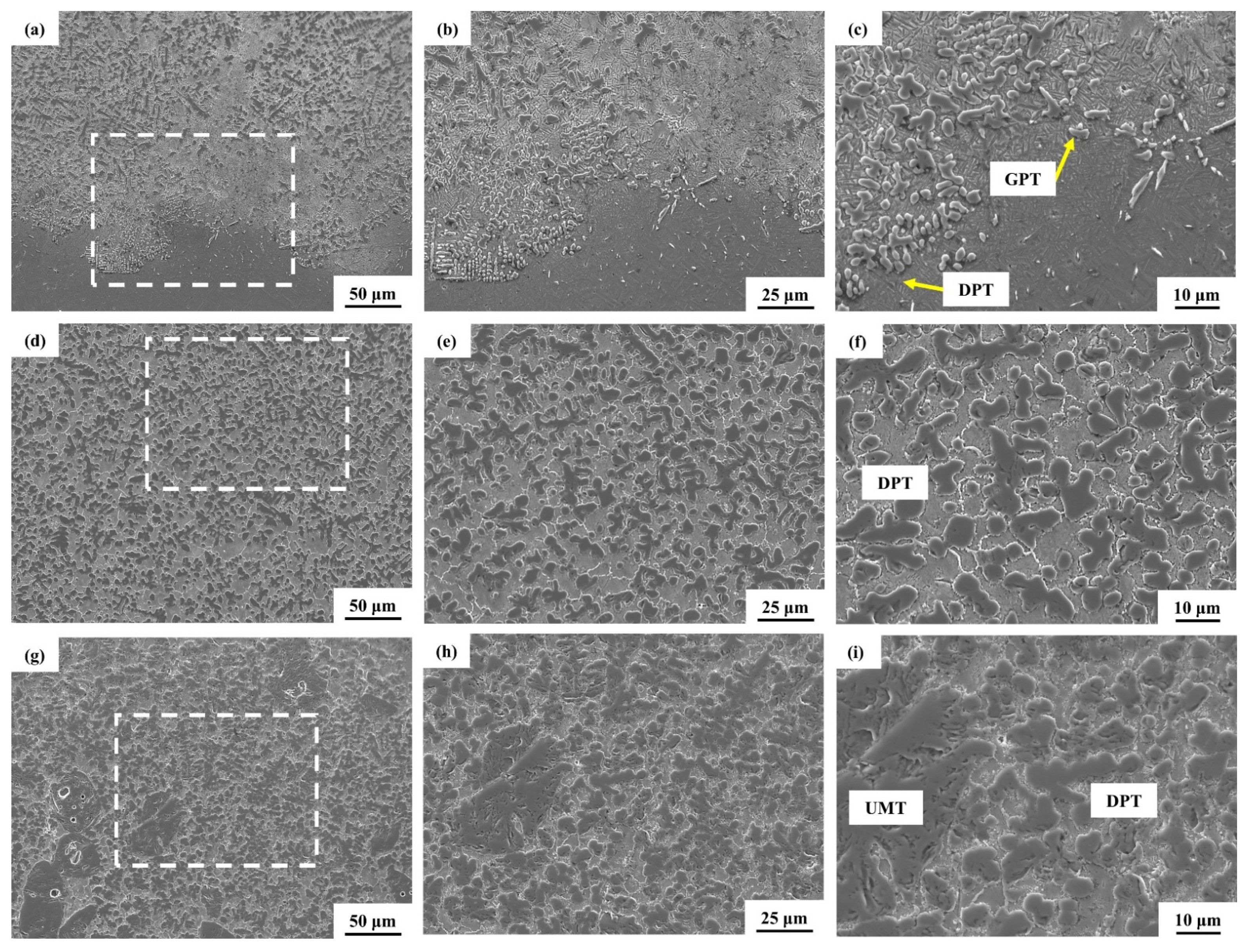
3.2. Laser Melting Deposition of TiC-Ti6Al4V
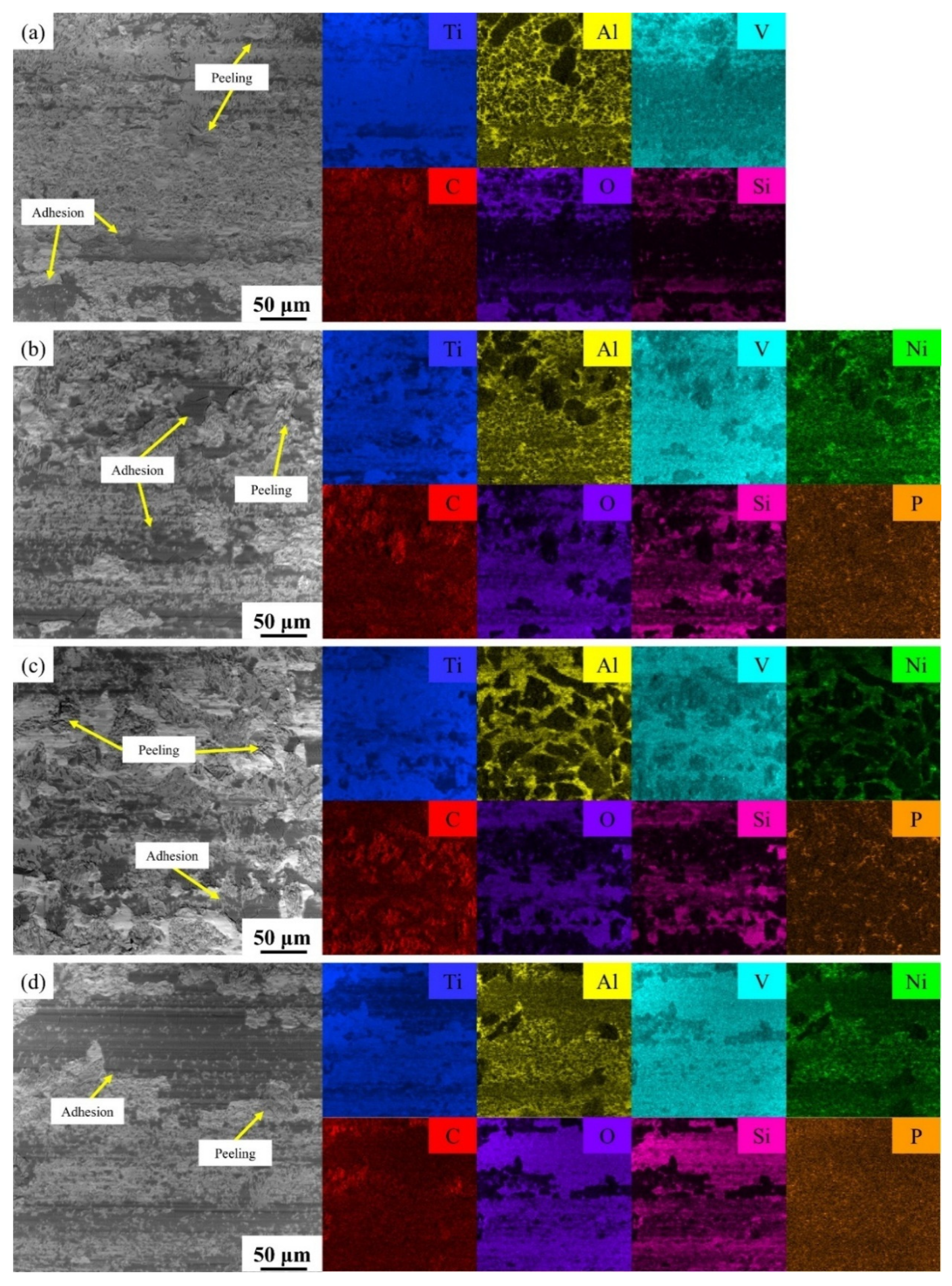
3.3. Hardness and Tribological Properties
4. Conclusions
- (1)
- Three metallized TiC samples with varying phosphorus concentrations were generated by altering chemical deposition formulations and processes. This resulted in surface plating thicknesses of 0.9 ± 0.2 µm, 2 ± 0.2 µm, and 3.5 ± 0.5 µm, respectively. The properties of the plating were significantly influenced by the phosphorus content. The surface plating of LP metallized ceramics was the thickest and exhibited the highest crystallinity, and it was characterized as microcrystalline;
- (2)
- Using untreated TiC and three metallized TiC variants combined with Ti6Al4V can produce coatings with consistent, continuous, dense, and non-overburned surfaces. The results demonstrate that the use of metallized TiC as a reinforcement reduced the occurrence of cracks. Specifically, TMC-MP and TMC-LP exhibited fewer cracks, with TMC-LP only showing cracks in the initial stage of processing;
- (3)
- The microhardness distribution of the coatings ranged from 720 HV0.5 to 1130 HV0.5. TMC-MP and TMC-LP exhibited a higher concentration of nickel, which contributes to the formation of robust Ti2Ni intermetallic compounds within the coating, resulting in a somewhat elevated microhardness. The primary wear processes of the four coatings include abrasive wear, adhesive wear, and oxidative wear. The average friction coefficients and wear rates were as follows: TMC-LP < TMC-MP < TMC-HP < TMC-Nickel-free. Ni-P metallization on the TiC surface can improve its wettability with Ti6Al4V, thereby enhancing the wear resistance of composite coatings. LP metallization was the most effective.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Revankar, G.D.; Shetty, R.; Rao, S.S.; Gaitonde, V.N. Wear resistance enhancement of titanium alloy (Ti–6Al–4V) by ball burnishing process. J. Mater. Res. Technol. 2017, 6, 13–32. [Google Scholar] [CrossRef]
- Philip, J.T.; Mathew, J.; Kuriachen, B. Tribology of Ti6Al4V: A Review. Friction 2019, 7, 497–536. [Google Scholar] [CrossRef]
- Ren, Z.Y.; Hu, Y.L.; Tong, Y.G.; Cai, Z.H.; Liu, J.; Wang, H.D.; Liao, J.Z.; Xu, S.; Li, L.K. Wear-resistant Nb Mo Ta W Ti high entropy alloy coating prepared by laser melting deposition technology on TC4 titanium alloy. Tribol. Int. 2023, 182, 108366. [Google Scholar] [CrossRef]
- Zhang, G.; Tang, J.; Yang, K.; Wang, R.; Chen, Y.; Xiong, Y.; Wu, C.; Li, Z.; Wang, Y.; Lin, H. Important contributions of metal interfaces on their tribological performances: From influencing factors to wear mechanisms. Compos. Struct. 2024, 337, 118027. [Google Scholar] [CrossRef]
- He, X.; Song, R.G.; Kong, D.J. Effects of TiC on the microstructure and properties of TiC/TiAl composite coating prepared by laser melting deposition technology. Opt. Laser Technol. 2019, 112, 339–348. [Google Scholar] [CrossRef]
- Mahamood, R.M.; Akinlabi, E.T.; Shukla, M.; Pityana, S. Scanning velocity influence on microstructure, microhardness and wear resistance performance of laser deposited Ti6Al4V/TiC composite. Mater. Des. 2013, 50, 656–666. [Google Scholar] [CrossRef]
- Cui, W.; Li, Y.; Li, F.; Qi, X.; Sun, X.; Pan, Z.; Niu, J. Wear and corrosion properties of in-situ TiC–TiB2 modified Ni-based composite coatings with different B/C ratios prepared by laser melting deposition technology. Ceram. Int. 2024, 50, 2424–2435. [Google Scholar] [CrossRef]
- Cai, Q.; Li, G.; Wu, B.; Xu, S.; Wang, L.; Guo, Y. Effect of TiC content on microstructure and properties of TiC/Ni60 coatings on Ti6Al4V alloy deposited by laser melting deposition technology. Opt. Laser Technol. 2024, 168, 109854. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.K.; Yu, D.S.; Sun, D.Q.; Li, H.M. A review paper on effect of the welding process of ceramics and metals. J. Mater. Res. Technol. 2020, 9, 16214–16236. [Google Scholar] [CrossRef]
- Wu, L.; Meng, L.; Wang, Y.; Zhang, S.; Bai, W.; Ouyang, T.; Lv, M.; Zeng, X. Effects of laser surface modification on the adhesion strength and fracture mechanism of electroless-plated coatings. Surf. Coat. Technol. 2022, 429, 127927. [Google Scholar] [CrossRef]
- Salicio-Paz, A.; Dalmau, A.; Grande, H.; Iriarte, A.; Sort, J.; Pellicer, E.; Fornell, J.; García-Lecina, E. Impact of the multilayer approach on the tribocorrosion behaviour of nanocrystalline electroless nickel coatings obtained by different plating modes. Wear 2020, 456, 203384. [Google Scholar] [CrossRef]
- Shen, Y.; Guo, J.; Wang, L.; Han, H.; Ma, Y.; Xin, B.; Wang, Z. Acceleration Mechanism of Triethanolamine in Electroless Bath for Pure Cobalt Deposition. J. Electrochem. Soc. 2023, 170, 112503. [Google Scholar] [CrossRef]
- Wang, S.; Sun, Y.; Li, G. Study on cobalt coating on ZTA particles by electroless plating and impact-abrasive wear behavior of ZTAp reinforced iron matrix composite. Wear 2022, 510, 204489. [Google Scholar] [CrossRef]
- Sun, P.; Dong, Z.; Chen, Y.; Yan, H.; Luo, C.; Song, H.; Hu, Z. Characterization of Ni coating layer of Al2O3 particles and their wettability behavior in Al2O3@ Ni/Al-10Si composites. Appl. Surf. Sci. 2020, 526, 146660. [Google Scholar] [CrossRef]
- Li, C.; Goei, R.; Li, Y.; Shi, J.; Li, B.; Liu, F.; Li, Y.; Gao, Y.; Li, S.; Zhao, S.; et al. Fabrication and wear property of NiCo coated ZrO2–Al2O3 ceramic particles reinforced high manganese steel-based composites. Wear 2022, 492, 204235. [Google Scholar] [CrossRef]
- Lou, M.; Chen, L.; Xu, K.; Zhang, G.; Chang, K. Tribocorrosion of TiC-based composites incorporating Ni and Co binders in saline solutions. Int. J. Refract. Hard Met. 2024, 119, 106519. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Chen, C. Microstructure and properties of Ni-Ti based gradient laser melting deposition technology layer of Ti6Al4V alloy by laser powder bed fusion. Addit. Manuf. 2024, 79, 103906. [Google Scholar]
- Huang, Z.; Nguyen, T.; Zhou, Y.; Qi, G. A low temperature electroless nickel plating chemistry. Surf. Coat. Technol. 2019, 372, 160–165. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, T.; Shi, H.; Liu, Q.; Wang, T. Fabrication of flexible copper patterns by electroless plating with copper nanoparticles as seeds. Appl. Surf. Sci. 2021, 547, 149220. [Google Scholar] [CrossRef]
- Sha, W.; Wu, X.M.; Sarililah, W. Scanning electron microscopy study of microstructural evolution of electroless nickel–phosphorus deposits with heat treatment. Mater. Sci. Eng. B 2010, 168, 95–99. [Google Scholar] [CrossRef]
- Chow, Y.M.; Lau, W.M.; Karim, Z.S. Surface properties and solderability behaviour of nickel–phosphorus and nickel–boron deposited by electroless plating. Surf. Interface Anal. 2001, 31, 321–327. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, F.; Li, J.; Chen, Z. Comparison of interfacial reactions and isothermal aging of cone Ni-P and flat Ni-P with Sn3.5Ag solders. Appl. Surf. Sci. 2023, 625, 157219. [Google Scholar] [CrossRef]
- Wu, M.; He, X.; Rafi-ud-Din; Ren, S.; Qin, M.; Qu, X. Effect of P and aging on microstructure and shear strength of Sn–2.5 Ag–2.0 Ni/Ni (P) solder joints. Mater. Chem. Phys. 2010, 121, 259–266. [Google Scholar] [CrossRef]
- Boakye, G.O.; Straume, E.O.; Kovalov, D.; Karlsdottir, S.N. Wear-reducing nickel-phosphorus and graphene oxide-based composite coatings: Microstructure and corrosion behavior in high temperature geothermal environment. Corros. Sci. 2022, 209, 110809. [Google Scholar] [CrossRef]
- He, P.; Huang, S.; Wang, H.; Huang, Z.; Hu, J.; Cheng, X.; Pan, C. Electroless nickel–phosphorus plating on silicon carbide particles for metal matrix composites. Ceram. Int. 2014, 40, 16653–16664. [Google Scholar] [CrossRef]
- Ye, Y.; Yang, J.; He, M.; Wang, Q.; Li, J.; Zhang, P.; Tu, X.; Li, W. Microstructural and wear resistance evolution of Alx (TiVZr) 100− x lightweight high-entropy alloys. Tribol. Int. 2024, 195, 109585. [Google Scholar] [CrossRef]
- Cui, G.; Li, N.; Li, D.; Chi, M. Study of optimized complexing agent for low-phosphorus electroless nickel plating bath. J. Electrochem. Soc. 2005, 152, C669. [Google Scholar] [CrossRef]
- Hu, B.; Sun, R.; Yu, G.; Liu, L.; Xie, Z.; He, X.; Zhang, X. Effect of bath pH and stabilizer on electroless nickel plating of magnesium alloys. Surf. Coat. Technol. 2013, 228, 84–91. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, T.; Zhang, R. Electroless deposition of NiMoP coating on Q235B steel and its Corrosion Resistance in Simulated Concrete Pore Solution. Int. J. Electrochem. Sci. 2023, 18, 100313. [Google Scholar]
- Shartal, K.M.; Kipouros, G.J. Electroless nickel phosphorus plating on AZ31. Met. Mater. Trans. B 2009, 40, 208–222. [Google Scholar] [CrossRef]
- Cui, C.; Du, H.; Liu, H.; Xiong, T. Corrosion behavior of the electroless Ni-P coating on the pore walls of the lotus-type porous copper. Corros. Sci. 2020, 162, 108202. [Google Scholar] [CrossRef]
- Yu, Q.; Zhou, T.; He, Y.; Liu, P.; Wang, X.; Jiang, Y.; Yan, J. Annealed high-phosphorus electroless Ni–P coatings for producing molds for precision glass molding. Mater. Chem. Phys. 2021, 262, 124297. [Google Scholar] [CrossRef]
- Tian, M.; Jian, Z.; Hai, R. Preparation and properties of thick nickel-phosphorus amorphous plating on SiCp/Al composite by double zincate pretreatment. J. Alloys Compd. 2022, 909, 164806. [Google Scholar] [CrossRef]
- Lelevic, A.; Walsh, F.C. Electrodeposition of NiP alloy coatings: A review. Surf. Coat. Technol. 2019, 369, 198–220. [Google Scholar] [CrossRef]
- Martyak, N.M.; Drake, K. Peak-profile analysis of electroless nickel coatings. J. Alloys Compd. 2000, 312, 30–40. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Liu, X.B.; Zhuang, S.G.; Yang, X.H.; Wang, M.; Sun, C.F. Preparation and high temperature tribological properties of laser in-situ synthesized self-lubricating composite coatings containing metal sulfides on Ti6Al4V alloy. Appl. Surf. Sci. 2019, 481, 209–218. [Google Scholar] [CrossRef]
- Li, Q.; Cui, J.; Yang, Y.; Zhao, Y.; Yu, C.; Wang, Q.; Zhang, P. Enhanced surface wettability modification of Al2O3 for laser cladding ceramic-metal composite coatings. Mater. Today Commun. 2024, 40, 109746. [Google Scholar] [CrossRef]
- Garay, J.E.; Anselmi-Tamburini, U.; Munir, Z.A. Enhanced growth of intermetallic phases in the Ni–Ti system by current effects. Acta Mater. 2003, 51, 4487–4495. [Google Scholar] [CrossRef]
- Xiong, Z.; Pang, X.; Liu, S.; Li, Z.; Misra, R.D.K. Hierarchical refinement of nickel-microalloyed titanium during additive manufacturing. Scr. Mater. 2021, 195, 113727. [Google Scholar] [CrossRef]
- Chen, L.; Yu, T.; Guan, C.; Zhao, Y. Microstructure and properties of metal parts remanufactured by laser cladding TiC and TiB2 reinforced Fe-based coatings. Ceram. Int. 2022, 48, 14127–14140. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Zhao, K.; Qi, C.; Shi, B.; Zhang, Y.; Yuan, S.; Zhan, X. Thermal analysis and microstructure evolution of TiC/Ti6Al4V functionally graded material by direct energy deposition. Mater. Sci. Eng. A 2024, 893, 146136. [Google Scholar] [CrossRef]
- Zheng, Y.; Yan, X.; Qiao, G.; Tang, Y.; Geng, Y.; Shao, Z.; Bai, Q. Enhanced wear resistance of TiC/Ti6Al4V composites through changing TiC morphologies in laser direct energy deposition. Addit. Manuf. 2024, 84, 104134. [Google Scholar] [CrossRef]
- Tang, M.; Zhang, L.; Zhang, N. Microstructural evolution, mechanical and tribological properties of TiC/Ti6Al4V composites with unique microstructure prepared by SLM. Mater. Sci. Eng. A 2021, 814, 141187. [Google Scholar] [CrossRef]
- Liu, S.; Shin, Y.C. The influences of melting degree of TiC reinforcements on microstructure and mechanical properties of laser direct deposited Ti6Al4V-TiC composites. Mater. Des. 2017, 136, 185–195. [Google Scholar] [CrossRef]
- Ma, G.; Yu, C.; Tang, B.; Li, Y.; Niu, F.; Wu, D.; Bi, G.; Liu, S. High-mass-proportion TiCp/Ti6Al4V titanium matrix composites prepared by directed energy deposition. Addit. Manuf. 2020, 35, 101323. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Lin, P.; Liu, H. Microstructure and mechanical properties of functionally graded TiCp/Ti6Al4V composite fabricated by laser melting deposition. Ceram. Int. 2017, 43, 16638–16651. [Google Scholar] [CrossRef]
- Zhang, F.; Deng, Y.; Zhou, X.; Wang, G.; Wang, Y.; Wang, M.; Tan, H. Effect of C addition on microstructure and mechanical properties of laser micro-alloying Ti–Al–V–C titanium matrix composites. J. Mater. Res. Technol. 2022, 20, 147–156. [Google Scholar] [CrossRef]
- Su, Y.Y.; Wang, Z.F.; Xie, J.C.; Xu, G.; Xing, F.; Luo, K.Y.; Lu, J.Z. Microstructures and mechanical properties of laser melting deposited Ti6Al4V/316L functional gradient materials. Mater. Sci. Eng. A 2021, 817, 141355. [Google Scholar] [CrossRef]
- Xi, L.; Ding, K.; Gu, D.; Guo, S.; Cao, M.; Zhuang, J.; Lin, K.; Okulov, I.; Sarac, B.; Eckert, J.; et al. Interfacial structure and wear properties of selective laser melted Ti/(TiC+ TiN) composites with high content of reinforcements. J. Alloys Compd. 2021, 870, 159436. [Google Scholar] [CrossRef]
- Chai, J.; Zhu, Y.; Wang, Z.; Shen, T.; Liu, Y.; Niu, L.; Li, S.; Yao, C.; Cui, M.; Liu, C. Microstructure and mechanical properties of SPS sintered Al2O3–ZrO2 (3Y)–SiC ceramic composites. Mater. Sci. Eng. A 2020, 781, 139197. [Google Scholar] [CrossRef]
- Dong, K.; Lu, F.; Huang, W.; Zhu, L. Residual stress and fracture toughness of thick 8YSZ-Al2O3 composite coatings via a modified Vickers indentation method. Vacuum 2020, 177, 109437. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, Y.; Cheng, W.Y.; Gu, J.; Chen, G. Microstructure and tribological behavior of CoCrFeNiMo0. 2/SiC high-entropy alloy gradient composite coating prepared by laser cladding. Surf. Coat. Technol. 2023, 467, 129681. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, J.; Chen, Y.; Hou, Z.; Zhao, Q.; Li, Y.; Zhu, L.; Zhang, F.; Zhao, Y. On enhancing wear resistance of titanium alloys by laser cladded WC-Co composite coatings. Int. J. Refract. Met. Hard Mat. 2022, 107, 105902. [Google Scholar] [CrossRef]
- Wang, P.; Liang, H.; Jiang, L.; Qian, L. Effect of nanoscale surface roughness on sliding friction and wear in mixed lubrication. Wear 2023, 530, 204995. [Google Scholar] [CrossRef]
- Zhang, B.; Ma, X.; Liu, L.; Wang, Y.; Yu, H.; Morina, A.; Lu, X. Reciprocating sliding friction behavior and wear state transition mechanism of cylinder liner and piston ring. Wear 2024, 546, 205293. [Google Scholar] [CrossRef]





| Constituents | Concentration | ||
|---|---|---|---|
| HP | MP | LP | |
| Nickel salt | 40 | 40 | 40 |
| Sodium hypophosphite (NaH2PO2·H2O) | 60 | 27 | 15 |
| Sodium citrate dihydrate (C6H5Na3O7·2H2O) | 48 | 60 | 50 |
| potassium sodium tartrate (NaKC4H4O6·4H2O) | 77 | / | / |
| Ammonium chloride NH4Cl | / | 32 | / |
| Disodium succinate C4H4Na2O4 | / | / | 74 |
| pH | 4.7 | 8 | 6.8 |
| Temperature and plating time | 65 °C and 2 h | 88 °C and 1 h | 85 °C and 1 h |
| Elements | H | C | Si | Fe | V | Al | Ti |
|---|---|---|---|---|---|---|---|
| wt.% | <0.0006 | <0.009 | ≤0.015 | ≤0.043 | 3.91 | 5.96 | Bal |
| Plating’s Parameters | Plating Type | ||
|---|---|---|---|
| HP | MP | LP | |
| Phosphorus content (wt.%) | 9.12 | 6.55 | 1.71 |
| Average thickness (µm) | 0.8 ± 0.25 | 2.1 ± 0.11 | 3.5 ± 0.34 |
| Ti, at. % | Al, at. % | V, at. % | C, at. % | Ni, at. % | P, at. % | |
|---|---|---|---|---|---|---|
| TMC-HP | 65.16 | 4.05 | 2.08 | 26.57 | 1.68 | 0.48 |
| TMC-MP | 65.11 | 3.61 | 1.87 | 25.67 | 3.02 | 0.72 |
| TMC-LP | 64.71 | 3.83 | 1.72 | 25.52 | 3.81 | 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Yang, Y.; Li, J.; Yu, C.; Du, X.; Zhao, H.; Chen, D.; Li, W.; Wang, Q.; Zhang, P. Interfacial Modulation of Laser-Deposited Ti6Al4V-TiC Wear-Resistant Coatings: Surface Ni-P Metallization of TiC Particles. Coatings 2025, 15, 629. https://doi.org/10.3390/coatings15060629
Wu Y, Yang Y, Li J, Yu C, Du X, Zhao H, Chen D, Li W, Wang Q, Zhang P. Interfacial Modulation of Laser-Deposited Ti6Al4V-TiC Wear-Resistant Coatings: Surface Ni-P Metallization of TiC Particles. Coatings. 2025; 15(6):629. https://doi.org/10.3390/coatings15060629
Chicago/Turabian StyleWu, Yiming, Yingfei Yang, Jie Li, Chuanyong Yu, Xinwei Du, Hu Zhao, Dexin Chen, Wei Li, Qiwei Wang, and Peng Zhang. 2025. "Interfacial Modulation of Laser-Deposited Ti6Al4V-TiC Wear-Resistant Coatings: Surface Ni-P Metallization of TiC Particles" Coatings 15, no. 6: 629. https://doi.org/10.3390/coatings15060629
APA StyleWu, Y., Yang, Y., Li, J., Yu, C., Du, X., Zhao, H., Chen, D., Li, W., Wang, Q., & Zhang, P. (2025). Interfacial Modulation of Laser-Deposited Ti6Al4V-TiC Wear-Resistant Coatings: Surface Ni-P Metallization of TiC Particles. Coatings, 15(6), 629. https://doi.org/10.3390/coatings15060629







